Abstract
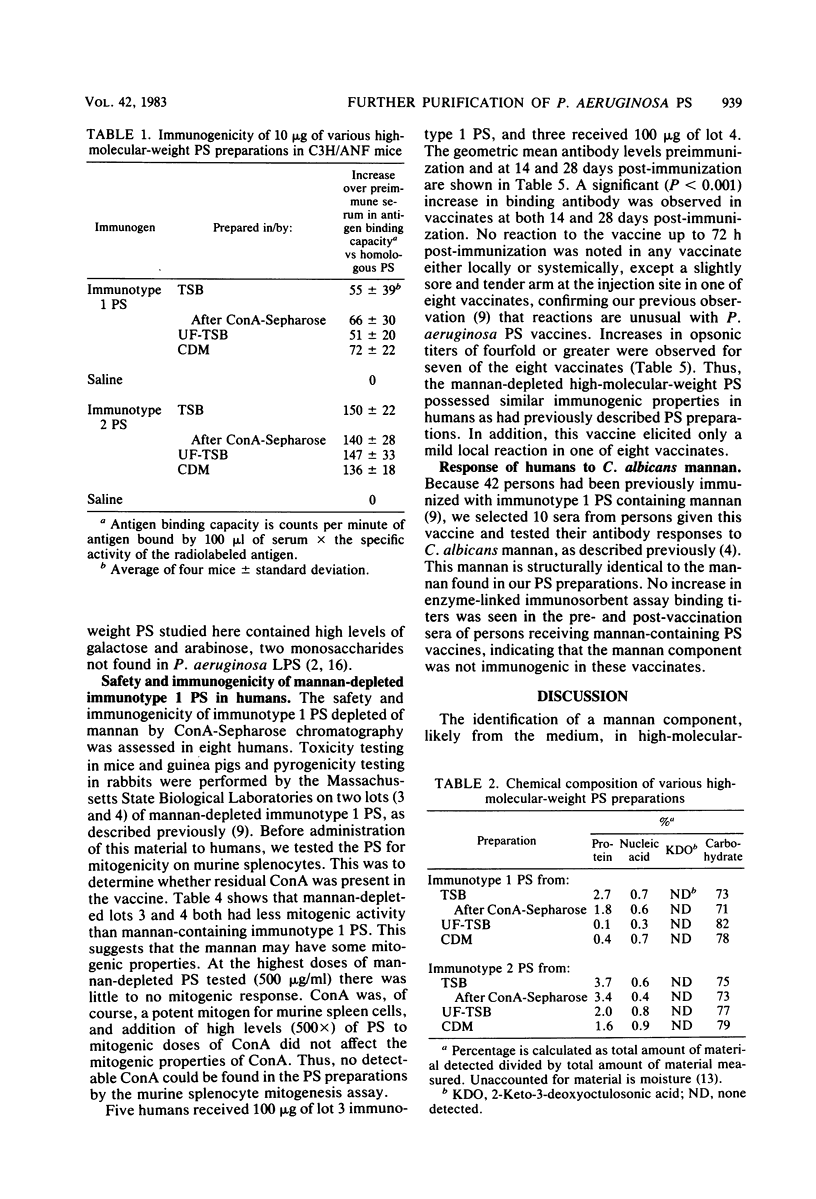
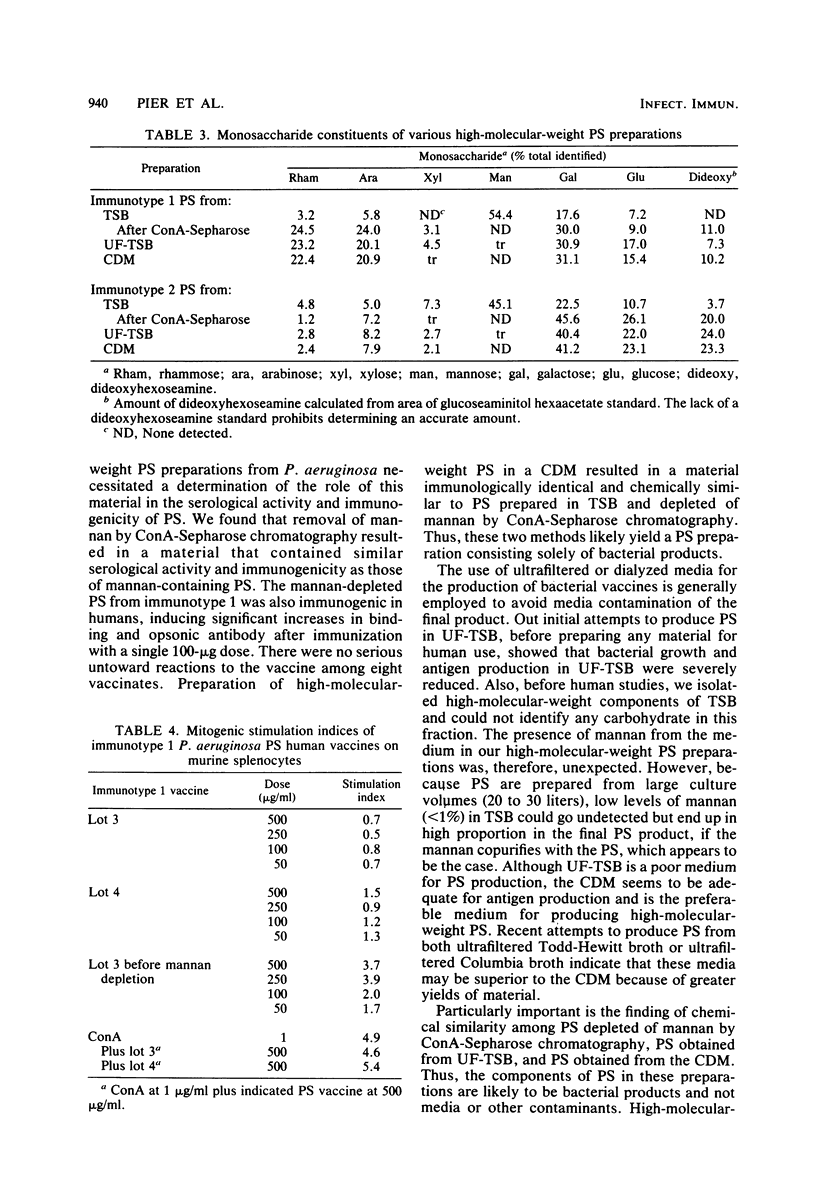
Previously published reports on high-molecular-weight polysaccharides from immunotype 1 and 2 of Pseudomonas aeruginosa indicated the presence of high levels of mannose in these preparations. This mannose has been found to be due to the presence of a yeastlike mannan in high-molecular-weight polysaccharide preparations. The source of the mannan was found to be the tryptic soy broth used to grow the bacteria. Mannan could be removed from the polysaccharide preparations by chromatography over columns of concanavalin A-Sepharose. The resulting polysaccharides had the same serological reactivity against rabbit antisera and the same immunogenic properties in mice as did the mannan-containing polysaccharides. Comparison of mannan-depleted polysaccharide with preparations of high-molecular-weight polysaccharide obtained from either ultrafiltered tryptic soy broth or a chemically defined medium showed that these polysaccharides were immunologically and chemically similar. Human immune responses to mannan-depleted polysaccharide from the immunotype 1 strain of P. aeruginosa were comparable with those previously seen in humans receiving mannan-containing polysaccharides. Thus, we found that P. aeruginosa high-molecular-weight polysaccharides prepared in either tryptic soy broth and then subjected to concanavalin A-Sepharose chromatography, ultrafiltered tryptic soy broth, or a chemically defined medium were immunologically and chemically comparable.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Horton D., Rodemeyer G., Haskell T. H. Analytical characterization of lipopolysaccharide antigens from seven strains of Pseudomonas aeruginosa. Carbohydr Res. 1977 May;55:35–47. doi: 10.1016/s0008-6215(00)84441-9. [DOI] [PubMed] [Google Scholar]
