Abstract
The gastric hormone gastrin regulates the expression of a variety of genes involved in control of acid secretion and also in the growth and organization of the gastric mucosa. One putative target is plasminogen activator inhibitor-2 (PAI-2), which is a component of the urokinase activator system that acts extracellularly to inhibit urokinase plasminogen activator (uPA) and intracellularly to suppress apoptosis. Previous studies have demonstrated that gastrin induces PAI-2 both in gastric epithelial cells expressing the gastrin (CCK-2) receptor and, via activation of paracrine networks, in adjacent cells that do not express the receptor. We have now sought to identify the response element(s) in the PAI-2 promoter targeted by paracrine mediators initiated by gastrin. Mutational analysis identified two putative response elements in the PAI-2 promoter that were downstream of gastrin-activated paracrine signals. One was identified as a putative MAZ site, mutation of which dramatically reduced both basal and gastrin-stimulated responses of the PAI-2 promoter by a mechanism involving PGE2 and the small GTPase RhoA. Yeast one-hybrid screening identified the other as binding the activating signal cointegrator-1 (ASC-1) complex, which was shown to be the target of IL-8 released by gastrin. RNA interference (RNAi) knockdown of two subunits of the ASC-1 complex (p50 and p65) inhibited induction of PAI-2 expression by gastrin. The data reveal previously unsuspected transcriptional mechanisms activated as a consequence of gastrin-triggered paracrine networks and emphasize the elaborate and complex cellular control mechanisms required for a key component of tissue responses to damage and infection.
Keywords: activating signal cointegrator-1, plasminogen activator inhibitor-2
in addition to stimulation of acid secretion, the gastric hormone gastrin activates mechanisms associated with tissue defense (17). In the stomach, as in other organs, tissue responses to damage, infection, or inflammation are recognized to involve multiple cell types that interact via paracrine extracellular messenger molecules including cytokines, growth factors, prostanoids, amines, and regulatory peptides. A growing body of evidence indicates that gastrin triggers the release of many of these agents, thereby activating complex paracrine cascades (20). Gastrin-regulated changes in gene expression have been relatively well characterized in the case of acid-control mechanisms, including for example the control of histidine decarboxylase and vesicular monoamine transporter-2 implicated in the synthesis and storage of histamine in enterochromaffin-like (ECL) cells (8, 15, 16, 25, 39). However, much less is known of the transcriptional mechanisms activated by gastrin involving tissue growth and organization. One recently identified target gene of gastrin is plasminogen activator inhibitor 2 (PAI-2), which is expressed mainly in chief, mucus, and ECL cells in the gastric mucosa (44, 45). It is an inhibitor of the urokinase plasminogen activator system (31, 50), which in the stomach is also increased by Helicobacter pylori and is associated with inhibition of cell invasion and suppression of apoptosis (44, 45).
Previous studies have demonstrated transcriptional regulation of PAI-2 by members of the CREB and AP-1 families of transcription factors following activation of PKC and MAP kinase (9, 10, 13, 14). Using a coculture system that allows study of paracrine mediators, we have shown that gastrin increased the expression of PAI-2 both in cells expressing the relevant receptor (the gastrin-cholecystokinin, or CCK-2, receptor) and in neighboring cells that do not express the receptor but respond to paracrine signals activated by gastrin; two relevant mediators were identified, namely IL-8 and cyclooxygenase-2 (COX-2) products (44). Similar mechanisms were shown to be activated by H. pylori (45). The direct effects of gastrin on CCK-2 receptor-expressing cells were mediated by the small GTPase RhoA and by NF-κB and involved the CRE and AP-1 response elements within the proximal 196-bp of the promoter. Disrupting the CRE and AP-1 promoter elements abolished the direct response to gastrin, but importantly the response to paracrine mediators persisted. Moreover, in contrast to direct activation, the increase in PAI-2 expression following gastrin-stimulated paracrine mediators did not require NF-κB although RhoA was involved.
In the present study we sought to elucidate the mechanisms involved in increased PAI-2 expression in cells responding to gastrin-activated paracrine signals (GAPS). We report here that gastrin regulates two distinct paracrine pathways linked to separate transcriptional mechanisms within the proximal 93 bp of the promoter.
MATERIAL AND METHODS
Cells, plasmids, and reagents.
AGS and AGS-GR cells were maintained as previously published (46). A reporter construct containing 196-bp of the human PA1–2 promoter (44) was used as a template in PCR to generate a fragment containing 93 bp of the promoter in the promoterless luciferase reporter vector pXP2 (37), referred to as PAI-2-93-luc wild-type (wt). PCR and recombinant PCR (22) were used to generate a panel of mutated constructs, referred to as PAI-2-93-luc mutants (m1-m12). Construct integrity was confirmed by dideoxy sequencing in both directions. An expression vector for constitutively active-RhoA (L63RhoA) was a gift from A. Hall (University College, London, UK). Heptadecapeptide amidated gastrin (G17) was purchased from Peninsula (St. Helens, Merseyside, UK); IL-8 and PGE2 were obtained from Calbiochem (Nottingham, UK). All other chemicals were obtained from Sigma (Poole, Dorset, UK).
Transient transfections and luciferase assays.
Experiments to study paracrine mechanisms mostly made use of coculture of AGS-GR and AGS cells. AGS-GR cells (2 × 105) were plated in medium containing 10% fetal bovine serum (full medium). The following day, medium was removed and cells were cotransfected with PAI-2-luc constructs (1.0 μg/well) together with a constitutively active Renilla luciferase reporter, phRL-SV40 (0.5 ng per well, Promega, Southampton, UK) by use of TransFast (Promega). In the coculture experiments, AGS cells, which lack the CCK-2 receptor, were transfected with the constructs followed by addition of AGS-GR cells after replacement of the medium as described previously (44, 45); 24 h following transfection, cells were incubated in serum-free medium with G17 for 8 h. In some experiments cells were cultured in Transwell filters to separate the two cell types as previously described (45). Luciferase activity was measured by dual luciferase assay (Promega) according to the manufacturer's instructions in a Lumat LB9507 luminometer (Berthold, Redbourne, Herts, UK). Results are presented as fold increase over unstimulated control, so the value of 1.0 signifies no change in luciferase activity.
Flow cytometry.
Cells were cultured as described above, recovered in suspension by mild digestion with trypsin, suspended in 2% paraformaldehyde (37°C, 15 min), permeabilized by the addition of methanol to a final concentration of 90%, and incubated on ice (1 h). Cells were then washed twice in 5% bovine serum albumin (BSA) in PBS, incubated with mouse anti-COX-2 (Santa Cruz) or mouse anti-IL-8 antibodies (R and D Systems, Oxford, UK; 4°C overnight with gentle shaking), washed twice in 4% donkey serum in PBS, and incubated in donkey anti-mouse antibody conjugated to FITC (Jackson Immunoresearch; 22°C, 1 h with gentle shaking). Finally, cells were washed twice in 5% BSA, and FACS analysis was carried out by use of a BD FACSVantage flow cytometer. Data were recorded and analyzed by using CellQuest Pro software as described previously; changes in the abundance of COX-2 or IL-8 were measured as an increase in the mean fluorescence intensity in treated and untreated cells (35).
