Abstract
The goal of this study was to investigate the role of the adipokine adiponectin (APN) in development of spontaneous colitis in IL-10 knockout (KO) mice. To this aim, we generated double IL-10 APN KO mice and compared their disease development to that of single IL-10 KO mice. Both IL-10 KO and double IL-10 APN KO mice spontaneously developed colitis of comparable severity. No significant differences in inflammatory infiltrate or crypt elongation were observed in colonic tissue obtained from IL-10 KO and double IL-10 APN KO mice at either 12 or 20 wk of age. A comparable increase in circulating levels of serum amyloid A and IFN-γ was observed in IL-10 KO and double IL-10 APN KO mice as disease progressed. In vitro stimulation of lymphocytes from mesenteric lymph nodes with anti-CD3 and anti-CD28 induced a significantly higher production of IL-17 and TNF-α in IL-10 KO and double IL-10 APN KO mice compared with their healthy littermates. No significant differences in cytokine production from lymphocytes or colonic mRNA expression of cytokines were observed between IL-10 KO and double IL-10 APN KO mice. Both IL-10 KO and double IL-10 APN KO mice had a similar decrease in body weight and bone mass compared with their respective healthy littermates. Finally, APN deficiency did not lead to development of insulin resistance, either in APN KO or double IL-10 APN KO mice. In conclusion, lack of APN does not play a significant role in the pathogenesis of spontaneous colonic inflammation in the IL-10 KO model.
Keywords: adipocytes, colon, cytokines, inflammation
adiponectin (APN) is an adipokine, a protein mostly produced by adipocytes, involved in modulation of insulin sensitivity, cardiovascular disease (CVD) and inflammatory responses (9). Murine and human APN are 82% identical at the amino acid level and share a common complex structure: APN monomers form trimers which further polymerize to generate complexes of varying molecular weight (13). Both murine and human APN exert their bioactivity by binding to specific membrane-bound receptors (ADIPOR1 and ADIPOR2) as well through molecular interactions with selected molecules, such as T-cadherin and various growth factors (27). The different molecular weight forms of APN may exert specific biological activities, although this issue has not yet been completely clarified.
Several reports indicate an anti-inflammatory function of APN, including effects on suppressing production of proinflammatory cytokines, such as TNF-α and IL-6, while increasing expression of anti-inflammatory ones, such as IL-10 and IL-1Ra (18). However, proinflammatory effects of APN have also been reported in various experimental systems (8). For example, APN induces chemokine production from colonic epithelial cells (22) and stimulates IL-6 synthesis in fibroblasts (20, 28). Thus the role of APN as a modulator of inflammation is likely multifaceted and highly context dependent.
Reduced circulating levels of APN are observed in subjects with the metabolic syndrome, Type 2 diabetes, or CVD compared with healthy individuals (13). It has been postulated that low APN is pathogenic in these metabolic conditions, favoring development of insulin resistance and atherosclerosis. In contrast, increased levels of APN are observed in several autoimmune and chronic inflammatory diseases (8). In particular, high levels of APN at both the mRNA and protein level are present in the mesenteric adipose tissue of Crohn's disease (CD) patients compared with controls (23, 32). In the same CD patient, APN expression is higher in inflamed compared with noninflamed adipose tissue, indicating the presence of an ongoing inflammatory reaction concomitant with high APN expression (32). The alterations of APN expression observed in CD patients suggest that this molecule might participate in modulation of colonic inflammation as well as influence extraintestinal manifestations of IBD, such as bone loss, arthritis, and others (11). It is therefore important to evaluate the potential involvement on APN in models of IBD to better understand the involvement of this adipokine in the pathogenesis of chronic colitis.
The use of experimental models of IBD, particularly in mice, has been instrumental in advancing our understanding of the pathogenesis of this complex disorder and in generating novel therapies (25). A variety of experimental models of IBD has been developed, each of which reproduces distinct aspects of human IBD (31). When studying the role of a molecule or pathway in IBD, it is therefore important to use different experimental approaches to encompass the whole pathophysiological spectrum. We and others have previously reported an altered response to colitis induced by administration of dextran sulfate sodium (DSS) in APN knockout (KO) mice (10, 21). However, although our laboratory (10) observed that APN deficiency was associated with protection from colonic inflammation in response to DSS, the opposite results were reported by Nishihara and colleagues (21). The reasons for this discrepancy are currently unclear.
