Abstract
Growing evidence indicates that nitrite, NO2−, serves as a circulating reservoir of nitric oxide (NO) bioactivity that is activated during physiological and pathological hypoxia. One of the intravascular mechanisms for nitrite conversion to NO is a chemical nitrite reductase activity of deoxyhemoglobin. The rate of NO production from this reaction is increased when hemoglobin is in the R conformation. Because the mammalian fetus exists in a low-oxygen environment compared with the adult and is exposed to episodes of severe ischemia during the normal birthing process, and because fetal hemoglobin assumes the R conformation more readily than adult hemoglobin, we hypothesized that nitrite reduction to NO may be enhanced in the fetal circulation. We found that the reaction was faster for fetal than maternal hemoglobin or blood and that the reactions were fastest at 50–80% oxygen saturation, consistent with an R-state catalysis that is predominant for fetal hemoglobin. Nitrite concentrations were similar in blood taken from chronically instrumented normoxic ewes and their fetuses but were elevated in response to chronic hypoxia. The findings suggest an augmented nitrite reductase activity of fetal hemoglobin and that the production of nitrite may participate in the regulation of vascular NO homeostasis in the fetus.
Keywords: kinetics, nitric oxide, vascular control, fetus
endothelium-derived nitric oxide (NO), a potent vasodilator, plays an important role in the maintenance of basal vascular tone in the mammalian fetus (20, 21, 24, 29, 55). From the endothelium, NO diffuses either abluminally into the smooth muscle to produce hyperpolarization with vasodilation or luminally into the blood. In blood, free NO is metabolized rapidly by several means including oxidation to nitrite (NO2−) in the plasma (54) and reaction with oxyhemoglobin (oxyHb) to produce nitrate (NO3−) and methemoglobin (MetHb) (16, 25) and by binding to the heme centers of deoxyhemoglobin (deoxy-Hb) to produce iron nitrosyl hemoglobin (HbNO) (11, 41).
The rate of NO reaction with oxyHb and deoxy-Hb is exceptionally rapid, and although encapsulation of hemoglobin within the erythrocyte slows NO access to hemoglobin by nearly 1,000-fold (34, 35), the half-life of free NO in blood is only fractions of a second (9, 26). As a result, physiological concentrations of free NO in blood are maintained in the subnanomolar range, making the measurement of free NO in blood impractical by currently available methods.
Nitrite and nitrate concentrations are often measured together and used as an index of NO in biological fluids. However, because nitrate concentrations are manyfold higher than nitrite and are influenced by diet, bacterial nitrate synthesis, denitryfing liver enzymes, and renal function, the nitrite concentration alone provides a more specific and accurate index of endothelial NO production (32, 33).
Until recently, nitrite was thought to be biologically inert at physiological concentrations. However, accumulating evidence now suggests that nitrite can be reduced to NO under hypoxic/ischemic conditions by one of several biochemical reactions including those catalyzed by xanthine oxidase, NO synthase, myoglobin, and hemoglobin and by nonenzymatic disproportionation (37).
The production of NO by reaction of nitrite with deoxy-Hb is as follows:
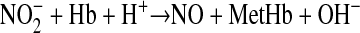 |
(1) |
In addition, a second reaction of nitrite with MetHb and NO has now been characterized that generates dinitrogen trioxide (N2O3), an NO-generating species that is capable of diffusional escape from the red blood cell, as shown (4):
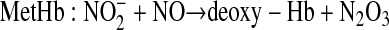 |
(2) |
These two reactions are potentially of great importance to the fetus because they provide an oxygen-sensitive mechanism for NO production in the vasculature and thus may play an important role in maintaining the low resistance to blood flow that is characteristic of the fetal circulation. The physiological relevance of these reactions is supported by studies demonstrating the hypoxia-sensitive vasodilatory effects of nitrite in the rat aorta (12, 31), human forearm (12, 13, 39), primate (46), rat brain (49), and sheep lung (30).
Based on the recent evidence that the nitrite-reductase properties of deoxy-Hb provide a physiological source of vasoactive NO and that the mammalian fetus is susceptible to hypoxic/ischemic insults, we thought it important to characterize the reaction rate of nitrite with fetal versus adult hemoglobin. In keeping with the law of mass action, the rate of NO production from reaction 1 is related directly to the concentrations of its substrates (nitrite, deoxy-Hb, and H+). However, the rate also is influenced by the allosteric R-T conformation of hemoglobin, being favored by the R (oxygenated) state as opposed to the T (deoxygenated) state (27, 28). As a result, the overall reaction rate varies by as much as 60-fold depending upon the level of hemoglobin oxygenation and approaches a maximum when O2 tensions approximate the P50 for O2 binding with hemoglobin (27, 28). Because normal fetal arterial oxyHb saturations (∼70–75%) are closer to P50 than those of the adult (∼98%), it is reasonable to hypothesize that the reaction between nitrite and deoxy-Hb is a more significant source of NO in the fetus than in the adult. In addition, biophysical data suggest that the increased nitrite reductase activity of R-state hemoglobin is driven by its low heme redox potential compared with the T state (22, 28). Therefore, because fetal hemoglobin is known to assume the R state more readily than adult hemoglobin (5), the above hypothesis can be further extended to state that fetal blood reduces nitrite more effectively than adult blood even at comparable oxyHb saturations.
The present study was undertaken to test the hypothesis that fetal sheep hemoglobin (sHbF) is a more efficient nitrite reductase than adult sheep hemoglobin (sHbA). We present experiments using purified hemoglobins and whole blood across a range of oxygen saturations, pH, and nitrite concentrations. In addition, we report the first measurements of nitrite in blood from chronically instrumented fetal and maternal sheep maintained under normoxic or chronically hypoxic conditions.
MATERIALS AND METHODS
All procedures involving animals were approved by the Loma Linda University Institutional Animal Care and Use Committee and adhered to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. All materials and chemicals were obtained from Sigma (Sigma-Aldrich, St. Louis, MO) unless otherwise specified.
Preparation of purified hemoglobins.
Purified hemoglobin solutions were prepared from blood lysed by three freeze-thaw cycles. Following equilibration of G-25 Sephadex columns with 3 vol of 0.1 mM PBS (pH 7.35), hemolysates were eluted through the column with the PBS buffer. Concentrations were determined using the Drabkin method (57), and hemoglobin was diluted to 4.0 mM (heme basis) by addition of PBS buffer.
To compare the oxygen affinities of sHbA and sHbF in PBS buffer, hemolysates were equilibrated by tonometry to a PO2 of 25 ± 3 Torr. Lysates were then transferred anaerobically to a quartz cuvette (80 μl, 1 cm pathlength) and absorptions measured at wavelengths from 450 to 700 nm (Cary 50 spectrophotometer; Varian, Palo Alto, CA). The resulting spectra were analyzed by global comparison to basis spectra for oxyHb, deoxy-Hb, and MetHb prepared from pure samples of either sHbF or sHbA. Samples with >2% MetHb were discarded from analysis.
When necessary, sHbA and sHbF were made anaerobic in a glovebox under a 2% to 4% H2 atmosphere of catalyst-deoxygenated nitrogen. Hemoglobins were reduced to the Fe2+ state by incubation with excess sodium hydrosulfite (dithionite) that was removed by passage through a sephadex G-25 column (Amersham Biosciences). Proteins were collected directly in quartz cuvettes and sealed inside the glovebox.