- Kasper D. L., Weintraub A., Lindberg A. A., Lönngren J. Capsular polysaccharides and lipopolysaccharides from two Bacteroides fragilis reference strains: chemical and immunochemical characterization. J Bacteriol. 1983 Feb;153(2):991–997. doi: 10.1128/jb.153.2.991-997.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lew M. A., Siber G. R., Donahue D. M., Maiorca F. Enhanced detection with an enzyme-linked immunosorbent assay of candida mannan in antibody-containing serum after heat extraction. J Infect Dis. 1982 Jan;145(1):45–56. doi: 10.1093/infdis/145.1.45. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B. Cross-protection by Pseudomonas aeruginosa polysaccharides. Infect Immun. 1982 Dec;38(3):1117–1122. doi: 10.1128/iai.38.3.1117-1122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Markham R. B., Eardley D. Correlation of the biologic responses of C3H/HEJ mice to endotoxin with the chemical and structural properties of the lipopolysaccharides from Pseudomonas aeruginosa and Escherichia coli. J Immunol. 1981 Jul;127(1):184–191. [PubMed] [Google Scholar]
- Pier G. B. Safety and immunogenicity of high molecular weight polysaccharide vaccine from immunotype 1 Pseudomonas aeruginosa. J Clin Invest. 1982 Feb;69(2):303–308. doi: 10.1172/JCI110453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Sidberry H. F., Sadoff J. C. High-molecular-weight polysaccharide antigen from Pseudomonas aeruginosa immunotype 2. Infect Immun. 1981 Nov;34(2):461–468. doi: 10.1128/iai.34.2.461-468.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Sidberry H. F., Sadoff J. C. Protective immunity induced in mice by immunization with high-molecular-weight polysaccharide from Pseudomonas aeruginosa. Infect Immun. 1978 Dec;22(3):919–925. doi: 10.1128/iai.22.3.919-925.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Sidberry H. F., Zolyomi S., Sadoff J. C. Isolation and characterization of a high-molecular-weight polysaccharide from the slime of Pseudomonas aeruginosa. Infect Immun. 1978 Dec;22(3):908–918. doi: 10.1128/iai.22.3.908-918.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S. G., Galbrath L. Studies of lipopolysaccharides from Pseudomonas aeruginosa. Eur J Biochem. 1975 Mar 17;52(2):331–343. doi: 10.1111/j.1432-1033.1975.tb04001.x. [DOI] [PubMed] [Google Scholar]




