Abstract
We have previously demonstrated that in a rat model of trauma-hemorrhage (T-H), glucosamine administration during resuscitation improved cardiac function, reduced circulating levels of inflammatory cytokines, and increased tissue levels of O-linked N-acetylglucosamine (O-GlcNAc) on proteins. The mechanism(s) by which glucosamine mediated its protective effect were not determined; therefore, the goal of this study was to test the hypothesis that glucosamine treatment attenuated the activation of the nuclear factor-κB (NF-κB) signaling pathway in the heart via an increase in protein O-GlcNAc levels. Fasted male rats were subjected to T-H by bleeding to a mean arterial blood pressure of 40 mmHg for 90 min followed by resuscitation. Glucosamine treatment during resuscitation significantly attenuated the T-H-induced increase in cardiac levels of TNF-α and IL-6 mRNA, IκB-α phosphorylation, NF-κB, NF-κB DNA binding activity, ICAM-1, and MPO activity. LPS (2 μg/ml) increased the levels of IκB-α phosphorylation, TNF-α, ICAM-1, and NF-κB in primary cultured cardiomyocytes, which was significantly attenuated by glucosamine treatment and overexpression of O-GlcNAc transferase; both interventions also significantly increased O-GlcNAc levels. In contrast, the transfection of neonatal rat ventricular myocytes with OGT small-interfering RNA decreased O-GlcNAc transferase and O-GlcNAc levels and enhanced the LPS-induced increase in IκB-α phosphorylation. Glucosamine treatment of macrophage cell line RAW 264.7 also increased O-GlcNAc levels and attenuated the LPS-induced activation of NF-κB. These results demonstrate that the modulation of O-GlcNAc levels alters the response of cardiomyocytes to the activation of the NF-κB pathway, which may contribute to the glucosamine-mediated improvement in cardiac function following hemorrhagic shock.
Keywords: nuclear factor-κB, cytokines, neonatal rat ventricular myocytes, O-linked N-acetylglucosamine
hypovolemia due to hemorrhage is a key factor in nearly half of the 150,000 deaths per year attributed to traumatic injury (34), and hemorrhage remains the primary cause of death on the battlefield in conventional warfare (2). Early deaths are determined by severe hemorrhage and central nervous system injuries, whereas late deaths are associated with inflammatory-mediated events, the development of sepsis, and multiple organ failure (1, 21, 37, 41). Multiple organ failure is typically characterized by the failure of at least two organ systems, such as lung, liver, and the cardiovascular system; the latter is associated with an especially high mortality rate (39). In a rat model of trauma-hemorrhage (T-H), we have demonstrated that treatment with glucosamine during resuscitation significantly improved cardiac function and peripheral organ perfusion and decreased the circulating levels of proinflammatory cytokines TNF-α and IL-6 (50).
Glucosamine is metabolized via the hexosamine biosynthesis pathway leading to the synthesis of uridine diphosphate N-acetylglucosamine (UDP-GlcNAc), which is a substrate for multiple glycosylation reactions catalyzed by various GlcNAc transferases, including a unique O-linked GlcNAc (O-GlcNAc) transferase (OGT) (8, 13). In contrast to all other protein glycosyl transferases, OGT is found in the nucleocytoplasmic compartment rather than the endoplasmic reticulum where it catalyzes the formation of a reversible posttranslational protein modification involving the attachment of GlcNAc via an O-linkage to specific serine and threonine residues. In mammalian cells, a variety of stress stimuli have been shown to increase the level of O-GlcNAc on nuclear and cytoplasmic proteins (52). The inhibition of this response increased sensitivity to stress, whereas the augmentation of the O-GlcNAc levels increased the tolerance to the same stress stimuli and improved cell survival (52). We have previously reported that increasing O-GlcNAc levels in both isolated cardiomyocytes and the intact heart improved tolerance to ischemic injury (7, 14, 24, 25).
Following T-H, glucosamine treatment not only improved cardiac function but also increased O-GlcNAc levels in multiple tissues, including the heart (50). This raised the possibility that the effect of glucosamine could be mediated via its effect on O-GlcNAc synthesis; however, since glucosamine also increases glucosamine-6-phosphate and UDP-GlcNAc levels, its effect could be mediated via a number of other pathways. Supporting the notion that increasing O-GlcNAc levels contributed to the protection seen with glucosamine following T-H, we showed that O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenylcarbamate (PUGNAc), an inhibitor of O-GlcNAcase, the enzyme that catalyzes the removal of O-GlcNAc from proteins, also increased tissue O-GlcNAc levels when administered during resuscitation and had a similar effect to glucosamine, improving cardiac function, as well as decreased the circulating levels of TNF-α and IL-6 (54).
A number of studies have shown that T-H-induced cardiac dysfunction was associated with increased TNF-α levels (32, 49). The nuclear factor-κB (NF-κB) signaling pathway plays a central role in regulating the release of many cytokines, including TNF-α and IL-6 (22, 31), and the activation of NF-κB has been implicated in organ dysfunction resulting from T-H (30, 32, 49). Therefore, the goal of this study was to test the hypothesis that the protection associated with glucosamine treatment during resuscitation was due at least, in part, to the attenuation of NF-κB signaling and that this was mediated via an increase in protein O-GlcNAc levels. We found that the in vivo administration of glucosamine following T-H attenuated the T-H-induced activation of NF-κB signaling in the heart and that glucosamine blocked LPS-induced TNF-α and IL-6 synthesis in isolated cardiomyocytes. We also demonstrate that OGT overexpression mimicked the effects of glucosamine treatment, whereas the transfection of cardiomyocytes with OGT small-interfering (si)RNA decreased O-GlcNAc levels and enhanced the response to LPS. Taken together, the results from these studies demonstrate that the modulation of O-GlcNAc levels alters the response of cardiomyocytes to the activation of the NF-κB pathway, which may contribute to the protection associated with glucosamine treatment.
MATERIALS AND METHODS
T-H shock model.