IL-10 is an anti-inflammatory cytokine primarily secreted by monocytes and lymphocytes (12). IL-10 KO mice spontaneously develop chronic intestinal inflammation, which is manifested by symptoms commonly associated with CD, including weight loss and osteopenia (2). Development of disease in IL-10 KO mice is largely dependent on the inappropriate immune response to intestinal antigens, mediated by effector CD4+ T cells. In IL-10 KO mice, colitis develops between 10–20 wk of age and is associated with excessive secretion of Th1 and Th17 cytokines.
Because APN is elevated in CD patients (23, 32), we evaluated the role of APN in the IL-10 KO model of chronic colitis that partially reproduces CD pathogenesis. To this aim we generated double IL-10 APN KO mice and compared them with single IL-10 KO mice. Our data indicate that APN deficiency does not play a significant role in modulating colonic inflammation in the IL-10 KO model.
MATERIALS AND METHODS
Experimental animals and treatments.
Care of mice followed institutional guidelines under a protocol aproved by the institutional Animal Committee of the University of Illinois at Chicago. Double IL-10 APN KO mice were generated by crossing heterozygote APN+/− mice on a C57BL/6J background with IL-10 KO mice also on a C57BL/6J background obtained from The Jackson Laboratories (Bar Harbor, ME). The progeny was genotyped for IL-10 and APN by PCR of genomic DNA. After genotyping, APN deficiency was confirmed in each mouse by measuring serum APN using a specific ELISA (R&D Systems, Minneapolis, MN). Mice were weighed weekly and monitored for appearance of diarrhea or rectal prolapse. Mice were euthanized by isoflurane inhalation and cervical dislocation at 12 or 20 wk of age. Littermates of various genotypes were used in each experiment.
Postmortem, the entire colon was excised and, after extensive washing in PBS to remove fecal matter, the colon was dissected. A 1-cm segment of the transverse colon was fixed in formalin, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin for histological analysis. Sections were evaluated in a blinded fashion by a pathologist (R. J. Cabay). A score (0–3) was given for inflammatory cell infiltration, based on the following criteria: grade 0, no change from normal tissue; grade 1, one or a few multifocal mononuclear cell infiltrates in the lamina propria; grade 2, intestinal lesions with several multifocal inflammatory cell infiltrates in the lamina propria, with no inflammation in the submucosa; grade 3, extensive leukocyte infiltration involving most of the intestinal section. A score (0–3) was given for crypt elongation, based on the following criteria: grade 0, no change from normal tissue; grade 1, minimal epithelial hyperplasia; grade 2, moderate hyperplasia; grade 3, marked hyperplasia.
Bone mineral density (BMD) and content (BMC) were evaluated by dual energy X-ray absorptiometry scanning of the whole mouse body, excluding the head.
Serum and tissue sample collection.
Blood samples were obtained from the retroorbital plexus under isoflurane anesthesia, and serum was prepared and stored at −70°C until analysis. Two segments of the colon were removed and snap frozen in liquid nitrogen and stored at −70°C until analysis. Lymphocytes were isolated from mesenteric lymph nodes (MLN). One MLN was removed, passed through a 100-μl strainer with 5 ml of RPMI (1% Pen/Strep), washed, and resuspended in 1 ml of RPMI. Lymphocytes were counted and cultured at 4 × 106/ml for 24 h at 37°C in 96-well plates in the presence or absence of plate-bound anti-CD3 and anti-CD28 antibodies, each at 10 μg/ml (BD Biosciences, San Jose, CA).
Miscellaneous measurements.
Adiponectin, leptin (R&D Systems), IL-6 (BD Biosciences), TNF-α, IFN-γ, and IL-17 (e-Bioscience, San Diego, CA) concentrations were determined by ELISA. The sensitivity of each assay is 15 pg/ml. Serum amyloid A levels were determined by ELISA (Biosource International, Camarillo, CA), with a sensitivity of 5 ng/ml. Blood glucose levels were measured in 4-h-fasted mice via a OneTouch glucose monitoring system (LifeScan). Insulin levels were evaluated by ELISA (Linco Research, St. Charles, MO).
