Abstract
Copper is a vital trace element required for normal growth and development of many organisms. To determine the roles for copper transporter 1 (Ctr1) in hepatic copper metabolism and the contribution of the liver to systemic copper homeostasis, we have generated and characterized mice in which Ctr1 is deleted specifically in the liver. These mice express less than 10% residual Ctr1 protein in the liver and exhibit a small but significant growth retardation, which disappears with age. Hepatic copper concentrations and the activities of copper-requiring enzymes are reduced; however, mild copper deficiency relative to Ctr1 protein deficit indicates compensatory mechanisms for copper metabolism. Copper concentrations of other organs did not alter despite the defect in hepatic copper uptake. Whereas biliary copper excretion is reduced, urinary copper concentration in these mice is higher than that of control mice. Our data indicate that Ctr1 plays a critical role in copper acquisition in the liver, and, when Ctr1 expression is compromised, compensatory mechanisms facilitate copper uptake and/or retention in the liver and excretion of copper via urine.
Keywords: liver-specific copper transporter 1 deletion, cuproenzymes, copper excretion, bile, urine
copper, a trace element, acts as an electron transfer agent because of its ability to donate or accept electrons through redox reactions. Thus copper is an essential cofactor for enzymes that perform critical functions in vital physiological processes, including energy generation, detoxification of superoxide anions, iron metabolism, and neurotransmitter biosynthesis (20, 25, 49). Copper also functions in a variety of other biological processes, such as immune response (15, 40), angiogenesis (3, 33), cardiovascular function (35, 46), and signaling (13, 35, 45, 46). Consistent with these vital roles for copper, copper-deficient animals exhibit a variety of symptoms, including defects in growth and development, cardiac failure, neurological dysfunctions, and anemia (17, 20, 25, 28, 38, 47–49). Patients with Menkes disease exhibit a defect in intestinal copper absorption and die in infancy because of copper deficiency (28). Genetic defects in copper incorporation into cytochrome c oxidase (CCO) also lead to fatal infantile disorders (47). Although copper is an essential micronutrient, it is highly toxic when accumulated in excess. Wilson disease is a human genetic disease associated with toxic accumulation of copper in the liver attributable to defects of a copper-transporting P-type ATPase (28, 48).
Since copper is an essential yet toxic micronutrient, organisms must acquire copper from the environment and maintain homeostatic metabolism. The copper transporter 1 (Ctr1) family of proteins plays a critical role for copper uptake across the plasma membrane in eukaryotes ranging from yeast to humans (7, 16, 20). Two Ctr1 members (Ctr1 and Ctr2) have been identified in the human and mouse genomes (24, 52). Several lines of evidence suggest that Ctr1 plays a critical role in cellular copper uptake in mammals. For example, human or mouse Ctr1 complements the function of yeast Ctr1 (24, 52). Mammalian cells transfected with a Ctr1 expression vector uptake more 64Cu compared with control cells (21). Given that Ctr1 knockout cells manifest a severe defect in copper delivery to all characterized copper-containing proteins (7, 22), Ctr1 is a major gateway for cellular copper. Although recent reports showed that Ctr2 stimulates copper transport in mammalian cells (1, 50), the functions of Ctr2 in mammals remain to be elucidated. Once copper is transported into the cell, it must be efficiently inserted into copper-requiring proteins without participation in harmful reactions. Indeed, copper is delivered to cuproenzymes via the target-specific cytoplasmic copper carrier molecules (copper chaperones) and assembly factors (5, 6, 42).
Characterizations of Ctr1 mRNA levels in mice and humans showed ubiquitous expression with high levels in the liver and kidney (24, 52). Mice completely deleted of the Ctr1 gene exhibit profound growth and developmental defects and die in utero in midgestation, demonstrating the important roles for Ctr1 in embryo development (19, 23). Interestingly, the steady-state copper levels of Ctr1 heterozygous (Ctr1+/−) mice were reduced approximately by half in the brain and spleen with no significant changes in other organs, including the liver and kidneys (23). Although these data demonstrate the critical role for Ctr1 in copper acquisition in the brain and spleen, the normal concentrations of copper in other organs of Ctr1+/− mice are puzzling. Characterization of copper transport in Ctr1-deleted mouse embryonic cells revealed that mammals possess Ctr1-independent copper transport system(s) (22). This alternate pathway may play an important role in copper absorption in certain organs and/or at specific developmental stages. When Ctr1 is deleted in intestinal epithelial cells, mice manifest severe copper deficiency in peripheral organs (31); however, these mice surprisingly accumulate biologically unavailable copper in enterocytes (31). Hence the roles for Ctr1 in copper acquisition at the organs and tissues in mammals remain largely unknown.
The liver rapidly and preferentially acquires copper (25, 49), but it is not known what determines this efficient copper uptake in the liver compared with other organs. Once copper enters into hepatocytes, copper is distributed to copper-requiring proteins, such as copper- and zinc-containing superoxide dismutase (Cu,Zn SOD) and CCO (16, 20). A significant portion of copper is also inserted into ceruloplasmin, a multicopper ferroxidase secreted from the liver into systemic circulation (12). Indeed, this ferroxidase contains greater than 95% of the plasma copper (12, 25). ATP7b, a copper-transporting P-type ATPase, delivers copper across the membrane of the trans-Golgi network for the incorporation into ceruloplasmin (28, 48). However, when copper levels are elevated, ATP7b travels from the secretory compartment to cytosolic vesicular structures, which appears to be an important mechanism for the excretion of excess copper into the bile (28, 48). Hence, the liver is known as a central organ for systemic copper homeostasis.
To define the roles for the Ctr1 in hepatic copper metabolism and the contribution of the liver to systemic copper homeostasis, we have generated and characterized transgenic mice in which Ctr1 is deleted specifically in the liver. Our data revealed that Ctr1 is a major player in hepatic copper acquisition. However, relatively mild copper deficiency in these mice where Ctr1 expression is reduced by more than 90% suggests that hepatocytes compensate for the Ctr1 deficit. Although the liver suffers copper deficiency, copper excretion into the bile is not completely blocked. This suggests that copper concentrations in the bile may not solely reflect the excretion of excess copper. Copper concentrations in other organs of these mice are normal. Elevated copper levels in the urine indicates that the renal excretion of copper might be an important mechanism for preventing excess copper accumulation in peripheral organs when hepatic uptake followed by biliary excretion of copper is reduced.