All animal experiments were approved by the University of Alabama Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Usage of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996).
T-H shock was induced in fasted male adult Sprague-Dawley rats (300 ± 25 g) as previously described in detail elsewhere (50, 54). Briefly, soft-tissue trauma was induced via a ventral midline laparotomy, followed by hemorrhage induced by removing blood via the left femoral artery to a mean arterial pressure (MAP) of 35–40 mmHg within 10 min, and a MAP was maintained at 35–40 mmHg for a total of 90 min. Animals were then resuscitated with Ringer lactate solution (4×) over a period of 60 min. Consistent with our previous study (50), 0.5 ml of 150 mM bolus of glucosamine was administered at 30 min after the onset of resuscitation followed by 2 ml of 150 mM of glucosamine mixed with resuscitation solution; in the vehicle-treated group, the same volume of normal saline was given. This protocol was used to try and mimic the situation, where a first responder would be able to initiate resuscitation but did not have access to or for other reasons would not be able to administer additional treatments. At the end of resuscitation, the animals were returned to cages and allowed to recover for 2 h. Animals undergoing sham operation underwent the same surgical procedure but were neither bled nor resuscitated.
Two hours after resuscitation or sham surgery, the animals were anesthetized with ketamine-HCl (100 mg/kg body wt), and cardiac function was assessed as previously described (50, 54). Tissues were then harvested for an assessment of O-GlcNAc levels and NF-κB activation as described in Biochemical measurements.
Cell culture studies.
Adult rat cardiomyocytes were isolated from normal rats as described previously (17). Two hours following the isolation, cardiomyocytes were pretreated with and without 5 mM glucosamine for 30 min before the exposure to LPS (1 μg/ml) for 6 h. Cell culture media was then harvested for measurement of TNF-α and IL-6 levels.
Neonatal rat ventricular myocytes (NRVMs) were isolated from 2- to 3-day-old neonatal Sprague-Dawley rats and cultured as described previously (5, 16, 36). A confluent monolayer of spontaneously beating NRVMs had formed within 1 to 2 days of isolation, and cells were pretreated with 5 mM glucosamine for 30 min followed by LPS (2 μg/ml) for 6 h. Additional groups of NRVMs were transfected with OGT adenovirus or OGT siRNA as previously described (5) for 48 h before LPS treatment. NRVMs were harvested with lysis buffer and stored at −80°C.
The mouse macrophage cell line RAW 264.7 was purchased from American Type Culture Collection. Cells were pretreated with 5 mM glucosamine for 30 min and with LPS (0.1 mg/ml) for 6 h. Cells were harvested with lysis buffer and stored at −80°C.
Biochemical measurements.
TNF-α and IL-6 gene expression was determined by QT-PCR (ABI PRISM 7900, Applied Biosystems, Foster City, CA) as previous described (49), and TNF-α and IL-6 concentrations were determined by ELISA (R&D Systems, Minneapolis, MN).
O-GlcNAc, TNF-α, ICAM-1, IκB-α, IκB-α phosphorylation, NF-κB levels of heart, and NRVMs were determined by Western blot analysis using anti-O-GlcNAc antibody CTD110.6, anti-TNF-α antibody, anti-rat ICAM-1 antibody (R&D system), NF-κB p65 antibody, and IκB-α and p-IκB-α antibodies (Cell Signaling). Whole heart and NRVM nuclei were isolated by NE-PER Nuclear and Cytoplasmic Extraction reagents kit (Pierce).
Cardiac NF-κB DNA binding activity was determined using the TransAM method, and a NF-κB p65 transcription factor assay kit was used to detect and quantify heart tissue NF-κB transcription factor activation according to the manufacturer's instructions (Active Motif, Carlsbad, CA). Cardiac myeloperoxidase (MPO) activity was measured as previously described (49).
Statistical analysis.
Data are expressed as means ± SE and compared by one-way ANOVA and Tukey's test. Statistically significant differences between groups were defined as P < 0.05.
RESULTS
Glucosamine treatment improves cardiac function, increases O-GlcNAc, and attenuates NF-κB activation.
Cardiac function and systemic hemodynamic parameters 2 h after the end of resuscitation are summarized in Table 1. Consistent with our earlier reports (50), MAP and cardiac contractility were depressed following T-H and resuscitation, and both were significantly improved in the glucosamine-treated group. Hemoglobin levels and %hematocrit were significantly depressed in both T-H groups, but there were no differences between the vehicle- and glucosamine-treated T-H groups, indicating that a similar level of blood loss was achieved in both groups. It is noteworthy, however, that while in vehicle-treated T-H group plasma lactate levels were markedly elevated, this response was significantly attenuated in the glucosamine-treated T-H group, such that plasma lactate levels were similar to those in the sham-operated groups.
Table 1.
Alterations in MAP, HR, ±dP/dt, and Hb at 2 h after T-H and resuscitation
| Sham-V | Sham-G | TH-V | TH-G | |
|---|---|---|---|---|
| MAP, mmHg | 126.8±2.7 | 122.6±1.8 | 46.7±8.4* | 81.5±4.4† |
| HR, beats/min | 408.8±2.6 | 406.0±65 | 277.3±19.5* | 386.1±15.0† |
| +dP/dt, mmHg/s | 16,910±2,108 | 17,063±328 | 6,388±745* | 11,619±712† |
| −dP/dt, mmHg/s | 9,323±976 | 10,572±248 | 3,668±1,203* | 6,593±402† |
| Hb, g/dl | 14.2±0.4 | 14.3±0.5 | 6.7±0.2* | 5.8±0.4* |
| Hct, % | 42.4±1.7 | 43.9±1.4 | 21.0±0.5* | 18.4±1.2* |
| Lactate, mg/dl | 10.2±0.5 | 6.8±0.7 | 73.4±17.3* | 10.8±1.4† |
Values are means ± SE (n = 5 rats/group). Sham-V, sham-operated rats (with vehicle); sham-G, sham-operated rats plus glucosamine; TH-V, trauma-hemorrhage (TH) treated with vehicle; TH-G, TH treated with glucosamine; MAP, mean arterial blood pressure; HR, heart rate; +dP/dt, maximum change of pressure over time; −dP/dt, minimum change of pressure over time; Hb, hemoglobin; Hct, hematocrit. Values were compared by one-way ANOVA and Tukey's test:
P < 0.05 vs. sham-V;
P < 0.05 vs. TH-V.