Anaerobic reactions of hemoglobins with excess nitrite.
UV-visible spectra and kinetic data were recorded on a HP8453 UV-Vis spectrophotometer (Hewlett-Packard) using quartz or special optical glass cuvettes with a 1-cm path length. Sheep Hb standard species for spectral deconvolution were prepared as previously described (23). Purified sHbA or sHbF (1 to 2 mM) samples were diluted with anaerobic phosphate buffer (100 mM; pH = 7.4) to indicated concentrations before each reaction. Oxygen saturation and concentration of sHbA and sHbF heme species were measured by visible absorption spectroscopy followed by the deconvolution of the spectrum by multiple linear regression analysis using standard spectra of the individual components (28). Solutions of 100 mM dithionite and 100 mM nitrite were prepared with helium degassed phosphate buffer and kept under helium. It is worth noting that although the use of spectrophotometry to study this reaction requires the use of nitrite concentrations in excess of hemoglobin, the reaction stoichiometry under these conditions is similar to that observed under more physiological conditions in whole blood and with hemoglobin in excess (12, 27).
Reaction kinetics of 50 or 100 μM fetal or adult deoxy-Hb (all Hb concentrations in terms of heme) with nitrite (1, 2.5, 5, and 10 mM) were monitored by absorption spectroscopy for the indicated time in a cuvette with (Fig. 1) or without (Fig. 2) 2.5 mM sodium dithionite. All reactions were run at 25°C in 0.1 M phosphate buffer (pH 7.4). Nitrite was added, using an airtight syringe, to a sealed anaerobic cuvette to initiate the reaction. Oxygen contamination was prevented by the application of positive helium pressure without a channel for gas escape.
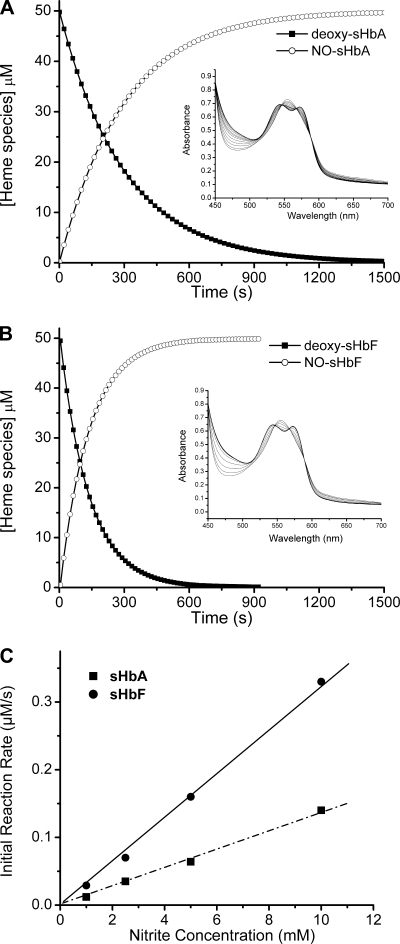
Fig. 1.
Reaction of nitrite with deoxy fetal sheep hemoglobin (deoxy-sHbF) and adult sheep hemoglobin (deoxy-sHbA). A: disappearance of sheep adult deoxyhemoglobin (deoxy-Hb) and the formation of iron nitrosyl-hemoglobin over time in the reaction of 50 μM deoxy-Hb with 10 mM nitrite at pH 7.4 and 25°C. Inset: spectra collected during the reaction selected at regular intervals of 60 s. B: same as in A for sHbF. C: initial rate of fetal and adult deoxy-Hb consumption for the reaction of 50 μM with increasing concentrations of nitrite (1–10 mM) at pH 7.4 and 25°C. All Hb concentrations are reported on a heme basis. NO, nitric oxide.
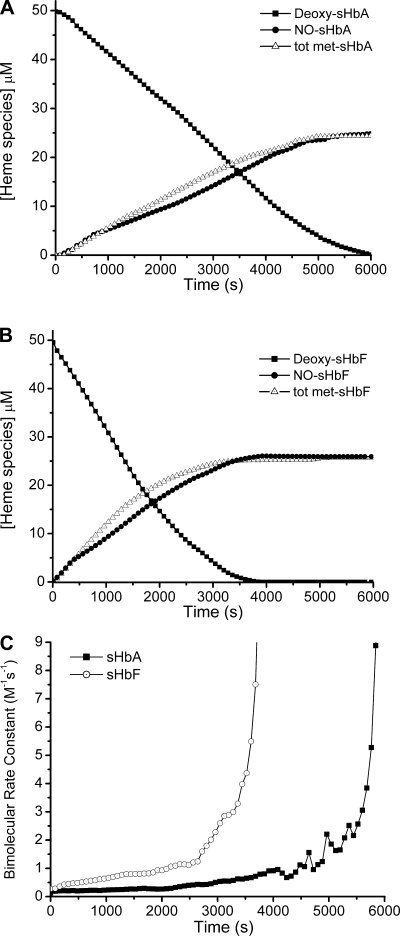
Fig. 2.
Reaction of anaerobic deoxy-sHbF and -sHbA with 1 mM nitrite in the absence of dithionite. A and B: disappearance of sheep adult and fetal deoxy-Hb and the formation of iron nitrosyl hemoglobin and total methemoglobin (Tot Met)-Hb over time in the reaction of 50 μM deoxy-Hb with 1 mM nitrite at pH 7.4 and 25°C. C: instantaneous bimolecular rate constants over time for the reaction in A and B calculated as the instantaneous reaction rate divided by the concentrations of deoxy-Hb and nitrite at that instant.
All reactions were analyzed using ProK software provided by Applied Photophysics (Surrey, UK). The data in Fig. 1 were analyzed using singular value decomposition followed by fitting of the reduced data matrix to a first-order kinetic model at all collected wavelengths and time points for determination of reaction half-times. ProK was also used to obtain initial bimolecular rate constants, fitting to a pseudo first-order process. The initial reaction rates were estimated from the slope of the tangent to the fitted deoxy-Hb decay curve at t = 0.
Nitrite metabolism in purified hemoglobin.
The oxygen saturations of fetal and adult purified hemoglobin solutions were adjusted to 50 ± 5% by tonometry with gas containing either 2% to 5% CO2 in a balance of N2 or air, as needed to lower or raise oxygen tensions, respectively. Gas tensions were adjusted in airtight containers sealed with a rubber septa to allow for anaerobic injection of nitrite and withdrawal of samples. Sodium nitrite dissolved in saline (0.17 ml of 0.2 mg/ml into 10 ml of hemoglobin solution) was injected anaerobically to achieve an initial nitrite concentration of 48 μM. Samples (0.4 ml each) were collected for measurement of nitrite concentrations at baseline and 1.5, 3, 6, 10, 15, 20, 40, 60, and 80 min following nitrite injection.
Nitrite metabolism in whole blood.
To compare adult and fetal nitrite metabolism as directly as possible, hemoglobin concentrations in whole blood obtained from low altitude maternal and fetal sheep were adjusted to 3.1 mM (heme basis) by the addition of normal saline. OxyHb saturations were adjusted to ∼50% by tonometry, and pH was adjusted to ∼7.4.