MATERIALS AND METHODS
Generation of mice deleted for Ctr1 specifically in the liver.
Liver-specific deletion of Ctr1 in mice was carried out using a Cre-loxp system. Ctr1F/F mice in which all four coding exons of Ctr1 are flanked by loxp recombination sites were described previously (31) and kindly provided by Dr. Dennis J. Thiele (Duke University). We crossed Ctr1F/F mice with Alb-Cre mice (37) (Jackson Laboratory), which express a Cre recombinase under the control of an albumin gene promoter, which obtains Ctr1F/+Alb-Cre mice. Ctr1F/+Alb-Cre mice were crossed with Ctr1F/F mice to generate Ctr1F/F, Ctr1F/FAlb-Cre, and Ctr1F/+Alb-Cre mice. Genotyping of these mice was conducted by PCR amplification of the DNA fragments of genomic DNA using the Ctr1- and Cre-specific primer sets (31) (Fig. 1A). Ctr1 heterozygous (Ctr1+/−) mice were described previously (23), and genotyping of these mice was carried out by PCR as described previously (23). Mice used in this study were maintained on the C57BL/6 genetic background. Animal experiments were conducted according to protocols approved by the IACUC at the University of Nebraska-Lincoln. Mice had free access to water and food (Harlan Teklad 7001, containing 24.26 mg copper/kg) and were kept under a 12-h light/dark cycle.
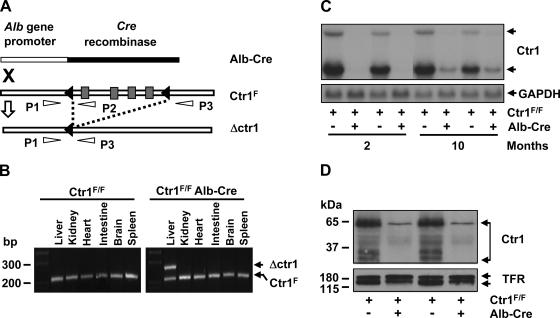
Fig. 1.
Deletion of Ctr1 in the liver. A: schematic depiction of chromosomal deletion of Ctr1 by recombination. Black triangles indicate loxp recombination sites that flank the four coding exons of Ctr1 (gray rectangles). A recombination event catalyzed by a Cre recombinase is indicated as dashed lines. P1, P2, and P3 indicate primers used for PCR genotyping of the loci. “X” indicates the intercross of mice possessing Ctr1F/F or a Cre recombinase gene fused with the albumin gene promoter. B: PCR analysis of recombination. Genomic DNA samples extracted from the organs of mice at 2 mo of age were subjected to PCR using P1, P2, and P3 primers. The fragments of 241 bp (PCR product of P1/P2 primer) and 281 bp (PCR product of P1/P3 primer) reflect Ctr1F and Ctr1 deletion (Δctr1), respectively. C: Northern blot analysis of Ctr1 transcripts. Total hepatic RNA isolated from 2 independent mice (1 male and 1 female) of each genotype was subjected to Northern blotting. A 32P-labeled DNA probe specific to mouse Ctr1 was used to detect mouse Ctr1 mRNA. A GAPDH-specific probe was used as a loading control as shown in the bottom panel. D: determination of Ctr1 protein expression by immunoblotting. Membrane proteins extracted from the liver of 2-mo-old mice were subjected to Western blot analysis of Ctr1 using anti-Ctr1 antibodies. Transferrin receptor (TFR) was probed as a loading control.
Measurement of metal levels.
Blood was collected from anesthetized mice, and then mice were perfused to obtained organ and tissue samples. To obtain serum fractions, whole blood samples were stored at 4°C overnight and then centrifuged at 1,000 g. The bile was obtained from gallbladders. Urine samples were collected in tubes directly from mice and weighed. Feces were collected from cages, dried using a dry oven, and then weighed. The samples were wet digested in 70% nitric acid and then diluted to 17.5% nitric acid with glass-distilled water. All plastic and glass wares used in metal analyses were soaked in 10% nitric acid and then washed thoroughly with glass-distilled water to prevent contamination of metal ions. Metal levels were measured by inductively coupled plasma mass spectrometry (ICP-MS).
Enzyme activity assays and immunoblotting.
Cell suspensions were obtained by homogenizing tissues with a homogenizer (Polytron) in buffer [10 mM Tris·HCl (pH 7.0), 250 mM NaCl, 1 mM EDTA, and protease inhibitor mixture (Complete Mini, Roche)] and then centrifugation at 300 g. Cells were lysed by freezing and thawing. For activity assays and immunoblotting of Cu,Zn SOD, cytosolic fractions were obtained by centrifugation of total cell lysates at 21,000 g for 15 min. Protein concentrations of samples were measured using the BCA assay kit (Pierce). Manganese-containing SOD was inactivated (30), and then Cu,Zn SOD activities were measured by determining the inhibition of nitrite formation from hydroxylamine in the presence of a superoxide-anion generator (32). Cu,Zn SOD activities were normalized to protein concentrations and then converted to the units using a standard curve of bovine liver Cu,Zn SOD (Sigma). For Western blotting, protein extracts were diluted in SDS-PAGE sample buffer, boiled, resolved by 15% SDS-PAGE, and then probed with antibodies against human Cu,Zn SOD (StressGen).
For CCO assays, cells were lysed in a 10 mM Tris·HCl buffer (pH 7.0) containing 250 mM sucrose, protease inhibitor mixture (Complete Mini, Roche), and 1% Tween 80 (Sigma). Total cell lysates were centrifuged at 21,000 g for 15 min, and supernatants were collected for enzyme assays and immunoblotting. CCO activity was detected as described previously (4). Immunoblotting was carried out with the use of antibodies against CCO subunit I (Molecular Probes). Anti-β-tubulin antibodies (Sigma) were used to probe tubulin as a loading control.
Determination of apo- and holoceruloplasmin in serum.