Western blots.
Protein was extracted from AGS cells in 2× Laemmli buffer containing protease and phosphatase inhibitors (Calbiochem). Western blotting was performed as previously described (44) by using goat anti-PAI-2 (Santa Cruz Biotechnology, Santa Cruz, CA) or polyclonal rabbit anti- activating signal cointegrator-1 (ASC-1) p65 (Bethyl Laboratories) antibodies.
EMSAs.
Nuclear extracts from AGS-GR or AGS cells were prepared using the Pierce NE-PER Nuclear and Cytoplasmic Extraction Kit (PerBio Science, Cramlington, UK) according the manufacturer's instructions. Complementary oligonucleotides spanning the regions −48 to −26 and −81 to −62 of the human PAI-2 promoter and containing 3-bp 5′ overhangs were annealed, and 5-pmol double-stranded product was labeled with [α-32P]dATP by using Klenow enzyme as previously described (49). Probe (10 fmol) was incubated with nuclear extract (10 μg) in a final volume of 20-μl binding buffer containing 10 mM Tris·HCl (pH 7.5), 50 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 1 mM DTT, 10% (vol/vol) glycerol, and 1 μg poly(dA·dT) as reported earlier (49). Binding reactions were performed for 20 min at room temperature. For competition assays, double-stranded wt or mutated competitor oligonucleotides (100-fold excess) were preincubated (20 min) with nuclear extracts prior to addition of probe. For immunodepletion experiments, nuclear extracts were preincubated (20 min) on ice with antibodies to Sp1-4, AP-2, ASC-1, TFIID (Santa Cruz Biotechnology), ZBP-89 (gift from J. Merchant, Ann Arbor, MI), MAZ (gift from K. Yokoyama, Ibaraki, Japan). Samples were separated on nondenaturing 6% (wt/vol) polyacrylamide gels, which were dried and exposed overnight to a phosphor screen, before visualization on a Phosphorimager (Bio-Rad, Hemel, Hempstead, UK) and analysis with Quantity One (Bio-Rad) image-analysis software.
Chromatin immunoprecipitation assays.
AGS-GR cells (1.5 × 107) were plated in full medium. The following day medium was removed and the cells were stimulated for 2 h with 5 nM G17 in serum-free medium, followed by fixation for 10 min with 1% formaldehyde. The fixation solution was removed and cells were rinsed with phosphate-buffered saline (PBS) and the reaction stopped by incubation with glycine stop-fix solution (Active Motif, Rixensart, Belgium) for 5 min. Fixed cells were rinsed with PBS and then scraped from the plate in 2 ml of ice-cold PBS containing 0.5 mM phenylmethylsulfonyl fluoride (PMSF) using a rubber policeman. Cells were pelleted by centrifugation at 720 RCF for 10 min at 4°C, resuspended in 1 ml of ice-cold lysis buffer containing 0.5 mM PMSF and 5 μl protease inhibitor cocktail (PIC, Active Motif) and dounced on ice for 15 strokes with a glass homogenizer (Anachem, Luton, UK). Nuclei were pelleted at 2400 RCF for 10 min at 4°C and resuspended in 350 μl of digestion buffer, and the chromatin was sheared to fragments of ∼200–1,500 bp by incubation for 10 min at 37°C in 10 U/ml enzymatic shearing cocktail (Active Motif). Sheared chromatin was precipitated by incubation for 4 h at 4°C with protein G magnetic beads (Active Motif) and 2 μg antibody against either ASC-1 or MAZ, according to the manufacturer's protocol. To take account of nonspecific binding of chromatin to the beads, parallel reactions were performed that contained 2 μg of nonimmune human IgG. Beads were washed by using a magnetic stand and chromatin was eluted according to the manufacturer's protocol. Cross links were reversed by addition of NaCl to 0.1 M and incubation at 95°C for 15 min; protein was removed by incubation with 10 μg/ml proteinase K for 1 h at 37°C, and the reaction was stopped by addition of 20 μl/ml proteinase K stop solution (Active Motif). PCR was performed on DNA coimmunoprecipitated by antibodies to ASC-1, MAZ, and negative control IgG, as well as input DNA that did not undergo chromatin immunoprecipitation (ChIP) but that was reverse cross-linked and treated with proteinase K. One set of positive control primers (forward, 5′-GATCAAAAGACAGAGGGAGAAAAAAA; reverse, 5′-TGTTCTCTGGTTATTCTCTGAGTTGCT) generated a 143-bp amplicon that corresponded to bases −80 to +63 of the PAI-2 gene. A second set of positive primers (forward, 5′-CCCCCAAAATTTCTTAAACCA; reverse, 5′-TCTCTGAGTTGCTGTCTGACG) generated a 223-bp amplicon corresponding to bases −174 to + 49 of PAI-2. Both positive control amplicons encompass the putative ASC-1 and MAZ binding sites in the PAI-2 proximal promoter. A negative control set of primers (forward 5′-GCAGGCACCCTTTACCATAA; reverse, 5′-GGGAGGACAGAAGGAAAACC) amplified a 186-bp fragment corresponding to bases −1589 to −1412 of the human VMAT2 promoter. This region does not contain putative MAZ or ASC-1 binding sites. PCR products were separated on 2% agarose gels.
Knockdown of ASC-1 by siRNA and immunocytochemistry.
Transfection of small interfering RNA (siRNA) for both p50 (ASCC1, Ambion, Huntingdon, UK) and p65 (TRIP4, Ambion) ASC-1 subunits was performed on cells together with the PAI-2 and Renilla luciferase vectors in suspension by use of Amaxa Nucleofection Apparatus (Amaxa, Koln, Germany), solution V, program B023, according the manufacturer's instruction. To validate siRNA transfection efficiency, transfected AGS cells were immunostained with polyclonal rabbit (Bethyl Laboratories) ASC-1 p65 antibody with the appropriate FITC-conjugated secondary antibody, raised in donkey (Jackson Immunoresearch, Soham, UK), by using Vectashield mounting medium with DAPI (Vector Laboratories, Peterborough, UK) to counterstain nuclei. Slides were examined with a Zeiss Axioplan-2 microscope (Zeiss Vision, Welwyn Garden City, UK), and images were captured with a JVC-3 charge-coupled device camera and KS300 software combined with a deconvolution software (Imaging Associates, Bicester, UK) as previously described (51).
Yeast one-hybrid screening.