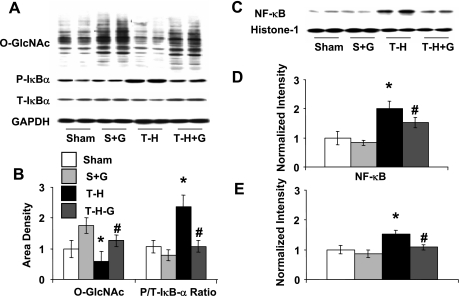
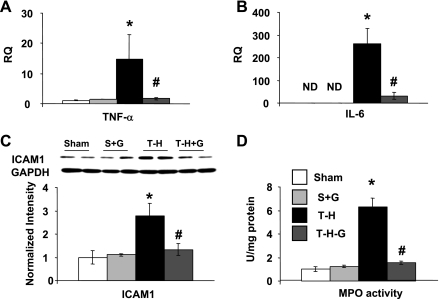
Following T-H, there was a significant decrease in O-GlcNAc levels and an increase in IκB-α phosphorylation that was associated with an increase in both nuclear levels of NF-κB and NF-κB DNA binding activity (Fig. 1) compared with those in the sham-operated controls. However, glucosamine treatment during resuscitation maintained O-GlcNAc levels and attenuated the increase in IκB-α phosphorylation and the subsequent NF-κB nuclear translocation. Consistent with the attenuation of NF-κB activation, glucosamine treatment also attenuated the T-H-induced increase in cardiac mRNA of TNF-α and IL-6, as well as ICAM-1 protein expression (Fig. 2). One consequence of an increase in ICAM-1 is neutrophil infiltration that can then lead to further injury. MPO activity was measured as an index of neutrophil infiltration, and, as shown in Fig. 2D, MPO activity increased fivefold 2 h after resuscitation, and this was significantly attenuated in the glucosamine-treated group.
Fig. 1.
Glucosamine treatment increases cardiac O-linked N-acetylglucosamine (O-GlcNAc) levels and attenuates NF-κB activation following trauma-hemorrhage (T-H) and resuscitation. A: immunoblots of O-GlcNAc, phospho (P)- and total (T)-IκB-α and GAPDH. B: mean densitometric analysis of immunoblots of CTD110 normalized to GAPDH and ratio of P- and T-IκB-α (P/T-IκB-α). C: immunoblots of nuclear extracts of NF-κB and histone-1. D: mean densitometric analysis of nuclear NF-κB protein levels normalized to histone-1. E: NF-κB DNA-binding activity (n = 5). Sham and S + G groups, rats subjected to sham surgery ± glucosamine treatment; T-H and T-H-G groups, rats subjected to T-H ± glucosamine treatment during resuscitation. Data are presented as means ± SE with n = 5 rats in each group. *P < 0.05 vs. sham; #P < 0.05 vs. T-H.
Fig. 2.
Glucosamine treatment attenuates cardiac TNF-α mRNA (A), IL-6 mRNA (B), ICAM-1 expression (C), and myeloperoxidase (MPO; D) activity following T-H and resuscitation. Data are presented as means ± SE with n = 5 rats in each group. RQ, relative quantification. *P < 0.05 vs. sham; #P < 0.05 vs. T-H.
Glucosamine and OGT overexpression attenuates NF-κB activation in cardiomyocytes.
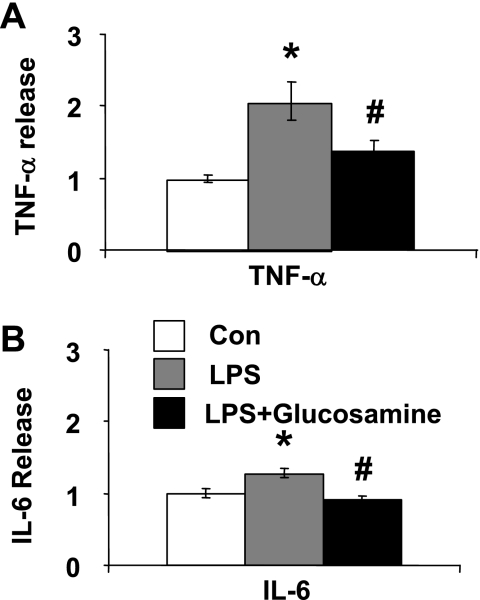
The results from our in vivo studies demonstrated that glucosamine treatment during resuscitation improved cardiac function and attenuated the activation of NF-κB signaling pathway in the heart. However, the decrease in cardiac NF-κB activation could be secondary to the decrease in systemic inflammatory mediators rather than a direct effect of glucosamine on the heart. Therefore, we examined the effect of glucosamine treatment on LPS-induced TNF-α and IL-6 release in isolated adult cardiomyocytes. Consistent with previous reports, LPS increased both TNF-α and IL-6 release (20) and this was significantly attenuated by glucosamine (Fig. 3).
Fig. 3.
Glucosamine treatment of cultured adult rat cardiomyocytes attenuates LPS-induced production of TNF-α (A) and IL-6 (B). Freshly isolated adult cardiomyocytes were treated with glucosamine 30 min before LPS (1 μg/ml); TNF-α and IL-6 levels in the media were determined 6 h after LPS administration. *P < 0.05 vs. control (Con); #P < 0.05 vs. LPS treated (n = 4–7 experiments in each group).
