Abstract
Matrix metalloproteinases (MMP) play an important role in pathogenesis of inflammatory bowel disease (IBD). Two known gelatinases, MMP-2 and MMP-9, are upregulated during IBD. Epithelial-derived MMP-9 is an important mediator of tissue injury in colitis, whereas MMP-2 protects against tissue damage and maintains gut barrier function. It has been suggested that developing strategies to block MMP-9 activity in the gut might be of benefit to IBD. However, given that MMP-2 and MMP-9 are structurally similar, such approaches would also likely inhibit MMP-2. Thus, to gain insight into outcome of inhibiting both MMP-2 and MMP-9, MMP-2−/−/MMP-9−/− double knockout mice (dKO) lacking both MMP-2 and MMP-9 were used in this study. Three models of murine colitis were used: dextran sodium sulfate (DSS), Salmonella typhimurium (S.T.), and trinitrobenzene sulfonic acid (TNBS). Our data demonstrate that MMP-2 and MMP-9 activities were highly upregulated in wild-type (WT) mice treated with DSS, S.T., or TNBS whereas dKO mice were resistant to the development of colitis. WT mice had extensive inflammation and tissue damage compared with dKO mice as suggested by histological assessment and myeloperoxidase activity. In conclusion, these results suggest an overriding role of MMP-9 in mediating tissue injury compared with the protective role of MMP-2 in development of colitis. Thus inhibition of MMP-9 may be beneficial in treatment of colitis even if resulting in inhibition of MMP-2.
Keywords: gelatinase, inflammation, myeloperoxidase activity, cytokines, chemokines
matrix metalloproteinases (MMPs), which include the collagenases, stromelysins, gelatinases, and membrane-type MMPs (MT-MMP), are a family of structurally related zinc-dependent proteases that are involved in cellular infiltration, cytokine activation, cell migration, tissue damage, remodeling, and repair (3, 36). MMPs are central to the regulation of extracellular matrix turnover, having the ability to cleave majority of extracellular matrix proteins. Importantly, dysregulated expression of MMPs has been shown to have a pathogenetic role in a number of diseases including arthritis, atherosclerosis, myocardial infarction, colorectal cancer, tumor invasion, and inflammatory bowel disease (IBD) (8, 18, 26, 54). MMP-2 and MMP-9 are the two MMPs referred to as gelatinases. MMP-2 and MMP-9 differ from other MMPs in terms of their structure as well as substrate specificity (39, 47). Although they have the basic structure of MMPs: a prodomain, a thiol group, a signal peptide, and a catalytic domain with Zn at its active center (41), both MMP-2 and MMP-9 have additional fibronectin repeats in the catalytic domain that distinguishes them from other MMPs. The matrix substrates of MMP-9 and MMP-2 are identical (type IV collagen, gelatin). Although MMP-9 is absent from most adult tissues, MMP-2 is constitutively expressed in almost all tissues. Several studies have shown that MMP-2 and MMP-9 are highly expressed in IBD inflamed colonic mucosa of IBD patients and are associated with disease activity (1, 20, 46, 55).
We and others have demonstrated that MMP-9 activity and protein expression is absent from normal colonic mucosa but is upregulated during experimental colitis in response to luminal toxin (dextran sodium sulfate, DSS) as well as bacteria Salmonella enterica serovar Typhimurium (S.T.) (4, 14, 19, 43). MMP-9−/− mice exposed to DSS or S.T. had dramatically reduced inflammation and mucosal injury and showed protection against acute colitis. Similar to MMP-9, MMP-2 protein expression and activity is highly upregulated during DSS- and S.T.-induced colitis (10). In contrast to MMP-9, MMP-2 served to protect from experimental colitis induced by chemicals (DSS) or bacteria (S.T.). MMP-2−/− mice were highly sensitive to experimental colitis, resulting in a significant increase in inflammation and mortality. Bone marrow chimera studies showed that epithelial, but not immune cell-derived, MMP-2 and MMP-9 mediated the effects on the development of colonic inflammation (4, 10). Together, these studies show that MMP-2 and MMP-9 have contrasting roles in the development of colitis despite structural similarities and upregulation of both during colitis. That MMP-9 drives colitis makes it a logical therapeutic target for IBD treatment. However, an in vivo dosing regimen capable of maintaining inhibition of MMP-9 would likely also block MMP-2 since the proteases have structurally similar active sites. Thus the aim of the present study is to determine the outcome of absence of both MMP-9 and MMP-2 in colitis using mice that are deficient in both MMP-2 and MMP-9.