Yeast one-hybrid screening was performed according to the manufacturer's instructions (BD Matchmaker one-hybrid library construction and screening kit). Briefly, double-stranded (ds) oligonucleotide comprising three tandem repeats of the gastrin response element (corresponding to bases −78 to −63 of the human PAI-2 promoter) was cloned into the target reporter vector pHis2 at EcoRI and MluI restriction sites, and integrity was confirmed by DNA sequencing. To ablate background histidine leakiness of the pHIS2 vector, the target reporter constructs were transformed into yeast strain Y187, plated on SD/-His/-Trp plates with increasing (10 to 60 mM) concentrations of 3-amino-1,2,4-triazole (3-AT), and incubated at 30°C for 1 wk. Total RNA was extracted from AGS-GR cells by use of Tri-Reagent (Sigma), and ds cDNA was prepared by using BD SMART technology (Clontech). The ds cDNA was purified by using a BD CHROMA SPIN TE-400 column (Clontech) and its size distribution was analyzed on a 1.2% agarose gel. The GAL4 AD fusion library was constructed by transforming Y187 with the pHIS2 target vector, SmaI-linearized pGADT7-Rec2 and the ds cDNA in a single step by using the BDMatchmaker library construction and screening kit protocol (Clontech). To select for one-hybrid interactions, transformants were plated on SD/-His/-Leu/-Trp agar plates with 45 mM 3-AT and incubated for 1 wk at 30°C, and positive colonies were replated onto SD/-His/-Leu/-Trp agar plates with 60 mM 3-AT for a further week at 30°C. To select colonies with an integrated library cDNA insert, yeast colony PCR was performed by using the BD Matchmaker AD LD-Insert Screening Amplimer Set and BD Advantage 2 PCR Polymerase Mix. The pGADT7-Rec2 vector was rescued from positive Y187 colonies by standard methods, transformed into Alpha-select Silver efficiency Escherichia coli competent cells (Bioline), and plated on Luria broth/ampicillin. Plasmids were isolated from E. coli using the Genelute plasmid miniprep kit (Sigma) and analyzed by DNA sequencing with a T7 sequencing primer.
Statistics.
Results are presented as means ± SE; comparisons were made by ANOVA or Student's t-tests as appropriate and were considered significant at P < 0.05.
RESULTS
A 93-bp sequence of the PAI-2 promoter is sufficient for responses to gastrin-activated paracrine signals.
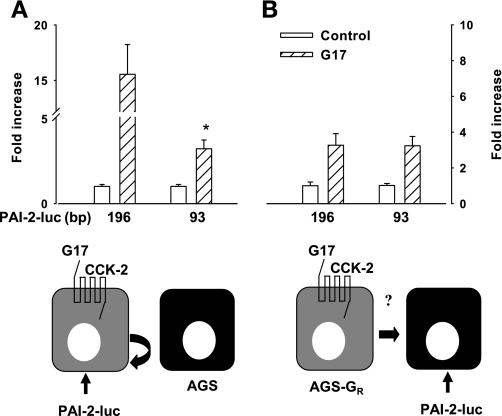
Initial experiments were directed at examining the activity of luciferase reporter constructs that either included (196 bp) or excluded (93 bp) the CRE and AP-1 sites previously implicated in the response to gastrin. Basal activity of the 93-bp construct was 62.4 ± 4.8% that of the 196-bp construct. When these constructs were expressed in cells with the CCK-2 receptor, there was a reduction of gastrin-stimulated activity in the 93-bp compared with the 196-bp construct (Fig. 1A), corresponding to loss of CRE and AP-1 sites. Importantly, however, an experimental protocol focusing on paracrine signaling responses in which cells not expressing the CCK-2 receptor were transfected with luciferase reporters and then cocultured with CCK-2 expressing cells showed that the responses of the 93-bp construct to GAPS were fully preserved compared with the 196-bp construct (Fig. 1B). Moreover, the response of the 93-bp promoter was identical in assays measuring the paracrine response compared with cells expressing the CCK-2 receptor (Fig. 1, A and B). The basal expression of PAI-2-luc reporters was similar when transfected into AGS cells and AGS-GR cells (not shown). To exclude possible effects due to direct intracellular communication between cocultured cells we previously showed paracrine stimulation of a 2.3 kb PAI-2-luc reporter using a Transwell culture system (45). Using the same approach in the present study, we found that gastrin stimulation of 93 bp-PAI-2-luc was 3.3 ± 1.1-fold higher than basal in cocultured cells compared with 3.2 ± 0.9-fold higher with use of Transwells (n = 3). The data therefore indicate that paracrine rather intracellular communication mediates responses to gastrin and that a 93-bp promoter sequence is sufficient for responses to GAPS.
Fig. 1.
For paracrine-mediated effects of gastrin, 93 bp of the plasminogen activator inhibitor-2 (PAI-2) promoter is sufficient. A: in cells expressing the CCK-2 receptor and transfected with PAI-2-luc vectors, there was a reduction in luciferase activity in response to G17 (1 nM, 8 h) of the 93-bp PAI-2 luciferase construct compared with the 196-bp construct, *P < 0.05, t-test, n = 3. B: in contrast, when the 93-bp construct was transfected into cells not expressing CCK-2 receptors, but cocultured with cells expressing this receptor, the response to G17 (attributable to the action of paracrine mediators) was similar for both constructs and was comparable to the response to the 93-bp construct according to the protocol illustrated in A. Schematic cartoons underneath A and B illustrate the experimental design in each case (see Fig. 7 for a summary of the relevant signaling pathways). Note that in A, AGS cells not expressing the CCK-2 receptor and not transfected with PAI-2-luc vectors are included to ensure that total cell numbers were the same in the 2 conditions; in these circumstances PAI-2-luc responses are expected to reflect either direct effects of gastrin or effects consequent on activation of autocrine signaling pathways.
Mutational analysis of the human 93-bp PAI-2 promoter identified two gastrin responsive elements.
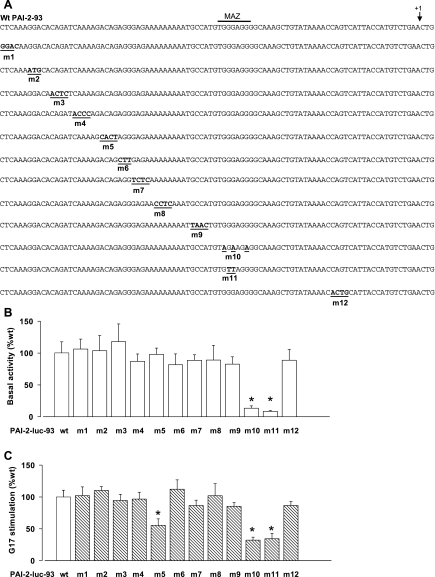
We then examined the effect of block mutations on basal activity within the 93-bp promoter region (Fig. 2A). Mutations within a putative MAZ site (m10-11) had a dramatic effect on basal luciferase activity (Fig. 2B) and exhibited reduced responsiveness to gastrin when set against the relevant control (wt, 2.7 ± 0.2; m10, 1.3 ± 0.2; m11, 1.4 ± 0.3 and Fig. 2C). An additional mutant (m5) in which the −72GACAGA−67 sequence was disrupted also reduced responses to gastrin by ∼50% (Fig. 2C). In both cases the residual responses to G17 were significantly different (P < 0.05) from control.
Fig. 2.