To measure the rates of nitrite metabolism and MetHb production, blood was placed in airtight flasks and equilibrated to 39°C (normal adult sheep body temperature) in a water bath. Following collection of baseline samples, sodium nitrite dissolved in saline (0.5 ml of 2 mg/ml into 30 ml of whole blood) was injected anaerobically into each flask and rapidly mixed to achieve an initial concentration of ∼480 μM. Samples for nitrite (0.4 ml) and MetHb (0.1 ml) measurement were collected at baseline and 1.5, 3, 6, 10, 15, 20, 40, 60, and 80 min following nitrite injection.
To study the effects of oxyHb saturation on initial rates of MetHb production, we performed similar experiments at oxyHb saturations ranging from <10% to >99% in both the fetal and adult samples. To study the effects of pH on initial rates of MetHb production, experiments were performed at oxyHb saturations of ∼70% in both the fetal and adult samples with pH adjusted, by the addition of HCl or NaOH, to vary between 7.0 and 7.7. Total hemoglobin and MetHb determinations were performed by hemoximetry (OSM 3; Radiometer, Copenhagen, Denmark), with initial production rates taken as the difference between concentrations at baseline and 3 min following the addition of nitrite.
For comparison of fetal and adult rates across the range of oxygen saturations, data were fit to a second-order polynomial equation (Prism v5.0 for Mac OS X; GraphPad Software). For comparison of fetal and adult rates across the studied range of pH, data were analyzed by linear regression and the slopes were compared using Prism v5.0.
HbNO production in whole blood.
As a measure of NO production from nitrite and hemoglobin in whole blood, we performed additional experiments to measure HbNO production following the addition of nitrite to fetal or maternal whole blood at 39°C with 3.1 mM heme, pH 7.4, and oxyHb saturations of ∼10% by methods described above. Aliquots (0.5 ml each) were collected for the measurement of HbNO at baseline and 30 s following the addition of nitrite to an initial concentration of 480 μM.
HbNO concentrations were determined by electron paramagnetic resonance (EPR) spectroscopy. Samples were snap frozen in quartz EPR tubes by submersion in liquid nitrogen immediately upon collection (<30 s) and stored on dry ice or at −70°C until assay. EPR was performed at 110K using a Bruker 4131VT temperature controller on an EMX 10/12 EPR spectrometer system (Bruker, Billerica, MA) at 9.4 GHz, 10 mW, 5 G modulation, 0.08-s time constant, and 84-s scan time over 600 G. Concentrations of HbNO were calculated by comparing the double integrals of the spectra with a standard sample.
Comparison of maternal and fetal blood nitrite concentrations.
Under inhalational anesthesia, pregnant mixed Western-breed ewes and their fetuses were instrumented with catheters in the brachial artery and veins as previously described (6, 56). At least 4 days following surgery, blood was collected into heparinized syringes from the ewes and their fetuses for nitrite analysis as described in Nitrite assay. Fetal age at the time of collection ranged from 125 to 135 days gestation (term, 147 ± 1 days). To assess the effects of chronic hypoxia on blood nitrite concentrations, we collected samples from sheep that had been quartered at low elevation (335 m) throughout pregnancy and from those maintained at high elevation (3,801 m) from 30 days of gestation onward, as previously described (45).
Nitrite assay.
Nitrite measurements were made using triiodide-based chemiluminescence as previously described (7, 44). The NO signal was quantified using NOANALYSIS 3.21 Liquid Software (Sievers). This method measures HbNO, NO, and nitrite combined but does not detect nitrate (44).
Data analyses.
Rate constants for the disappearance of nitrite from whole blood and purified hemoglobin were determined by fitting the data to a monoexponential decay equation (Prism v5.0 for Mac OS X; Graphpad Software). To detect significant changes from baseline after the addition of nitrite, one-way ANOVA was performed with Tukey's and Bonferroni's post hoc analysis to detect significance at specific time points. To detect significant differences between fetus and ewe and the effects of long-term hypoxic exposure, a two-way ANOVA was performed with Bonferroni's post hoc analysis (Prism v5.0). Data are presented as means ± SE.
RESULTS
Oxygen affinities of sHbA and sHbF.
Oxygen saturations of purified sHbA and sHbF in PBS at a PO2 of ∼25 Torr are compared in Table 1. At comparable oxygen tensions, sHbF had a characteristically higher oxygen affinity than adult hemoglobin.
Table 1.
Comparison of O2 affinities for purified sHbA and sHbF
| Po2, Torr | HbO2, % | |
|---|---|---|
| sHbA | 25.4±0.9 | 41.6±1.9 |
| sHbF | 24.3±0.5 | 60.4±1.2* |
Values are means ± SD; n = 3 experiments/group.
Significant difference from adult sheep hemoglobin (sHbA) (P = 0.015, unpaired t-test). sHbF, fetal sheep hemoglobin.
Reactions of nitrite with sHbA and sHbF in the presence and absence of dithionite.
As has been shown previously, nitrite reacts with deoxy-Hb, forming HbNO and MetHb (28). However, in the presence of excess sodium dithionite the newly generated MetHb is readily reduced to deoxy-Hb that once more reacts with nitrite (22, 51). We used this method to compare directly the reactivity of sHbA and sHbF with nitrite in anaerobic conditions. We tested the possibility that sodium dithionite directly reduces nitrite to NO and found no significant reactivity within the time frame of these reactions up to a concentration of 10 mM dithionite, a finding consistent with previous work (22, 51).
In Fig. 1, A and B, 50 μM adult and fetal deoxy-Hb (concentrations in terms of heme), respectively, were reacted with 10 mM nitrite in the presence of 2.5 mM dithionite at 25°C. The insets contain selected spectra collected between 450 and 700 nm every 60 s during the reaction. The reaction spectra show well-defined isobestic points indicating the presence of two major species identified as deoxy-Hb and HbNO. The concentrations of the single species (black and white circles for deoxy-Hb and HbNO, respectively) at each time point were obtained using global analysis of spectra and were plotted as a function of time. With the use of this method, the data could be fitted well by either a single exponential (first-order reaction rate) or a sigmoidal function [consistent with an apparent zero-order allosteric modulation of the reaction rate (both R2= 0.99) (28)].
Figure 2 shows the concentration of deoxy-Hb, nitrosyl-Hb, and total MetHb as a function of time measured by the deconvolution of spectra obtained for the reaction of 50 μM adult and fetal deoxy-Hb, respectively (Fig. 2, A and B), with 1 mM nitrite in the absence of dithionite. At this low nitrite concentration (compared with 10 mM shown in Fig. 1), under anaerobic conditions the shape of the curves demonstrates a sigmoidal kinetic profile characterized by an early lag (slow) phase, followed by a rapid linear phase, and finally another slow phase. As previously shown for this reaction (27), we obtained equimolar formation of MetHb and HbNO. These species are known to increase the degree of R-state stabilization of the remaining free ferrous hemes and correlate with an increase of the bimolecular rate of reaction. In Fig. 2C, we compared the instantaneous bimolecular rate constant for sHbA and SHbF. Both present an exponentially propagating curve, however, fetal Hb has on average a twofold higher value. In addition, under strictly anaerobic conditions, sheep hemoglobin reaction with nitrite demonstrates the same allosteric autocatalytic behavior found for human hemoglobin (27, 28).
sHbF is a more efficient nitrite reductase than sHbA.