RNA expression analysis.
Total RNA was isolated from frozen colon tissue samples using TRIzol and reverse transcribed. Gene expression levels were assessed by real-time quantitative RT-PCR using specific primers from Applied Biosystems. Amplification of the housekeeping gene GAPDH was performed for each sample to allow normalization. Data normalized to GAPDH were compared using the ΔΔCt method and expressed as fold induction of gene expression in IL-10 KO samples vs. control samples.
Statistical analysis.
Statistical analyses were performed using XLStat software (Addinsoft, Paris, France). Significance of differences was determined by factorial ANOVA (Fisher's pairwise comparison) and paired Student's t-test. Data are expressed as means ± SE. Differences were considered significant at P < 0.05.
RESULTS
Serum APN levels.
In agreement with previous studies (15), serum APN levels were significantly reduced in both male and female APN+/− compared with APN+/+ mice. As shown in Table 1, on average serum APN levels were 58% lower in APN+/− compared with APN+/+ males and 50% lower in APN+/− compared with APN+/+ females.
Table 1.
Serum APN levels
Values are means ± SE. Serum adiponectin (APN) levels were measured in male and female APN+/+ and APN+/− mice. N = 9 for APN+/+ and N = 13 for APN+/− mice.
P < 0.001 APN+/− vs. respective APN+/+.
However, no significant differences were observed between APN+/− and APN+/+ mice for any of the other parameters evaluated. Therefore, results from both genotypes were combined into a single group [wild-type (WT)]. Furthermore, no significant differences in circulating APN levels were observed between IL-10+/+ and IL-10−/− mice at either 12 or 20 wk of age when matched by age and APN genotype (+/+ or +/−) (data not shown).
Development of colitis in IL-10 KO and double IL-10 APN KO mice.
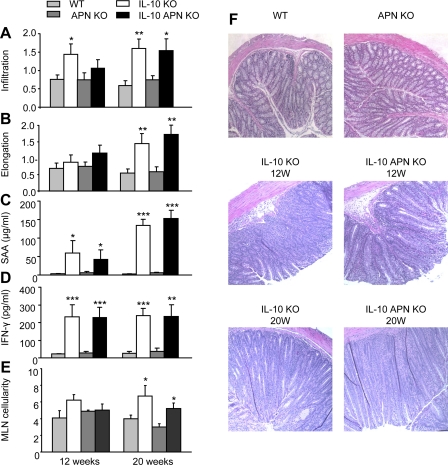
Double IL-10 APN KO mice were generated and disease development was compared with that of single IL-10 KO mice, at an early phase (12 wk) and a late phase (20 wk) of disease. Both IL-10 KO and double IL-10 APN KO mice developed a significant inflammatory infiltrate in the colon at 12 and 20 wk (Fig. 1A). Severity of the inflammatory infiltrate was not significantly different between the two groups. In the early phase of disease, both IL-10 KO and double IL-10 APN KO displayed minimal crypts hyperplasia (Fig. 1B), whereas in the late phase a marked hyperplastic response was observed in both groups compared with their respective controls. Representative figures of colonic samples from each group are shown in Fig. 1F.
Fig. 1.
Inflammatory infiltrate, colonic hyperplasia, and serum markers of inflammation. Inflammatory cell infiltration (A) and crypt elongation (B) were evaluated in colonic tissue at week 12 and 20. Levels of serum amyloid A (SAA; C) and IFN-γ (D) were measured in serum at the same time points. E: cellularity of the mesenteric lymph nodes. F: representative histological samples of colonic tissue from the indicated groups. Data are means ± SE. N = 10 for wild-type (WT) and IL-10 knockout (KO) mice and N = 14 for adiponectin (APN) KO and double IL-10 APN KO mice. *P < 0.05, **P < 0.01, and ***P < 0.001 WT vs. IL-10 KO mice and APN KO vs. double IL-10 APN KO mice.