MATERIALS AND METHODS
Experimental animals.
The Animal Care Committee of Emory University, Atlanta approved all procedures performed on animals, which were in accordance with the “Guide for the Care and Use of Laboratory Animals” [DHEW Publication No. (NIH) 85-23, Revised 1996, Office of Science and Health Reports, DRR/NIH, Bethesda, MD 20205].. MMP-2−/− mice (C57/B6 background) were a kind gift from Dr. Lynn Matrisian (Vanderbilt University) (10, 17). MMP-9−/− mice were purchased from Jackson Laboratories (Bar Harbor, ME) and bred at our facility (4). The homozygous MMP-2−/− mice of C57/B6 background with disruption of the MMP-2 gene (10, 17, 40) were backcrossed with the homozygous MMP-9−/− mice of C57/B6 background with disruption of the MMP-9 gene to generate homozygous MMP-2−/−/MMP-9−/− double knockout mice (dKOs). Genotyping was done by PCR analysis using genomic DNA isolated from a small piece of tail. For MMP-2 wild-type (WT) mice, we used the PCR primers 5′-CAACGATGGAGGCACGAGTG-3′ and 5′-GCCGGGGAACTTGATCATGG-3′, and for MMP-2−/− we used the primers 5′-GACCACCAAGCGAAACAT-3′ and 5′-CAAGAAGGCGATAGAAGG-3′. For MMP-9 wild types, we used 5′-GCATACTTGTACCGCTATGG-3′ and 5′-TGTGATGTTATGATGGTCCC-3′ primers. For the MHP-9−/−, we used 5′-ATGATTGAACAAGATGGATTGCAC-3′ and 5′-TTCGTCCAGATCATCCTGATCGAC-3′ primers. The lack of MMP-2 and MMP-9 protein was confirmed by Western blot analysis. WT and MMP-2−/−/MMP-9−/− dKO littermates used in the study were around 8 wk old at the beginning of the experimental protocol and were maintained on a 12:12-h dark-light cycle with free access to pelleted nonpurified diet and tap water under conditions of controlled temperatures (25 ± 2°C).
Induction of DSS colitis.
Colitis was induced in two groups of age- and sex-matched male and female WT and MMP-2−/−/MMP-9−/− dKO littermates by oral administration of DSS (ICN Biomedicals, Aurora, OH) at 3% (wt/vol) in tap water ad libitum for 7 days. Age-matched male and female WT and MMP-2−/−/MMP-9−/− dKO littermates receiving tap water served as control. Mice were observed daily and evaluated for changes in body weight and the development of clinical symptoms. N = 6 mice/group.
S.T. infection.
Gut-restricted S.T. infection was induced as described previously (2, 4). To prepare S.T. inocula, bacteria (S.T. SL3201) were grown overnight at 37°C in 10 ml of Luria-Bertani broth in a 20-ml container with shaking (150 rpm) and were then used to inoculate fresh medium (1:100) and were grown under the same conditions for 2–3 h until an optical density at 550 nm of 0.35–0.6 was reached. Bacterial cultures were then diluted in normal saline, and the colony-forming units were enumerated by plating a dilution series of the inoculum. Water and food were withdrawn 4 h before treatment with 7.5 mg of streptomycin (75 μl of sterile water containing streptomycin or 75 μl of sterile water by gavage). Afterward, animals were supplied with food and water ad libitum. At 20 h after streptomycin treatment, food and water were withdrawn again for 4 h before mice were infected with 108 colony-forming units of S.T. (50-μl suspension in phosphate-buffered saline) or treated with vehicle. Thereafter food and water were offered immediately. Mice were euthanized after 48 h by CO2 inhalation, and tissue samples were processed as described for the DSS colitis model (34).
Induction of TNBS colitis.