We compared the efficiencies of nitrite reduction by deoxy-Hb by varying the concentration of nitrite (1–10 mM) at constant Hb concentration. In Fig. 1C, the initial rate of deoxy-Hb consumption, at constant Hb concentration, is plotted as a function of nitrite concentration. The initial reaction rates are directly proportional to the concentration of nitrite, as expected from previous reports (27, 28), resulting in a linear increase in reaction rate. However, in all cases tested, sHbF was found to possess about twofold faster initial reaction rates. In Table 2, we report the calculated half-time, t1/2, corresponding to the time required to consume one-half of the deoxy-Hb. The data confirm that the deoxy-Hb reactivity is directly proportional to the concentration of nitrite.
Table 2.
Effect of nitrite concentration on the rate of reaction of sHbA and sHbF
| [NO2−], mM |
t1/2, s |
|
|---|---|---|
| sHbA | sHbF | |
| 1.0 | 2,621±330 | 1,168±153 |
| 2.5 | 1,120±104 | 572±38 |
| 5 | 673±56 | 208±13 |
| 10 | 202±11 | 97±5 |
Data are means ± SD of 3 separate mixing experiments. The observed time (t1/2) corresponding to the time required to convert one-half of the reactant, deoxy-Hb, to the product, iron nitrosyl hemoglobin, is inversely proportional to the concentration of nitrite.
An average bimolecular rate constant was calculated using the ratio between the slope of the linear fit and the deoxy-Hb concentration. Nitrite concentrations, which were in excess, were assumed to remain constant. The rate constants for sHbA and sHbF were 0.28 ± 0.04 and 0.68 ± 0.07 M−1s−1, respectively.
Metabolism of nitrite in hemoglobin solutions and whole blood.
We compared the rate of nitrite metabolism in five fetal and five adult whole blood samples in vitro after comparable concentrations of hemoglobin (fetal, 3.10 ± 0.06 mM; and adult, 3.08 ± 0.06 mM), pH (fetal, 7.41 ± 0.02; and adult, 7.40 ± 0.01), and oxyHb saturations (fetal, 48 ± 4%; and adult, 51 ± 3%) had been achieved. As shown in Fig. 3A, following injection of nitrite to an initial concentration of 480 μM, nitrite concentrations decreased significantly faster in fetal compared with adult blood. Although the reduction of nitrite by deoxy-Hb is second-order overall (first order in nitrite and first order in hemoglobin), the rate of nitrite disappearance in these experiments was pseudo first-order in nitrite due to excess hemoglobin concentrations. Over the 80-min period of study, the half-life of nitrite in fetal blood averaged 2.5 ± 0.2 min, significantly shorter than the half-life of 8.4 ± 0.8 min in adult blood (P < 0.001). The bimolecular rate constants, determined from the rate of nitrite disappearance over the first 3 min of the reaction and 1.55 mM deoxyheme concentration (3.1 mM heme at 50% oxygen saturation), were 4.2 ± 0.8 M−1s−1 and 1.5 ± 0.6 M−1s−1 for fetal and adult whole blood, respectively (P < 0.01). As shown in Fig. 3B, MetHb concentrations also increased more rapidly following the addition of nitrite to fetal compared with adult blood. Thereafter, MetHb concentrations reached a plateau at similar values and then decreased more rapidly in fetal blood. This finding was anticipated due to the relatively high activity of MetHb reductase enzymes in fetal sheep blood (47).
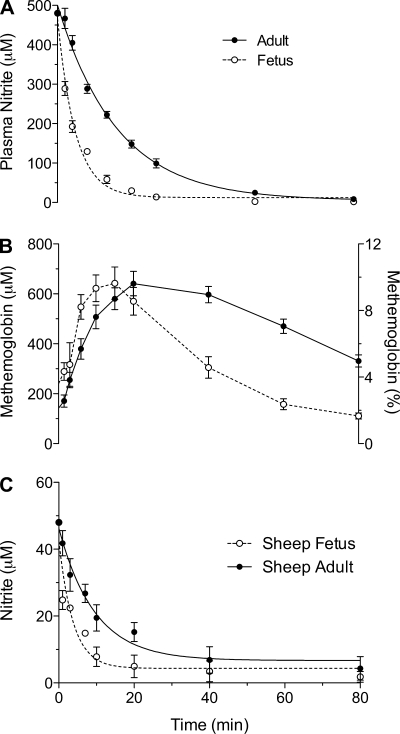
Fig. 3.
Nitrite metabolism in fetal and adult whole blood and purified Hb. A: time course of disappearance of nitrite from maternal and fetal whole blood measured in vitro at 39°C after Hb concentrations; oxyHb saturations and pH are adjusted to be comparable. At time zero, 7.2 μmol of sodium nitrite are introduced to 10 ml blood. Nitrite disappears from fetal blood with an apparent half-time of 2.6 ± 0.9 min, significantly shorter than maternal blood, 6.7 ± 1.8 min (P < 0.001). B: more rapid initial increase in MetHb in fetal blood and a subsequently more rapid return to baseline dependent on high fetal MetHb reductase activity. C: time course of the disappearance of nitrite introduced into purified Hb solutions. The loss of nitrite is 1.9-fold faster in fetal Hb solutions than in maternal solutions.
In addition, as noted in Fig. 3C, nitrite disappearance was more rapid in purified hemoglobin solutions prepared from fetal as opposed to maternal blood. After the introduction of nitrite to an initial concentration of 48 μM, the rate of disappearance was 1.9-fold faster in sHbF solutions (P < 0.01). The disappearance again was pseudo first-order in nitrite, and the apparent half-times for sHbF and sHbA were 2.6 ± 0.9 min and 6.7 ± 1.8 min, respectively.
Effect of oxygenation and pH on MetHb production.
The initial rates of MetHb production as a function of oxyHb saturation are shown in Fig. 4A. An optimal HbO2 saturation was observed for both maternal and fetal blood, with highest rates occurring in the 50–70% range of HbO2 saturation and in the oxygen tension range of 15 to 50 Torr. Notably, within this range initial MetHb production rates averaged more than twofold higher in fetal than in adult blood. Fit of the fetal and adult data to a second-order polynomial for the sake of comparison resulted in an R2 of 0.80 and 0.68, respectively, with fetal rates reaching a peak of 58.8 ± 5.5 μmol/min at 57% HbO2 and adult rates reaching a peak of 24.2 ± 1.8 μmol/min at 60% HbO2.
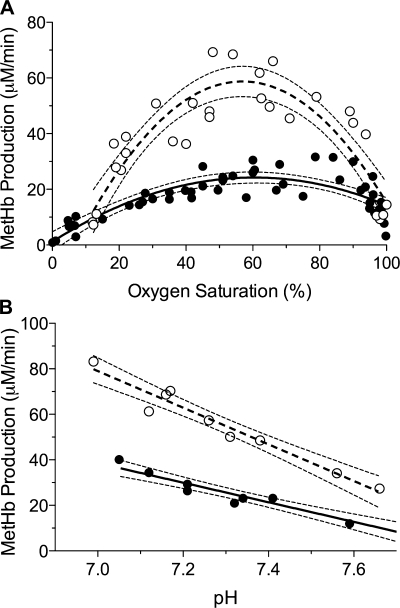
Fig. 4.