Serum samples (2 μl) were subjected to SDS-PAGE either with or without sample heating and addition of dithiothreitol (DTT) (100 mM) in SDS-PAGE sample buffer as described previously (44). Ceruloplasmin was detected by immunoblotting using anticeruloplasmin antibodies (44) kindly provided by Dr. Jonathan D. Gitlin (Washington University School of Medicine).
Western blotting of Ctr1.
Cells dissociated from the liver using a tissue homogenizer (Polytron) were lysed by freezing and thawing in a lysis buffer containing 10 mM Tris·HCl (pH 7.0), 250 mM NaCl, 1 mM EDTA, and protease inhibitor mixture (Complete Mini, Roche). Membrane fractions were collected by centrifugation of total cell lysates at 21,000 g for 15 min. Proteins were extracted by dissolving the pellets in the same buffer containing 1% Triton X-100 followed by centrifugation at 21,000 g for 5 min. Samples were denatured in SDS sample buffer without reducing agent for 15 min at 37°C, resolved by SDS-PAGE, and then transferred to a nitrocellulose membrane. Ctr1 was detected by chemiluminescence using anti-Ctr1 antibodies (31) kindly provided by Dr. Dennis J. Thiele (Duke University) and horseradish peroxidase (HRP)-conjugated anti-rabbit IgG antibodies (Santa Cruz Biotechnology). Transferrin receptor was probed to determine equal loading using antitransferrin receptor antibodies (Zymed Laboratories) and HRP-conjugated anti-mouse IgG antibodies (GE Healthcare).
Northern blot analysis.
Total RNA was isolated using the TRIzol Reagent (Invitrogen) according to the manufacturer's specifications. Twenty micrograms total RNA was separated by electrophoresis on a 1% formaldehyde-agarose gel and transferred to a membrane. Ctr1 and GAPDH mRNA levels were determined by using 32P-labeled mouse Ctr1 and rat GAPDH coding sequences as probes.
Statistical analysis.
Data were presented as means ± SD, and statistical comparisons of control and experimental groups were performed using unpaired, two tailed Student's t-test. P < 0.05 was considered to be significant.
RESULTS
Generation of mice deleted for Ctr1 specifically in the liver.
To determine the roles for Ctr1 in hepatic copper acquisition, we have generated Ctr1F/F mice expressing a Cre recombinase specifically in the liver. In Ctr1F/F mice, the loxp recombination sites were inserted in front and back of the four coding exons of Ctr1 (31) (Fig. 1A). These mice did not exhibit any significant change in growth or copper metabolism compared with wild-type control mice (31). The Alb-Cre transgenic mice express a Cre recombinase under the control of the promoter and upstream enhancer of the rat albumin gene, which leads to a hepatocyte-specific expression of the Cre recombinase (37). The genotypes of pups generated from these intercrosses (Ctr1F/+, Ctr1F/F, Ctr1F/+Alb-Cre, Ctr1F/FAlb-Cre) showed the expected Mendelian ratios, suggesting no defects of in utero growth and development of Ctr1F/FAlb-Cre mice (data not shown). Given that previous data indicated that recombination by Alb-Cre occurs progressively with age and reaches the maximum efficiency after 6 wk from birth (36), we characterized recombination of the Ctr1F alleles in 2-mo-old Ctr1F/F Alb-Cre mice and Ctr1F/F mice. We also characterized 10-mo-old mice to determine the copper metabolism of aged Ctr1F/FAlb-Cre mice. Analyses of recombination of Ctr1F alleles by the PCR using the P1, P2, and P3 primers (31) (Fig. 1A) revealed that Ctr1 deletion occurs specifically in the liver in an Alb-Cre-dependent manner (Fig. 1B). Northern blot analyses of Ctr1 expression in the liver of Ctr1F/FAlb-Cre mice indicated that Ctr1 mRNA is undetectable at 2 mo after birth and reduced by ∼90% at the age of 10 mo (Fig. 1C). Western blotting of Ctr1 demonstrated that Ctr1 expression in 2-mo-old Ctr1F/FAlb-Cre mice is less than 10% compared with control mice (Fig. 1D).
Growth retardation.
Ctr1F/FAlb-Cre mice did not manifest any obvious abnormality until 1 mo after birth; however, growth of Ctr1F/FAlb-Cre mice at 2 and 3 mo of age were retarded when compared with Ctr1F/F mice for both sexes (Fig. 2A). However, the difference in body weight disappeared at 10 mo of age (Fig. 2A). Analyses of the ratios of wet weight of major organs (including the liver, kidney, heart, spleen, and brain) to body weight revealed no significant difference between Ctr1F/FAlb-Cre mice and Ctr1F/F mice, suggesting an overall growth retardation of Ctr1F/FAlb-Cre mice rather than a problem in the specific organs (Fig. 2B). Although body size of Ctr1F/FAlb-Cre mice was smaller than that of control mice, these mice did not exhibit any noticeable abnormality in breeding, nursing, behavior, and life span up to ∼10–12 mo of age (data not shown).
Fig. 2.
Growth retardation of mice deleted for Ctr1 in the liver. A: body weights of Ctr1F/F and Ctr1F/FAlb-Cre mice were measured at 1, 2, 3, and 10 mo of age. B: relative wet weights of organs compared with total body weight were determined in mice at the age of 2 mo. Each bar represents the mean ± SD of the data of at least 10 male (M) or female (F) mice. *P < 0.05; **P < 0.005, Student's t-test.
Reduced copper concentrations in the liver and systemic circulation.