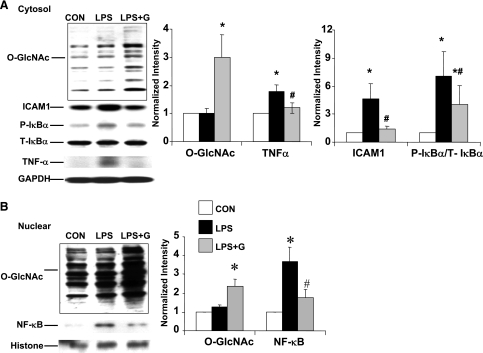
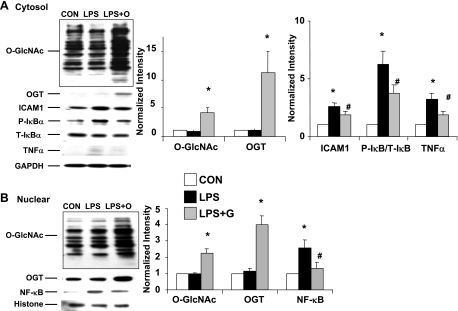
We have previously shown that following T-H, increasing O-GlcNAc levels with the O-GlcNAcase inhibitor PUGNAc had similar effects to glucosamine. These results were consistent with the notion that the protection associated with glucosamine was mediated via an increase in O-GlcNAc; however, the protection associated with glucosamine treatment could be mediated via pathways other than O-GlcNAc, such as gangliosides (29) or cell surface N-glycans (45). Therefore, we compared the effect of glucosamine treatment with OGT overexpression on the response of NRVMs to LPS (Figs. 4 and 5). Consistent with the results in adult cardiomyocytes (Fig. 3), LPS treatment in NRVMs increased IκB-α phosphorylation, ICAM-1, and TNF-α expression and this was significantly attenuated by glucosamine treatment (Fig. 4A). LPS treatment alone had no effect on the overall cytoplasmic O-GlcNAc levels; however, glucosamine plus LPS significantly increased O-GlcNAc levels compared with untreated control cells (2.9 ± 0.7 vs. 1.0 ± 0.3, P < 0.05) (Fig. 4A).
Fig. 4.
Glucosamine treatment increases O-GlcNAc levels and attenuates LPS-induced NF-κB activation in neonatal rat ventricular myocytes (NRVMs). A: cytoplasmic protein levels of O-GlcNAc, P- and T-IκB-α, ICAM-1, TNF-α, and GAPDH. O, O-GlcNAc transferase (OGT) adenovirus. B: nuclear protein levels of O-GlcNAc, NF-κB, and histone-1. NRVMs were treated with glucosamine 30 min before LPS (2 μg/ml) for 6 h. Left: typical immunoblots. Right: mean densitometric analysis normalized to GAPDH or histone-1. Data are normalized to control groups and presented as means ± SE of n = 4–7 experiments in each group. *P < 0.05 vs. Con; #P < 0.05 vs. LPS treated.
Fig. 5.
OGT overexpression increases O-GlcNAc levels and attenuates LPS-induced NF-κB activation in NRVMs. A: cytoplasmic protein levels of O-GlcNAc, OGT, P- and T-IκB-α, ICAM-1, TNF-α, and GAPDH. B: nuclear protein levels of O-GlcNAc, OGT, NF-κB, and histone-1. NRVMs were transfected with OGT adenovirus 48 h before LPS (2 μg/ml) for 6 h. Left: typical immunoblots. Right: mean densitometric analysis normalized to GAPDH or histone-1. Data are normalized to control groups and presented as means ± SE of n = 4–7 experiments in each group. *P < 0.05 vs. Con; #P < 0.05 vs. LPS treated.
NRVMs transfected with OGT adenovirus exhibited a 6.9 ± 1.7-fold increase in OGT expression compared with controls (P < 0.05), and this was associated with a 4.2 ± 0.8-fold increase in O-GlcNAc levels (P < 0.05) (Fig. 5A). Similar to glucosamine treatment, the OGT-mediated increase in O-GlcNAc was also associated with the attenuation of the LPS-induced increase in IκB-α phosphorylation, ICAM-1, and TNF-α expression (Fig. 5B). Both glucosamine and OGT overexpression also attenuated the LPS-induced increase in NF-κB (Figs. 4B and 5B), and this was associated with a two- to threefold increase in nuclear O-GlcNAc levels. One limitation of these experiments is that an empty adenoviral vector was not used as a control. However, since both glucosamine and OGT adenoviral transfection resulted in a similar blunted response to LPS-induced activation of NF-κB, we believe it is highly unlikely that the results from OGT overexpression are a consequence of nonspecific effects due to adenoviral transfection.
It should be noted that while we did not directly assess apoptosis, LPS treatment had no obvious impact on cell viability over the time course of these experiments in either adult or neonatal cardiomyocytes (data not shown).
Decreased OGT expression enhances the response to LPS.
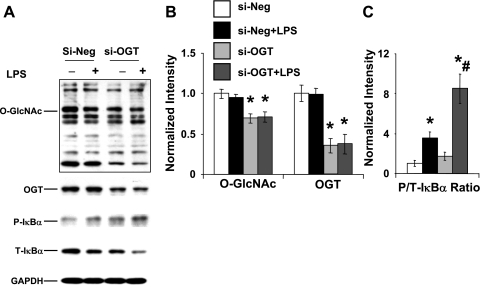
Transfection of NRVMs with OGT siRNA significantly decreases O-GlcNAc levels by ∼60% and OGT levels by >50% compared with scrambled siRNA cells, and this response was unaffected by LPS treatment (Fig. 6, A and B). Consistent with the results in Figs. 4 and 5, LPS treatment significantly increased IκB-α phosphorylation; however, this response was markedly augmented in the OGT siRNA group (Fig. 6, A and C).
Fig. 6.
Decreased OGT expression in NRVMs reduced O-GlcNAc levels and enhanced LPS-induced increase in IκB-α phosphorylation. A: immunoblots of O-GlcNAc, OGT, P- and T-IκB-α. B: mean densitometric analysis of O-GlcNAc and OGT normalized to GAPDH. C: mean densitometric analysis of P/T-IκB-α. Cells were transfected with either OGT siRNA (si-OGT) or scrambled siRNA (si-Neg) 48 h before treatment with LPS (2 μg/ml) for 6 h. Data are normalized to Si-Neg group and presented as means ± SE of n = 5 experiments per group. *P < 0.05 vs. Con; #P < 0.05 vs. LPS treated.
