Abstract
Genetic rodent models of type 2 diabetes are routinely utilized in studies of diabetes-related cardiovascular disease; however, these models frequently exhibit abnormalities that are not consistent with diabetic complications. The aim of this study was to develop a model of type 2 diabetes that exhibits evidence of cardiovascular dysfunction commonly seen in patients with diabetes with minimal nondiabetes-related pathologies. Young male rats received either control (Con), high-fat (HF; 60%), or Western (Wes; 40% fat, 45% carbohydrate) diets for 2 wk after which streptozotocin (2 × 35 mg/kg ip 24 h apart) was administered to induce diabetes (Dia). Blood glucose levels were higher in Con + Dia and Wes + Dia groups compared with the HF + Dia group (25 ± 1, 25 ± 2, and 15 ± 1 mmol/l, respectively; P < 0.05) group. Liver, kidney, and pancreatic dysfunction and cardiomyocyte lipid accumulation were found in all diabetic animals. Despite lower heart rates in Con + Dia and HF + Dia groups, arterial and left ventricular pressures were not different between any of the experimental groups. All three diabetic groups had diastolic dysfunction, but only HF + Dia and Wes + Dia groups exhibited elevated diastolic wall stress, arterial stiffness (augmentation index), and systolic dysfunction (velocity of circumferential shortening, systolic wall stress). Surprisingly, we found that left ventricular dysfunction and arterial stiffness were more pronounced in the HF + Dia than the Con + Dia group and was similar to the Wes + Dia group despite significantly lower levels of hyperglycemia compared with either group. In conclusion, the HF + Dia group exhibited a stable, modest level of hyperglycemia, which was associated with cardiac dysfunction comparable with that seen in moderate to advanced stages of human type 2 diabetes.
Keywords: cardiac function, high-fat diet, Western diet
the prevalence of diabetes and impaired fasting glucose is reaching epidemic proportions in the United States and encompasses 35% of the adult population (12). This comprises 26% that exhibits impaired fasting glucose levels (i.e., prediabetic) and 9% diabetic; of the latter population, 90–95% suffers from type 2 diabetes (3). The increased incidence of cardiovascular disease is the single most important factor contributing to higher mortality rates associated with diabetes. Clinical and experimental data also suggest that, in addition to increased vascular disease, diabetes also leads to defects at the level of the myocardium, which may contribute to the increased incidence of heart failure observed in the diabetic population (46). Thus the need for an animal model that closely resembles the cardiac dysfunction seen in human type 2 diabetes is of increasing importance.
Several murine and rodent models of diabetes currently exist, each with associated advantages and disadvantages. The experimental model most commonly used to study the effect of diabetes on cardiac function is the streptozotocin (STZ)-induced model, in which the glucosamine-nitrosourea compound STZ is administered at a relatively high dose in mice and rats to induce an insulin-deficient phenotype that is characterized by frank, hyperglycemia analogous to that of uncontrolled type 1 diabetes. Although this model can be useful for studies of the effects of acute hyperglycemia, the severity of hyperglycemia and subsequent multiple organ damage, dehydration, and emaciation are not typical of what is observed in even poorly controlled human type 1 diabetes, and the relevance of this model in the context of type 2 diabetes is questionable.
Several models of type 2 diabetes are commercially available and include the db/db mouse and Zucker diabetic fatty (ZDF; fa/fa), Goto Kakizaki (GK), and Otsuka Long Evans Tokushima fatty rats. The number of reports of the impact of diabetes on cardiac function in these models is growing; however, the obesity, insulin resistance, and diabetes seen in these inbred models are typically the result of gene mutations, such as the leptin receptor (OB-r), not commonly found in humans. In addition, a number of limitations are present in these models that are not related to diabetes per se. For example, female ZDF rats are obese but do not develop diabetes, and the progression to diabetes in db/db mice and ZDF rats is due to a failure to increase β-cell mass, whereas human type 2 diabetes results from increased islet amyloid formation (10). Although hyperleptinemia is often associated with insulin resistance and diabetes, the systemic leptin receptor defect seen in db/db mice and fa/fa rats is not considered to be a typical characteristic of human type 2 diabetes. In addition, leptin has been implicated in myriad processes including angiogenesis, tumorigenesis, immunity, hyperinsulinemia, bone formation, blood pressure, and reproduction that are often dependent on a functioning leptin receptor (1). Finally, we recently reported that in the widely used ZDF model of type 2 diabetes there was a significant incidence of hydronephrosis in both lean and obese homozygous ZDF rats (28). Hydronephrosis is characterized by the dilation of the renal pelvis, compression of the papilla, and atrophy of the renal parenchyma (49); these features bear no relationship to diabetic nephropathy. We also found that there was a significant association between left ventricular (LV) end-systolic wall stress (LVESδ) and the presence of hydronephrosis (28), which raises serious questions regarding the validity of the ZDF rat as a model for studying the cardiovascular, or indeed renal, consequence of diabetes.
It is apparent, therefore, that there is a need for not only a reproducible, cost-effective model of type 2 diabetes that recapitulates many characteristics of the disease progression in humans but for one that also includes indexes of cardiovascular dysfunction such as large artery stiffness (29, 52) and LV dysfunction (13). A few studies have suggested that the combination of a high-fat (HF) diet with a relatively low dose of STZ may lead to type 2 diabetic phenotype (20, 26, 37, 44, 55); however, there is little consistency with regard to the type and duration of diets or the appropriate dose of STZ. The duration of diabetes was also relatively short in these studies, and there were no assessments of cardiovascular function. Therefore, the goal of this study was to evaluate the HF diet plus STZ model of diabetes as a model suitable for assessing the cardiovascular complications of type 2 diabetes. We compared two different diets, one that was primarily HF (60%) and one that more closely mimics the so-called Western (Wes) diet combining moderately HF with high carbohydrate (40% fat, 45% carbohydrate). Animals were treated with STZ after consuming the diets for 2 wk, and cardiovascular function was assessed 12 wk later. We found that the combination of a HF diet and moderate dose of STZ elicited a moderate diabetic phenotype with moderate hyperglycemia, diastolic, and systolic dysfunction and arterial stiffness similar to that typically observed in human type 2 diabetes, whereas consumption of a Wes diet resulted in severe hyperglycemia but a similar level of cardiac and arterial dysfunction to HF-fed animals. In addition, we also found that consumption of HF and Wes diets in diabetic animals resulted in a greater level of cardiac and endothelial dysfunction than that seen in animals that received a control diet, suggesting that the combination of hyperglycemia and dietary fat is more detrimental to endothelial and cardiovascular function than either intervention alone.
METHODS
Study protocol.
This protocol was approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham. Four-week-old, male Sprague-Dawley rats (Charles River, Wilmington, MA) were housed two per cage, maintained on a 12-h:12-h light-dark cycle, and provided with food and tap water ad libitum. Animals were randomized to receive control (Con; 12% kcal fat/19% protein/69% carbohydrate; and 3.87 kcal/g), HF (60%/19%/21%; 5.2 kcal/g), or Wes (40%/15%/45%; 4.62 kcal/g) diets prepared and analyzed by TestDiet (Richmond, IN). Saturated fat content was higher in both HF (28% wt/wt lard) and Wes (10%) compared with Con (0.4%). The contribution of simple sugars to the carbohydrate was also different between diets with a higher level of sucrose in Wes (31% wt/wt) compared with HF (6%) and Con (17%). A detailed list of the macro- and micronutrient composition of the three diets can be found in the supplemental material with the online version of this article.