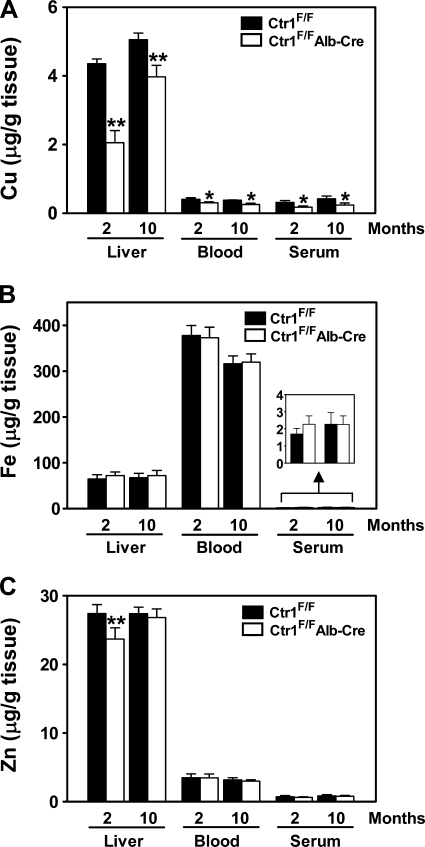
If Ctr1 plays a critical role in hepatic acquisition of copper, Ctr1 expression should correlate with copper levels in the liver. To test this, we dissected the liver of Ctr1F/F and Ctr1F/FAlb-Cre mice and measured the copper levels by ICP-MS. As shown in Fig. 3A, hepatic copper concentrations of 2- and 10-mo-old Ctr1F/FAlb-Cre mice were reduced significantly (48% and 79% of control mice, respectively). Although the reduction of copper concentrations did not exactly reflect Ctr1 protein expression levels shown in Fig. 1D, these data clearly demonstrate that Ctr1 is required for normal copper acquisition in the liver. The steady-state levels of zinc were also reduced, but iron levels were slightly increased (no statistically significant difference) (Fig. 3, B and C). We also measured the copper, iron, and zinc levels in systemic circulation to determine whether the reduced copper uptake in the liver affects the metabolism of these metal ions at the organismal level. The copper concentrations in whole blood and serum of Ctr1F/F Alb-Cre mice were reduced significantly (76% and 57% of control mice, respectively, at 2 mo of age and 68% and 57%, respectively, at 10 mo of age) (Fig. 3A). The serum iron level was slightly higher in Ctr1F/FAlb-Cre mice relative to that of control mice (no statistically significant difference) (Fig. 3B). In contrast, iron concentration in whole blood (Fig. 3B) was similar between the control and Ctr1F/FAlb-Cre groups, suggesting normal hemoglobin synthesis in Ctr1F/FAlb-Cre mice. No significant difference in zinc concentrations in blood and serum was observed (Fig. 3C).
Fig. 3.
Metal concentrations in the liver, blood, and serum. A portion of the large lob of the liver, whole blood, and serum were collected from 2-mo (female) and 10-mo-old (male) Ctr1F/F and Ctr1F/FAlb-Cre mice. Samples were digested in nitric acid, and then copper (Cu) (A), iron (Fe) (B), and zinc (Zn) (C) concentrations were measured by inductively coupled plasma mass spectrometry (ICP-MS). Each bar represents the mean ± SD of data obtained from at least 6 mice. *P < 0.005; **P < 0.001, Student's t-test.
Reduced activities of copper-requiring enzymes.
The cytosolic SOD (Cu,Zn SOD) and CCO are well-characterized cuproenzymes. Since Ctr1 deletion in the liver results in reduced steady-state accumulation of copper in the liver (Fig. 3A), we predicted that Ctr1F/FAlb-Cre mice would exhibit defects in the activities of these copper-requiring enzymes. Indeed, the activities of both Cu,Zn SOD and CCO enzymes in the liver of 2-mo-old Ctr1F/FAlb-Cre mice are reduced to 42% and 53% relative to the control mice, respectively (Fig. 4, A and B, lane 4, top). These enzyme activities correlated well with copper concentrations in the liver (Fig. 3A). No significant reduction of the activities of these enzymes in Ctr1F/+Alb-Cre mice in which Ctr1 deletion may occur only at one allele demonstrated that the deficits of Cu,Zn SOD and CCO activities are the consequence of Ctr1 deletion rather than ectopic expression of a Cre recombinase in the liver (Fig. 4, A and B, lane 3, top). Consistent with our previous data (23), the activities of these enzymes in the liver of Ctr1 heterozygous (Ctr1+/−) mice were similar to those of control Ctr1F/F mice (Fig. 4, A and B, lane 2, top). In contrast to previous reports indicating a reduced stability of Cu,Zn SOD protein and copper-containing subunits of CCO in cells suffering severe copper deficiency (5, 39), we could not observe significant reduction of protein levels of these enzymes in the liver of Ctr1F/FAlb-Cre mice (Fig. 4, A and B, middle). Only a small reduction of Cu,Zn SOD protein was observed in Ctr1F/FAlb-Cre mice (Fig. 4A, lane 4, middle). These data suggest that reduction of copper up to about 50% of normal concentrations does not affect the steady-state protein levels of these enzymes, and the reduced activities of Cu,Zn SOD and CCO in Ctr1F/F Alb-Cre mice are the consequence of a defect in copper insertion into these proteins.
Fig. 4.
Activities, copper incorporation, and expression levels of copper-dependent enzymes synthesized in the liver. Cu,Zn-superoxide dismutase (SOD1) (A) and cytochrome c oxidase (CCO) (B) activities were measured in cytosolic fractions and total protein extracts of the liver, respectively. Each bar represents the mean ± SD of 4 female mice of indicated genotypes at 2 mo of age. Immunoblots are shown below enzyme assay data. Tubulin was probed as a loading control. Data were compared with those of control mice (Ctr1F/F) by Student's t-test. *P < 0.0001. C: determination of copper insertion into ceruloplasmin (Cp). Serum samples (2 μl) were subjected to Western blotting using anticeruloplasmin antibodies. To differentiate apo- and holoceruloplasmin, serum samples were diluted in a nonreducing SDS sample buffer at room temperature and then resolved by SDS-PAGE at 4°C (top). To probe total ceruloplasmin, serum samples were denatured in a SDS sample buffer containing DTT (100 mM) at 95°C for 5 min and then resolved by SDS-PAGE at room temperature (bottom).
