Abstract
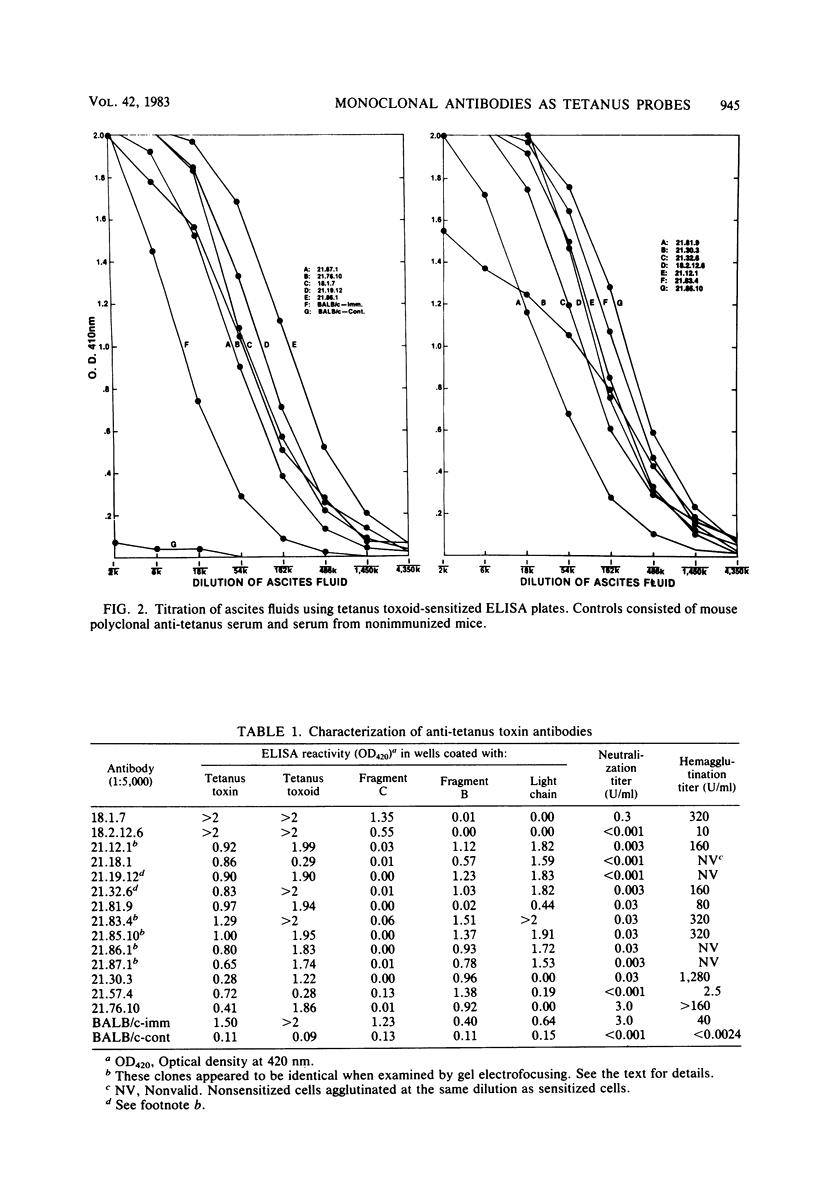
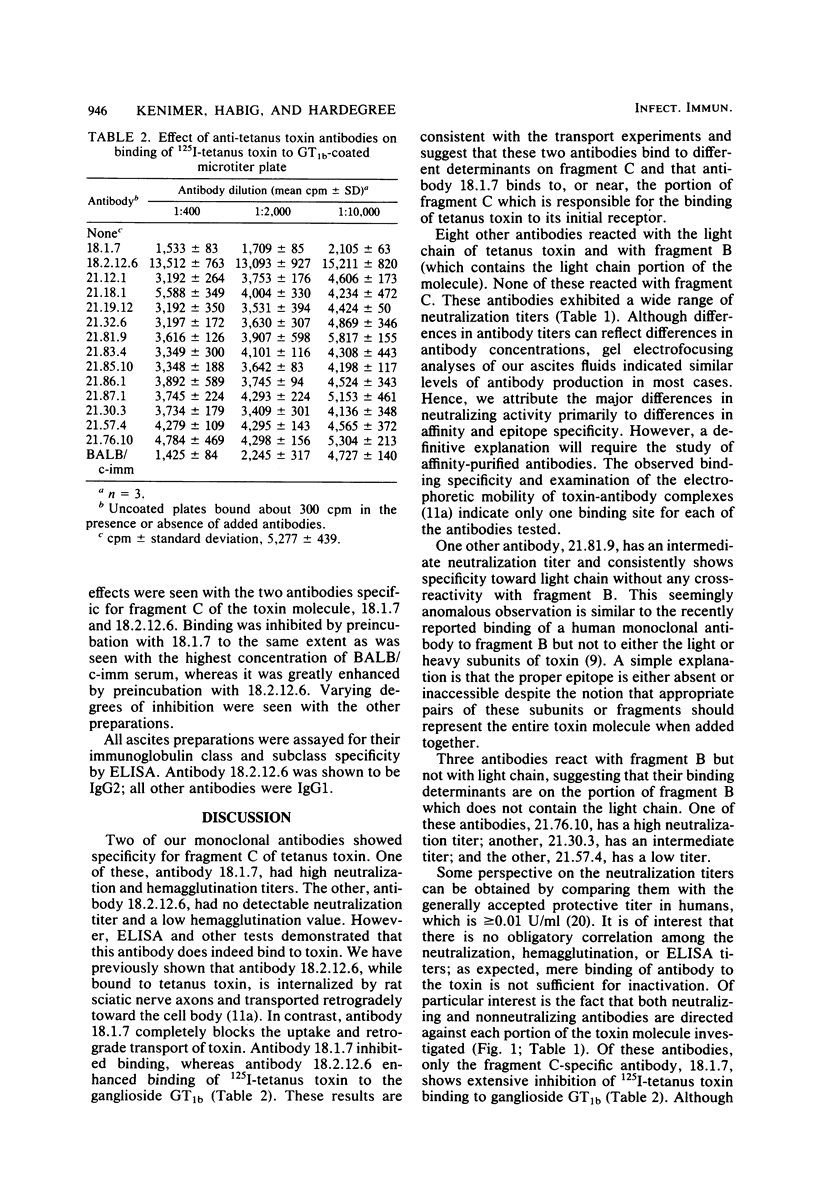
Monoclonal antibodies specific for fragment B, fragment C, and light chain of tetanus toxin were prepared by fusion of P3X63Ag8 BALB/c myeloma cells with spleen cells from BALB/c mice immunized with tetanus toxoid or fragment B. Hybridoma colonies were assayed for antibody production by an enzyme-linked immunosorbent assay. Fourteen positive clones were identified, cloned by limiting dilution, and injected intraperitoneally into mice to obtain ascites fluids. Thirteen of the monoclonal antibodies were of the immunoglobulin G1 subclass and one was immunoglobulin G2. Two of the antibodies were directed against sites on fragment C, nine were directed against the light chain, and three were directed against the portion of fragment B which does not comprise the light chain of tetanus toxin. At least one antibody in each group exhibited significant toxin neutralization activity. However, only one of these neutralizing antibodies strongly inhibited the binding of 125I-tetanus toxin to ganglioside-coated plates. These data indicate that interference with receptor recognition is not the only means of neutralizing tetanus toxin. Monoclonal antitoxins as potential therapeutic and prophylactic reagents are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnert-Hilger G., Bizzini B., Goretzki K., Müller H., Völckers C., Habermann E. Monoclonal antibodies against tetanus toxin and toxoid. Med Microbiol Immunol. 1983;172(2):123–135. doi: 10.1007/BF02124513. [DOI] [PubMed] [Google Scholar]
- Barile M. F., Hardegree M. C., Pittman M. Immunization against neonatal tetanus in New Guinea. 3. The toxin-neutralization test and the response of guinea-pigs to the toxoids as used in the immunization schedules in New Guinea. Bull World Health Organ. 1970;43(3):453–459. [PMC free article] [PubMed] [Google Scholar]