The gradual progress of colonic inflammation was associated with elevation in circulating levels of the acute-phase protein serum amyloid A (SAA) in both IL-10 KO and double IL-10 APN KO mice (Fig. 1C). Serum levels of IFN-γ also correlated with colitis severity, with markedly increased levels in both IL-10 KO and double IL-10 APN KO at 12 and 20 wk compared with their respective controls (Fig. 1D). No significant differences in SAA or IFN-γ levels were observed between IL-10 KO and double IL-10 APN KO mice. As shown in Fig. 1E, a significant enlargement of MLN was observed at 20 wk of age in both IL-10 KO and double IL-10 APN KO mice with no significant differences between the two groups.
Mortality was observed in 3 of 34 IL-10 KO mice and 1 of 52 IL-10 APN KO mice (not significant). Finally, 1 of 34 IL-10 KO and 1 of 52 IL-10 APN KO mice developed rectal prolapse (not significant).
Cytokine production by mesenteric lymphocytes.
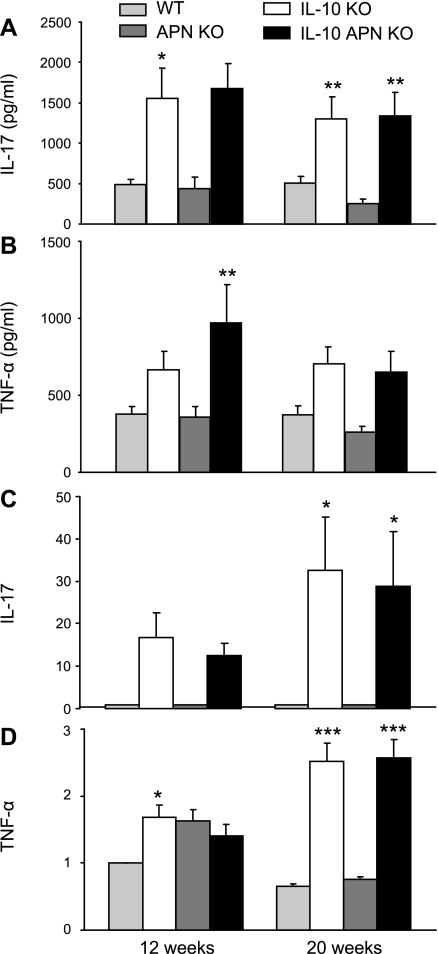
Lymphocytes obtained from MLN were cultured in vitro with and without stimulation with anti-CD3 and anti-CD28 antibodies to evaluate cytokine production. As shown in Fig. 2A, stimulated lymphocytes from both IL-10 KO and double IL-10 APN KO mice produced significantly higher levels of IL-17 and TNF-α at 12 and 20 wk compared with lymphocytes from their respective healthy littermates. However, no significant differences were observed between IL-10 KO and double IL-10 APN KO mice in terms of IL-17 or TNF-α production. Comparable data were obtained when IL-6 and IFN-γ were measured (data not shown). No significant differences for any of the cytokines were observed among the different groups in unstimulated MLN cultures (data not shown).
Fig. 2.
Cytokine production by mesenteric lymphocytes and colonic gene expression. Lymphocytes were isolated from MLN of WT, APN KO, IL-10, KO and double IL-10 APN KO mice at 12 and 20 wk and stimulated with anti-CD3 and anti-CD28. Levels of IL-17 (A) and TNF-α (B) were measured in the supernatant. Colonic expression of IL-17 (C) and TNF-α (D) was determined by RT-PCR. Data in C and D are presented as values normalized to GAPDH. Data are means ± SE. N = 10 for A and B and N = 5 for C and D. *P < 0.05, **P < 0.01, and ***P < 0.001 WT vs. IL-10 KO mice and APN KO vs. IL-10 APN KO mice.
Colonic expression of cytokines.
To analyze cytokine expression at the site of inflammation, various cytokines were analyzed by RT-PCR of RNA extracted from colonic tissue. Colonic gene expression of IL-17 and TNF-α was increased in both IL-10 KO and double IL-10 APN KO mice compared with their healthy controls (Fig. 2, C and D). However, no significant differences were observed between IL-10 KO and double IL-10 APN KO mice in terms of IL-17 or TNF-α expression. A similar trend was observed for IFN-γ (data not shown).