Colitis was induced in two groups of age- and sex-matched male and female WT and MMP-2−/−/MMP-9−/− dKO littermates, by colonic injection of 150 mg/kg body wt of trinitrobenzene sulfonic acid (TNBS; Sigma, St. Louis, MO) dissolved in 50% ethanol. Age-matched male and female WT and MMP-2−/−/MMP-9−/− dKO littermates administered colonic injection of 50% ethanol served as control. Colonic inflammation was assessed 48 h after TNBS administration. N = 10 mice/group.
Protein extraction and Western blot analysis.
As described previously (4), for Western blot analysis, colon tissues obtained as described above were homogenized and extracted with lysis buffer. Samples were then centrifuged at 12,000 rpm for 10 min at 4°C and the resulting supernatant was used for assays. The total protein concentration of all samples was measured by the Bradford method using Protein assay reagent (Bio-Rad, Hercules, CA). Total protein (40 μg) was boiled for 5 min in Laemmli's sample buffer (Bio-Rad) and electrophoresed in 10% SDS-PAGE gels. Proteins were transferred to nitrocellulose (Bio-Rad), and the membrane was then blocked in 5% nonfat dry milk for 1 h. Incubation was performed overnight at 4°C with antibodies for MMP-2 (7.5 μg/ml), and MMP-9 (1:1,000) (Abcam, Cambridge, MA). Subsequently, the membranes were washed with Tris-NaCl-Tween 20 and incubated with a goat anti-mouse (1:4,000) and/or with a goat anti-rabbit (1:2,500) IgG horseradish peroxidase conjugate (Bio-Rad) for 1 h at room temperature. Membranes were developed with Western Lightning Chemiluminescence Reagent plus (Perkin Elmer, Boston, MA) and quantified by image analysis (45).
Clinical activity score.
Assessment of body weights, stool consistency, and the presence of occult or gross blood by a guaiac test (Hemoccult Sensa; Beckman Coulter, Fullerton, CA) were determined daily for each mouse. Colitis was quantified with a clinical score, as described by Cooper et al. (5), using the parameters of weight loss, stool consistency, and fecal blood. Briefly, no weight loss was considered as 0 point; weight loss of 1–5% was scored 1 point, loss of 5–10% as 2 points, and 10–20% weight loss as 3 points; and a loss of more than 20% of the weight was scored as 4. The stool character was characterized as normal (0), soft with well-formed pellets (1), soft without pellets (2), or diarrhea (4). For occult blood, no blood was scored 0, positive Hemoccult scored as 2 points, and gross bleeding was scored 4. The total score was added to get a clinical activity score ranging from 0 to 12. Six days after the induction of colitis, mice were euthanized by CO2 and hypothermia. The abdominal cavity was exposed by a midline laparotomy, and the entire colon was removed from the cecum to the anus. The colon was flushed with cold phosphate-buffered saline and opened longitudinally for morphological studies. The length and weight of the colon were measured and tissue obtained from each colon was processed for further assays.
Histological assessment of colitis.
Colonic specimens obtained as described above were fixed in formalin and coded for blind microscopic assessment of mucosal lesions (descending colon for DSS colitis and cecum for S.T. colitis). Sections were stained with hematoxylin and eosin. Microscopic sections were analyzed and histological scoring was performed as described by Cooper et al. (5) based on three variables according to the severity of the induced damage. Briefly, for inflammation, rare inflammatory cells in the lamina propria were counted as 0, increased numbers of granulocytes in the lamina propria as 1, and confluence of inflammatory cells extending into the submucosa as 2; a score of 3 was given for transmural extension of the infiltrate. For crypt damage, intact crypt was scored 0, loss of one-third basal was counted as 1, loss of two-thirds basal was counted as 2, entire crypt loss was scored 3, change of epithelial surface with erosion was scored 4, and a score of 5 was given for confluent erosion. For evaluation of ulcers, an absence of ulcer was scored 0, one or two foci of ulcerations were scored as 1, three or foci of ulcerations were scored as 2, and confluent/extensive ulceration was scored 3. These values were added to give a total histological score of 11.
MPO activity in the colon.