Rate of MetHb production in vitro at varying oxyHb saturations and pH. The initial rate of MetHb production in whole fetal (○) and adult (•) blood after introducing nitrite (1.44 mM initial concentration) is taken as a useful index of rates of nitrite reduction and NO formation. A: rates for fetal blood exceed those for adult blood by nearly 2-fold, and both rates are maximal in the 55–65% oxyHb saturation range. Lines and 95% confidence intervals represent a best-fit second-order polynomial equation with R2 = 0.90 for fetus (dashed line) and 0.80 for adult (solid line). B: in both fetal and adult blood, the rate of MetHb production was inversely proportional to pH, with slopes of −5.2 ± 0.5 and −4.0 ± −0.4, respectively (P = 0.06).
The effect of pH on the rates of nitrite metabolism by whole fetal and adult sheep blood in vitro is demonstrated in Fig. 4B. An inverse relationship between pH and the rate of nitrite disappearance was observed for both fetal and adult blood (R2 = 0.95 and 0.93, respectively). pH tended to have a greater effect on fetal compared with adult rates, demonstrated by the marginal difference in linear regression slopes (−5.2 ± 0.5 and −4.0 ± 0.4, respectively), but the difference was not statistically significant (P = 0.06).
HbNO production in whole blood.
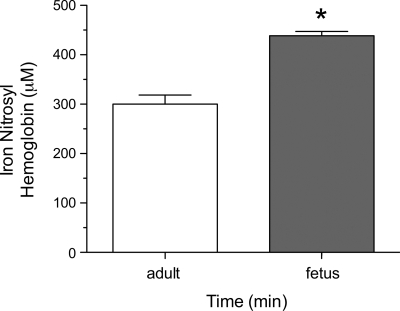
The data in Fig. 5 represent the production of HbNO following the addition of nitrite (480 μM initial concentration) to whole fetal and adult sheep blood at ∼10% oxyHb saturation and pH 7.4. Baseline HbNO concentrations were <1 μM in both fetal and adult blood. Thirty seconds following nitrite addition, fetal concentrations (438 ± 8 μM; n = 3) were significantly higher than adult concentrations (300 ± 18 μM; n = 3; P < 0.01), indicating a more rapid reaction of nitrite with fetal compared with adult hemoglobin.
Fig. 5.
Production of iron nitrosyl hemoglobin after introduction of nitrite into adult or fetal whole blood. Thirty seconds following addition of nitrite (500 μM initial concentration) to whole blood at ∼10% HbO2, fetal (n = 3) iron nitrosyl hemoglobin concentrations, measured by electron paramagnetic resonance, are significantly greater than those measured in adult blood (n = 3; *significant difference from adult, P < 0.01).
Fetal and maternal blood nitrite concentrations and the effect of chronic hypoxia.
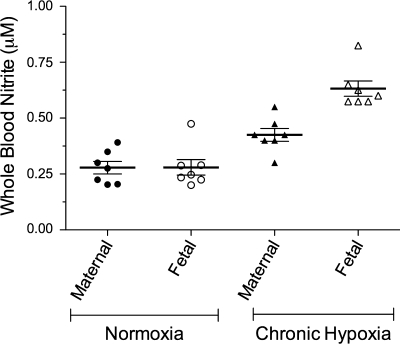
We measured nitrite concentrations in blood from seven low-altitude and seven high-altitude maternal-fetal pairs. Average concentrations for the ewes and fetal sheep are shown in Fig. 6 for normoxic and chronically hypoxic groups. In the low-altitude group, maternal and fetal concentrations were not measurably different. However, in the high-altitude group, fetal nitrite concentrations were significantly higher in fetal blood compared with that of the ewes (0.60 ± 0.05 vs. 0.38 ± 0.01 μM, fetal, maternal, respectively). In addition, high altitude was associated with a significant increase in nitrite concentrations of both ewes and fetuses compared with their low-altitude counterparts, suggesting a hypoxemic adaptive response.
Fig. 6.
Nitrite levels in whole blood samples taken from pregnant sheep and their fetuses at 125–135 days gestation. Maternal and fetal concentrations are comparable in low altitude sheep (normoxia). At high altitude (chronic hypoxia; maternal arterial Po2, 50–60 Torr), both fetal and maternal nitrite concentrations are significantly increased relative to their low altitude counterparts, and fetal nitrite concentrations are greater than those of their mothers.
DISCUSSION
In the present study, we present evidence that nitrite is reduced to NO by fetal sheep blood at a rate twice that of the adult even when hemoglobin concentration, oxyHb saturations, pH, temperature, and nitrite are controlled. This property is demonstrated to be intrinsic to the fetal hemoglobin molecule itself. In addition, consistent with previous reports for adult human hemoglobin, the reduction of nitrite by sHbF and sHbA is shown to be dependent upon hemoglobin allosteric conformation, such that the reaction approaches a maximum at ∼50% oxyHb saturation. Despite this more rapid metabolism of nitrite in fetal blood, basal levels of nitrite did not differ when collected from ewes and their fetuses living under normoxic conditions. This combination of findings indicates there is increased constituent production of nitrite in the fetus that balances its continuing more rapid loss and is consistent with an increased rate of NO and nitrite production in the fetus. The findings also suggest that nitrite, by reaction with deoxy-Hb, is an important source of NO in the fetus and, as such, plays an important role in maintaining vascular relaxation and low resistance to flow that characterize the fetal circulation. The elevated baseline levels of nitrite in chronically hypoxic fetuses suggest a yet further increased role for nitrite during long-term hypoxic stress.
Reaction of nitrite with hemoglobin.
The reaction between nitrite and deoxy-Hb was first described by Brooks (8) in 1935 and was investigated further by Doyle et al. (15) in 1981. At that time this reaction was considered to be of biological significance only following the ingestion of toxic amounts of nitrite. Recently, however, a growing number of reports have provided evidence that nitrite, at near physiological concentrations, is a hypoxia-dependent source of vasoactive concentrations of NO in the human forearm (10, 12, 13), primate (46), rat brain (49), pulmonary vasculature of the newborn lamb (30), and in isolated arterial ring bioassays (53).
When oxygen concentrations are controlled, the initial rate of reaction between nitrite and deoxy-Hb (reaction 1) bears a direct relationship to the concentrations of the reactants. However, the reaction rate is also modulated by allosteric changes in the hemoglobin molecule, proceeding more rapidly when hemoglobin is in the R state compared with the T state (27, 28). Because the R state is favored by the binding of oxygen to hemoglobin, which by definition decreases the relative concentration of deoxyheme centers available to react, the allosteric effects on the reaction rate are in opposition to the deoxy-Hb mass action effects. As a result, the maximal reaction rate is thought to occur when the oxyHb saturation is ∼50% (27, 28). The current experiments support this concept for both fetal and adult sheep whole blood in that the initial rate of MetHb production was observed to be maximal as HbO2 approached 50% (Fig. 4A).