After 2 wk, animals were randomized to receive either vehicle (Veh; 0.1 mol/l citrate buffer, pH 4.5; Con + Veh, n = 10; HF + Veh, n = 9; and Wes + Veh, n = 9) or 2 × 35 mg/kg ip STZ (Sigma, St. Louis, MO) injections given 24 h apart [Con + diabetes (Dia), n = 10; HF + Dia, n = 9; and Wes + Dia, n = 10]. The use of duplicate STZ injections was the result of preliminary work, which found that a single 35 mg/kg dose of STZ did not cause any change in blood glucose even up to 3 mo postinjection, whereas the administration of 45 and 55 mg/kg STZ produced severe hyperglycemia that was more indicative of uncontrolled type 1 diabetes (data not shown). Animals were then closely monitored for a further 12 wk until euthanization. There were four nonresponders to STZ in Con + Dia and one each in HF + Dia and Wes + Dia groups. The remainder of the animals displayed a sustained increase in blood glucose; therefore, the sample sizes in these groups for all subsequent analyses have been reduced (Con + Dia, n = 6; HF + Dia, n = 8; and Wes + Dia, n = 9).
Blood/plasma analysis.
Random whole blood samples were collected from the retro-orbital plexus under light isoflurane anesthesia early in the light cycle. Glucose and glycated hemoglobin (HbA1c) levels were measured in whole blood using the Accu-Chek Advantage (Roche Diagnostics, Basel, Switzerland) and DCA2000+ (Bayer Healthcare, Elkhart, IN) analyzers, respectively. Serum insulin levels were determined using a commercially available ultrasensitive ELISA kit (Mercodia, Winston-Salem, NC). Blood for the remaining analyses was collected at euthanization from the inferior vena cava. A standard biochemistry panel was analyzed in plasma using the VetScan Chemistry Analyzer (Abaxis, Union City, CA). Xanthine oxidase (XO) activity was assayed by a fluorometric method (7) using pterine as a substrate; the product, isoxanthopterin, was measured using a plate-based spectrofluorometer (Fluostar Optima; BMG Labtech, Durham, NC) with excitation at 345 nm and emission at 390 nm. Allopurinol was used to confirm specificity of XO activity.
Insulin tolerance test.
Insulin sensitivity was measured using an insulin tolerance test (ITT) in Veh-treated, nondiabetic rats. Rats were fasted overnight and then bled from the tail tip to obtain a baseline glucose level. Insulin (0.8 U/kg body wt in 0.9% saline ip) was administered, and blood samples were taken from the tail tip for glucose measurements at 15-min intervals for 120 min. The rate of glucose disappearance (kITT) was determined during the linear decay phase (0 to 60 min).
Hemodynamic and echocardiographic measurements.
Rats were anesthetized with 2% isoflurane, and echocardiography was performed (Agilent Sonos 5500; Philips, Bothell, WA) as previously described (39). Animals were then intubated and mechanically ventilated with 2% isoflurane in 100% oxygen. Heparin (100 Units) was administered through the right external jugular vein, and the right carotid artery was isolated. A 2F-combined conductance catheter-micromanometer (Millar, Houston, TX) was advanced retrogradely to the ascending aorta, where arterial pressure waveforms were collected before the catheter was advanced into the LV. Simultaneous pressure and volume measurements were collected at 1,000 Hz both at baseline and during reduced loading conditions caused by a transient (3–5 s) occlusion of the inferior vena cava at the level of the apex of the heart by a balloon catheter inserted into the right femoral vein. End-systolic and -diastolic volumes were calculated using the Bullet formula (14) with long- and short-axis dimensions obtained using a B-mode video; these values were then used to calibrate the raw data from the conductance catheter. Pressure-volume relations were analyzed using cardiac pressure-volume analysis software (PVAN 3.0; Millar). Pulse pressure was calculated as the difference between maximum and minimum aortic pressures. Augmentation index (AIx), a measure of arterial stiffness, was calculated as the height of the reflected aortic pressure wave as a percentage of pulse pressure using customized software (SphygmoCor 8.0; AtCor Medical, Sydney, Australia).
Immunohistochemistry.
To determine myocyte cross-sectional area, horizontal short-axis sections through the mid-LV were Formalin fixed and then immersed in 25% sucrose, embedded in optimum cutting temperature (OCT) compound, frozen in methylbutane over liquid nitrogen, sectioned at 5 μm, and probed for laminin (Abcam, Cambridge, MA). Images of tissue in cross-sectional orientation from the anterior, posterior, and free walls of the LV endocardium were acquired (40× objective), total field size was measured, and myocytes were counted within the field to determine average area. Myocyte apoptosis was examined using terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL; Promega, Madison, WI) as per the manufacturer's instructions. Positive controls were generated using DNase I (Invitrogen, Carlsbad, CA), and negative controls used DNase I without the rTDT enzyme. All data were analyzed using epifluorescence microscopy and digital image analysis software.
Collagen and lipofuscin analyses.
Horizontal short-axis sections through the mid-LV were Formalin fixed, paraffin embedded, sectioned at 5-μm thickness, and stained with Picric Acid Sirius Red F3BA or Armed Forces Institute of Pathology stains for collagen and lipofuscin, respectively. Myocardial interstitial collagen and lipofuscin volume percentage (including both interstitial and small vessel perivascular regions) were quantitatively evaluated with light microscopy (20× objective) using a 540 nm (green) filter and digital image analysis software in 20 fields containing cardiomyocytes in a longitudinal orientation within the LV midwall.
Oil red O staining.
For examination of intracellular lipid accumulation in cardiomyocytes, horizontal short-axis sections through the mid-LV were Formalin fixed and then immersed in 25% sucrose, embedded in OCT compound, frozen in methylbutane over liquid nitrogen, sectioned at 5 μm, and stained with oil red O and counterstained with hematoxylin-eosin. Representative images of tissue in cross-sectional orientation from the LV midwall were acquired with light microscopy (40× objective) using digital image analysis software.
Statistical analysis.
A two-way ANOVA was used to determine differences between groups for all variables except the insulin tolerance test (kITT), for which a one-way ANOVA was utilized; Tukey's post hoc analysis was used where appropriate. Data that were not normally distributed and/or of unequal variance underwent either log or rank transformations, and the subsequent analyses were performed on the transformed data. Differences between diets in the response to STZ treatment were evaluated using the χ2-test. Partial correlation analyses were used to determine interactions between measures of cardiac function. Values are presented as means ± SE, and significance was established at P < 0.05.
RESULTS
Biochemical parameters.
STZ was administered after 2 wk of dietary intervention. The number of animals that exhibited a significant increase in blood glucose following STZ was higher in HF (8/9) and Wes (9/10) compared with Con (6/10); however, these differences in response rates did not achieve statistical significance (χ2 = 3.47; P = 0.176). The data shown in the remainder of results are presented only from those animals that responded to STZ.