Mutation of the proximal 93-bp promoter region indicates that the paracrine-mediated actions of gastrin target multiple sites. A: the series of mutants studied included those with deletion of putative MAZ site (m10-11) and the GACAGA sequence (m5). The consensus MAZ binding site is indicated by a horizontal bar and the start of transcription by an arrow. B: basal expression of the wild-type (wt) and m1 to m12 promoter-reporter constructs. Results are expressed as relative to the basal luciferase activity of the wt PAI-2-93-luc construct; note the effect of m10 and m11 on basal expression. C: responses to G17 (1 nM, 8 h) of the wt and mutant promoter-reporter constructs. Results are expressed relative to the response of wt PAI-2-93-luc. *P < 0.05, ANOVA; n = 3.
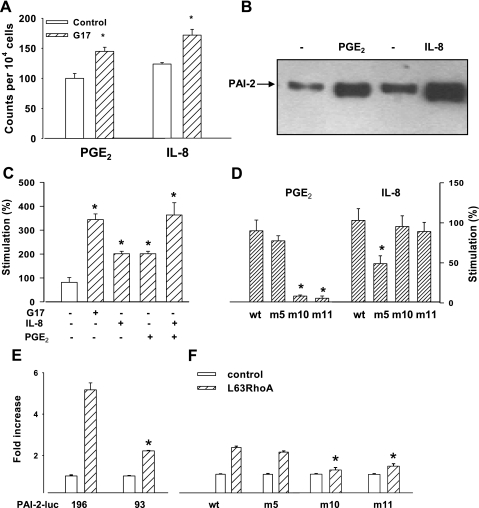
Previous studies suggested that PGE2 and IL-8 were putative GAPS (44); consistent with this, we showed that gastrin increased COX-2 and IL-8 in cells expressing the gastrin receptor (Fig. 3A). Moreover, PGE2 and IL-8 increased endogenous PAI-2 protein when added to AGS cells (Fig. 3B). We then examined the action of these on cells transfected either with the wt or the m5 and m10-11 mutated 93-bp constructs. Both IL-8 and PGE2 stimulated the wt PAI-2-93-luc promoter but there was no evidence of synergistic interaction (Fig. 3C). The response to IL-8 was reduced but not abolished in the m5 mutant but not mutants disrupting the putative MAZ site. Conversely, the MAZ mutants abolished the response to PGE2 whereas the m5 mutant was not significantly different from control (Fig. 3D). The data therefore indicate that gastrin-triggered prostaglandin and IL-8 release each act on PAI-2 expression through distinct promoter sequences in the 93-bp proximal region of the PAI-2 promoter.
Fig. 3.
Effects of PGE2, IL-8, and RhoA on wt and mutant PAI-2 promoter constructs. A: flow cytometric analysis shows that gastrin increased COX-2 and IL-8 in AGS cells expressing the CCK-2 receptor. B: Western blot shows that the abundance of wt PAI-2 protein is increased in AGS cells treated with PGE2 and IL-8. C: PGE2 (28 μM, 6 h) and IL-8 (125 ng/ml, 6 h) stimulated expression of wt PAI-2-93-luc. D: responses to PGE-2 are significantly reduced in m10 and m11 but not m5, whereas the response to IL-8 is reduced in m5 but not in m10 and m11. E: cotransfection of AGS cells with L63RhoA (0.5 μg/well) stimulates PAI-2-93-luc to a lesser extent than PAI-2-196 (*P < 0.05, t-test, n = 3). F: responses to L63RhoA of PAI-2-93-luc were significantly reduced by mutations of the MAZ site (m10-11) but not of the GACAGA site (m5). *P < 0.05, ANOVA; n = 3.
Previous work has shown that the small GTPase RhoA partially mediates the effect of gastrin on PAI-2 expression by actions both on AP-1 sites and also indirectly by stimulating the expression of COX-2 responsible for the production of PGE2 (44, 45). We therefore examined the effects of a constitutively active RhoA plasmid (L63RhoA) on the m5 and m10-11 constructs. Cotransfection studies showed that RhoA stimulated both 196- and 93-bp luciferase although the latter response was reduced (Fig. 3E). Mutation of the putative MAZ site reduced responses to RhoA by ∼50%, but the residual responses were still significantly different (P < 0.05) from control whereas disruption of the sequence −72 to −67 (m5) had only a modest and not statistically significant effect on responses to RhoA, consistent with the idea that RhoA acts primarily on the pathway that links gastrin to the putative MAZ site via prostanoids (Fig. 3F).
Identification of MAZ as a binding protein for the PAI-2 promoter.
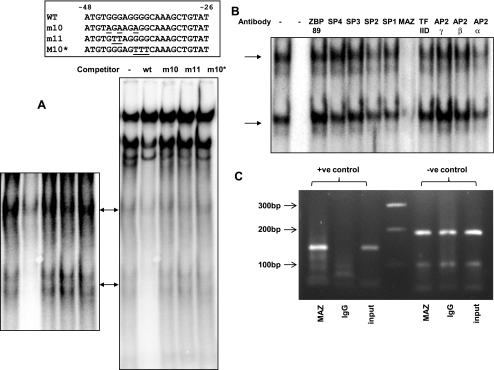
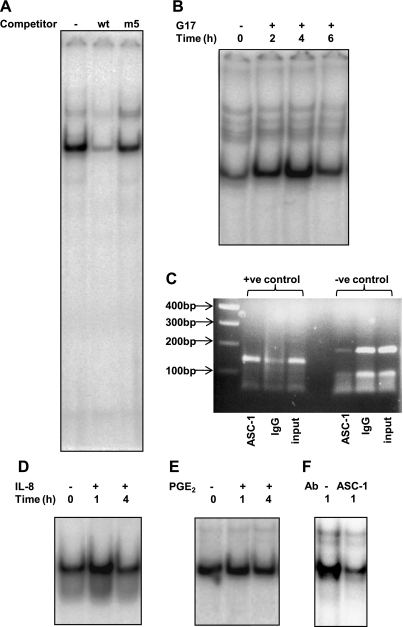
To identify putative DNA binding proteins in nuclear extracts of AGS cells, we used EMSAs with probes containing the sequences of the putative GAPS response elements. In nuclear extracts of unstimulated cells we identified two complexes that exhibited specific binding to a probe covering the sequence −48 to −26, which includes the putative MAZ site. Competition experiments using wt sequences and three mutations spanning the putative MAZ site within this probe supported the hypothesis that the MAZ site was required for binding (Fig. 4A). Moreover, in immunoneutralization assays using antibody to MAZ there was depletion of both bands (Fig. 4B) whereas a variety of antibodies directed toward other transcription factors including ZBP89, SP1-SP4, TFIID, AP2α, AP2β, or AP2γ showed no effect (Fig. 4B). There was no change in the intensities of the two bands following stimulation with gastrin for up to 8 h (data not shown). To demonstrate binding of MAZ to the endogenous PAI-2 promoter, we performed ChIP assays. Positive control primers amplified a 143-bp product from DNA immunoprecipitated with a MAZ antibody, but not with control IgG. Moreover, the positive control primers amplified the target in the MAZ sample more strongly relative to the input DNA than did the negative control primer set (Fig. 4C).