Glucosamine attenuates response of macrophages to LPS.
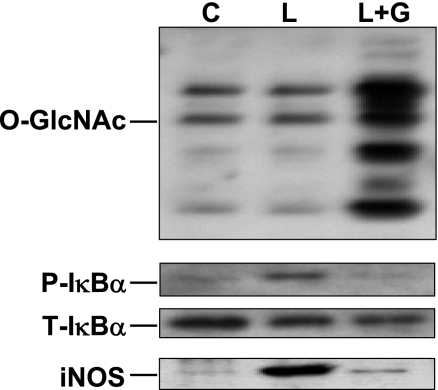
Macrophage activation is an important contributing factor not only to the systemic inflammatory response seen following T-H and resuscitation but also to the subsequent progression of tissue injury. Therefore, we examined the effect of glucosamine treatment on the LPS-induced IκB-α phosphorylation and inducible nitric oxide synthase (iNOS) expression in the macrophage cell line, RAW 264.7. As shown in Fig. 7, similar to the results in cardiomyocytes, LPS-induced IκB-α phosphorylation was attenuated by glucosamine treatment, and this was associated with increased O-GlcNAc levels. As expected, LPS markedly increased iNOS expression, and this was also substantially attenuated with glucosamine treatment.
Fig. 7.
Glucosamine treatment of RAW 264.7 cells increased cardiac O-GlcNAc levels and attenuated LPS-induced P-IκB-α and inducible nitric oxide synthase (iNOS) protein expression. Cells were pretreated with 5 mM glucosamine for 30 min and before treatment with LPS (0.1 μg/ml, 6 h). C, control; L, LPS treated.
DISCUSSION
Advances in intensive care and patient management have improved short-term survival following severe injury (4, 40); however, the overall hospital mortality for adult trauma patients admitted to the intensive care unit is almost 20% (46). Cardiac dysfunction is a frequent complication associated with severe trauma and is an important factor contributing to the high mortality associated with multiorgan failure (39). Rodent models of hemorrhagic shock recapitulate many of the responses seen in patients, including depressed cardiac function and impaired peripheral organ perfusion and function in addition to increased inflammatory response (10, 49). We have previously reported that following T-H increasing tissue levels of O-GlcNAc, either by augmenting synthesis with glucosamine or inhibiting degradation with the O-GlcNAcase inhibitor PUGNAc, improved cardiac function and peripheral organ perfusion, decreased end-organ injury, and reduced the circulating levels of inflammatory cytokines (50, 54). However, the mechanism underlying this protection had not been identified. We show here, for the first time, that an in vivo administration of glucosamine during resuscitation markedly attenuated the activation of the NF-κB pathway in the heart and that this can be replicated by glucosamine treatment of isolated cardiomyocytes.
The fact that glucosamine and PUGNAc, both of which increase O-GlcNAc levels but via different mechanisms, improved the outcomes following T-H and resuscitation in vivo (50, 54), support the notion that the observed protection is mediated via O-GlcNAc. Nevertheless, glucosamine treatment can affect a number of pathways other than O-GlcNAc (29, 45), and PUGNAc can also inhibit other β-hexosamindases (28, 38) and thus may alter the processing of glycoconjugates in addition to O-GlcNAc. Therefore, we also examined whether altering cardiomyocyte OGT expression would modulate NF-κB activation and found that increasing OGT levels mimicked the effects of glucosamine, not only increasing O-GlcNAc levels but also attenuating LPS-induced activation of NF-κB. Conversely, decreasing OGT expression significantly enhanced the response to LPS. Taken together, these results suggest that the improvement in cardiac function seen with glucosamine treatment following T-H is due at least in part to the attenuation of NF-κB pathway activation mediated by an increase in protein O-GlcNAc levels. Since glucosamine is administered at relatively high concentrations, it is conceivable that its protection could be due to an independent buffering effect, which attenuates lactate acidosis, thus improving cardiac function and decreasing the inflammatory response. However, the fact that O-GlcNAcase inhibition with PUGNAc has almost identical effects to glucosamine strongly suggests that this is not the case (54).
In addition to attenuating NF-κB activation in cardiomyocytes, we also show preliminary evidence that glucosamine attenuates LPS-induced NF-κB activation in macrophages and that this is also associated with an increase in O-GlcNAc levels (Fig. 7). This observation suggests that the activation of O-GlcNAc formation will also attenuate NF-κB signaling in cells that contribute to the systemic inflammatory response that follows severe trauma such as hemorrhagic shock. The activation of NF-κB plays an important role in the systemic increase in inflammatory mediators, including TNF-α and IL-6, which we have shown are both decreased by glucosamine and PUGNAc treatment. An excessive production of nitric oxide as a result of increased iNOS expression is another important factor contributing to both increased inflammation as a well as end-organ injury that occurs following hemorrhagic shock (15). Thus, while additional studies are needed to demonstrate a more definitive link between O-GlcNAc and the regulation of NF-κB signaling in macrophages, these results suggest that increases in O-GlcNAc levels not only afford direct protection to parenchymal tissue but also attenuate the activation of systemic inflammatory responses to trauma.