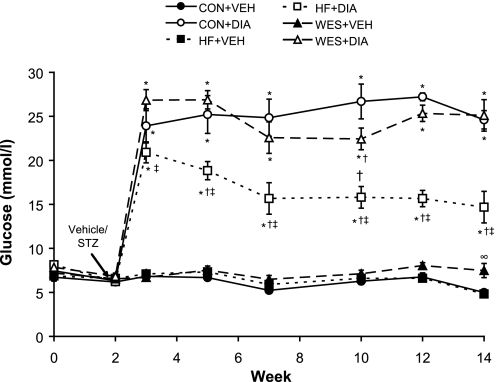
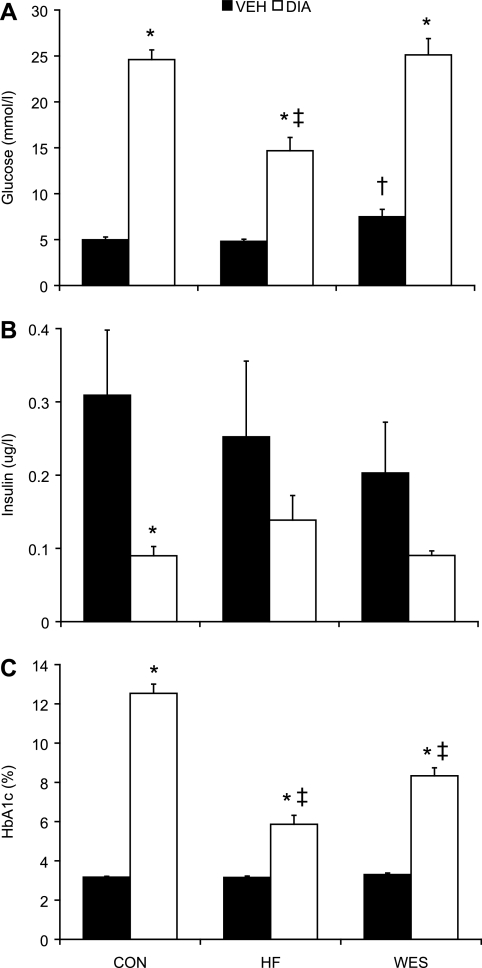
Blood glucose following STZ treatment peaked 1 wk after the administration, and this response was enhanced in Con + Dia and Wes + Dia compared with HF + Dia (P < 0.05; Fig. 1) groups. Elevated blood glucose levels in diabetic animals reached a plateau ∼5 wk following STZ and remained significantly higher compared with Veh-treated groups at all time points (P < 0.05; Fig. 1); the higher level in Con + Dia and Wes + Dia groups compared with the HF + Dia group remained sustained over the course of the protocol (P < 0.05). At euthanization, 12 wk after STZ treatment, hyperglycemia was significantly higher in Con + Dia and Wes + Dia groups compared with the HF + Dia group (25 ± 1, 25 ± 2, and 15 ± 1 mmol/l, respectively; P < 0.05; Fig. 2). In Veh-treated animals, glucose levels were higher in Wes + Veh than in either Con + Veh or HF + Veh (7.5 ± 0.8, 5.0 ± 0.3, and 5.0 ± 0.3 mmol/l, respectively; P < 0.05) groups. HbA1c was significantly elevated in all diabetic animals compared with Veh-treated controls (P < 0.05; Fig. 2); within the diabetic groups, HbA1c levels were significantly higher in the Con + Dia compared with the HF + Dia and Wes + Dia groups. Insulin levels were reduced in diabetic animals to almost half that of Veh-treated controls on the same diet, but this difference was statistically significant only in Con + Veh versus Con + Dia animals due to the large variability in Veh-treated animals (Fig. 2). The high variability in insulin levels in the nondiabetic animals may be a consequence of the measurements being performed in fed animals.
Fig. 1.
Time course of random blood glucose levels following vehicle (Veh) or streptozotocin [STZ; diabetes (Dia)] treatment. Values are means ± SE. Con, control diet; HF, high-fat diet; Wes, Western diet. *P < 0.05 vs. respective Veh treatment; †P < 0.05 vs. Con + Dia; ‡P < 0.05 vs. Wes + Dia; ∞P < 0.05 vs. HF + Veh.
Fig. 2.
Random blood glucose (A), plasma insulin (B), and HbA1c (C) levels 12 wk following Veh or STZ (Dia) treatment. Values are means ± SE. *P < 0.05 vs. respective Veh treatment; †P < 0.05 vs. Con + Veh; ‡P < 0.05 vs. Con + Dia.
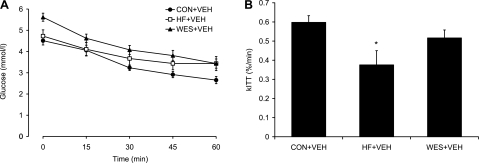
Since glucose levels were elevated in the Wes + Veh group, ITTs were performed in all Veh-treated groups. We found significantly lower insulin sensitivity in the HF + Veh group with a blunted blood glucose response during the ITT compared with the Con + Veh group (Fig. 3). Surprisingly, however, there was no significant difference in insulin sensitivity between the Wes + Veh and Con + Veh groups despite the higher baseline glucose level in the Wes + Veh group.
Fig. 3.
Blood glucose response (A) and rate of glucose disappearance (kITT; B) following intraperitoneal injection of insulin (0.8 U/kg body wt) during the insulin tolerance test in Veh animals. Values are means ± SE. *P < 0.05 vs. Con + Veh.
As expected, pancreatic function was affected by STZ, but not diet, with decreased plasma amylase in all diabetic groups (P < 0.05; Table 1). Diabetic animals also showed evidence of liver dysfunction with increased plasma levels of alanine aminotransferase (P < 0.05). Diabetic rats had higher kidney weights and blood urea nitrogen (BUN) levels in the presence of lower creatinine levels, which is indicative of renal enlargement and hyperfiltration typically reported in the early stages of diabetes (6).
Table 1.
Blood and plasma biochemical parameters
| Con + Veh | Con + Dia | HF + Veh | HF + Dia | Wes + Veh | Wes + Dia | |
|---|---|---|---|---|---|---|
| Renal function | ||||||
| Kidney wt/TL, mg/mm | 29.6±1.2 | 48.6±3.4* | 29.5±3.5 | 44.2±2.7* | 32.8±1.3 | 54.8±2.8* |
| Creatinine, mg/dl | 0.62±0.04 | 0.47±0.02* | 0.70±0.04 | 0.52±0.03* | 0.77±0.08 | 0.56±0.05* |
| Blood urea nitrogen, mg/dl | 18.2±1.0 | 35.2±1.1* | 20.6±0.6 | 23.7±1.4† | 18.8±1.0 | 26.4±1.4*† |
| Pancreatic function | ||||||
| Amylase, U/l | 793±56 | 517±74* | 880±36 | 572±34* | 855±86 | 615±46* |
| Liver function | ||||||
| Alanine aminotransferase, U/l | 27.7±2.2 | 65.0+12.9* | 34.5±6.4 | 56.0±6.1* | 27.6±3.5 | 53.0±6.8* |
| Bilirubin, mg/dl | 0.31±0.02 | 0.37±0.03 | 0.33±0.02 | 0.42±0.04 | 0.36±0.02 | 0.44±0.05 |
| Electrolytes | ||||||
| Calcium, mg/dl | 11.1±0.2 | 10.7±0.3 | 11.8±0.6 | 10.8±0.3 | 10.9±0.2 | 11.5±0.8 |
| Phosphorus, mg/dl | 7.4±0.3 | 7.6±0.9 | 7.6±0.7 | 8.5±0.3 | 9.0±1.4 | 10.6±1.2 |
| Sodium, mmol/l | 147±1 | 140±2* | 145±3 | 150±1*† | 143±1 | 145±2 |
| Potassium, mmol/l | 7.2±0.3 | 7.5±0.0 | 6.7±0.3 | 7.6±0.4 | 6.6±0.3 | 7.6±0.2 |
| Total protein, g/dl | 6.0±0.2 | 5.1±0.2* | 6.4±0.3 | 5.5±0.2* | 6.4±0.3 | 5.8±0.3 |
| Oxidative stress | ||||||
| Xanthine oxidase activity, uU/ml | 3,207±169 | 4,640±755* | 3,416±288 | 3,261±242† | 2,905±211 | 3,443±179† |
Values are means ± SE. Con, control diet; HF, high-fat diet; Dia, diabetes; Wes, Western diet; TL, tibia length.
P < 0.05 vs. respective vehicle (Veh) treatment;
P < 0.05 vs. Con + Dia.
We also measured XO activity to determine whether our model of type 2 diabetes was characterized by an increased level of superoxide production and found that XO activity was not affected by diet in Veh-treated animals but was significantly higher in Con + Dia compared with nondiabetic controls (P < 0.05) and either the Wes + Dia or HF + Dia diets. A subsequent linear regression analysis revealed a significant interaction between BUN and XO activity (r = 0.401; P < 0.05), suggesting an association between renal dysfunction and superoxide production.
Body weight.