Fig. 4.
Analysis of the MAZ binding site. A: wt and mutant MAZ double-stranded (ds) oligonucleotides (sequences inset, 100× excess) were used to compete with radiolabeled wt probe binding to nuclear extracts from G17-stimulated AGS-GR cells. MAZ site-specific binding complexes are indicated by arrows. Left, whole gel; right, enhancement of MAZ site-specific complexes. B: effects of transcription factor antibodies on wt probe binding to nuclear extracts from G17-stimulated AGS-GR cells. Lane 1, no antibody, lane 2, no extract, lanes 3-12, antibodies as indicated. C: PCR analysis of chromatin immunoprecipitation (ChIP) assay. Lane 1, positive (+ve) control primers (143-bp amplicon) with MAZ immunoprecipitated DNA as template. Lane 2, positive control primers with IgG immunoprecipitated DNA as template. Lane 3, positive control primers with unprecipitated input DNA as template. Lane 4, 100-bp marker. Lanes 5-7, templates as for lanes 1-3 but with negative (−ve) control primers (186-bp amplicon).
Yeast one-hybrid screening identifies ASC-1 as previously unknown target of gastrin.
Using a probe spanning the putative GACAGA (−72 to −67) site, we identified a single major binding complex (Fig. 5A), which on the basis of competition experiments was specific for this site. The intensity of the band increased on stimulation by gastrin for up to 4 h (Fig. 5B). In keeping with the data from luciferase assays, IL-8 (Fig. 5D) but not PGE2 (Fig. 5E) also increased the intensity of this band.
Fig. 5.
Analysis of the −81 to −59 region of the PAI-2 promoter. A: wt and mutant ds oligonucleotides (100× excess) were used to compete with radiolabeled wt probe binding to nuclear extracts from gastrin-stimulated AGS-GR cells. B: time course of wt probe binding to nuclear extracts from gastrin-stimulated AGS-GR cells. Lane 1: unstimulated cells; lanes 2-4: cells stimulated with G17 (1 nM) for times indicated. Representative data from 3 replicate experiments. C: PCR analysis of ChIP assay. Lane 1, 100-bp marker. Lane 2, positive control primers (143-bp amplicon) with ASC-1 immunoprecipitated DNA as template. Lane 3, positive control primers with IgG immunoprecipitated DNA as template. Lane 4, positive control primers with unprecipitated input DNA as template. Lanes 5-7, templates as for lanes 2-4 but with negative control primers (186-bp amplicon). D: time course of wt probe binding to nuclear extracts from gastrin-stimulated AGS-GR cells. Lane 1, unstimulated cells; lanes 2-3, cells stimulated with IL-8 (125 ng/ml) for times indicated. E: time course of wt probe binding to nuclear extracts from gastrin-stimulated AGS-GR cells. Lane 1: unstimulated cells; lanes 2-3: cells stimulated with PGE-2 (28 μM) for the times indicated. F: effects of ASC-1 antibody on wt probe binding to nuclear extracts from G17-stimulated AGS-GR cells. Lane 1, control rabbit IgG; lane 2, ASC-1 antibody.
To identify the nuclear factor binding the GAPS response element and mediating IL-8 signaling in the PAI-2 promoter, we screened an AGS cell cDNA library by using three tandem copies of the region that spans bases −78 to −63 of the promoter as bait. Sequence analysis of the positive pGADT7-Rec2-containing clones that also showed growth with high levels (45 mM) of 3-AT on SD/-His/-Leu/-Trp dropout plates indicated that one contained the in-frame 3′ sequence of the human activating signal cointegrator-1 complex-p50 subunit (ASCC1) cDNA. Further positive clones contained partial open reading frames for gastric cancer antigen Zg14 and ADP-ribosylation factor-like 3 (ARL3). We focused on ASCC1 since the ASC-1 complex is a well-known DNA-dependent transcriptional regulator and has already been shown to be expressed in human gastric gland cells (33). The ASC-1 complex contains multiple proteins, including p65, that are believed to be important for the DNA binding affinity of the complex. In immunoneutralization assays using an antibody against ASC-1 we showed depletion of the intensity of the gastrin-sensitive band using a probe spanning the −59 to −81 sequence of the promoter (Fig. 5F). To demonstrate binding of components of the ASC-1 complex to the endogenous PAI-2 promoter we performed ChIP assays. Positive control primers amplified a 143-bp product from DNA immunoprecipitated with an ASC-1 antibody, but only weakly with control IgG. Additionally, the positive control primers amplified the target in the ASC-1-precipitated sample much more strongly relative to the input DNA than did the negative control primer set (Fig. 5C). Similar results were obtained by using positive control primers that produced a 223-bp amplicon encompassing the putative ASC-1 binding site (not shown).
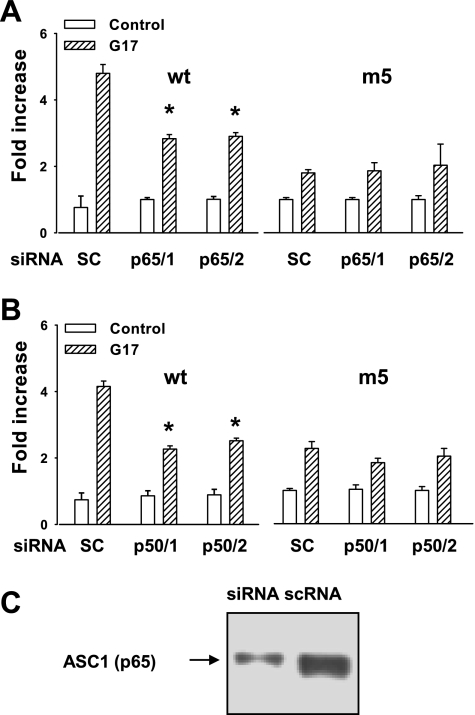
To further test the hypothesis that GAPS act through the ASC-1 complex, we transfected siRNA against both the p50 and the p65 subunits of the ASC-1 complex together with either the wt or m5 mutants of the PAI-2 promoter. The efficacy of the RNA interference (RNAi) treatment was established by immunocytochemical analysis, which showed reduced protein expression in the p65 silenced cells after gastrin stimulation (scrambled control, 71.4.0 ± 14.3%; ASC siRNA, 19.2 ± 2.5% cells positive for p65). RNAi using two different sequences (identified as p65/1 and p65/2 in Fig. 6A) both significantly reduced the action of gastrin in stimulating the wt promoter; however, in the case of the m5 mutant the response to gastrin was reduced compared with the wt sequence (consistent with the observations described above) and was not significantly changed after siRNA treatment with either p65/1 or p65/2 (Fig. 6A). Similarly, using two different siRNAs for p50 (identified as p50/1 and p50/2 in Fig. 6B), we found significantly reduced responses to gastrin of the wt 93-bp PAI-luc compared with control, whereas with the m5 mutant the response to gastrin was not significantly altered by siRNA treatment with either p50/1 or p50/2 (Fig. 6B). In case of both p65 and p50 siRNA, the residual responses to G17 were nevertheless still significantly greater than control (P < 0.05). The efficacy of the siRNA treatment was demonstrated by Western blot, which showed substantial depletion of ASC-1 protein in siRNA-treated cells compared with control (Fig. 6C).