Much of the work examining the role of O-GlcNAc in regulating cellular function has been in the context of various chronic disease states (9, 11, 13, 27, 43, 47); however, it is increasing clear that protein O-GlcNAcylation is an important mechanism involved in signal transduction and influences a wide range of cellular processes, including cell cycle, cell growth, apoptosis, and cell survival (27, 44, 51). The importance of O-GlcNAc synthesis in regulating cell survival was first reported by Zachara et al. (52); we have subsequently shown that ischemia-reperfusion increased O-GlcNAc levels in a glucose-dependent manner (7) and that the augmentation of this significantly improved cell survival, whereas the attenuation of the response reduced survival (5, 7). Further support for a cardioprotective role of O-GlcNAc was demonstrated by Jones et al. (19), who showed that the treatment of mice with PUGNAc increased O-GlcNAc levels and decreased infarct size. The activation of NF-κB also occurs following ischemia-reperfusion, and the resulting increase in TNF-α levels has been implicated in contributing to the development of cardiac injury (31). Thus the attenuation of NF-κB signaling may also be a contributing factor in the protective effect of PUGNAc reported by Jones et al. (19).
Consequently, there is now considerable evidence that the acute elevation of protein O-GlcNAc levels is protective at the cellular, organ, and organismal level; however, the specific mechanisms associated with this protection remain to be determined. Increased levels of heat shock proteins HSP70 and HSP40 (52); O-GlcNAc modification of VDAC, a component of the mitochondrial permeability transition pore (19); and increased mitochondrial Bcl-2 levels (5) have all been linked to O-GlcNAc-mediated cellular protection. The fact that we observed a decrease in inflammatory cytokines TNF-α and IL-6 with glucosamine and PUGNAc treatment following T-H and resuscitation (50, 54) raised the possibility that the attenuation of NF-κB signaling may be another contributing factor to the protective effects of increasing O-GlcNAc in vivo. The results shown here demonstrate that glucosamine treatment during resuscitation attenuated the activation of NF-κB in the heart, leading to decreased mRNA levels of IL-6 and TNF-α as well as lower ICAM-1 expression levels.
This could be due in part to a decreased systemic inflammatory response; however, our results demonstrated that glucosamine also blunted LPS-induced NF-κB activation in isolated cardiomyocytes. We also found that increasing OGT expression mimicked glucosamine treatment, confirming that the effect of glucosamine on NF-κB activation was indeed mediated via increased O-GlcNAc levels. These results are also supported by recent studies, which demonstrate that in an in vivo vascular injury model, both glucosamine and PUGNAc attenuated the expression of downstream proinflammatory targets of NF-κB, decreasing neutrophil and monocyte infiltration, and reducing neointimal formation (48). Increased O-GlcNAc levels have been shown to attenuate apoptosis and necrosis following ischemia-reperfusion (6, 24). Although we did not specifically assess the apoptosis, cell viability was unaffected 6 h following LPS treatment. Therefore, additional studies will be required to determine whether the O-GlcNAc-mediated attenuation of NF-κB activation will also decrease cardiomyocyte injury.
NF-κB is known to be activated by more than 400 different stimuli; here we used LPS, which initiates NF-κB activation via binding to Toll-like receptor-4 (26). While the upstream signaling pathways differ depending on the specific stimuli, in classical NF-κB signaling there is a convergence at the IκB kinase (IKK) complex leading to the phosphorylation of IκB-α. As we only examined one activator of NF-κB, it is possible that the O-GlcNAc-mediated attenuation of IκB-α phosphorylation is specific for LPS; however, a recent study in vascular smooth muscle cells demonstrated that increased O-GlcNAc levels attenuated the TNF-α-induced activation of NF-κB (48). This suggests that the effect of increased O-GlcNAcylation on NF-κB signaling is likely mediated at the level of, or downstream from, IKK.
While these data support the notion that O-GlcNAc regulates NF-κB activation, the question remains as to the mechanism by which this occurs. James et al. (18) reported that an increase in hexosamine biosynthesis pathway flux was associated with increased O-GlcNAc modification of the p65 subunit of NF-κB, which could be a potential mechanism. The nuclear translocation of NF-κB is dependent on the phosphorylation of IκB-α, which is subsequently targeted for degradation by the proteasome. Thus it is also possible that O-GlcNAcylation of IκB-α may prevent its phosphorylation, thereby preventing its degradation. Alternatively, increased O-GlcNAc levels decrease proteasome activity (53), which could decrease IκB-α degradation and thus indirectly attenuate NF-κB translocation. It should also be noted that the phosphorylation of serine and threonine residues of IKK is required for their activation, and the acetylation of these residues prevents phosphorylation and inhibits NF-κB signaling (33). Thus it is also possible that O-GlcNAcylation of IKK could also block the activation of NF-κB. Clearly, further studies are warranted to determine the mechanism(s) by which increased flux through OGT inhibits NF-κB activation.
In this study, we investigated only a single time point following T-H and resuscitation. It could be argued that investigating a single time point is not sufficient to conclude a potential role of glucosamine in the observed effects on NF-κB following T-H. However, previous studies have shown that if a pharmacological agent was effective in improving cell and organ function early after the insult, i.e., at 2 h after resuscitation, that agent also improved cell and organ function at later time points, i.e., 24 and 48 h, and that it also improved the survival of animals subjected to T-H and the induction of subsequent sepsis (3, 12, 42). Thus, based on those studies, it would appear that the salutary effects of glucosamine on NF-κB and other parameters would persist even if one examined those effects at 24 or 48 h after glucosamine treatment. In support of this, a recent study demonstrated that glucosamine administered during resuscitation improved 24-h survival rates following T-H (35). Interestingly, 24 h following resuscitation, in untreated animals, protein O-GlcNAc levels remained depressed in the liver and lung but not the heart, suggesting tissue-specific differences in O-GlcNAc turnover rates. Future studies looking at O-GlcNAc levels and NF-κB activation, in different tissues at different time points following resuscitation, would provide valuable insight into the role of O-GlcNAcylation and the regulation of NF-κB signaling.