There were no differences in body weight between groups before the beginning of the dietary intervention or at the time of Veh or STZ treatments (data not shown). Following STZ treatment, diabetic animals exhibited a stunted growth pattern such that 12 wk after STZ all diabetic animals were significantly lighter and had shorter tibias than their Veh-treated, age- and diet-matched controls (Table 2). There were no significant differences in body weight between diabetic animals on different diets, whereas nondiabetic animals that received either the HF or Wes diets were ∼15% heavier (P < 0.05) than those that received the Con diet.
Table 2.
Body weight and left ventricular morphology and fibrosis
| Con + Veh | Con + Dia | HF + Veh | HF + Dia | Wes + Veh | Wes + Dia | |
|---|---|---|---|---|---|---|
| Body weight, g | 525±21 | 270±15* | 608±26† | 314±15* | 620±25† | 341±18* |
| TL, mm | 50.9±0.6 | 46.7±0.8* | 51.2±0.5 | 47.9±0.5* | 51.3±0.6 | 48.7±0.3* |
| Heart wt/TL, mg/mm | 26.2±0.7 | 19.6±0.8* | 27.6±1.4 | 22.9±1.0* | 28.2±0.7 | 25.8±1.3‡ |
| LV wt/TL, mg/mm | 18.7±0.6 | 13.6±0.5* | 19.4±0.7 | 16.0±0.8* | 19.6±0.4 | 17.6±0.7*‡ |
| PWd, mm | 2.1±0.1 | 2.1±0.2 | 2.2±0.1 | 2.0±0.1 | 2.1±0.1 | 1.9±0.1 |
| LVEDD, mm | 7.5±0.3 | 6.8±0.2 | 7.8±0.1 | 7.8±0.4‡ | 7.1±0.5 | 8.1±0.2*‡ |
| LVEDD/PWd | 3.8±0.4 | 3.3±0.2 | 3.6±0.2 | 3.9±0.3 | 3.5±0.3 | 4.5±0.3*‡ |
| LVESD, mm | 4.9±0.3 | 4.3±0.4 | 4.9±0.3 | 5.3±0.4 | 4.0±0.5 | 5.6±0.2 |
| Myocyte CSA, μm2 | 432±29 | 481±61 | 422±39 | 405±50 | 401±52 | 334±37 |
| Myocardial vol % collagen, % area | 2.6±0.1 | 2.9±0.3 | 2.6±0.2 | 2.6±0.1 | 3.3±0.2†§ | 3.0±0.1 |
Values are means ± SE. LV, left ventricular; PWd, posterior wall thickness; LVEDD, LV end-diastolic diameter; LVESD, LV end-systolic diameter; CSA, myocyte cross-sectional area; Vol % collagen, interstitial collagen volume percentage.
P < 0.05 vs. respective Veh treatment;
P < 0.05 vs. Con + Veh;
P < 0.05 vs. Con + Dia;
P < 0.05 vs. HF + Veh.
LV morphology.
We found no indication of gross cardiac hypertrophy as assessed by either heart or LV weight to tibia length. Indeed, when normalized to tibia length, LV weight was lower in all diabetic groups compared with their respective controls (Table 2), and this was not accompanied by differences in posterior wall thickness (PWd) or myocyte cross-sectional area. Both heart and LV weights were higher in the Wes + Dia group (P < 0.05) and appear to be the result of eccentric remodeling as evidenced by higher LV end-diastolic dimension and ratio of LV chamber to wall thickness (LVEDD/PWd) in the Wes + Dia group (P < 0.05).
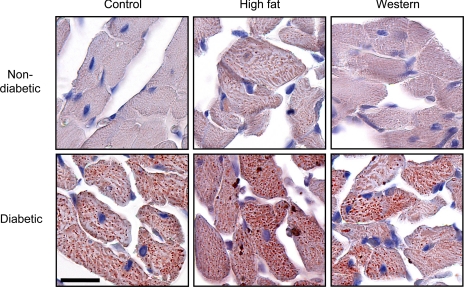
Veh-treated animals did not exhibit any changes in LV morphology although, surprisingly, the Wes + Veh group had ∼25% more interstitial collagen than both the HF + Veh and Con + Veh groups (P < 0.05; Table 2). Diabetes had no effect on collagen volume percentage. Intracellular lipid accumulation in cardiomyocytes, indicated by enhanced oil red O staining, was higher in HF + Veh compared with either the Con + Veh or Wes + Veh groups (Fig. 4); lipid droplets were evident in all diabetic groups and were more pronounced in the HF + Dia group. Lipofuscin content was analyzed in the LV midwall to determine the extent of cumulative oxidative stress and lipotoxicity (45); negligible levels of lipofuscin were found (0.01–0.02% of total area), and this was not different between groups (data not shown).
Fig. 4.
Intramyocardial lipid accumulation in diabetic and nondiabetic animals. Representative pictures from 5 individual experiments using oil red O staining (red droplets) and counterstained with hematoxylin-eosin (×40 magnification) are shown. Scale bar, 20 μm.
Contrary to recent reports (15, 17, 41), we did not find any apoptotic cardiomyocytes, regardless of treatment; however, we did detect <1 apoptotic cell/mm2, but these were typically capillary endothelial cells and this number was not different between groups (data not shown). We subsequently performed Western blot analysis of cleaved caspase-3 and found no difference between groups (data not shown), thus confirming our TUNEL data.
LV function and arterial compliance.
Although heart rates were lower in the Con + Dia and HF + Dia groups (P < 0.05), mean arterial, pulse, and LV end-systolic and end-diastolic pressures were not affected by either diet or diabetes (Table 3). It should be noted that although we used the lowest level of anesthesia possible, given the invasive nature of these experiments, it is likely that these parameters are lower than those observed in conscious animals. However, since the level of anesthesia was constant across all animals, this should not contribute to any differences between groups.
Table 3.
Cardiac function and arterial stiffness
| Con + Veh | Con + Dia | HF + Veh | HF + Dia | Wes + Veh | Wes + Dia | |
|---|---|---|---|---|---|---|
| Heart rate, beats/min | 289±16 | 240±11* | 307±13 | 235±9* | 308±22 | 285±11‡ |
| MAP, mmHg | 83.8±6.1 | 63.0±5.7 | 95.2±7.7 | 82.7±7.4 | 61.4±8.7† | 73.1±9.2 |
| Pulse pressure, mmHg | 27.5±1.8 | 23.1±2.0 | 30.0±2.3 | 29.0±1.8 | 29.8±2.5 | 27.0±2.5 |
| Ejection fraction, % | 67±4 | 66±6 | 65±4 | 66±4 | 71±6 | 65±4 |
| LVEDP, mmHg | 3.1±1.2 | 5.9±2.4 | 5.5±0.7 | 7.9±1.5 | 5.1±1.2 | 5.9±1.4 |
| LVESP, mmHg | 73.3±9.1 | 65.4±2.6 | 77.5±5.5 | 84.1±9.1 | 72.8±7.9 | 80.2±6.5 |
| FS, % | 34.9±2.6 | 37.1±3.6 | 36.6±3.8 | 31.9±1.7 | 42.9±5.3 | 30.6±1.9* |
| LVEDσ, kdyn/cm2 | 2.9±1.2 | 4.5±1.8 | 4.8±0.8 | 7.9±1.7* | 4.6±1.1 | 6.2±1.4 |
| dP/dtmax, mmHg/s | 5,220±839 | 3,927±330 | 5,327±689 | 4,757±624 | 6,152±1,034 | 4,999±921 |
| dP/dtmin, mmHg/s | −6,363±1,251 | −3,945±321 | −5,950±684 | −5,243±844 | −5,607±872 | −5,191±757 |
| Ees, mmHg/ml | 0.13±0.03 | 0.16±0.04 | 0.14±0.03 | 0.10±0.02 | 0.20±0.05 | 0.10±0.02 |
| Emax, mmHg/ml | 0.24±0.07 | 0.29±0.08 | 0.38±0.09 | 0.27±0.04 | 0.42±0.12 | 0.20±0.04 |
Values are means ± SE. MAP, mean arterial pressure; LVEDP, LV end-diastolic pressure; LVESP, LV end-systolic pressure; FS, fractional shortening; LVEDσ, LV end-diastolic wall stress; dP/dtmax and dP/dtmin, maximal and minimal change in pressure over time, respectively; Ees, end-systolic volume elastance; Emax, maximal elastance.