Neutrophil infiltration into colon was quantified by measuring myeloperoxidase (MPO) activity as described previously (4, 11). Briefly, a portion of colon was homogenized in 1:20 (wt/vol) of 50 mM phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethyl ammonium bromide (Sigma, St. Louis, MO) on ice by use of a Polytron homogenizer. The homogenate was sonicated for 10 s, freeze-thawed three times, and centrifuged at 14,000 rpm for 15 min. The supernatant (14 μl) was added to 1 mg/ml of o-dianisidine hydrochloride (Sigma) and 0.0005% hydrogen peroxide, and the change in absorbance at 460 nm was measured. One unit of MPO activity was defined as the amount that degraded 1 μmol of peroxidase per minute at 25°C. The results were expressed as absorbance per gram of tissue.
Measurement of cytokines.
Proinflammatory cytokines were measured by ELISA in medium collected from colonic organ culture. Colitis was induced by DSS in two groups of age- and sex-matched male and female WT and MMP-2−/−/MMP-9−/− dKO littermates. Age-matched male and female WT and MMP-2−/−/MMP-9−/− dKO littermates receiving tap water served as control. Colons were dissected from mice and flushed with cold saline to remove fecal matter. Each colon was cut into 1-cm lengths and washed in HBSS with penicillin-streptomycin and cultured in serum-free RPMI-1640 (Mediatech, Manassas, VA), supplemented with penicillin-streptomycin. Cultures were incubated at 37°C in 5% CO2 for 24 h. After 24 h harvestation, supernatants were centrifuged and interleukin (IL)-6 and keratinocyte-derived chemokine (KC) levels were measured by ELISA (12) via a DuoSet ELISA Development kit (R&D Systems, Minneapolis, MN).
Statistical analysis.
The data are presented as means ± SE. Groups were compared by Student's t-test. P values <0.05 was considered statistically significant.
RESULTS
MMP-2−/−/MMP-9−/− dKO mice are resistant to DSS-induced colitis.
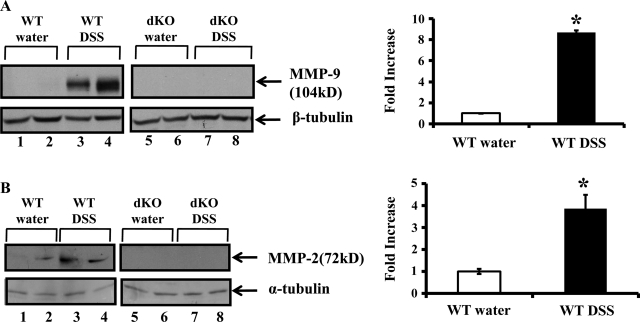
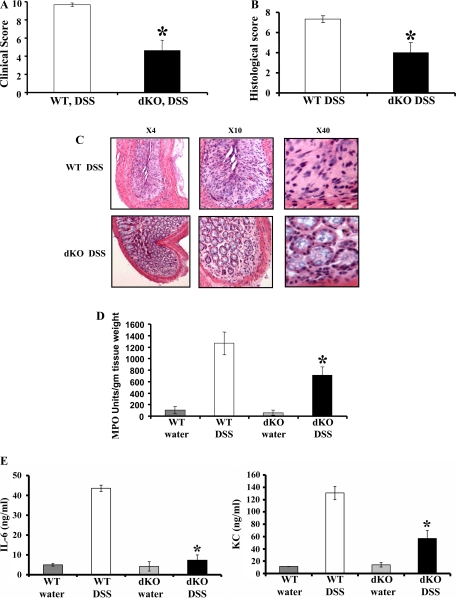
To investigate the relative role of MMP-9 and MMP-2 in the pathogenesis of colitis, we created C57/ B6 MMP-2−/−/MMP-9−/− dKO mice with targeted deletion of MMP-2 and MMP-9 (4, 17). These mice exhibit a normal phenotype. We administered 3% DSS in drinking water to age- (8 wk) and sex matched C57/B6 WT and homozygous MMP-2−/−/MMP-9−/− dKO mice. Both WT and MMP-2−/−/MMP-9−/− dKO mice exposed to DSS for 7 days developed signs of colitis. Mice were euthanized after 7 days of DSS and protein lysates were prepared from the colon mucosal stripping for Western blot analysis as described in methods. Western blot analysis showed that MMP-2−/−/MMP-9−/− dKO mice with or without the treatment of DSS lacked MMP-9 and MMP-2 protein expression (Fig. 1, A and B, lanes 5–8). WT mice given water showed no MMP-9 protein expression but constitutive MMP-2 protein expression (Fig. 1, A and B, respectively, lanes 1–2). WT mice treated with DSS showed increased MMP-9 and MMP-2 protein expression compared with WT control mice (Fig. 1, A and B, respectively, lanes 3–4). Densitometric analysis shown by the graphs revealed a 3.67 ± 0.22-fold increase in MMP-9 protein expression and a 3.87 ± 0.63-fold increase in MMP-2 protein expression in WT mice treated with DSS