In conclusion, we have shown that the in vivo administration of glucosamine attenuates the activation of NF-κB in the heart induced by T-H and resuscitation, and this was associated with improved cardiac function, decreased systemic inflammatory response, and increased tissue O-GlcNAc levels. This was recapitulated at the cellular level where glucosamine attenuated LPS-induced activation of NF-κB and its downstream targets. We also demonstrated that an increased expression of OGT mimicked the effect of glucosamine both with regard to the attenuation of NF-κB as well as the increase in O-GlcNAc levels, supporting the notion that the response to glucosamine is mediated via increased flux through OGT and increased O-GlcNAc formation. A role for O-GlcNAc in mediating NF-κB signaling was further supported by the observation that the decreased OGT expression enhanced LPS-induced IκB-α phosphorylation. We also found that glucosamine attenuated the LPS-induced phosphorylation of IκB-α phosphorylation and iNOS expression in macrophages, demonstrating that the in vivo administration of glucosamine may exert its protective effect not only at the level of the heart and other parenchymal tissues but also by the attenuation of inflammatory responses in circulating mediators such as macrophages. We have yet to identify the specific molecular mechanism by which changes in O-GlcNAc status modulate NF-κB activation; however, given the critical role of NF-κB in mediating the cardiovascular response to different stress stimuli, these results suggest that strategies designed to acutely increase O-GlcNAc levels may represent a valuable new therapeutic approach for not only T-H but also sepsis and ischemic injury.
GRANTS
This work was supported by National Institutes of Health Grants R01-HL-076175 (to R. B. Marchase), R37-GM-39519 (to I. H. Chaudry), and R01-HL-067464 (to J. C. Chatham).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Acosta JA, Yang JC, Winchell RJ, Simons RK, Fortlage DA, Hollingsworth-Fridlund P, Hoyt DB. Lethal injuries and time to death in a level I trauma center. J Am Coll Surg 186: 528–533, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Alam HB, Koustova E, Rhee P. Combat casualty care research: from bench to the battlefield. World J Surg 29, Suppl 1: S7–S11, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Angele MK, Schwacha MG, Ayala A, Chaudry IH. Effect of gender and sex hormones on immune responses following shock. Shock 14: 81–90, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Barringer ML, Thomason MH, Kilgo P, Spallone L. Improving outcomes in a regional trauma system: impact of a level III trauma center. Am J Surg 192: 685–689, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein O-GlcNAc and increased mitochondrial Bcl-2. Am J Physiol Cell Physiol 294: C1509–C1520, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein O-GlcNAc and increased mitochondrial Bcl-2. Am J Physiol Cell Physiol 294: C1509–C1520, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc. Am J Physiol Cell Physiol 292: C178–C187, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Chatham JC, Not LG, Fulop N, Marchase RB. Hexosamine biosynthesis and protein O-glycosylation: the first line of defense against stress, ischemia, and trauma. Shock 29: 431–440, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Chou TY, Hart GW, Dang CV. c-Myc is glycosylated at threonine 58, a known phosphorylation site and a mutational hot spot in lymphomas. J Biol Chem 270: 18961–18965, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Choudhry MA, Schwacha MG, Hubbard WJ, Kerby JD, Rue LW, Bland KI, Chaudry IH. Gender differences in acute response to trauma-hemorrhage. Shock 24, Suppl 1: 101–106, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Copeland RJ, Bullen JW, Hart GW. Cross-talk between GlcNAcylation and phosphorylation: roles in insulin resistance and glucose toxicity. Am J Physiol Endocrinol Metab 295: E17–E28, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diodato MD, Knoferl MW, Schwacha MG, Bland KI, Chaudry IH. Gender differences in the inflammatory response and survival following haemorrhage and subsequent sepsis. Cytokine 14: 162–169, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Fulop N, Marchase RB, Chatham JC. Role of protein O-linked N-acetyl-glucosamine in mediating cell function and survival in the cardiovascular system. Cardiovasc Res 73: 288–297, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fulop N, Zhang Z, Marchase RB, Chatham JC. Glucosamine cardioprotection in perfused rat heart associated with increased O-linked N-acetylglucosamine protein modification and altered p38 activation. Am J Physiol Heart Circ Physiol 292: H2227–H2236, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hierholzer C, Billiar TR. Molecular mechanisms in the early phase of hemorrhagic shock. Langenbecks Arch Surg 236: 302–308, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunton DL, Lucchesi PA, Pang Y, Cheng X, Dell'Italia LJ, Marchase RB. Capacitative calcium entry contributes to nuclear factor of activated T-cells nuclear translocation and hypertrophy in cardiomyocytes. J Biol Chem 277: 14266–14273, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Hunton DL, Zou LY, Pang Y, Marchase RB. Adult rat cardiomyocytes exhibit capacitative calcium entry. Am J Physiol Heart Circ Physiol 286: H1124–H1132, 2004. [DOI] [PubMed] [Google Scholar]
- 18.James LR, Tang D, Ingram A, Ly H, Thai K, Cai L, Scholey JW. Flux through the hexosamine pathway is a determinant of nuclear factor kappaB-dependent promoter activation. Diabetes 51: 1146–1156, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Jones SP, Zachara NE, Ngoh GA, Hill BG, Teshima Y, Bhatnagar A, Hart GW, Marban E. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation 117: 1172–1182, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Kapadia S, Lee J, Torre-Amione G, Birdsall HH, Ma TS, Mann DL. Tumor necrosis factor-alpha gene and protein expression in adult feline myocardium after endotoxin administration. J Clin Invest 96: 1042–1052, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keel M, Trentz O. Pathophysiology of polytrauma. Injury 36: 691–709, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Liang Y, Zhou Y, Shen P. NF-kappaB and its regulation on the immune system. Cell Mol Immunol 1: 343–350, 2004. [PubMed] [Google Scholar]
- 24.Liu J, Marchase RB, Chatham JC. Increased O-GlcNAc levels during reperfusion leads to improved functional recovery and reduced calpain proteolysis. Am J Physiol Heart Circ Physiol 293: H1391–H1399, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Pang Y, Chang T, Bounelis P, Chatham JC, Marchase RB. Increased hexosamine biosynthesis and protein O-GlcNAc levels associated with myocardial protection against calcium paradox and ischemia. J Mol Cell Cardiol 40: 303–312, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Liu SF, Malik AB. NF-κB activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol 290: L622–L645, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Love DC, Hanover JA. The hexosamine signaling pathway: deciphering the “O-GlcNAc code”. Sci STKE 2005: re13, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Macauley MS, Whitworth GE, Debowski AW, Chin D, Vocadlo DJ. O-GlcNAcase uses substrate-assisted catalysis: kinetic analysis and development of highly selective mechanism-inspired inhibitors. J Biol Chem 280: 25313–25322, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Masson E, Wiernsperger N, Lagarde M, El Bawab S. Glucosamine induces cell-cycle arrest and hypertrophy of mesangial cells: implication of gangliosides. Biochem J 388: 537–544, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonald MC, Mota-Filipe H, Paul A, Cuzzocrea S, Abdelrahman M, Harwood S, Plevin R, Chatterjee PK, Yaqoob MM, Thiemermann C. Calpain inhibitor I reduces the activation of nuclear factor-kappaB and organ injury/dysfunction in hemorrhagic shock. FASEB J 15: 171–186, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Meldrum DR Tumor necrosis factor in the heart. Am J Physiol Regul Integr Comp Physiol 274: R577–R595, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Meldrum DR, Shenkar R, Sheridan BC, Cain BS, Abraham E, Harken AH. Hemorrhage activates myocardial NFkappaB and increases TNF-alpha in the heart. J Mol Cell Cardiol 29: 2849–2854, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Mukherjee S, Keitany G, Li Y, Wang Y, Ball HL, Goldsmith EJ, Orth K. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science 312: 1211–1214, 2006. [DOI] [PubMed] [Google Scholar]
- 34.National Heart, Lung, and Blood Institute. Hypovolemic circulatory collapse: mechanisms to improve resuscitation outcomes. RFA No. HL-03-015, 2003.