P < 0.05 vs. respective Veh treatment;
P < 0.05 vs. HF + Veh;
P < 0.05 vs. HF + Dia.
AIx, a measure of arterial stiffness, was significantly higher in HF + Dia and Wes + Dia groups (Fig. 5), indicating that afterload was elevated through decreased vascular compliance in the absence of any change in arterial pressure. There was no evidence of arterial stiffness in the Con + Dia group; indeed, AIx values were equivalent to that seen in Veh-treated groups.
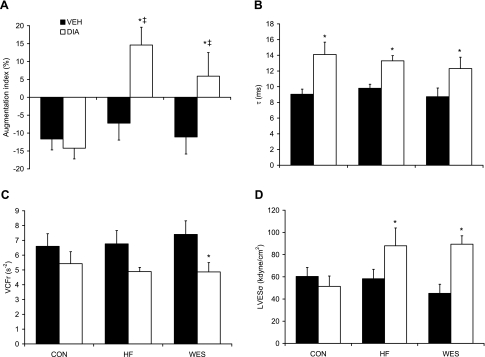
Fig. 5.
Augmentation index (A), time constant of isovolumic relaxation (τ; B), velocity of circumferential shortening (VCFr; C), and left ventricular end-systolic wall stress (LVESσ; D) 12 wk following Veh or STZ (Dia) treatment. Values are means ± SE. *P < 0.05 vs. respective Veh treatment; ‡P < 0.05 vs. Con + Dia.
Load-dependent systolic function, indicated by velocity of circumferential shortening corrected for heart rate (VCFr), was impaired in Wes + Dia groups (P < 0.05; Fig. 5). There was a trend toward lower VCFr in the HF + Dia group (P = 0.065) compared with respective nondiabetic controls; however, there were no differences in the load-dependent maximal change in pressure over time (Table 3). LVESδ was also significantly elevated in the Wes + Dia and HF + Dia groups (P < 0.05), and there was a strong, negative association with VCFr (r = 0.436; P < 0.05). Load-independent systolic function was also impaired with a trend toward lower LV end-systolic volume elastance in the HF + Dia (P = 0.079) and Wes + Dia (P = 0.082) compared with the HF + Veh and Wes + Veh groups, respectively.
In the absence of any change in LV end-diastolic pressure, end-diastolic wall stress was elevated in the HF + Dia group (P < 0.05; Table 3) with a trend toward higher values in the Wes + Dia group (P = 0.089) compared with respective nondiabetic controls. There were no differences between groups in the load-dependent minimal change in pressure over time, but the time constant of isovolumic relaxation (τ), a preload-independent marker of active myocyte relaxation (47), was elevated in all diabetic groups (P < 0.05; Fig. 5 and supplemental Fig. 1).
DISCUSSION
It is well established that diabetes increases the incidence of heart failure, and clinical and experimental data also suggest that this is a consequence, at least in part, to defects at the level of the myocardium. However, much of the experimental data examining the impact of diabetes on cardiac function has been based on severe, insulin-deficient, STZ-induced, diabetic models that are frequently associated with marked weight loss and dehydration or on genetic models of type 2 diabetes where other nondiabetic-related pathologies may contribute to alterations in cardiovascular function. This highlights the need for reproducible, cost-effective models of diabetes that more closely mimic the disease progression in humans, particularly with regard to the presence of cardiovascular abnormalities such as large artery stiffness and LV dysfunction. We report here, for the first time, that the combination of a HF (60%) diet with a low to moderate dose of STZ (2 × 35 mg/kg ip) elicited a stable, modest level of hyperglycemia (14 mmol/l glucose, 6% HbA1c) and lower insulin levels in young Sprague-Dawley rats that was associated with cardiac dysfunction and arterial stiffness; these findings are comparable with characteristics observed in the moderate-advanced stages of human type 2 diabetes (3, 4, 13, 25). It is noteworthy that, in contrast with more severe rodent models of diabetes (23, 31, 54), we did not see any changes in ejection fraction, LV end-systolic pressure, or LV end-diastolic pressure; this supports the notion that this model recapitulates the cardiovascular dysfunction characteristic of that seen in patients with type 2 diabetes without additional complications.
Surprisingly, we found that rats on the HF diet exhibited a blunted glucose response following STZ treatment compared with STZ-treated animals that received either the Con or Wes diets; indeed, glucose levels in Con and Wes diet-fed animals were similar to those seen in recent studies using high doses of STZ to induce a type 1 diabetic phenotype (18, 24). The different responses to STZ in these groups could be a consequence of decreased sensitivity of pancreatic β-cells in the HF group to STZ. Future studies examining the effect of HF and Wes diet on pancreatic β-cell function before and after STZ treatment may provide some additional insight into the differences seen here. Interestingly, although we observed LV diastolic dysfunction in all diabetic groups, arterial stiffness and systolic abnormalities were present only in diabetic animals that received either the HF or Wes diets. These results suggest that the combination of hyperglycemia and elevated dietary fat is more detrimental to cardiovascular function than either intervention alone.
The initial goal of this study was to develop a model of diabetes that exhibited similar cardiac dysfunction to that seen in patients with type 2 diabetes. We based our protocol on the findings of several previous studies that reported a type 2 diabetic phenotype with the combination of a HF diet and low dose of STZ (20, 26, 37, 44, 55). These studies varied in dietary composition; therefore, we compared two different diets: a HF (60%) diet and a Wes diet that combined moderately HF with high carbohydrate (40% fat, 45% carbohydrate). In these earlier studies there was also no consistency in the dose of STZ; therefore, in preliminary studies we examined 35, 45, and 55 mg/kg doses of STZ. We found that a single 35 mg/kg dose of STZ had no effect on blood glucose in any diet group even up to 3 mo posttreatment. In contrast, 45 and 55 mg/kg STZ produced severe hyperglycemia that was more indicative of the uncontrolled severe insulin-deficient diabetes usually found with STZ treatment. We found that two 35 mg/kg ip doses of STZ separated by 24 h resulted in a reproducible increase in blood glucose that was stable for at least 12 wk.
The majority of the previous studies that utilized the HF/low dose STZ model reported little or no effect of STZ in animals that consumed normal rat chow. In contrast, however, we found that 60% of our Con-fed, STZ-treated rats developed marked hyperglycemia, and although this was lower than the 90% response in the STZ-treated HF and Wes diet-fed groups, this difference in response rate did not achieve statistical significance. Surprisingly, in those animals that exhibited a response to STZ, the extent of hyperglycemia was significantly attenuated in the HF group compared with either Con or Wes diet-fed rats despite comparable plasma insulin levels. This difference was significant 3 wk after STZ treatment and was sustained for the remainder of the study. At euthanization, the lower HbA1c levels in the HF + Dia group, compared with the Con + Dia group, were consistent with a sustained lower level of hyperglycemia.
The modest increases in glucose and HbA1c and concomitant decreases in insulin and body weight in the HF + Dia group suggest a level of diabetes analogous to either undiagnosed or poorly controlled human type 2 diabetes. The factors contributing to the differential response of the HF and Wes diet-fed animals to STZ remain to be determined. The protein levels were moderately lower in the Wes diet (15.3% vs. 18.6%); however, in the Con diet protein levels were identical to the HF diet, whereas the response to STZ was similar to the Wes diet. One of the major differences between the HF and Wes diets was the level of sucrose, which was approximately fivefold higher in the Wes diet (6% vs. 31%), which may be an important contributing factor to the increased sensitivity to STZ. We did not record food intake during this study although we did not subjectively observe any differences in consumption between the three dietary groups. Due to the large variation in carbohydrate and fat content, the diets were not isocaloric and differences in energy intake may have contributed to the responses observed; however, we do not believe that the enhanced response to STZ with the Wes diet can be attributed to increased caloric intake alone since the HF diet was more energy dense than the Wes diet.