compared with WT mice treated with water (means ± SE, 6 mice per group; P < 0.05) (Fig. 1, A and B, respectively). We next assessed the clinical signs of disease according to a previously described grading system (4, 5). Almost all the WT mice developed diarrhea after day 5. These mice were hemo-occult positive starting from day 4 and exhibited frank bleeding on day 7 of DSS administration. Interestingly, MMP-2−/−/MMP-9−/− dKO mice were protected from DSS-induced colitis. Clinical score, based on the three parameters of weight loss, stool character, and fecal blood, was 4.6 ± 1.2 MMP-2−/−/MMP- 9−/− dKO mice compared with WT mice having a score of 9.7 ± 0.2 as shown in Fig. 2A (means ± SE, 6 mice per group; P value is <0.05). DSS-induced colitis is characterized by the presence of inflammation of the colon manifested by crypt destruction, mucosal damage, epithelial erosions, and infiltration of inflammatory cells into the mucosal tissue. Tissues collected from WT and MMP-2−/−/MMP-9−/− dKO mice exposed to DSS were examined histologically and compared with those from normal controls. Figure 2B shows a mean histological score of 4 ± 1.7 in MMP-2−/−/MMP-9−/− among dKO mice given DSS compared with a mean histological score of 7.4 ± 0.6 for WT mice given DSS (means ± SE, 6 mice per group; P < 0.05). Interestingly, MMP-2−/−/MMP-9−/− dKO mice treated with DSS showed less inflammation and colonic damage compared with WT mice treated with DSS (Fig. 2C). WT mice had extensive ulceration with almost complete loss of crypt architecture and increased infiltration of neutrophils. The ulcers were not only greater in number but involved larger surface area compared with MMP-2−/−/MMP-9−/− dKO mice (Fig. 2C). Histological signs of inflammation were not detected in the water control groups (data not shown). To confirm the histological finding of granulocyte infiltration, the myeloperoxidase assay was performed. MMP-2−/−/MMP-9−/− dKO mice showed significantly less MPO activity of 708.85 ± 143.93 units/g tissue wt compared with WT mice having MPO activity of 1,267.05 ± 198.85 units/g tissue wt (means ± SE, 6 mice per group; P < 0.05) (Fig. 2D). Thus the data obtained supported the results obtained from clinical analysis and confirmed the resistance toward the development of colitis as a synergistic effect of targeted MMP-9 and MMP-2 deletion.
Fig. 1.
Matrix metalloproteinase (MMP)-2 and MMP-9 protein expression is upregulated during dextran sodium sulfate (DSS) colitis. Mice were given water or 3% DSS (wt/vol) for 7 days, after which time mice were euthanized. Colon was harvested and processed for Western blot. A and B: representative Western blots of protein from the colon of wild-type (WT) mice given water (lanes 1–2) or DSS (lanes 3–4) and MMP-2−/−/MMP-9−/− double knockout (dKO) mice given water (lanes 5–6) or DSS (lanes 7–8). Blots were probed with anti-MMP-9 (1:1,000) or anti-MMP-2 (7.5 μg/ml) (Abcam, Cambridge, MA). Western blot was quantified by scanning densitometry and represented as fold increase of MMP-9/β-tubulin (A) and MMP-2/α-tubulin (B) in WT mice treated with DSS compared with WT mice treated with water in adjacent graphs. Values are representative of 3 individual experiments, means ± SE; n = 6.
Fig. 2.