Body weight, serum leptin levels, and bone mass in IL-10 and double IL-10 APN KO mice.
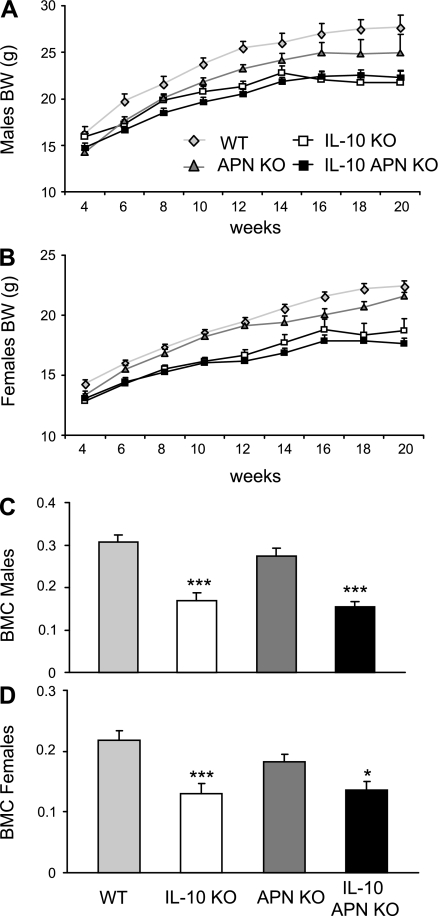
IL-10 deficiency is associated with reduced body weight (17). Body weight was evaluated weekly in IL-10 KO, double IL-10 APN KO mice and their respective littermates starting at age 4 wk. Growth retardation became evident in both IL-10 KO and double IL-10 APN KO mice compared with their respective controls between 4 and 6 wk of age, with both sexes equally affected (Fig. 3, A and B). Reduced body weight in both IL-10 KO and double IL-10 APN KO mice was associated with significantly lower circulating levels of leptin compared with their respective controls (Table 2). Furthermore, male APN KO mice had significantly lower body weight compared with their WT littermates up to 16 wk of age, when the difference became not significant (Fig. 3A). Female APN KO mice were significantly smaller than WT littermates only at 4 wk of age (Fig. 3B).
Fig. 3.
Body weight and bone mineral content. WT, IL-10 KO, APN KO, and double IL-10 APN KO males (A) and females (B) were weighed weekly starting at age 4 wk. Both male and female IL-10 KO and double IL-10 APN KO mice were significantly smaller than WT and APN KO mice, respectively, at all time points after age 6 wk. C and D: whole-body BMC was evaluated by dual-energy X-ray absorptiometry (DEXA) in males (C) and females (D). Data are means ± SE. N = 18–22 for A and B and N = 7–10 for C and D. ***P < 0.001 WT vs. IL-10 KO mice and APN KO vs. IL-10 APN KO mice.
Table 2.
Serum leptin levels
| 12 wk | 20 wk | |
|---|---|---|
| WT | 4.3±0.8 | 5.7±1.0 |
| IL-10 KO | 1.8±0.3* | 2.9±0.6† |
| APN KO | 4.1±0.7 | 4.1±0.7 |
| IL-10 APN KO | 1.2±0.2§ | 2.3±0.5† |
Values are means ± SE. Serum leptin levels were measured at 12 and 20 wk of age. N = 12 for wild-type (WT) and IL-10 knockout (KO) mice and N = 15 for APN KO and double IL-10 APN KO mice.
P < 0.05,
P < 0.001 vs. WT; ‡P < 0.05;
P < 0.01 vs. APN KO mice.