As expected, after 12 wk of diabetes all diabetic groups exhibited diastolic dysfunction compared with their nondiabetic control groups, as indicated by significant increases in the time constant of relaxation, τ (Fig. 5B). Interestingly, however, even though serum glucose was at a lower level in the HF + Dia compared with the Con + Dia groups, evidence of LV systolic abnormalities such as LVESδ and arterial stiffness as indicated by increased AIx (Fig. 5A) was present in the HF + Dia but not the Con + Dia group. We also found that systolic dysfunction in the Wes + Dia group was impaired to a similar extent as the HF + Dia group despite evidence of eccentric remodeling and higher serum glucose levels in the Wes + Dia group. In the absence of diabetes we found no significant functional alterations in systolic function between groups. Blood glucose levels were not different in the HF + Veh compared with the Con + Veh group; however, there was a significant decrease in insulin sensitivity (Fig. 3). Conversely, in the Wes + Veh group blood glucose levels were significantly increased compared with the Con + Veh group (7.5 ± 0.8 vs. 5.0 ± 0.3 mmol/l, respectively; P < 0.05), but insulin sensitivity was not affected (Fig. 3). We assessed insulin sensitivity using an ITT, and additional examination of glucose tolerance may have provided additional useful information between dietary treatment groups. Thus both HF and Wes diets alone had modest but variable effects on metabolic status; however, none of these alterations provides an obvious explanation for the LV functional abnormalities seen in their respective diabetic groups. The mechanisms underlying the effects of Wes on blood glucose and the pancreatic response to STZ were not determined in this study, and we are currently investigating these findings in our laboratory. We postulate that the combination of high dietary fat and increased sucrose levels in Wes causes endoplasmic reticulum (ER) and/or oxidative stress in β-cells, thus increasing the sensitivity of the pancreas to STZ and aging.
Surprisingly, we found that LV dysfunction and arterial stiffness was more pronounced in the HF + Dia than the Con + Dia group despite lower level of hyperglycemia. Furthermore, it is also noteworthy that cardiovascular dysfunction was not exacerbated in the Wes + Dia compared with the HF + Dia group despite increased hyperglycemia. These data suggest that diets high in fat appear to exacerbate the adverse effects of hyperglycemia on endothelial and cardiovascular function. Cardiomyocyte lipid accumulation was evident in all diabetic animals but did not appear to be substantially different between diets. Unfortunately, measurements of plasma levels of fatty acids were not determined; thus we cannot ascertain whether the increase in lipid accumulation parallel changes in circulating lipid levels or if differences in plasma lipids could contribute to the dissociation between the level hyperglycemia and the degree of cardiac dysfunction.
Thus our data demonstrate dissociation between the level of blood glucose and the development of cardiac dysfunction, suggesting that factors other than hyperglycemia contribute to impaired cardiac function in vivo. Previous studies suggest that high dietary fat causes LV mitochondrial damage, impaired calcium handling (38), ER stress (43), and altered substrate utilization (11, 51). Since these factors are also known to occur with hyperglycemia and diabetes (9), we propose that the combination of hyperglycemia and high dietary fat results in a substrate overload that causes excessive upregulation of these pathologies that is more deleterious to LV function than either intervention alone.
Type 2 diabetes is often associated with endothelial dysfunction and arterial stiffness, and both are independent predictors of adverse cardiovascular outcomes that result in or contribute to secondary complications of diabetes (8, 29, 52). The underlying cause of arterial stiffness is likely to include factors such as the formation of advanced glycation end-products, dyslipidemia, autonomic nerve dysfunction, and oxidative stress (52). Although arterial stiffness was elevated in HF + Dia and Wes + Dia groups, plasma XO activity was only increased in the Con + Dia group. While this suggests that oxidative stress may not be a contributing factor to arterial stiffness in these models, XO activity is a specific indicator of superoxide production, and we cannot rule out the possibility that increases in lipid peroxidation, protein oxidation, or other free radicals may play a role in the endothelial dysfunction observed in this study.
It is often reported that diabetes is associated with cardiac hypertrophy (13, 16, 17); however, in contrast, we found that LV weight relative to tibia length was actually lower in all three diabetic groups compared with age-matched, nondiabetic controls (Table 2). Body weight was also lower in all diabetic animals compared with age-matched controls; however, this was a result of attenuated growth rather than weight loss and is consistent with previous studies of STZ-induced diabetes in young rats and mice (22, 26, 30, 35, 44). Decreased body weight has been reported in adult (3 to 4 mo) rat models of fat-fed, STZ-induced diabetes (20, 55), which is consistent with clinical studies that demonstrate unintentional weight loss in patients with poor glucose control (19, 21, 50). We also found no differences in LV wall thickness or myocyte cross-sectional area, demonstrating a lack of hypertrophy also at the cardiomyocyte level. It should be noted that studies examining cardiomyocyte size in diabetic animals have elicited equivocal findings (31, 40), whereas cardiomyocytes isolated from patients with and without diabetes were found to be of similar sizes (27). Thus, taken together, these data suggest that diabetes alone does not lead to cardiac hypertrophy. The discrepancy with other experimental studies may be due, at least in part, to the frequent use of body weight for normalization of heart or LV weight rather than skeletal size, indicated by tibia length (5, 53), and suggests that the reports of cardiac hypertrophy in patients with diabetes may be a consequence of other frequent comorbidities such as hypertension.
A number of human and animal studies (15, 17, 20, 42) have reported that diabetes leads to an increase in cardiomyocyte apoptosis; however, we found no evidence of increased apoptosis in any of our diabetic groups. The number of apoptotic cardiomyocytes at any one time may be very small, and thus the absence of apoptosis seen after 12 wk of diabetes does not mean that myocyte loss might not have occurred at an earlier time point. Indeed, Fiordaliso et al. (15) reported highest levels of cardiomyocyte apoptosis 3 days following STZ treatment, which significantly decreased 28 days following STZ. This suggests that STZ treatment may lead to an acute increase in apoptosis, possibly due to the sudden onset of metabolic dysfunction, or perhaps as a direct consequence of STZ toxicity, but that a sustained period of hyperglycemia does not contribute to further apoptosis. We cannot rule out the possibility that there might have been an early increase in apoptosis shortly following STZ treatment in this study; however, the fact that there was no evidence of replacement fibrosis in any diabetic groups would suggest that this was not the case. It is important to note that although we did not observe an increase in apoptosis with diabetes alone, this does not preclude the possibility that diabetes may increase the susceptibility of cardiomyocytes to apoptosis in response to another stress such as hypertension or infarction. Indeed, the increase in apoptosis reported in patients with diabetes could be due to additional factors including age and overt heart failure (17).
One limitation of this study is that the level of diabetes we have described and the associated complications may be specific to the strain of rat since subtle differences are known to exist between the Sprague-Dawley rat and other popular strains (33). It is also important to note that STZ causes an abrupt onset of diabetes rather than the insidious onset seen in humans following a period of insulin resistance. We did find decreased insulin sensitivity in the HF + Veh group, but not the Wes + Veh group, after 2 wk of feeding (data not shown) that was still evident at 14 wk (Fig. 3); thus we are confident that ingestion of a HF (60%) diet results in insulin resistance. However, the duration of insulin resistance before STZ treatment was clearly relatively short, and there is evidence to suggest that insulin resistance initiates pathways that are subsequently exacerbated by diabetes and may contribute to cardiac dysfunction (36, 48).