MMP-2−/−/MMP-9−/− dKO mice are resistant to DSS-induced colitis. WT C57/B6 and MMP-2−/−/MMP-9−/− dKO mice, 8 wk old, were weighed and given water or DSS (3% wt/vol) for 7 days. Mice were euthanized, and colon was processed for histology. A: disease severity was assessed and expressed in terms of clinical activity score. Clinical score was calculated by using parameters of stool consistency, weight loss, and fecal blood. Each bar represents means ± SE, *P < 0.05. B: mean histological score of MMP-2−/−/MMP-9−/− dKO mice compared with WT. Each bar represents means ± SE, *P < 0.05. C: representative sections from 2 individual experiments. N = 6 for DSS group with magnifications ×4, ×10, and ×40. Colon specimens from descending colon were fixed in formalin, and histological score was calculated on hematoxylin and eosin (H&E) sections based on 3 variables: inflammatory cells, crypt damage, and ulcers. Colons were snap frozen in liquid nitrogen, and myeloperoxidase (MPO) activity was measured (D) as an index of neutrophil infiltration into the injured tissue. Each bar represents means ± SE; n = 6 animals for each group. *P < 0.05. E: mice were given water or 3% DSS (wt/vol) for 7 days, after which time mice were euthanized. After 24 h, supernatants were collected from colonic organ culture and were centrifuged, and interleukin (IL)-6 and keratinocyte-derived chemokine (KC) levels were measured by ELISA. Each bar represents mean ± SE, *P < 0.05 and n = 6 for each group.
We next measured IL-6 and IL-8 (KC) in the serum of WT and MMP-2−/−/MMP-9−/− dKO mice given DSS or water. Proinflammatory cytokine (IL-6) and chemokine (KC) were measured by ELISA after inducing colitis by DSS in two groups of age- and sex-matched male and female WT and MMP-2−/−/MMP-9−/− littermates. Age-matched male and female WT and MMP-2−/−/MMP-9−/− littermates receiving tap water served as control. Figure 2E shows that there was significantly decreased (P < 0.05) expression of IL-6 among MMP-2−/−/MMP-9−/− dKO mice (7.4 ± 2.6) compared with WT mice (43.5 ± 1.6) treated with DSS. Similarly, Fig. 2E shows decreased expression (P < 0.005) of chemokine KC among MMP-2−/−/MMP-9−/− dKO mice (56.67 ± 13.4) compared with WT mice (130.75 ± 10.8) in the DSS-treated group.
MMP-2−/−/MMP-9−/− dKO mice are resistant to salmonella- induced colitis.
The bacteria-induced colitis model was used as a second model to confirm the data obtained by the DSS-induced colitis model. S.T. strain SL3201 was used for this model and was administered orally after pretreatment of mice with streptomycin. In this model, S.T. induces clinical and histological features of enterocolitis predominantly involving the cecum (2, 4). We chose this model since it recapitulates several aspects of clinical and histological human S.T. infection as well as acute flares of IBD, wherein mucosa-pathogen interaction is thought to play an important role in the pathogenesis of inflammation. The characteristic histological feature of gut-restricted S.T. enteritis includes neutrophil infiltration of the intestinal mucosa, the hallmark of infectious colitis as well as acute flares of IBD. Mice were euthanized at 48 h after the administration of S.T. and colonic tissue was removed, weighed with the contents, and photographed. Western blot analysis of cecum tissues showed that there was no protein expression of MMP-9 and MMP-2 among the MMP-2−/−/MMP-9−/− dKO mice (Fig. 3, A and B, respectively, lanes 7–10) with or without the treatment of S.T. WT