Ceruloplasmin is a serum glycoprotein containing six copper ions per molecule that are incorporated during its synthesis in the liver (12). To determine the effects of hepatic Ctr1 deletion on copper insertion into ceruloplasmin and its secretion, we probed apo- and holoceruloplasmin in serum by SDS-PAGE followed by Western blot analysis using anticeruloplasmin antibodies as described previously (44). Consistent with copper deficiency in the liver, a major species of ceruloplasmin in serum of Ctr1F/FAlb-Cre mice migrated at ∼115 kDa corresponding to the apo form (44) (Fig. 4C, lane 4, top); however, long exposure of the same blot showed several bands that migrated smaller than ∼115 kDa size (data not shown), suggesting some residual synthesis of holoceruloplasmin. Serum samples that were obtained from mice of other genotypes contained several species that migrated between 80 to 115 kDa (Fig. 4C, lanes 1–3, top), indicating ceruloplasmin species that contain one to six copper ions. Complete denaturation of serum by heating (95°C, 5 min) in a sample buffer containing a reducing agent (DTT, 100 μM) followed by Western blotting indicated that total ceruloplasmin levels were similar to each other among these serum samples (Fig. 4C, bottom). These data indicate that Ctr1-mediated copper acquisition is necessary for maturation of ceruloplasmin in the liver. Collectively, deletion of Ctr1 in the liver leads to defects in copper delivery to all characterized copper-requiring proteins.
Concentrations of copper, iron, and zinc in other organs.
Reduced hepatic copper uptake might result in excess copper accumulation in other organs and tissues. Alternatively, if copper uptake into hepatocytes is necessary for the synthesis and secretion of copper-carrier complexes that deliver copper to other organs, hepatic copper deficiency would result in copper deficiency in other organs. Given that holoceruloplasmin was reduced in the Ctr1F/FAlb-Cre mice relative to Ctr1F/F control mice (Fig. 4C) and ceruloplasmin plays an important role for iron metabolism (12), the deficits of enzymatically active ceruloplasmin might lead to a perturbation of iron metabolism. To address these questions, we measured copper, iron, and zinc levels in major organs of mice deleted for Ctr1 in the liver. The only difference we observed between Ctr1F/F Alb-Cre and Ctr1F/F mice was the slight reduction of iron levels in the spleen of Ctr1F/FAlb-Cre mice at the age of 2 mo (no statistically significant difference) (data not shown). No significant difference in copper and iron concentrations in tissues, including the intestine (duodenum), was observed between Ctr1F/F and Ctr1F/FAlb-Cre mice (data not shown). This suggests that the intestinal uptake of copper and iron from the diet is not regulated in response to the reduced hepatic copper uptake and subsequent deficits of holoceruloplasmin in serum.
Changes in copper excretion to the bile and urine.
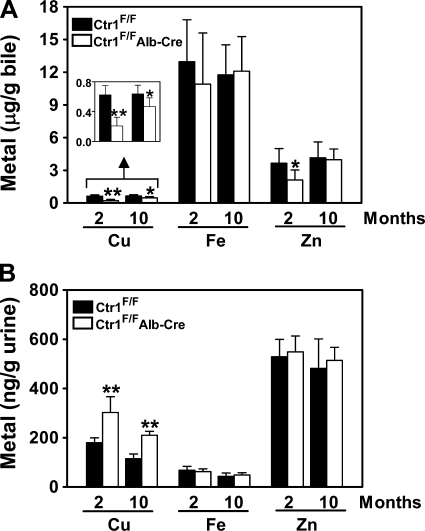
We predicted a correlation between the hepatic copper concentrations and copper excretion into bile. Indeed, Ctr1F/FAlb-Cre mice contained significantly lower copper levels in bile compared with Ctr1F/F mice (Fig. 5A). It is also interesting to note that zinc concentration in bile is reduced when Ctr1 is deleted in the liver (Fig. 5A), which is consistent with reduced zinc accumulation in the liver (Fig. 3C). Despite significant reduction of copper uptake and excretion in the liver of Ctr1F/FAlb-Cre mice, the copper concentrations in other organs and tissues are similar to those of control mice (data not shown). This data suggests that Ctr1F/FAlb-Cre mice maintain systemic copper homeostasis using alternative mechanisms. Given that a significant portion of copper absorbed from the diet is initially distributed to both the liver and kidneys (25, 26, 49), we determined whether the kidneys of Ctr1F/FAlb-Cre mice excrete excess copper to the urine. Indeed, the concentration of copper in the urine of Ctr1F/FAlb-Cre mice was significantly elevated compared with Ctr1F/F mice with no change in iron and zinc concentrations (Fig. 5B), suggesting roles for renal copper excretion in systemic copper homeostasis.
Fig. 5.
Excretion of copper, iron, and zinc into bile and urine. Bile (A) and urine (B) samples of 2-mo and 10-mo-old mice were collected into acid-washed tubes, weighed, mixed with nitric acid, and then copper (Cu), iron (Fe), and zinc (Zn) levels were measured by ICP-MS. Each bar represents the mean ± SD of the data obtained from at least 6 mice. *P < 0.05; **P < 0.0001, Student's t-test.
DISCUSSION
Characterization of copper metabolism in mice deleted for Ctr1 specifically in the liver demonstrates that Ctr1 plays a critical role in hepatic copper acquisition. However, copper deficiency was relatively mild considering that Ctr1 expression is reduced by more than 90%. This indicates that the mechanisms of copper homeostasis compensate for Ctr1 deficit at least partially. Moreover, excess copper excretion in the urine when Ctr1 is deleted in the liver provides a new insight into how mammals maintain systemic copper homeostasis.
Mice deleted for Ctr1 in the liver exhibit defects in growth at 2 and 3 mo of age but not at 1 and 10 mo of age. This is consistent with the observation that the recombination of loxp sites in mice carrying an Alb-Cre transgene occurs progressively with age with maximum efficiency after 6 wk from birth (36). Younger animals likely require more copper for rapid growth. Moreover, at 10 mo of age, Ctr1 deletion in Ctr1F/F Cre-Alb mice was less efficient and defects in copper acquisition were less severe when compared with those at 2 mo of age. This could explain the normal body weight of Ctr1F/FCre-Alb mice at older age. The underlying reason for the higher residual expression of Ctr1 mRNA in Ctr1F/FCre-Alb mice at 10 mo of age is not clear. It is possible that the liver in 10-mo-old mice might contain elevated levels of nonparenchymal cells that do not express a Cre recombinase. Second, expression and/or activities of Cre recombinase could be low at this age. Third, this might reflect a selective growth disadvantage of hepatocytes lacking Ctr1 and gradual overgrowth of those that have not deleted Ctr1.