Inflammatory bowel disease is associated with bone loss (24). The presence of colitis is an important contributor to osteopenia in IL-10 KO mice, with increased mechanical fragility and decreased bone formation (6). The degree of osteopenia was investigated in IL-10 KO and double IL-10 APN KO mice by evaluation of BMD and BMC of the whole body as well as the tibia and spine separately at 12 and 20 wk of age. Both IL-10 KO and double IL-10 APN KO mice had a significantly lower BMC compared with their respective littermates, with a comparable effect observed in males and females (Fig. 3, C and D). However, no significant differences were observed between IL-10 KO and double IL-10 APN KO mice. No differences were observed among the various groups when BMD was evaluated (data not shown). Separate analysis of tibia and spine generated results comparable to those obtained when the whole body was examined (data not shown).
Blood glucose and insulin levels.
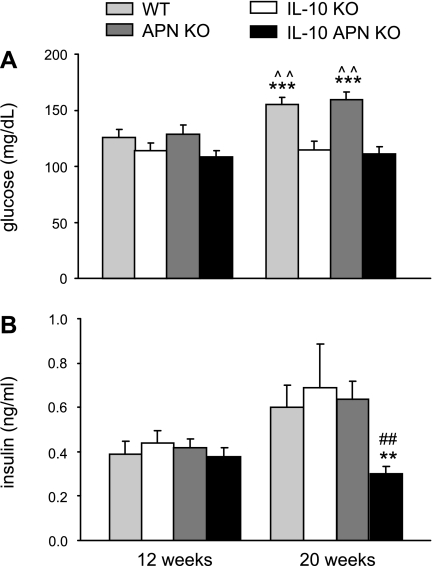
Because APN regulates insulin sensitivity, glucose and insulin levels were evaluated in IL-10 KO and double IL-10 APN KO mice and their respective controls after a 4-h fast. As shown in Fig. 4, A and B, at 12 wk of age, levels of glucose and insulin were comparable in each of the four experimental groups. At 20 wk, WT and APN KO mice had a comparable and significant age-related increase in serum glucose, whereas glycemia did not increase significantly in either IL-10 KO or double IL-10 APN KO mice. Furthermore, 20-wk-old double IL-10 APN KO mice had significantly lower serum insulin levels compared with each of the other groups at the same time point.
Fig. 4.
Glucose and insulin levels. Glucose and insulin levels were measured in serum of fasted mice. Data are means ± SE. N = 10 for WT and IL-10 KO mice and N = 14 for APN KO and double APN KO mice. **P < 0.01, and ***P < 0.001 WT vs. IL-10 KO mice and APN KO vs. IL-10 APN KO mice. ^^P < 0.01 12 wk vs. 20 wk of age. ##P < 0.01 IL-10 KO vs. IL-10 APN KO mice.
DISCUSSION
The present report demonstrates that APN deficiency does not play a crucial role in the pathogenesis of spontaneous chronic colitis in the IL-10 KO model. In fact, no significant differences in disease severity, cytokine production, body weight loss, and osteopenia were observed between IL-10 KO and double IL-10 APN KO mice.
On the basis of data obtained in the DSS model (10, 21) and on the described activities of APN as both a pro- and anti-inflammatory protein (8, 18), either protection or increased inflammation could have developed in double IL-10 APN KO mice compared with single IL-10 KO mice. However, no significant differences in terms of inflammatory infiltrate or expression of proinflammatory cytokines was observed in colonic tissue between the two groups. Furthermore, because APN binds to and inhibits the activity of several growth factors involved in mucosal repair (30), an increased hyperplasia of the colonic epithelial layer was expected in double IL-10 APN KO mice, as a consequence of enhanced cell proliferation (10). Instead, the chronic inflammatory process caused comparable regenerative hyperplasia in both IL-10 KO and double IL-10 APN KO mucosa, leading to an analogous thickening of the intestinal wall in the two groups. A significant association between polymorphisms of either APN or one of its receptors, ADIPOR1, is associated with decreased colorectal cancer risk in humans (14). Although the mechanisms linking APN to colon cancer are not currently understood, our data suggest that, in the IL-10 KO model, APN does not exert a major influence on colonic inflammation and mucosal hyperplasia, two parameters that favor development of colon cancer.