mice without the treatment of S.T. showed no protein expression of MMP-9 (Fig. 3A, lanes 1–2) and showed constitutive protein expression of MMP-2 (Fig. 3B, lanes 1–2). In contrast, WT mice infected with S.T. showed increased protein expression of MMP-9 and MMP-2 (Fig. 3, A and B, respectively, lanes 3–6). Densitometric analysis shown by the graphs a revealed 16.17 ± 0.12-fold increase in MMP-9 protein expression and a 4.65 ± 1.04-fold increase in MMP-2 protein expression in WT mice treated with S.T. compared with WT mice treated without S.T. (means ± SE, 6 mice per group; P < 0.05) (Fig. 3, A and B, respectively). The cecum was processed for histology and MPO activity. MMP-2−/−/MMP-9−/− dKO mice showed relatively normal ceca and significantly reduced inflammatory infiltrates. In contrast, ceca of all the WT mice infected with S.T. appeared pale and shriveled to a small size and were filled with purulent exudates. This clinical observation was reflected by histological score (Fig. 3C) and leukocyte infiltration with loss of crypts as well as ulcerations (Fig. 3D). Figure 3C shows a mean histological score of 3.34 ± 0.34 in MMP-2−/−/MMP-9−/− dKO mice treated with S.T. compared with a mean histological score of 7.0 ± 0.01 for WT mice treated with S.T. (means ± SE, 6 mice per group; P < 0.05). MMP-2−/−/MMP-9−/− dKO mice treated with S.T. showed less inflammation and damage compared with WT mice treated with S.T. (Fig. 3D). MMP-2−/−/MMP-9−/− dKO mice had reduced MPO activity of 136.86 ± 0.01 units/g tissue wt compared with WT mice 2,148.48 ± 0.15 units/g tissue wt (means ± SE, 6 mice per group; P < 0.05) (Fig. 3E). Taken together, these data suggest that MMP-9−/−/MMP-2−/− dKO mice are protected from S.T.-induced colitis.
Fig. 3.
MMP-2−/−/MMP-9−/− dKO mice are resistant to Salmonella typhimurium (S.T.)-induced colitis. WT C57/B6 and MMP-2−/−/MMP-9−/− dKO mice 8 wk old were weighed and treated with or without S.T. for 48 h. Mice were euthanized and cecum was processed for histology. Disease severity was assessed and expressed in terms of histological score. A and B: representative Western blots of protein from the colon of WT without S.T. treatment (lanes 1–2) or with the treatment of S.T. (lanes 3–6) and MMP-2−/−/MMP-9−/− dKO mice without S.T. treatment (lanes 7–8) or with the treatment of S.T. (lanes 9–10) probed with anti-MMP-9 (1:1,000) and anti-MMP-2 (7.5 μg/ml) (Abcam, Cambridge, MA). Each lane shows protein (30 μg/lane) from an individual mouse with or without DSS treatment. Western blot was quantified by scanning densitometry and represented as fold increase of MMP-9/β-tubulin (A) and MMP-2/α-tubulin (B) in WT mice treated with S.T. compared with WT mice treated without S.T. in adjacent graphs. Values are representative of 3 individual experiments, means ± SE; n = 6; P < 0.05. C: mean histological score of MMP-2−/−/MMP-9−/− dKO mice compared with WT. D: representative sections from 2 individual experiments; n = 6 for S.T. group and n = 4 for control group with magnifications ×4, ×10, and ×40. Cecum specimens were fixed in formalin and histological score was assessed by using H&E sections based on 3 variables: inflammatory cells, crypt damage, and ulcers. Ceca were snap frozen in liquid nitrogen and myeloperoxidase activity was measured (E) as an index of neutrophil infiltration into the injured tissue. Each bar represents means ± SE; n = 6 animals for each group. *P < 0. 05.
MMP-2−/−/MMP-9−/− dKO mice are resistant to TNBS-induced colitis.