Fig. 6.
Knockdown of the ASC-1 complex inhibits luciferase activity of the wt 93-bp promoter but not the m5 mutant. A: 2 independent small interfering RNAs (siRNAs) to p65 subunit (p65/1 and p65/2, 30 nM, 48 h) significantly reduce luciferase expression of the wt but not m5 PAI-2-93-luc, compared with scrambled control siRNA (SC, scRNA). B: 2 siRNAs to p50 subunit (p50/1 and p50/2, 30 nM, 48 h) also inhibit luciferase expression of the wt but not the m5 PAI-2-93-luc construct. C: Western blot shows depletion of ASC-1 protein by siRNA treatment compared with scRNA. *P < 0.05, ANOVA; n = 3.
DISCUSSION
The main findings of the present study are that gastrin stimulates PAI-2 expression in cells that do not express the CCK-2 receptor via paracrine mechanisms directed at both a MAZ cis-regulatory element that is downstream of prostaglandin stimulation and a previously unrecognized gastrin response element within the 93-bp of the PAI-2 promoter that binds the p50 subunit of ASC-1 and is downstream of IL-8. Although it is well recognized that gastrin activates paracrine mechanisms in the gastric mucosa (17, 20), little has been done until now to distinguish between transcriptional responses that are a consequence of direct effects (i.e., on cells expressing the CCK-2 receptor) and those that are a consequence of activation of paracrine-signaling networks. Plasminogen activator inhibitor-2 is a good model for study of the relevant mechanisms since it is induced by gastrin both in cells expressing the CCK-2 receptor, e.g., ECL cells, and in adjacent cells that do not express the receptor, e.g., mucus cells (44, 45). The biological consequences of gastrin-stimulation of PAI-2 are inhibition of apoptosis and suppression of uPA activity, thereby limiting thrombolysis, which suggests that PAI-2 is a component of the system by which gastrin promotes mucosal protection.
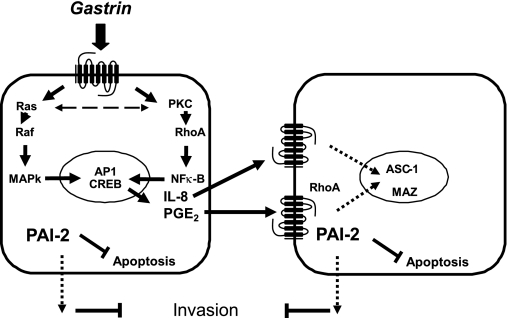
In studies of transcriptional responses to hormones, growth factors, cytokines, and other regulatory factors, it is often convenient to assume that regulated gene expression is an exclusive property of cells expressing the relevant receptor. But in mixed cell populations it is also clear that activation of one receptor may induce a paracrine cascade, allowing other cells to respond and providing the capacity for both amplification of the response and integration with other signaling pathways. In the case of many G protein-coupled receptors, particularly those that act via Gαq/11, liberation of EGF receptor ligands appears to be a common mechanism for activation of a paracrine signaling cascade (38, 40), and it is clear that gastrin also acts by liberation of EGF-R ligands such as heparin-binding EGF in several different systems (46, 48). Induction of COX-2 and liberation of PGE2 is also now recognized as an important component of the response to a variety of hormones (including gastrin), cytokines, and other paracrine mediators (47). In addition to both liberation of EGF receptor ligands, and activation of COX-2, it is well established that gastrin exerts effects via release of histamine (21); although less intensively explored, there is also evidence for gastrin stimulation of IL-8 (23) and FGF (35). We have sought to develop experimental systems that allow the separate study of transcriptional responses secondary to paracrine mechanisms. In particular we have studied responses of luciferase reporter constructs transfected into either AGS cell clones that express the CCK-2 receptor or those that do not but are cocultured with CCK-2-expressing cells (44). The response of the former can be considered the total response: i.e., the sum of both direct intracellular signaling pathway and of paracrine activated pathways; the response of the latter is attributable solely to paracrine pathways. The direct intracellular response includes roles for PKC, Ras, Raf, MAPKase, RhoA, NF-κB, CREB, and AP-1, whereas the paracrine pathway includes roles for RhoA and, as we now show, MAZ and ASC-1 (Fig. 7).
Fig. 7.
Schematic representation of regulation of PAI-2 expression by gastrin. Gastrin regulates PAI-2 expression in both CCK-2 receptor-expressing cells by acting directly through its receptor (left) and in neighboring cells, via paracrine mediators released from CCK-2 receptor expressing cells (right). Direct regulation involves the CRE and AP-1 sites via PKC, Ras, Raf, RhoA, and the NF-κB pathways. The paracrine mediators IL-8 and PGE2 are released in response to gastrin stimulation; IL-8 acts through a GACAGA site via the ASC-1 complex, whereas PGE2 targets the MAZ site via the small GTPase RhoA.
Gastrin stimulation of COX-2 in cells expressing the CCK-2 receptor has been reported by many groups (3, 41, 43). There is evidence that induction of COX-2 by gastrin in vivo is relevant to cytoprotection and ulcer healing (7, 30). However, specific changes in gene expression in response to gastrin that are dependent on this pathway are unclear and more needs to be done to characterize the relevant downstream receptors and signaling pathways. Nevertheless, the present data identify MAZ-mediated PAI-2 expression as one such target. The MAZ site in PAI-2 appears important for basal expression, but there is also a proportionate reduction in the response to gastrin. Interestingly, a previous study implicated MAZ as a mediator of gastrin-stimulated trefoil factor (TFF)-1 expression in AGS cells (28). Moreover like PAI-2, TFF-1 is thought to be important in host defense. Earlier studies of MAZ (Myc-associated zinc finger protein, also known as ZF87 or Pur-1) have established that it is widely distributed and its targets include control of insulin expression (6, 27). We suggest that it is now timely to consider its role in the specific context of control of expression of genes involved in gastric cytoprotection.
The stimulation of IL-8 by gastrin has now been reported by several groups (23, 42, 44). The role of IL-8 in H. pylori gastritis, particularly as a neutrophil chemoattractant has been well documented (11). Moreover, previous studies have shown expression of IL-8 receptors on AGS cells, i.e., CXCR1 and CXCR2 (5). Even so, the role of IL-8 as a downstream mediator of the effects of gastrin has been largely neglected. Our previous work established increased expression of PAI-2 in response to both H. pylori and gastrin compatible with a convergence of actions on this gene (45). The present observations now identify the ASC-1 complex as a potential mediator in this pathway. Previous work has shown ASC-1 to be important for activation of transcription in several systems and to consist of four subunits: p50, p200, p100, and the central subunit p65 (26). Disruption of this complex interferes with its DNA binding and results in loss of function (33). The p65 subunit was previously identified as thyroid receptor interacting protein 4 (TRIP4) (32) and was shown to be a novel transcriptional coactivating molecule for nuclear receptors (29). Silencing at least two subunits of ASC-1, p50 and p65, inhibited gastrin-stimulated PAI-2 expression, indicating that ASC-1 acts as a transcription activator complex to regulate transcription.