- 35.Not LG, Brocks CA, Marchase RB, Chatham JC. O-GlcNAc agonist treatment improves survival, reduces inflammation and organ damage 24 hours after trauma-hemorrhage in rats (Abstract). FASEB J 22: 1227, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pang Y, Hunton DL, Bounelis P, Marchase RB. Hyperglycemia inhibits capacitative calcium entry and hypertrophy in neonatal cardiomyocytes. Diabetes 51: 3461–3467, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Peng R, Chang C, Gilmore D, Bongard F. Epidemiology of immediate and early trauma deaths at an urban level I trauma center. Am Surg 64: 950–954, 1998. [PubMed] [Google Scholar]
- 38.Perreira M, Kim EJ, Thomas CJ, Hanover JA. Inhibition of O-GlcNAcase by PUGNAc is dependent upon the oxime stereochemistry. Bioorg Med Chem 14: 837–846, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Regel G, Grotz M, Weltner T, Sturm JA, Tscherne H. Pattern of organ failure following severe trauma. World J Surg 20: 422–429, 1996. [DOI] [PubMed] [Google Scholar]
- 40.Sampalis JS, Denis R, Lavoie A, Frechette P, Boukas S, Nikolis A, Benoit D, Fleiszer D, Brown R, Churchill-Smith M, Mulder D. Trauma care regionalization: a process-outcome evaluation. J Trauma 46: 565–579, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, Pons PT. Epidemiology of trauma deaths: a reassessment. J Trauma 38: 185–193, 1995. [DOI] [PubMed] [Google Scholar]
- 42.Schneider CP, Nickel EA, Samy TS, Schwacha MG, Cioffi WG, Bland KI, Chaudry IH. The aromatase inhibitor, 4-hydroxyandrostenedione, restores immune responses following trauma-hemorrhage in males and decreases mortality from subsequent sepsis. Shock 14: 347–353, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Shaw P, Freeman J, Bovey R, Iggo R. Regulation of specific DNA binding by p53: evidence for a role for O-glycosylation and charged residues at the carboxy-terminus. Oncogene 12: 921–930, 1996. [PubMed] [Google Scholar]
- 44.Slawson C, Housley MP, Hart GW. O-GlcNAc cycling: how a single sugar post-translational modification is changing the way we think about signaling networks. J Cell Biochem 97: 71–83, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Stanley P A method to the madness of N-glycan complexity? Cell 129: 27–29, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Ulvik A, Wentzel-Larsen T, Flaatten H. Trauma patients in the intensive care unit: short- and long-term survival and predictors of 30-day mortality. Acta Anaesthesiol Scand 51: 171–177, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Wells L, Whalen SA, Hart GW. O-GlcNAc: a regulatory post-translational modification. Biochem Biophys Res Commun 302: 435–441, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Xing D, Feng W, Not LG, Miller AP, Zhang Y, Chen YF, Majid-Hassan E, Chatham JC, Oparil S. Increased protein O-GlcNAc modification inhibits inflammatory and neointimal responses to acute endoluminal arterial injury. Am J Physiol Heart Circ Physiol 295: H335–H342, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang S, Zheng R, Hu S, Ma Y, Choudhry MA, Messina JL, Rue LW 3rd, Bland KI, Chaudry IH. Mechanism of cardiac depression after trauma-hemorrhage: increased cardiomyocyte IL-6 and effect of sex steroids on IL-6 regulation and cardiac function. Am J Physiol Heart Circ Physiol 287: H2183–H2191, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Yang S, Zou LY, Bounelis P, Chaudry I, Chatham JC, Marchase RB. Glucosamine administration during resuscitation improves organ function after trauma hemorrhage. Shock 25: 600–607, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Zachara NE, Hart GW. O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim Biophys Acta 1673: 13–28, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Zachara NE, O'Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem 279: 30133–30142, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Zhang F, Su K, Yang X, Bowe DB, Paterson AJ, Kudlow JE. O-GlcNAc modification is an endogenous inhibitor of the proteasome. Cell 115: 715–725, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Zou LY, Yang S, Chaudry IH, Marchase RB, Chatham JC. PUGNAc administration during resuscitation improves organ function following trauma-hemorrhage. Shock 27: 402–408, 2007. [DOI] [PubMed] [Google Scholar]