It is worth noting that the difference between fetal and adult whole blood rates of MetHb production disappears at very high and low oxygen saturations (Fig. 4A). This is in contrast with our study of purified hemoglobins in excess nitrite under conditions of anoxia (Figs. 1 and 2). It is possible that fetal MetHb reductase enzymes more effectively reduce MetHb compared with the adult, causing an underestimation of MetHb production. However, this is not likely given the relatively slow rate of these enzymes compared with the rate of nitrite reaction with hemoglobin (2 to 3 orders of magnitude difference) (47). It is also possible that fetal MetHb production is underestimated due to the reaction with nitrite and NO, which results in the reduction of MetHb back into the ferrous state (reaction 2 in introduction) as recently described (4, 18). This possibility is not supported by the experiments using sodium dithionite to maintain anoxia (Fig. 1). Dithionite does not react with nitrite, nor does it alter the kinetics of the initial reaction between nitrite and deoxy-Hb (22, 51). However, because it prevents MetHb from accumulating by rapidly reducing it to deoxy-Hb, the influence of reaction 2 over deoxy-Hb and MetHb concentrations would be minimal, and N2O3 production would be prevented. Finally, there is the possibility that the results of experiments conducted using purified hemoglobin and excess nitrite do not accurately describe the reactions that take place in whole blood due to the compartmentalization of the hemoglobin within the red blood cell. This possibility seems to contradict the calculated bimolecular rate constants of the purified hemoglobin and whole blood experiments in the present studies. This comparison demonstrates that the whole blood reaction rates might have been predicted with reasonable accuracy based on the expected increase in whole blood bimolecular rates (28) due to these experiments being performed at 50% oxyHb saturation versus 0% for the purified hemoglobin experiments. Nonetheless, it is worth noting that others have demonstrated evidence of a putative NO-labile intermediate during the reaction of purified deoxy-Hb with nitrite (2, 36, 49). This intermediate appears to be quenched as nitrite-to-hemoglobin ratios approach 1. Although it is expected that the production of this putative intermediate would be secondary to the primary reaction between nitrite and deoxy-Hb measured in the present studies, future studies at near-physiological nitrite-to-hemoglobin ratios are needed to assess its role in the fetus compared with the adult.
The question arises as to what mechanisms might account for the more rapid metabolism of nitrite by fetal compared with adult blood. The difference was observed even if pH, oxyHb saturation, hemoglobin concentration, and temperature were comparable in the two types of blood. sHbF, like that of humans, has γ-subunits rather than β-subunits. As a result of this substitution, the R state is preferentially favored in fetal hemoglobin, independent of pH and oxyHb saturations. Because nitrite reduction involves a one-electron oxidation of the heme iron, the ability of R state hemoglobin to react with nitrite more rapidly than T state hemoglobin is thought to be due to the lower heme redox potential of the R conformation (22, 28). Applied to the differences we observed between fetal and adult hemoglobin, it is possible that the fetal heme centers have an inherently lower redox potential than their adult counterparts, independent of allosteric conformation. However, this possibility is discounted by previous studies that have found no difference between the redox potentials of fetal and adult hemoglobins (1, 19). Thus it seems more likely that the increased efficiency of nitrite reduction by fetal hemoglobin observed in the current experiments is due to a larger portion of the fetal hemoglobins being in the R state at a given saturation and pH compared with adult hemoglobin.
Effect of pH.
The ratio of R and T states of both fetal and adult hemoglobins is influenced by pH, a phenomenon known as the Bohr effect. As a result, the stability of the T state increases as pH decreases, creating a direct relationship between oxygen affinity and pH. Within the range of physiological pH, the magnitude of the Bohr effect is thought to be equal in both fetal and adult hemoglobins (3, 40, 42, 50). Because H+ is a required substrate for nitrite reduction by hemoglobin (reaction 1), the effects of pH on the rate of nitrite reduction are due to both the Bohr effect, which decreases the reaction rate as pH falls by stabilizing the T state, and the stronger mass action effect of H+ concentration, which increases the rate as pH falls, resulting in an overall inverse relationship between pH and nitrite reductase activity (28). As shown in Fig. 4B, these factors combined result in a similar relationship between pH and nitrite reduction for fetal and adult blood across the physiological range of pH, although the effect was slightly more pronounced in fetal blood.
Maternal and fetal nitrite concentrations and chronic hypoxia.
To our knowledge, fetal blood nitrite concentrations have not been previously reported, although total nitrogen oxides (NO, nitrite, and nitrate, of which nitrite is typically <2%) have been compared in fetal and maternal blood with the finding of either no difference (14, 59) or elevated levels in the fetus (38, 43, 58). The present study revealed no difference between blood nitrite concentrations of healthy fetuses and their mothers. With the consideration that the present finding that fetal blood metabolizes nitrite twice as fast as that of the mother, it would appear that fetal endothelial NO synthase (eNOS) activity, the primary source of circulating NO2−, is greater than that of the adult. However, the characterization of the movement of nitrite between the maternal, placental, and fetal compartments is necessary to address this issue more fully.
The production of NO by NO synthase enzymes constitutes an important cardiovascular response of the fetus to acute hypoxic stress by augmenting the compensatory increase in blood flow to the brain and heart (29, 48, 52). The cardiovascular responses of the fetal sheep to chronic mild hypoxia include decreases in cardiac output along with decreased resistance to flow in the brain, heart, and adrenals, such that oxygen delivery to these organs is maintained at the expense of peripheral tissues. The role of NO in these chronic responses is not fully understood, although fetal whole body NO production also appears to be upregulated in response to chronic hypoxia, as evidenced by higher circulating concentrations of nitrite/nitrate in both ewes and fetuses acclimatized at high elevation (3,820 m) for the latter two-thirds of gestation compared with sea level controls (59). The current findings are in agreement since blood nitrite concentrations, which are a more accurate indication of eNOS activity than total nitrite and nitrate (33), were significantly elevated in both adult and fetal hypoxic sheep. The current findings are also consistent with recent reports from the Tibetan population demonstrating blood nitrite concentrations on the order of 10-fold greater than those of sea level populations (17). An increased rate of nitrite reduction to NO by fetal hemoglobin may serve to enhance effects of increased eNOS activity by increasing the production of NO via the nitrite reductase pathway, thereby increasing overall NO bioavailability.
Summary and Conclusions
Hemoglobin reduces nitrite to NO with the formation of MetHb, and this process is twice as rapid in fetal than in adult blood when studied under comparable conditions in vitro. The greater fetal rate is dependent on the properties of the fetal hemoglobin molecule rather than differences in plasma constituents, red cell membranes, pH, and 2,3-diphosphoglycerate levels. Furthermore, in both adult and fetal blood, this reaction proceeds most rapidly when hemoglobin is in the range of 40–60% oxyHb saturation. Therefore, the reduction of nitrite to NO in the fetus is favored by properties intrinsic to the hemoglobin molecule and by the fact that normal fetal arteriovenous oxyHb concentrations (∼75% to 45%) span the range where nitrite reduction is most favored.