In recent years it has become evident that the mucosal microenvironment plays an important role in regulating epithelial function. Interactions between different epithelial cells and between epithelial and mesenchymal cells mediated by paracrine factors are key determinants of normal mucosal organization in development, in wound healing, and in the progression to cancer that occurs with prolonged inflammation and H. pylori infection. In view of the hostile nature of the gastric environment it is not surprising that multiple defense mechanisms have evolved that allow the tissue to respond to infection and damage by minimizing acid-peptic autodigestion and ulcer formation. The induction of PAI-2 is thought to be one component of this response (17). In very many other tissues induction of PAI-2 is also thought to be a response to damage, infection, or inflammation (4, 12, 52). In addition, PAI-2 expression has been linked to cancer (1, 2), and it seems possible that whereas acute induction is protective, long-term upregulation is potentially a contributory factor in tumorigenesis. For these reasons an understanding of the multiple mechanisms by which PAI-2 expression is controlled are likely to be of relevance in many different circumstances.
Together the data indicate how complex interacting extracellular signals can control the transcriptional responses of a single gene and how different regulatory elements within the promoter can be linked to different signaling pathways. Particularly in the case of genes that are induced in multiple cell types within a given tissue, we suggest that careful consideration should always be given to the need to examine paracrine pathways downstream of a single extracellular mediator. At the level of signaling pathways, much remains to be done to understand how the various regulatory mechanisms interact; for example, the mechanisms controlling the abundance and posttranslational modifications to MAZ and ASC-1 in this system remain unknown. More specifically, in the context of PAI-2 regulation we would suggest that the multiple pathways shown to determine expression in gastric epithelial cells indicate that there are many opportunities for integration of different inputs in varying physiological and pathological circumstances between different cell populations in the gastric mucosa.
GRANTS
This work was supported by the Medical Research Council.
Acknowledgments
We thank Prof. Graham Dockray for helpful discussions and for critical review of the manuscript, Professor Alan Hall for gift of the RhoA plasmid, and Drs. Kazunari Yokoyama and Juanita Merchant for the MAZ and ZBP-89 antibodies, respectively.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Andreasen PA, Egelund R, Petersen HH. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol Life Sci 57: 25–40, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreasen PA, Kjoller L, Christensen L, Duffy MJ. The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer 72: 1–22, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Ansorge N, Juttner S, Cramer T, Schmidt WE, Hocker M, Schmitz F. An upstream CRE-E-box element is essential for gastrin-dependent activation of the cyclooxygenase-2 gene in human colon cancer cells. Regul Pept 144: 25–33, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Antalis TM, Costelloe E, Muddiman J, Ogbourne S, Donnan K. Regulation of the plasminogen activator inhibitor type-2 gene in monocytes: localization of an upstream transcriptional silencer. Blood 88: 3686–3697, 1996. [PubMed] [Google Scholar]
- 5.Backhed F, Torstensson E, Seguin D, Richter-Dahlfors A, Rokbi B. Helicobacter pylori infection induces interleukin-8 receptor expression in the human gastric epithelium. Infect Immun 71: 3357–3360, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bossone SA, Asselin C, Patel AJ, Marcu KB. MAZ, a zinc finger protein, binds to c-MYC and C2 gene sequences regulating transcriptional initiation and termination. Proc Natl Acad Sci USA 89: 7452–7456, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brzozowski T, Konturek PC, Konturek SJ, Pajdo R, Schuppan D, Drozdowicz D, Ptak A, Pawlik M, Nakamura T, Hahn EG. Involvement of cyclooxygenase (COX)-2 products in acceleration of ulcer healing by gastrin and hepatocyte growth factor. J Physiol Pharmacol 51: 751–773, 2000. [PubMed] [Google Scholar]
- 8.Catlow K, Ashurst HL, Varro A, Dimaline R. Identification of a gastrin response element in the vesicular monoamine transporter type 2 promoter and requirement of 20 S proteasome subunits for transcriptional activity. J Biol Chem 282: 17069–17077, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Costa M, Shen Y, Medcalf RL. Overexpression of a dominant negative CREB protein in HT-1080 cells selectively disrupts plasminogen activator inhibitor type 2 but not tissue-type plasminogen activator gene expression. FEBS Lett 482: 75–80, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Cousin E, Medcalf RL, Bergonzelli GE, Kruithof EK. Regulatory elements involved in constitutive and phorbol ester-inducible expression of the plasminogen activator inhibitor type 2 gene promoter. Nucleic Acids Res 19: 3881–3886, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crabtree JE, Wyatt JI, Trejdosiewicz LK, Peichl P, Nichols PH, Ramsay N, Primrose JN, Lindley IJ. Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J Clin Pathol 47: 61–66, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darnell GA, Schroder WA, Gardner J, Harrich D, Yu H, Medcalf RL, Warrilow D, Antalis TM, Sonza S, Suhrbier A. SerpinB2 is an inducible host factor involved in enhancing HIV-1 transcription and replication. J Biol Chem 281: 31348–31358, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Dear AE, Costa M, Medcalf RL. Urokinase-mediated transactivation of the plasminogen activator inhibitor type 2 (PAI-2) gene promoter in HT-1080 cells utilises AP-1 binding sites and potentiates phorbol ester-mediated induction of endogenous PAI-2 mRNA. FEBS Lett 402: 265–272, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Dear AE, Medcalf RL. The novel anti-tumour agent oxamflatin differentially regulates urokinase and plasminogen activator inhibitor type 2 expression and inhibits urokinase-mediated proteolytic activity. Biochim Biophys Acta 1492: 15–22, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Dimaline R, Evans D, Forster ER, Sandvik AK, Dockray GJ. Control of gastric corpus chromogranin A messenger RNA abundance in the rat. Am J Physiol Gastrointest Liver Physiol 264: G583–G588, 1993. [DOI] [PubMed] [Google Scholar]