- Berzofsky J. A., Hicks G., Fedorko J., Minna J. Properties of monoclonal antibodies specific for determinants of a protein antigen, myoglobin. J Biol Chem. 1980 Dec 10;255(23):11188–11191. [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boquet P., Duflot E. Tetanus toxin fragment forms channels in lipid vesicles at low pH. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7614–7618. doi: 10.1073/pnas.79.24.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Gigliotti F., Insel R. A. Protective human hybridoma antibody to tetanus toxin. J Clin Invest. 1982 Dec;70(6):1306–1309. doi: 10.1172/JCI110730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardegree M. C., Barile M. F., Pittman M., Maloney C. J., Schofield F., Maclennan R. Immunization against neonatal tetanus in New Guinea. 4. Comparison of tetanus antitoxin titres obtained by haemagglutination and toxin neutralization in mice. Bull World Health Organ. 1970;43(3):461–468. [PMC free article] [PubMed] [Google Scholar]
- Helting T. B., Ronneberger H. J., Vollerthun R., Neubauer V. Toxicity of papain-digested tetanus toxin. Pathological effect of fragment B in the absence of spastic paralysis. J Biol Chem. 1978 Jan 10;253(1):125–129. [PubMed] [Google Scholar]
- Helting T. B., Zwisler O. Structure of tetanus toxin. I. Breakdown of the toxin molecule and discrimination between polypeptide fragments. J Biol Chem. 1977 Jan 10;252(1):187–193. [PubMed] [Google Scholar]
- Holmes N. J., Parham P. Enhancement of monoclonal antibodies against HLA-A2 is due to antibody bivalency. J Biol Chem. 1983 Feb 10;258(3):1580–1586. [PubMed] [Google Scholar]