Whereas mice completely deleted for Ctr1 die during mid-gestation (19, 23), deletion of Ctr1 specifically in the liver results in mild but significant growth defect. The residual expression of Ctr1 (∼10% of control) in the liver of Ctr1F/F Alb-Cre mice might explain the residual copper accumulation and relatively mild growth retardation. Deficits in cuproproteins that acquire copper via Ctr1 likely cause the growth defect of copper-deficient animals and embryonic death of Ctr1 knockout mice. However, our analysis of the ratios of wet weight of major organs to body weight indicates that growth retardation of Ctr1F/FAlb-Cre is not restricted to the liver although the liver is the only organ exhibiting copper deficiency. These data suggest that homeostatic copper acquisition in the liver is an important factor determining the growth of other organs and tissues. Alternatively, given that Ctr1 protein plays a role in fibroblast growth factor signal pathways (10), reduction of Ctr1 protein in the liver of Ctr1F/FAlb-Cre mice might lead to a defect in the mechanism of growth control that is dependent on normal functions of the liver.
Ctr1 is abundant in the canaliculi of the liver (18), but the functional significance of this distribution is not yet known. Since mice deleted for Ctr1 in the liver accumulate less copper both in the liver and bile, it is unlikely that Ctr1 plays a role in reabsorption of copper secreted into bile. Instead, our data suggest that Ctr1 functions for the acquisition of copper supplied via the portal vein and/or hepatic artery. Interestingly, it was shown that mice deleted for Ctr1 specifically in the intestine accumulate excess copper in intestinal epithelial cells that is biologically unavailable; despite this, mice manifest the expected phenotypes of copper deficiency in peripheral organs and tissues (31). However, given that the copper concentrations in the liver are reduced when Ctr1 is deleted, the excess accumulation of copper in the enterocytes when Ctr1 is deleted in these cells is a tissue-specific response. Characterization of the functions of Ctr1 in other organs and tissues would define whether Ctr1-dependent copper acquisition is carried out in other organs and whether any alternative copper uptake system complements Ctr1. Ctr2 (1, 22), divalent metal transporter (DCT1, Nramp2) (8), and/or Ctr1-independent copper uptake pathway identified in mouse embryos deleted for Ctr1 (22) could play a role in copper acquisition in the liver. However, given that the liver deleted for Ctr1 manifests copper deficiency, it is certain that copper uptake in the liver via Ctr1-independent pathway(s) could not replace the function of Ctr1. Secondly, reduced excretion of copper into bile might compensate for deficits of Ctr1-mediated acquisition. It is known that ATP7b copper-transporting P-type ATPases are mobilized from the secretory pathway to cytosolic vesicle-like compartments in response to excess copper (28, 48). When copper acquisition is insufficient, copper excretion to bile via ATP7b is likely inhibited. However, in Ctr1F/FAlb-Cre mice, copper excretion into bile remains 34% of control despite the fact that copper concentrations in the liver are less than 48% of control. Hence, copper excretion into bile may not reflect only the clearance of excess copper. Thirdly, subcellular location and/or activities of Ctr1 might be regulated in response to copper deficiency. Although the liver and kidneys have ∼20-fold higher levels of mRNA relative to other organs (24), Ctr1 protein expression in organs and tissues does not correlate well with mRNA levels (18). Consistently, the abundance of Ctr1 protein is influenced by the physiological state such as lactation and copper-deficient diet in a tissue-specific manner (18). It was also shown that elevated extracellular copper triggers endocytosis and turnover of Ctr1 in mammalian cell lines (9, 34). When Ctr1 expression is reduced resulting from Ctr1 deletion, the remaining Ctr1 might be more stable and functionally active in copper translocation across the plasma membrane.
Ceruloplasmin contains the most copper in the blood plasma under normal physiological condition (12). However, several lines of evidence indicate that ceruloplasmin does not play a significant role for copper metabolism in peripheral organs and tissues (11, 12, 29). We also could not observe any perturbation of copper acquisition in other organs of mice deleted for Ctr1 in the liver despite the fact that a majority of ceruloplasmin in serum of these mice does not contain copper. It is known that copper absorbed from the diet binds with amino acids and proteins (e.g., albumin and macroglobulin) in the blood plasma (26). Thus copper in the plasma of Ctr1F/FAlb-Cre mice is likely the amino acid-, albumin-, and/or macroglobulin-bound forms.
Copper-dependent ferroxidase activities of ceruloplasmin are believed to facilitate the release of iron from reticuloendothelial cells after the catabolism of senescent erythrocytes (12), which is supported by excess accumulation of iron in the liver and spleen when functional ceruloplasmin is defective (11, 39). Because the holoceruloplasmin levels were significantly low in Ctr1F/FAlb-Cre mice when compared with control mice, we thought that these mice would exhibit similar defects in iron metabolism observed in mice lacking ceruloplasmin. However, the iron deficiency in the spleen along with higher iron levels in serum of mice deleted for Ctr1 in the liver was different from the iron distribution patterns observed in aceruloplasminemia patients and mouse model. It was shown that mice suffering perinatal copper deficiency exhibit reduced iron accumulation in the spleen and higher iron levels in the liver along with reduced ceruloplasmin activities (41), which is similar to the phenotype of mice deleted for Ctr1 specifically in the liver.
Interactions between copper and zinc in their metabolism have been demonstrated. Several cases of zinc-induced copper deficiency have been reported (14, 51). Physiological concentrations of zinc affect the kinetics of copper uptake and transport in the Caco-2 human intestinal cells (43). Indeed, zinc has been used for the treatment of Wilson disease, an inherited disease of excess copper accumulation in the liver and brain (2). Despite these ample experimental data, the mechanisms underlying the interactions between zinc and copper have not been elucidated. Our data demonstrated that Ctr1 deletion in the liver reduces both copper and zinc levels in the liver and bile. However, given that 50-fold but not 10-fold higher concentrations of zinc significantly (>25%) inhibit Ctr1-mediated copper transport (21), Ctr1-mediated zinc transport unlikely occurs at physiological zinc concentrations. It is possible that cellular redox status (attributable to deficits of Cu,Zn SOD) or energy levels (attributable to deficits of CCO) may indirectly affect hepatic zinc uptake followed by excretion into bile.