Another potential limitation is that diabetes was induced in relatively young (6 wk) rats, and although this is a common age for such studies, this represents a juvenile rather than adult animal. On the other hand, given the increasing incidence of obesity, insulin resistance, and type 2 diabetes in children (32), studies of diabetes in younger animals are likely to be increasingly relevant. Although the majority of diagnosed cases of juvenile type 2 diabetes occur in obese patients, recent evidence suggests that type 2 diabetes is also evident in nonobese children (34). Thus, although obesity may not precede development of diabetes in our model, it is clear that type 2 diabetes is a disease of polygenic onset, of which obesity is only one characteristic. It is also worth noting that popular rodent models of type 2 diabetes, such as the GK (2) and ZDF (18, 28) rats, also do not exhibit obesity before or following the development of diabetes.
It should also be noted that the diabetic animals in this study exhibited growth retardation following the induction of diabetes, which is not commonly seen in children with diabetes due to early diagnosis and subsequent glucose control. However, we do not believe that slower growth resulted in the cardiovascular pathologies reported since the major functional variables used in this study, such as AIx, wall stress, τ, and VCFr, are independent of body size and we typically find no differences in these indexes between rats of different sizes. Although we have demonstrated cardiac, liver, and kidney dysfunction in our diabetic model, alterations in normal growth patterns may have resulted in other pathologies that are independent of diabetes per se and therefore not representative of the human disease progression. One important advantage of the type of diabetic model described here, combining dietary manipulation with a low to moderate dose of STZ, is that the interactions of age and the duration of insulin resistance before the onset of diabetes on the development cardiac dysfunction can be more readily investigated than in more commonly used genetic models of diabetes such as ZDF rats and db/db mice, although it is unclear whether the moderate level of hyperglycemia and subsequent cardiac dysfunction would be apparent if this protocol were applied to older animals.
In conclusion, we have shown that the combination of a HF (60%) diet combined with a moderate dose of STZ elicits a moderate level of hyperglycemia, cardiac dysfunction, and arterial stiffness comparable with that observed in patients with type 2 diabetes. Consumption of a Wes diet resulted in a more severe hyperglycemia but a similar level of cardiac and arterial dysfunction to HF-fed animals. In contrast with many other studies, we did not find any evidence of cardiomyocyte apoptosis or hypertrophy, suggesting that these may be a consequence of more severe and longer periods of diabetes or secondary to additional pathologies such as hypertension, infarction, or heart failure. The fact that LV dysfunction and arterial stiffness was more pronounced in the HF + Dia than the Con + Dia group despite a lower level of hyperglycemia suggests that diets high in fat appear to exacerbate the adverse effects of hyperglycemia on endothelial and cardiovascular function. Finally, this study suggests that using protocols similar to those described here may represent valuable new models for study of the complex interactions between diet and diabetes on cardiovascular function that avoid the problems inherent in many commercially available genetic rodent models of type 2 diabetes.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-77100 and HL-67464.
Acknowledgments
We gratefully acknowledge the technical assistance of Laura Cain, Charlye Brocks, Pamela Powell, and Scott Sweeney.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ahima RS, Osei SY. Leptin signaling. Physiol Behav 81: 223–241, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Akimoto Y, Hart GW, Wells L, Vosseller K, Yamamoto K, Munetomo E, Ohara-Imaizumi M, Nishiwaki C, Nagamatsu S, Hirano H, Kawakami H. Elevation of the post-translational modification of proteins by O-linked N-acetylglucosamine leads to deterioration of the glucose-stimulated insulin secretion in the pancreas of diabetic Goto-Kakizaki rats. Glycobiology 17: 127–140, 2007. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 30: S42–S47, 2007. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Standards of Medical Care in Diabetes—2007. Diabetes Care 30: S4–S41, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Animal Models of Diabetic Complications Consortium. Validation of models of cardiovascular disease in diabetes (Online). http://www.amdcc.org/shared/showFile.aspx?docID=26&docTypeID=3 [4 June 2003].
- 6.Bak M, Thomsen K, Christiansen T, Flyvbjerg A. Renal enlargement precedes renal hyperfiltration in early experimental diabetes in rats. J Am Soc Nephrol 11: 1287–1292, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Beckman JS, Parks DA, Pearson JD, Marshall PA, Freeman BA. A sensitive fluorometric assay for measuring xanthine dehydrogenase and oxidase in tissues. Free Radic Biol Med 6: 607–615, 1989. [DOI] [PubMed] [Google Scholar]
- 8.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol 23: 168–175, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Brownlee M Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Cefalu WT Animal models of type 2 diabetes: clinical presentation and pathophysiological relevance to the human condition. ILAR J 47: 186–198, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Christoffersen C, Bollano E, Lindegaard ML, Bartels ED, Goetze JP, Andersen CB, Nielsen LB. Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology 144: 3483–3490, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, Saydah SH, Williams DE, Geiss LS, Gregg EW. Prevalence of diabetes and impaired fasting glucose in adults in the U. S population: National Health and Nutrition Examination Survey 1999–2002. Diabetes Care 29: 1263–1268, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Dawson A, Morris AD, Struthers AD. The epidemiology of left ventricular hypertrophy in type 2 diabetes mellitus. Diabetologia 48: 1971–1979, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Dell′Italia LJ, Blackwell GG, Pearce DJ, Thorn B, Pohost GM. Assessment of ventricular volumes using cine magnetic resonance in the intact dog. A comparison of measurement methods. Invest Radiol 29: 162–167, 1994. [DOI] [PubMed] [Google Scholar]
- 15.Fiordaliso F, Li B, Latini R, Sonnenblick EH, Anversa P, Leri A, Kajstura J. Myocyte death in streptozotocin-induced diabetes in rats is angiotensin II-dependent. Lab Invest 80: 513–527, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Fredersdorf S, Thumann C, Ulucan C, Griese DP, Luchner A, Riegger GA, Kromer EP, Weil J. Myocardial hypertrophy and enhanced left ventricular contractility in Zucker diabetic fatty rats. Cardiovasc Pathol 13: 11–19, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circ Res 87: 1123–1132, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Fulop N, Mason MM, Dutta K, Wang P, Davidoff AJ, Marchase RB, Chatham JC. Impact of Type 2 diabetes and aging on cardiomyocyte function and O-linked N-acetylglucosamine levels in the heart. Am J Physiol Cell Physiol 292: C1370–C1378, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Gerhardt CM, Klingensmith GJ. New-onset diabetes in an obese adolescent: diagnostic dilemmas. Nat Clin Pract Endocrinol Metab 4: 578–583, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh S, Qi D, An D, Pulinilkunnil T, Abrahani A, Kuo KH, Wambolt RB, Allard M, Innis SM, Rodrigues B. Brief episode of STZ-induced hyperglycemia produces cardiac abnormalities in rats fed a diet rich in n-6 PUFA. Am J Physiol Heart Circ Physiol 287: H2518–H2527, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Gregg EW, Gerzoff RB, Thompson TJ, Williamson DF. Trying to lose weight, losing weight, and 9-year mortality in overweight U. S. adults with diabetes. Diabetes Care 27: 657–662, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Hamblin M, Friedman DB, Hill S, Caprioli RM, Smith HM, Hill MF. Alterations in the diabetic myocardial proteome coupled with increased myocardial oxidative stress underlies diabetic cardiomyopathy. J Mol Cell Cardiol 