The persistent acute-phase response associated with both human and murine colonic inflammation is well documented (1, 29). Previous studies reported an increase in circulating levels of proinflammatory cytokines, together with high levels of acute-phase proteins, in models of chronic colonic inflammation (3, 5, 26). Furthermore, in subjects with active IBD, inflammatory markers, like acute-phase proteins, are significantly increased (4). A comparable increase in circulating SAA and IFN-γ was detected in IL-10 KO and double IL-10 APN KO mice with colitis, indicating that APN deficiency does not play a significant role in modulating these responses during colonic inflammation. This observation is at variance with results reported in obese subjects and patients with Type 2 diabetes, in which an inverse correlation between APN and biomarkers of inflammation, such as C-reactive protein or SAA, has been widely described (13). These data suggest that APN modulates inflammatory responses during metabolic diseases but plays a less important role during colonic inflammation.
It has been amply demonstrated that APN regulates production of various cytokines from a variety of cell types (9). In the present study, stimulation of mesenteric lymphocytes induced production of comparable amounts of proinflammatory cytokines in IL-10 KO and double IL-10 APN KO mice. Moreover, lymphocytes from WT and APN KO mice produced comparable amounts of IL-17, IL-6, TNF-α, and IFN-γ when stimulated with anti-CD3 and anti-CD28. These data indicate that APN does not have a critical influence in the activation of T cells either in the presence or absence of IL-10.
In addition to its role as a potential regulator of inflammatory responses, reports also indicate effects of APN in regulation of bone metabolism, insulin resistance and energy expenditure (13, 16, 19), parameters that are associated with and influenced by the inflammatory response. Our data indicate that APN deficiency does not significantly influence bone density, glucose, insulin, or leptin levels either in the presence or absence of chronic intestinal inflammation. In fact, BMC as well as serum glucose, insulin, and leptin levels were affected in a comparable way in IL-10 KO and double IL-10 APN KO mice compared with their respective healthy controls. These data do not exclude the possibility that augmented levels of APN, as observed for example in APN transgenic mice, might influence bone or glucose metabolism, as recently demonstrated by Ealey et al. (7).
In conclusion, despite its recognized role as a modulator of inflammatory responses both in vivo and in vitro (8, 18), APN deficiency does not significantly modulate inflammation in the IL-10 KO model of spontaneous chronic colitis. However, our data do not exclude the possibility that the high levels of APN observed in CD (23, 32) might contribute to altering the metabolic and inflammatory profile of these patients.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-061483 (to G. Fantuzzi).
Acknowledgments
The authors would like to acknowledge and express gratitude to Lawrence Chan, from the Baylor College of Medicine (Houston, TX), who provided APN KO mice.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest 98: 1010–1020, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blumberg RS, Saubermann LJ, Strober W. Animal models of mucosal inflammation and their relation to human inflammatory bowel disease. Curr Opin Immunol 11: 648–656, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Davidson NJ, Leach MW, Fort MM, Thompson-Snipes L, Kuhn R, Muller W, Berg DJ, Rennick DM. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J Exp Med 184: 241–251, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Beer FC, Mallya RK, Fagan EA, Lanham JG, Hughes GR, Pepys MB. Serum amyloid-A protein concentration in inflammatory diseases and its relationship to the incidence of reactive systemic amyloidosis. Lancet 2: 231–234, 1982. [DOI] [PubMed] [Google Scholar]