- 16.Dimaline R, Sandvik AK, Evans D, Forster ER, Dockray GJ. Food stimulation of histidine decarboxylase messenger RNA abundance in rat gastric fundus. J Physiol 465: 449–458, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dockray G, Dimaline R, Varro A. Gastrin: old hormone, new functions. Pflügers Arch 449: 344–355, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Dockray GJ, Varro A, Dimaline R, Wang T. The gastrins: their production and biological activities. Annu Rev Physiol 63: 119–139, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Hersey SJ, Sachs G. Gastric acid secretion. Physiol Rev 75: 155–189, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Higuchi R, Krummel B, Saiki RK. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res 16: 7351–7367, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hiraoka S, Miyazaki Y, Kitamura S, Toyota M, Kiyohara T, Shinomura Y, Mukaida N, Matsuzawa Y. Gastrin induces CXC chemokine expression in gastric epithelial cells through activation of NF-κB. Am J Physiol Gastrointest Liver Physiol 281: G735–G742, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Hocker M, Raychowdhury R, Plath T, Wu H, O'Connor DT, Wiedenmann B, Rosewicz S, Wang TC. Sp1 and CREB mediate gastrin-dependent regulation of chromogranin A promoter activity in gastric carcinoma cells. J Biol Chem 273: 34000–34007, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Jung DJ, Sung HS, Goo YW, Lee HM, Park OK, Jung SY, Lim J, Kim HJ, Lee SK, Kim TS, Lee JW, Lee YC. Novel transcription coactivator complex containing activating signal cointegrator 1. Mol Cell Biol 22: 5203–5211, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy GC, Rutter WJ. Pur-1, a zinc-finger protein that binds to purine-rich sequences, transactivates an insulin promoter in heterologous cells. Proc Natl Acad Sci USA 89: 11498–11502, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan ZE, Wang TC, Cui G, Chi AL, Dimaline R. Transcriptional regulation of the human trefoil factor, TFF1, by gastrin1. Gastroenterology 125: 510–521, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Kim HJ, Yi JY, Sung HS, Moore DD, Jhun BH, Lee YC, Lee JW. Activating signal cointegrator 1, a novel transcription coactivator of nuclear receptors, and its cytosolic localization under conditions of serum deprivation. Mol Cell Biol 19: 6323–6332, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komori M, Tsuji S, Sun WH, Tsujii M, Kawai N, Yasumaru M, Kakiuchi Y, Kimura A, Sasaki Y, Higashiyama S, Kawano S, Hori M. Gastrin enhances gastric mucosal integrity through cyclooxygenase-2 upregulation in rats. Am J Physiol Gastrointest Liver Physiol 283: G1368–G1378, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Kruithof EK, Tran-Thang C, Gudinchet A, Hauert J, Nicoloso G, Genton C, Welti H, Bachmann F. Fibrinolysis in pregnancy: a study of plasminogen activator inhibitors. Blood 69: 460–466, 1987. [PubMed] [Google Scholar]
- 32.Lee JW, Choi HS, Gyuris J, Brent R, Moore DD. Two classes of proteins dependent on either the presence or absence of thyroid hormone for interaction with the thyroid hormone receptor. Mol Endocrinol 9: 243–254, 1995. [DOI] [PubMed] [Google Scholar]
- 33.Lee YS, Kim HJ, Lee HJ, Lee JW, Chun SY, Ko SK, Lee K. Activating signal cointegrator 1 is highly expressed in murine testicular Leydig cells and enhances the ligand-dependent transactivation of androgen receptor. Biol Reprod 67: 1580–1587, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Noble PJ, Wilde G, White MR, Pennington SR, Dockray GJ, Varro A. Stimulation of gastrin-CCKB receptor promotes migration of gastric AGS cells via multiple paracrine pathways. Am J Physiol Gastrointest Liver Physiol 284: G75–G84, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Nordeen SK Luciferase reporter gene vectors for analysis of promoters and enhancers. Biotechniques 6: 454–458, 1988. [PubMed] [Google Scholar]
- 38.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 402: 884–888, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Raychowdhury R, Fleming JV, McLaughlin JT, Bulitta CJ, Wang TC. Identification and characterization of a third gastrin response element (GAS-RE3) in the human histidine decarboxylase gene promoter. Biochem Biophys Res Commun 297: 1089–1095, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Schafer B, Gschwind A, Ullrich A. Multiple G-protein-coupled receptor signals converge on the epidermal growth factor receptor to promote migration and invasion. Oncogene 23: 991–999, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Slice LW, Hodikian R, Zhukova E. Gastrin and EGF synergistically induce cyclooxygenase-2 expression in Swiss 3T3 fibroblasts that express the CCK2 receptor. J Cell Physiol 196: 454–463, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Subramaniam D, Ramalingam S, May R, Dieckgraefe BK, Berg DE, Pothoulakis C, Houchen CW, Wang TC, Anant S. Gastrin-mediated interleukin-8 and cyclooxygenase-2 gene expression: differential transcriptional and posttranscriptional mechanisms. Gastroenterology 134: 1070–1082, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Subramaniam D, Ramalingam S, May R, Dieckgraefe BK, Berg DE, Pothoulakis C, Houchen CW, Wang TC, Anant S. Gastrin-mediated interleukin-8 and cyclooxygenase-2 gene expression: differential transcriptional and posttranscriptional mechanisms. Gastroenterology 134: 1070–1082, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Varro A, Hemers E, Archer D, Pagliocca A, Haigh C, Ahmed S, Dimaline R, Dockray GJ. Identification of plasminogen activator inhibitor-2 as a gastrin-regulated gene: role of Rho GTPase and menin. Gastroenterology 123: 271–280, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Varro A, Noble PJ, Pritchard DM, Kennedy S, Hart CA, Dimaline R, Dockray GJ. Helicobacter pylori induces plasminogen activator inhibitor 2 (PAI-2) in gastric epithelial cells through NF-κB and RhoA: implications for invasion and apoptosis. Cancer Res 64: 1695–1702, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Varro A, Noble PJ, Wroblewski LE, Bishop L, Dockray GJ. Gastrin-cholecystokinin(B) receptor expression in AGS cells is associated with direct inhibition and indirect stimulation of cell proliferation via paracrine activation of the epidermal growth factor receptor. Gut 50: 827–833, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wallace JL, Devchand PR. Emerging roles for cyclooxygenase-2 in gastrointestinal mucosal defense. Br J Pharmacol 145: 275–282, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang TC, Dangler CA, Chen D, Goldenring JR, Koh T, Raychowdhury R, Coffey RJ, Ito S, Varro A, Dockray GJ, Fox JG. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology 118: 36–47, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Watson F, Kiernan RS, Deavall DG, Varro A, Dimaline R. Transcriptional activation of the rat vesicular monoamine transporter 2 promoter in gastric epithelial cells: regulation by gastrin. J Biol Chem 276: 7661–7671, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Wohlwend A, Belin D, Vassalli JD. Plasminogen activator-specific inhibitors produced by human monocytes/macrophages. J Exp Med 165: 320–339, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wroblewski LE, Noble PJ, Pagliocca A, Pritchard DM, Hart CA, Campbell F, Dodson AR, Dockray GJ, Varro A. Stimulation of MMP-7 (matrilysin) by Helicobacter pylori in human gastric epithelial cells: role in epithelial cell migration. J Cell Sci 116: 3017–3026, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Yamanaka H, Obata K, Fukuoka T, Dai Y, Kobayashi K, Tokunaga A, Noguchi K. Induction of plasminogen activator inhibitor-1 and -2 in dorsal root ganglion neurons after peripheral nerve injury. Neuroscience 132: 183–191, 2005. [DOI] [PubMed] [Google Scholar]