42: 884–895, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joffe II, Travers KE, Perreault-Micale CL, Hampton T, Katz SE, Morgan JP, Douglas PS. Abnormal cardiac function in the streptozotocin-induced non-insulin-dependent diabetic rat: noninvasive assessment with Doppler echocardiography and contribution of the nitric oxide pathway. J Am Coll Cardiol 34: 2111–2119, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Pang Y, Chang T, Bounelis P, Chatham JC, Marchase RB. Increased hexosamine biosynthesis and protein O-GlcNAc levels associated with myocardial protection against calcium paradox and ischemia. J Mol Cell Cardiol 40: 303–312, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Loimaala A, Groundstroem K, Majahalme S, Nenonen A, Vuori I. Impaired myocardial function in newly onset Type 2 diabetes associates with arterial stiffness. Eur J Echocardiogr 7: 341–347, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Luo J, Quan J, Tsai J, Hobensack CK, Sullivan C, Hector R, Reaven GM. Nongenetic mouse models of non-insulin-dependent diabetes mellitus. Metabolism 47: 663–668, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Maier S, Lange V, Simm A, Walter U, Kirstein M. Insulin fails to modulate the cardiac L-type Ca2+ current in Type II diabetes patients—a possible link to cardiac dysfunction in diabetes mellitus. Diabetologia 44: 269, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Marsh SA, Powell PC, Agarwal A, Dell′italia LJ, Chatham JC. Cardiovascular dysfunction in Zucker obese and Zucker diabetic fatty rats: the role of hydronephrosis. Am J Physiol Heart Circ Physiol 293: H292–H298, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Meigs JB, O'Donnell CJ, Tofler GH, Benjamin EJ, Fox CS, Lipinska I, Nathan DM, Sullivan LM, D'Agostino RB, Wilson PWF. Hemostatic markers of endothelial dysfunction and risk of incident type 2 diabetes: the Framingham Offspring Study. Diabetes 55: 530–537, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Mori DM, Baviera AM, de Oliveira Ramalho LT, Vendramini RC, Brunetti IL, Pepato MT. Temporal response pattern of biochemical analytes in experimental diabetes. Biotechnol Appl Biochem 38: 183–191, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Nemoto O, Kawaguchi M, Yaoita H, Miyake K, Maehara K, Maruyama Y. Left ventricular dysfunction and remodeling in streptozotocin-induced diabetic rats. Circ J 70: 327–334, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295: 1549–1555, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Osadchii O, Norton G, Deftereos D, Woodiwiss A. Rat strain-related differences in myocardial adrenergic tone and the impact on cardiac fibrosis, adrenergic responsiveness and myocardial structure and function. Pharmacol Res 55: 287–294, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Otabe S, Nakayama H, Fukutani T, Yuan X, Wada N, Hashinaga T, Kato T, Inada C, Yamada K. Clinical and genetic features of childhood-onset Type 2 diabetes in Japan. Acta Diabetol 44: 181–185, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Ottlecz A, Bensaoula T, Eichberg J, Peterson RG. Angiotensin-converting enzyme activity in retinas of streptozotocin-induced and Zucker diabetic rats. The effect of angiotensin II on Na+,K+-ATPase activity. Invest Ophthalmol Vis Sci 37: 2157–2164, 1996. [PubMed] [Google Scholar]
- 36.Park SY, Cho YR, Kim HJ, Higashimori T, Danton C, Lee MK, Dey A, Rothermel B, Kim YB, Kalinowski A, Russell KS, Kim JK. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes 54: 3530–3540, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Reed MJ, Meszaros K, Entes LJ, Claypool MD, Pinkett JG, Gadbois TM, Reaven GM. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism 49: 1390–1394, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Relling DP, Esberg LB, Fang CX, Johnson WT, Murphy EJ, Carlson EC, Saari JT, Ren J. High-fat diet-induced juvenile obesity leads to cardiomyocyte dysfunction and upregulation of Foxo3a transcription factor independent of lipotoxicity and apoptosis. J Hypertens 24: 549–561, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Ryan TD, Rothstein EC, Aban I, Tallaj JA, Husain A, Lucchesi PA, Dell′Italia LJ. Left ventricular eccentric remodeling and matrix loss are mediated by bradykinin and precede cardiomyocyte elongation in rats with volume overload. J Am Coll Cardiol 49: 811–821, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Sakata S, Lebeche D, Sakata Y, Sakata N, Chemaly ER, Liang L, Nakajima-Takenaka C, Tsuji T, Konishi N, del Monte F, Hajjar RJ, Takaki M. Transcoronary gene transfer of SERCA2a increases coronary blood flow and decreases cardiomyocyte size in a Type 2 diabetic rat model. Am J Physiol Heart Circ Physiol 292: H1204–H1207, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Sheu JJ, Chang LT, Chiang CH, Sun CK, Chang NK, Youssef AA, Wu CJ, Lee FY, Yip HK. Impact of diabetes on cardiomyocyte apoptosis and connexin43 gap junction integrity. Int Heart J 48: 233–245, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Shiomi T, Tsutsui H, Ikeuchi M, Matsusaka H, Hayashidani S, Suematsu N, Wen J, Kubota T, Takeshita A. Streptozotocin-induced hyperglycemia exacerbates left ventricular remodeling and failure after experimental myocardial infarction. J Am Coll Cardiol 42: 165–172, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Son NH, Park TS, Yamashita H, Yokoyama M, Huggins LA, Okajima K, Homma S, Szabolcs MJ, Huang LS, Goldberg IJ. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J Clin Invest 117: 2791–2801, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res 52: 313–320, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Terman A, Dalen H, Eatin J, Neuzil J, Brunk U. Aging of cardiac myocytes in culture: oxidative stress, lipofuscin accumulation, and mitochondrial turnover. Ann NY Acad Sci 1019: 70–77, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Tribouilloy C, Rusinaru D, Mahjoub H, Tartiere JM, Kesri-Tartiere L, Godard S, Peltier M. Prognostic impact of diabetes mellitus in patients with heart failure and preserved ejection fraction. A prospective five-year study. Heart 94: 1450–1455, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Varma S, Owen R, Smucker M, Feldman M. Is tau a preload-independent measure of isovolumetric relaxation? Circulation 80: 1757–1765, 1989. [DOI] [PubMed] [Google Scholar]
- 48.Vasanji Z, Cantor EJF, Juric D, Moyen M, Netticadan T. Alterations in cardiac contractile performance and sarcoplasmic reticulum function in sucrose-fed rats is associated with insulin resistance. Am J Physiol Cell Physiol 291: C772–C780, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Wen JG, Frokiaer J, Jorgensen TM, Djurhuus JC. Obstructive nephropathy: an update of the experimental research. Urol Res 27: 29–39, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Williamson DF Weight loss and mortality in persons with type-2 diabetes mellitus: a review of the epidemiological evidence. Exp Clin Endocrinol Diabetes 106 Suppl 2: 14–21, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Wilson CR, Tran MK, Salazar KL, Young ME, Taegtmeyer H. Western diet, but not high fat diet, causes derangements of fatty acid metabolism and contractile dysfunction in the heart of Wistar rats. Biochem J 406: 457–467, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woodman RJ, Watts GF. Measurement and application of arterial stiffness in clinical research: focus on new methodologies and diabetes mellitus. Med Sci Monit 9: RA81–RA89, 2003. [PubMed] [Google Scholar]
- 53.Yin FC, Spurgeon HA, Rakusan K, Weisfeldt ML, Lakatta EG. Use of tibial length to quantify cardiac hypertrophy: application in the aging rat. Am J Physiol Heart Circ Physiol 243: H941–H947, 1982. [DOI] [PubMed] [Google Scholar]
- 54.Yu X, Tesiram YA, Towner RA, Abbott A, Patterson E, Huang S, Garrett MW, Chandrasekaran S, Matsuzaki S, Szweda LI, Gordon BE, Kem DC. Early myocardial dysfunction in streptozotocin-induced diabetic mice: a study using in vivo magnetic resonance imaging (MRI). Cardiovasc Diabetol 6: 6–13, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang F, Ye C, Li G, Ding W, Zhou W, Zhu H, Chen G, Luo T, Guang M, Liu Y, Zhang D, Zheng S, Yang J, Gu Y, Xie X, Luo M. The rat model of type 2 diabetic mellitus and its glycometabolism characters. Exp Anim 52: 401–407, 2003. [DOI] [PubMed] [Google Scholar]