- 5.De Villiers WJ, Varilek GW, de Beer FC, Guo JT, Kindy MS. Increased serum amyloid a levels reflect colitis severity and precede amyloid formation in IL-2 knockout mice. Cytokine 12: 1337–1347, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Dresner-Pollak R, Gelb N, Rachmilewitz D, Karmeli F, Weinreb M. Interleukin 10-deficient mice develop osteopenia, decreased bone formation, and mechanical fragility of long bones. Gastroenterology 127: 792–801, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Ealey KN, Kaludjerovic J, Archer MC, Ward WE. Adiponectin is a negative regulator of bone mineral and bone strength in growing mice. Exp Biol Med (Maywood) 233: 1546–1553, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Fantuzzi G Adiponectin and inflammation: consensus and controversy. J Allergy Clin Immunol 121: 326–330, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Fantuzzi G Adipose tissue, adipokines, inflammation. J Allergy Clin Immunol 115: 911–919, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Fayad R, Pini M, Sennello JA, Cabay RJ, Chan L, Xu A, Fantuzzi G. Adiponectin deficiency protects mice from chemically induced colonic inflammation. Gastroenterology 132: 601–614, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Garaulet M, Hernández-Morante JJ, de Heredia FP, Tébar FJ. Adiponectin, the controversial hormone. Public Health Nutr 10: 1145–1150, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Ho AS, Moore KW. Interleukin-10 and its receptor. Ther Immunol 1: 173–185, 1994. [PubMed] [Google Scholar]
- 13.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 116: 1784–1792, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaklamani VG, Wisinski KB, Sadim M, Gulden C, Do A, Offit K, Baron JA, Ahsan H, Mantzoros C, Pasche B. Variants of the adiponectin (ADIPOQ) and adiponectin receptor 1 (ADIPOR1) genes and colorectal cancer risk. JAMA 300: 1523–1531, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Froguel P, Nagai R, Kimura S, Kadowaki T, Noda T. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem 277: 25863–25866, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 6: 55–68, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75: 263–274, 1993. [DOI] [PubMed] [Google Scholar]
- 18.Lago F, Dieguez C, Gomez-Reino J, Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol 3: 716–724, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Lenchik L, Register TC, Hsu FC, Lohman K, Nicklas BJ, Freedman BI, Langefeld CD, Carr JJ, Bowden DW. Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone 33: 646–651, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Liao W, Yu C, Wen J, Jia W, Li G, Ke Y, Zhao S, Campell W. Adiponectin induces interleukin-6 production and activates STAT3 in adult mouse cardiac fibroblasts. Biol Cell 2008. [Epub ahead of print]. [DOI] [PubMed]
- 21.Nishihara T, Matsuda M, Araki H, Oshima K, Kihara S, Funahashi T, Shimomura I. Effect of adiponectin on murine colitis induced by dextran sulfate sodium. Gastroenterology 131: 853–861, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Ogunwobi OO, Beales IL. Adiponectin stimulates proliferation and cytokine secretion in colonic epithelial cells. Regul Pept 134: 105–113, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Paul G, Schaffler A, Neumeier M, Furst A, Bataillle F, Buechler C, Muller-Ladner U, Scholmerich J, Rogler G, Herfarth H. Profiling adipocytokine secretion from creeping fat in Crohn's disease. Inflamm Bowel Dis 12: 471–477, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Roux C, Abitbol V, Chaussade S, Kolta S, Guillemant S, Dougados M, Amor B, Couturier D. Bone loss in patients with inflammatory bowel disease: a prospective study. Osteoporos Int 5: 156–160, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Shih DQ, Targan SR. Immunopathogenesis of inflammatory bowel disease. World J Gastroenterol 14: 390–400, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh UP, Singh S, Taub DD, Lillard JW Jr. Inhibition of IFN-gamma-inducible protein-10 abrogates colitis in IL-10−/− mice. J Immunol 171: 1401–1406, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi T, Adachi Y, Ohtsuki Y, Furihata M. Adiponectin receptors, with special focus on the role of the third receptor, T-cadherin, in vascular disease. Med Mol Morphol 40: 115–120, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Tang CH, Chiu YC, Tan TW, Yang RS, Fu WM. Adiponectin enhances IL-6 production in human synovial fibroblast via an AdipoR1 receptor, AMPK, p38, and NF-kappa B pathway. J Immunol 179: 5483–5492, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut 55: 426–431, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Lam KS, Xu JY, Lu G, Xu LY, Cooper GJ, Xu A. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem 280: 18341–18347, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Wirtz S, Neurath MF. Mouse models of inflammatory bowel disease. Adv Drug Deliv Rev 59: 1073–1083, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto K, Kiyohara T, Murayama Y, Kihara S, Okamoto Y, Funahashi T, Ito T, Nezu R, Tsutsui S, Miyagawa JI, Tamura S, Matsuzawa Y, Shimomura I, Shinomura Y. Production of adiponectin, an anti-inflammatory protein, in mesenteric adipose tissue in Crohn's disease. Gut 54: 789–796, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]