- Holmgren J., Elwing H., Fredman P., Svennerholm L. Polystyrene-adsorbed gangliosides for investigation of the structure of the tetanus-toxin receptor. Eur J Biochem. 1980 May;106(2):371–379. doi: 10.1111/j.1432-1033.1980.tb04583.x. [DOI] [PubMed] [Google Scholar]
- Kozbor D., Roder J. C., Chang T. H., Steplewski Z., Koprowski H. Human anti-tetanus toxoid monoclonal antibody secreted by EBV-transformed human B cells fused with murine myeloma. Hybridoma. 1982;1(3):323–328. doi: 10.1089/hyb.1.1982.1.323. [DOI] [PubMed] [Google Scholar]
- Kozbor D., Roder J. C. Requirements for the establishment of high-titered human monoclonal antibodies against tetanus toxoid using the Epstein-Barr virus technique. J Immunol. 1981 Oct;127(4):1275–1280. [PubMed] [Google Scholar]
- Levine L., McComb J. A., Dwyer R. C., Latham W. C. Active-passive tetanus immunization. Choice of toxoid, dose of tetanus immune globulin and timing of injections. N Engl J Med. 1966 Jan 27;274(4):186–190. doi: 10.1056/NEJM196601272740404. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Yoneda M. Isolation and purification of two antigenically active, "complimentary" polypeptide fragments of tetanus neurotoxin. Infect Immun. 1975 Nov;12(5):1147–1153. doi: 10.1128/iai.12.5.1147-1153.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi J., Yoshida T., Sato Y., Nagaoka F., Kondo S., Matuhasi T. Requirement of at least two distinct monoclonal antibodies for efficient neutralization of tetanus toxin in vivo. Naturwissenschaften. 1982 Dec;69(12):597–598. doi: 10.1007/BF00396359. [DOI] [PubMed] [Google Scholar]
- Morris N. P., Consiglio E., Kohn L. D., Habig W. H., Hardegree M. C., Helting T. B. Interaction of fragments B and C of tetanus toxin with neural and thyroid membranes and with gangliosides. J Biol Chem. 1980 Jul 10;255(13):6071–6076. [PubMed] [Google Scholar]