Deletion of Ctr1 specifically in the liver allowed us to determine whether hepatic uptake and biliary excretion is a major mechanism for systemic copper homeostasis in mammals. Given that patients with Wilson disease accumulate copper in the liver and several other organs (28, 48), it has been assumed that copper uptake from the diet and subsequently into the liver is not regulated in response to hepatic copper status and there is no efficient compensatory route for copper excretion besides bile. However, mice deleted for Ctr1 in the liver do not accumulate excess copper in other organs and tissues. Downregulation of copper uptake at the intestine could be a mechanism of this systemic copper homeostasis. However, the fecal copper concentrations of Ctr1F/FAlb-Cre mice are not different from those of Ctr1F/F control mice (data not shown), but a caveat of this experiment is that fecal copper reflects the combination of both unabsorbed dietary copper and secreted copper via saliva, stomach fluid, and bile (26). Moreover, the concentrations of copper in the duodenum were not altered either. These data suggest no regulation of copper absorption from the intestine. In contrast, the enhanced copper concentrations in the urine of Ctr1F/FAlb-Cre mice indicate a compensatory copper excretion via the kidneys. This copper excretion pathway could play a critical role for systemic copper homeostasis under conditions of compromised hepatic copper uptake and presumably under normal physiological conditions as well.
Previous studies on animals growing with a copper-restricted diet revealed that the kidneys are more resistant to copper deficiency relative to other organs (39, 41). The kidneys may possess the capability for an active control of copper homeostasis. Although the expression of two copper-transporting ATPases (ATP7a and ATP7b) exhibits some organ-specific expression patterns, such as predominant expression of ATP7b in hepatocytes, many organs express both ATP7a and ATP7b (28). Immunohistochemistry revealed that renal cells express both ATP7a and ATP7b, and their subcellular locations are altered as a function of serum copper levels (27). Given that the liver and kidneys are two major organs that initially take up copper absorbed at the intestine (25, 49), it is likely that the kidneys receive more copper when Ctr1 in the liver is deleted. Copper-dependent regulation of these copper-transporting ATPases might promote excretion of copper into the urine, which could be an underlying mechanism for copper homeostasis in mice deleted for Ctr1 in the liver. Next, given that Ctr1 is localized to renal tubules, specifically the apical part of the tubule epithelium (18), the regulation of Ctr1-mediated absorption of copper on the luminal side of renal tubules could also determine the excretion of copper into the urine in response to excess copper in systemic circulation.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant DK79209 (to J. Lee), NIH Grant P20-RR-17675 (Nebraska Redox Biology Center), and funds provided through the Hatch Act in the University of Nebraska Agricultural Research Division.
Acknowledgments
We thank Dr. Dennis J. Thiele (Duke University) for Ctr1F/F mice and anti-Ctr1 antibodies and Dr. Jonathan D. Gitlin (Washington University School of Medicine) for anticeruloplasmin antibodies. We also thank Dr. Joseph R. Prohaska (University of Minnesota-Duluth) for helpful discussions and critical reading of this manuscript and Dr. Ted Huston (University of Michigan) for ICP-MS. We thank Lee laboratory members for technical assistance and suggestions.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bertinato J, Swist E, Plouffe LJ, Brooks SP, L'abbé MR. Ctr2 is partially localized to the plasma membrane and stimulates copper uptake in COS-7 cells. Biochem J 409: 731–740, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Brewer GJ Zinc acetate for the treatment of Wilson's disease. Expert Opin Pharmacother 2: 1473–1477, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Brewer GJ Copper control as an antiangiogenic anticancer therapy: lessons from treating Wilson's disease. Exp Biol Med (Maywood) 226: 665–673, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Capaldi RA, Marusich MF, Taanman JW. Mammalian cytochrome-c oxidase: characterization of enzyme and immunological detection of subunits in tissue extracts and whole cells. Methods Enzymol 260: 117–132, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Cobine PA, Pierrel F, Winge DR. Copper trafficking to the mitochondrion and assembly of copper metalloenzymes. Biochim Biophys Acta 1763: 759–772, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Culotta VC, Klomp LWJ, Strain J, Casareno RLB, Krems B, Gitlin JD. The copper chaperone for superoxide dismutase. J Biol Chem 272: 23469–23472, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Dancis A, Yuan DS, Haile D, Askwith C, Eide D, Moehle C, Kaplan J, Klausner RD. Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell 76: 393–402, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388: 482–488, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Guo Y, Smith K, Lee J, Thiele DJ, Petris MJ. Identification of methionine-rich clusters that regulate copper-stimulated endocytosis of the human Ctr1 copper transporter. J Biol Chem 279: 17428–17433, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Haremaki T, Fraser ST, Kuo YM, Baron MH, Weinstein DC. Vertebrate Ctr1 coordinates morphogenesis and progenitor cell fate and regulates embryonic stem cell differentiation. Proc Natl Acad Sci USA 104: 12029–12034, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris ZL, Durley AP, Man TK, Gitlin JD. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc Natl Acad Sci USA 96: 10812–10817, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellman NE, Gitlin JD. Ceruloplasmin metabolism and function. Annu Rev Nutr 22: 439–458, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Hirayama T, Alonso JM. Ethylene captures a metal! Metal ions are involved in ethylene perception and signal transduction. Plant Cell Physiol 41: 548–555, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman HN, Phyliky RL, Fleming CR. Zinc-induced copper deficiency. Gastroenterology 94: 508–512, 1988. [DOI] [PubMed] [Google Scholar]
- 15.Hopkins RG, Failla ML. Transcriptional regulation of interleukin-2 gene expression is impaired by copper deficiency in Jurkat human T lymphocytes. J Nutr 129: 596–601, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol 4: 176–185, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Klevay LM Cardiovascular disease from copper deficiency-a history. J Nutr 130: 489S–492S, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Kuo YM, Gybina AA, Pyatskowit JW, Gitschier J, Prohaska JR. Copper transport protein (Ctr1) levels in mice are tissue specific and dependent on copper status. J Nutr 136: 21–26, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuo YM, Zhou B, Cosco D, Gitschier J. The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc Natl Acad Sci USA 98: 6836–6841, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J, Adle D, Kim H. Molecular mechanisms of copper homeostasis in yeast. Topics Curr Genet 14: 1–36, 2006. [Google Scholar]
- 21.Lee J, Pena MM, Nose Y, Thiele DJ. Biochemical characterization of the human copper transporter Ctr1. J Biol Chem 277: 4380–4387, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Lee J, Petris MJ, Thiele DJ. Characterization of mouse embryonic cells deficient in the ctr1 high affinity copper transporter. Identification of a Ctr1-independent copper transport system. J Biol Chem 277: 40253–40259, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Lee J, Prohaska JR, Thiele DJ. Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc Natl Acad Sci USA 98: 6842–6847, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J, Prohaska JR, Dagenais SL, Glover TW, Thiele DJ. Isolation of a murine copper transporter gene, tissue specific expression and functional complementation of a yeast copper transport mutant. Gene 254: 87–96, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Linder MC Biochemistry of Copper. New York: Plenum Press, 1991.