Despite the more rapid metabolism of nitrite in fetal blood, its concentrations are comparable in normoxic ewes and their fetuses and even higher in chronically hypoxic fetuses compared with their mothers. Although this finding may indicate a rapid constituent production of nitrite in the fetus that balances the more rapid reduction to NO by hemoglobin, it may also be due to movement of nitrite to the fetus from the placenta or to decreased fetal tissue metabolism or renal clearance of nitrite, none of which have been studied in the fetus. Given the hypoxic in utero environment of the mammalian fetus (relative to the adult) and the recent paradigm that nitrite serves as a reservoir for NO bioactivity under hypoxic conditions, the current findings suggest that the characterization of the role of nitrite in regulating fetal NO homeostasis may provide insight into the remarkable tolerance of the fetus to hypoxic stress.
GRANTS
This work was partially supported by NIH Grants HL-058091 and HL-078706 (to D. B. Kim-Shapiro) and HD-03807 (to L. D. Longo).
Acknowledgments
We acknowledge the expert technical assistance of Shannon L. Bragg, Laura S. Kirby, and Douglas P. Hatran. A. B. Blood and G. G. Power designed the research, analyzed some of the data, and wrote the article. S. T. Verma and J. Lo performed some of the research experiments. L. D. Longo contributed blood samples from chronically instrumented sheep and contributed helpful suggestions to the article. M. S. Joshi, I. Azarov, and D. B. Kim-Shapiro helped design experiments involving EPR, performed the EPR analysis, and contributed to writing the article. M. Tiso and M. T. Gladwin designed the spectrophotometry experiments, performed that research, analyzed that data, and contributed to writing the article.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abraham EC, Taylor JF. Oxidation-reduction potentials of human fetal hemoglobin and gamma chains. Effects of blocking sulfhydryl groups. J Biol Chem 250: 3929–3935, 1975. [PubMed] [Google Scholar]
- 2.Angelo M, Singel DJ, Stamler JS. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc Natl Acad Sci USA 103: 8366–8371, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonini E, Wyman J, Brunori M, Fronticelli C, Bucci E, Reichlin M, Fanelli AR. The oxygen Bohr effect of human fetal hemoglobin. Arch Biochem Biophys 108: 569–572, 1964. [DOI] [PubMed] [Google Scholar]
- 4.Basu S, Grubina R, Huang J, Conradie J, Huang Z, Jeffers A, Jiang A, He X, Azarov I, Seibert R, Mehta A, Patel RP, King SB, Hogg N, Ghosh A, Gladwin MT, Kim-Shapiro DB. Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nat Chem Biol 3: 785–794, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Benesch RE, Maeda N, Benesch R. 2,3-Diphosphoglycerate and the relative affinity of adult and fetal hemoglobin for oxygen and carbon monoxide. Biochim Biophys Acta 257: 178–182, 1972. [DOI] [PubMed] [Google Scholar]
- 6.Blood AB, Hunter CJ, Power GG. Adenosine mediates decreased cerebral metabolic rate and increased cerebral blood flow during acute moderate hypoxia in the near-term fetal sheep. J Physiol 553: 935–945, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blood AB, Power GG. In vitro and in vivo kinetic handling of nitrite in blood: effects of varying hemoglobin oxygen saturation. Am J Physiol Heart Circ Physiol 293: H1508–H1517, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Brooks J The action of nitrite on haemoglobin in the absence of oxygen. Proc R Soc Med 123: 368–382, 1937. [Google Scholar]
- 9.Butler AR, Megson IL, Wright PG. Diffusion of nitric oxide and scavenging by blood in the vasculature. Biochim Biophys Acta 1425: 168–176, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Cannon RO 3rd, Schechter AN, Panza JA, Ognibene FP, Pease-Fye ME, Waclawiw MA, Shelhamer JH, Gladwin MT. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J Clin Invest 108: 279–287, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassoly R, Gibson Q. Conformation, co-operativity and ligand binding in human hemoglobin. J Mol Biol 91: 301–313, 1975. [DOI] [PubMed] [Google Scholar]
- 12.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9: 1498–1505, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Dejam A, Hunter CJ, Tremonti C, Pluta RM, Hon YY, Grimes G, Partovi K, Pelletier MM, Oldfield EH, Cannon RO 3rd, Schechter AN, Gladwin MT. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation 116: 1821–1831, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Di Iorio R, Marinoni E, Emiliani S, Villaccio B, Cosmi EV. Nitric oxide in preeclampsia: lack of evidence for decreased production. Eur J Obstet Gynecol Reprod Biol 76: 65–70, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Doyle MP, Pickering RA, DeWeert TM, Hoekstra JW, Pater D. Kinetics and mechanism of the oxidation of human deoxyhemoglobin by nitrites. J Biol Chem 256: 12393–12398, 1981. [PubMed] [Google Scholar]
- 16.Eich RF, Li T, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN Jr, Olson JS. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry 35: 6976–6983, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Erzurum SC, Ghosh S, Janocha AJ, Xu W, Bauer S, Bryan NS, Tejero J, Hemann C, Hille R, Stuehr DJ, Feelisch M, Beall CM. Higher blood flow and circulating NO products offset high-altitude hypoxia among Tibetans. Proc Natl Acad Sci USA 104: 17593–17598, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez BO, Lorkovic IM, Ford PC. Mechanisms of ferriheme reduction by nitric oxide: nitrite and general base catalysis. Inorg Chem 43: 5393–5402, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Flohe L, Uehleke H. The oxidation-reduction potential of human haemoglobin F. Life Sci 5: 1041–1045, 1966. [DOI] [PubMed] [Google Scholar]
- 20.Giussani DA, Gardner DS, Cox DT, Fletcher AJ. Purinergic contribution to circulatory, metabolic, and adrenergic responses to acute hypoxemia in fetal sheep. Am J Physiol Regul Integr Comp Physiol 280: R678–R685, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Green LR, Bennet L, Hanson MA. The role of nitric oxide synthesis in cardiovascular responses to acute hypoxia in the late gestation sheep fetus. J Physiol 497: 271–277, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grubina R, Basu S, Tiso M, Kim-Shapiro DB, Gladwin MT. Nitrite reductase activity of hemoglobin s (sickle) provides insight into contributions of heme redox potential versus ligand affinity. J Biol Chem 283: 3628–3638, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Grubina R, Huang Z, Shiva S, Joshi MS, Azarov I, Basu S, Ringwood LA, Jiang A, Hogg N, Kim-Shapiro DB, Gladwin MT. Concerted nitric oxide formation and release from the simultaneous reactions of nitrite with deoxy- and oxyhemoglobin. J Biol Chem 282: 12916–12927, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Harris AP, Helou S, Gleason CA, Traystman RJ, Koehler RC. Fetal cerebral and peripheral circulatory responses to hypoxia after nitric oxide synthase inhibition. Am J Physiol Regul Integr Comp Physiol 281: R381–R390, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Herold S, Exner M, Nauser T. Kinetic and mechanistic studies of the NO*-mediated oxidation of oxymyoglobin and oxyhemoglobin. Biochemistry 40: 3385–3395, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Huang KT, Han TH, Hyduke DR, Vaughn MW, Van Herle H, Hein TW, Zhang C, Kuo L, Liao JC. Modulation of nitric oxide bioavailability by erythrocytes. Proc Natl Acad Sci USA 98: 11771–11776, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang KT, Keszler A, Patel N, Patel RP, Gladwin MT, Kim-Shapiro DB, Hogg N. The reaction between nitrite and deoxyhemoglobin. Reassessment of reaction kinetics and stoichiometry. J Biol Chem 280: 31126–31131, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest 115: 2099–2107, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunter CJ, Blood AB, White CR, Pearce WJ, Power GG. Role of nitric oxide in hypoxic cerebral vasodilatation in the ovine fetus. J Physiol 549: 625–633, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunter CJ, Dejam A, Blood AB, Shields H, Kim-Shapiro DB, Machado RF, Tarekegn S, Mulla N, Hopper AO, Schechter AN, Power GG, Gladwin MT. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nat Med 10: 1122–1127, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Isbell TS, Gladwin MT, Patel RP. Hemoglobin oxygen fractional saturation regulates nitrite-dependent vasodilation of aortic ring bioassays. Am J Physiol Heart Circ Physiol 293: H2565–H2572, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Kleinbongard P, Dejam A, Lauer T, Jax T, Kerber S, Gharini P, Balzer J, Zotz RB, Scharf RE, Willers R, Schechter AN, Feelisch M, Kelm M. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic Biol Med 40: 295–302, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Godecke A, Schrader J, Schulz R, Heusch G, Schaub GA, Bryan NS, Feelisch M, Kelm M. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med 35: 790–796, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Lancaster JR Jr. A tutorial on the diffusibility and reactivity of free nitric oxide. Nitric Oxide 1: 18–30, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Liao JC, Hein TW, Vaughn MW, Huang KT, Kuo L. Intravascular flow decreases erythrocyte consumption of nitric oxide. Proc Natl Acad Sci USA 96: 8757–8761, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luchsinger BP, Rich EN, Yan Y, Williams EM, Stamler JS, Singel DJ. Assessments of the chemistry and vasodilatory activity of nitrite with hemoglobin under physiologically relevant conditions. J Inorg Biochem 99: 912–921, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7: 156–167, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Lyall F, Young A, Greer IA. Nitric oxide concentrations are increased in the fetoplacental circulation in preeclampsia. Am J Obstet Gynecol 173: 714–718, 1995. [DOI] [PubMed] [Google Scholar]
- 39.Maher AR, Milsom AB, Gunaruwan P, Abozguia K, Ahmed I, Weaver RA, Thomas P, Ashrafian H, Born GV, James PE, Frenneaux MP. Hypoxic modulation of exogenous nitrite-induced vasodilation in humans. Circulation 117: 670–677, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Meschia G, Hellegers A, Blechner JN, Wolkoff AS, Barron DH. A comparison of the oxygen dissociation curves of the bloods of maternal, fetal and newborn sheep at various pHs. Q J Exp Physiol Cogn Med Sci 46: 95–100, 1961. [DOI] [PubMed] [Google Scholar]
- 41.Morris RJ, Gibson QH. The role of diffusion in limiting the rate of ligand binding to hemoglobin. J Biol Chem 255: 8050–8053, 1980. [PubMed] [Google Scholar]
- 42.Nechtman CM, Huisman TH. Comparative studies of oxygen equilibria of human adult and cord blood red cell hemolysates and suspensions. Clin Chim Acta 10: 165–174, 1964. [DOI] [PubMed] [Google Scholar]
- 43.Norris LA, Higgins JR, Darling MR, Walshe JJ, Bonnar J. Nitric oxide in the uteroplacental, fetoplacental, and peripheral circulations in preeclampsia. Obstet Gynecol 93: 958–963, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Pelletier MM, Kleinbongard P, Ringwood L, Hito R, Hunter CJ, Schechter AN, Gladwin MT, Dejam A. The measurement of blood and plasma nitrite by chemiluminescence: pitfalls and solutions. Free Radic Biol Med 41: 541–548, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Pena JP, Tomimatsu T, Hatran DP, McGill LL, Longo LD. Cerebral blood flow and oxygenation in ovine fetus: responses to superimposed hypoxia at both low and high altitude. J Physiol 578: 359–370, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pluta RM, Dejam A, Grimes G, Gladwin MT, Oldfield EH. Nitrite infusions to prevent delayed cerebral vasospasm in a primate model of subarachnoid hemorrhage. JAMA 293: 1477–1484, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Power GG, Bragg SL, Oshiro BT, Dejam A, Hunter CJ, Blood AB. A novel method of measuring reduction of nitrite-induced methemoglobin applied to fetal and adult blood of humans and sheep. J Appl Physiol 103: 1359–1365, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Reller MD, Burson MA, Lohr JL, Morton MJ, Thornburg KL. Nitric oxide is an important determinant of coronary flow at rest and during hypoxemic stress in fetal lambs. Am J Physiol Heart Circ Physiol 269: H2074–H2081, 1995. [DOI] [PubMed] [Google Scholar]
- 49.Rifkind JM, Nagababu E, Barbiro-Michaely E, Ramasamy S, Pluta RM, Mayevsky A. Nitrite infusion increases cerebral blood flow and decreases mean arterial blood pressure in rats: a role for red cell NO. Nitric Oxide 16: 448–456, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Rudolph AM, Heymann MA. The fetal circulation. Annu Rev Med 19: 195–206, 1968. [DOI] [PubMed] [Google Scholar]
- 51.Salhany JM Kinetics of reaction of nitrite with deoxy hemoglobin after rapid deoxygenation or predeoxygenation by dithionite measured in solution and bound to the cytoplasmic domain of band 3 (SLC4A1). Biochemistry 47: 6059–6072, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Santos AC, Yun EM, Bobby PD, Noble G, Arthur GR, Finster M. The effects of bupivacaine, l-nitro-l-arginine-methyl ester, and phenylephrine on cardiovascular adaptations to asphyxia in the preterm fetal lamb. Anesth Analg 85: 1299–1306, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Schopfer FJ, Baker PR, Giles G, Chumley P, Batthyany C, Crawford J, Patel RP, Hogg N, Branchaud BP, Lancaster JR Jr, Freeman BA. Fatty acid transduction of nitric oxide signaling nitrolinoleic acid is a hydrophobically stabilized nitric oxide donor. J Biol Chem 280: 19289–19297, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Shiva S, Wang X, Ringwood LA, Xu X, Yuditskaya S, Annavajjhala V, Miyajima H, Hogg N, Harris ZL, Gladwin MT. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat Chem Biol 2: 486–493, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Smolich JJ NO modulates fetoplacental blood flow distribution and whole body oxygen extraction in fetal sheep. Am J Physiol Regul Integr Comp Physiol 274: R1331–R1337, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Tomimatsu T, Pena JP, Longo LD. Fetal hypercapnia and cerebral tissue oxygenation: studies in near-term sheep. Pediatr Res 60: 711–716, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Yang BK, Vivas EX, Reiter CD, Gladwin MT. Methodologies for the sensitive and specific measurement of S-nitrosothiols, iron-nitrosyls, and nitrite in biological samples. Free Radic Res 37: 1–10, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Yang D, Lang U, Greenberg SG, Myatt L, Clark KE. Elevation of nitrate levels in pregnant ewes and their fetuses. Am J Obstet Gynecol 174: 573–577, 1996. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L, Xiao D, Bouslough DB. Long-term high-altitude hypoxia increases plasma nitrate levels in pregnant ewes and their fetuses. Am J Obstet Gynecol 179: 1594–1598, 1998. [DOI] [PubMed] [Google Scholar]