- 26.Linder MC, Wooten L, Cerveza P, Cotton S, Shulze R, Lomeli N. Copper transport. Am J Clin Nutr 67: 965S–971S, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Linz R, Barnes NL, Zimnicka AM, Kaplan JH, Eipper B, Lutsenko S. Intracellular targeting of copper-transporting ATPase ATP7A in a normal and Atp7b-/- kidney. Am J Physiol Renal Physiol 294: F53–F61, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. Function and regulation of human copper-transporting ATPases. Physiol Rev 87: 1011–1046, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Meyer LA, Durley AP, Prohaska JR, Harris ZL. Copper transport and metabolism are normal in aceruloplasminemic mice. J Biol Chem 276: 36857–36861, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Nebot C, Moutet M, Huet P, Xu JZ, Yadan JC, Chaudiere J. Spectrophotometric assay of superoxide dismutase activity based on the activated autoxidation of a tetracyclic catechol. Anal Biochem 214: 442–451, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Nose Y, Kim BE, Thiele DJ. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab 4: 235–244, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Oyanagui Y Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal Biochem 142: 290–296, 1984. [DOI] [PubMed] [Google Scholar]
- 33.Pan Q, Kleer CG, van Golen KL, Irani J, Bottema KM, Bias C, De Carvalho M, Mesri EA, Robins DM, Dick RD, Brewer GJ, Merajver SD. Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res 62: 4854–4859, 2002. [PubMed] [Google Scholar]
- 34.Petris MJ, Smith K, Lee J, Thiele DJ. Copper-stimulated endocytosis and degradation of the human copper transporter, hCtr1. J Biol Chem 278: 9639–9646, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Plane F, Wigmore S, Angelini GD, Jeremy JY. Effect of copper on nitric oxide synthase and guanylyl cyclase activity in the rat isolated aorta. Br J Pharmacol 121: 345–350, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Postic C, Magnuson MA. DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis 26: 149–150, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, Lindner J, Cherrington AD, Magnuson MA. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem 274: 305–315, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Prohaska JR Long-term functional consequences of malnutrition during brain development: copper. Nutrition 16: 502–504, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Prohaska JR, Brokate B. Lower copper, zinc-superoxide dismutase protein but not mRNA in organs of copper-deficient rats. Arch Biochem Biophys 393: 170–176, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Prohaska JR, Lukasewycz OA. Copper deficiency suppresses the immune response of mice. Science 213: 559–561, 1981. [DOI] [PubMed] [Google Scholar]
- 41.Prohaska JR, Lukasewycz OA. Effects of copper deficiency on the immune system. Adv Exp Med Biol 262: 123–143, 1990. [DOI] [PubMed] [Google Scholar]
- 42.Pufahl RA, Singer CP, Peariso KL, Lin SJ, Schmidt PJ, Fahrni CJ, Culotta VC, Penner-Hahn JE, O'Halloran TV. Metal ion chaperone function of the soluble Cu(I) receptor Atx1. Science 278: 853–856, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Reeves PG, Briske-Anderson M, Johnson L. Physiologic concentrations of zinc affect the kinetics of copper uptake and transport in the human intestinal cell model, Caco-2. J Nutr 128: 1794–1801, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Sato M, Gitlin JD. Mechanisms of copper incorporation during the biosynthesis of human ceruloplasmin. J Biol Chem 266: 5128–5134, 1991. [PubMed] [Google Scholar]
- 45.Schlief ML, West T, Craig AM, Holtzman DM, Gitlin JD. Role of the Menkes copper-transporting ATPase in NMDA receptor-mediated neuronal toxicity. Proc Natl Acad Sci USA 103: 14919–14924, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shiva S, Wang X, Ringwood LA, Xu X, Yuditskaya S, Annavajjhala V, Miyajima H, Hogg N, Harris ZL, Gladwin MT. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat Chem Biol 2: 486–493, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Shoubridge EA Cytochrome c oxidase deficiency. Am J Med Genet 106: 46–52, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Tao TY, Gitlin JD. Hepatic copper metabolism: insights from genetic disease. Hepatology 37: 1241–1247, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Uauy R, Olivares M, Gonzalez M. Essentiality of copper in humans. Am J Clin Nutr 67: 952S-959S, 1998. [DOI] [PubMed] [Google Scholar]
- 50.van den Berghe PV, Folmer DE, Malingré HE, van Beurden E, Klomp AE, van de Sluis B, Merkx M, Berger R, Klomp LW. Human copper transporter 2 is localized in late endosomes and lysosomes and facilitates cellular copper uptake. Biochem J 407: 49–59, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Willis MS, Monaghan SA, Miller ML, McKenna RW, Perkins WD, Levinson BS, Bhushan V, Kroft SH. Zinc-induced copper deficiency: a report of three cases initially recognized on bone marrow examination. Am J Clin Pathol 123: 125–131, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Zhou B, Gitschier J. hCTR1: a human gene for copper uptake identified by complementation in yeast. Proc Natl Acad Sci USA 94: 7481–7486, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]