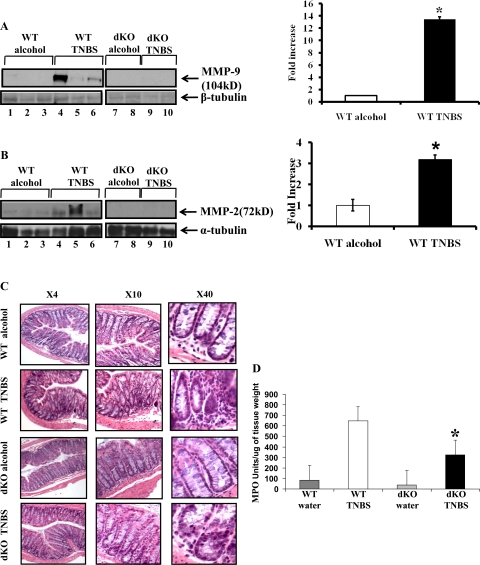
TNBS-induced colitis was used as a third model to support the data from DSS-induced colitis model and S.T.-induced colitis model. Colitis was induced by use of TNBS (150 mg/kg body wt) as described in methods. Western blot analysis showed that MMP-2−/−/MMP-9−/− dKO mice with or without the treatment of TNBS lacked MMP-9 and MMP-2 protein expression (Fig. 4, A and B, lanes 7–10). WT mice treated with vehicle showed no expression of MMP-9 protein but demonstrated constitutive expression of MMP-2 (Fig. 4, A and B, respectively, lanes 1–3). Both MMP-2 and MMP-9 expression increased with TNBS treatment (Fig. 4, A and B, respectively, lanes 4–6). Densitometric analysis revealed a 13.45 ± 0.3-fold increase in MMP-9 protein expression and a 3.19 ± 0.2-fold increase in MMP-2 protein expression in WT mice treated with TNBS compared with WT mice treated with vehicle. (means ± SE 6 mice per group; P < 0.005) (Fig. 4, A and B, respectively). MMP-2−/−/MMP-9−/− dKO mice developed significantly less inflammation compared with WT mice that received TNBS. Tissues collected from MMP-2−/−/MMP-9−/− dKO mice and WT mice exposed to TNBS were examined histologically and compared with those from controls. MMP-2−/−/MMP-9−/− dKO mice showed less inflammation with less damage to crypt architecture compared with WT mice (Fig. 4C). There was also some infiltration of neutrophils in controls, likely due to ethanol, which damages the mucosal barrier (Fig. 4C). The histological findings were supported by the MPO assay. MMP-2−/−/MMP-9−/− dKO mice had significantly reduced MPO (325.11 ± 58.1 units/g tissue wt) compared with WT mice administered TNBS 646.1 ± 106.1 units/g tissue wt (MPO units means ± SE, 10 mice per group; P < 0.05) (Fig. 4D). Thus these data corroborated the results obtained from the DSS-induced colitis model and the S.T.-induced colitis model and confirmed the resistance of MMP-9−/−/MMP-2−/− dKO mice to the development of colitis.
Fig. 4.
MMP-2−/−/MMP-9−/− dKO mice are resistant to trinitrobenzene sulfonic acid (TNBS)-induced colitis. WT C57/B6 and MMP-2−/−/MMP-9−/− dKO mice 8 wk old were weighed and treated with or without TNBS for 48 h. Mice were euthanized, and colon was processed for Western blot and histology. A and B: representative Western blots of protein from the colon of WT mice given vehicle (lanes 1–3) or TNBS (lanes 4–6) and MMP-2−/−/MMP-9−/− dKO mice given vehicle (lanes 7–8) or TNBS (lanes 9–10). Western blot was quantified by scanning densitometry and represented in adjacent graphs as the fold increase of MMP-9/β-tubulin (A) or MMP-2/α-tubulin (B) in WT mice treated with TNBS compared with WT mice treated with vehicle. Values are representative of 3 individual experiments, means ± SE; n = 10. Disease severity was assessed and expressed in terms of histological score. C: representative photomicrographs from 2 individual experiments; n = 10 for TNBS group and n = 10 for control group with magnifications ×4, ×10, and ×40. Colon were snap frozen in liquid nitrogen and myeloperoxidase activity was measured (D) as an index of neutrophil infiltration into the injured tissue. Each bar represents mean ± SE; n = 10 animals for each group. *P < 0.05.
DISCUSSION
This study examined the phenotype of mice lacking both MMP-9 and MMP-2 in three distinct models of experimental colitis (DSS, S.T., and TNBS). We observed that such MMP-9−/−//MMP-2−/− dKO mice were protected from the development of colitis measured by clinical, histological, and biochemical parameters. Thus, if specific inhibition of MMP-9 proves unfeasible, inhibition of both MMP-9 and MMP-2 may still be therapeutically useful. Although developing pharmacological approaches that can effectively achieve potent block of these MMPs in the gut may be difficult, our results support that, at least if successful, the approach would have therapeutic potential.
Our previous data showed that MMP-2 deficiency led to barrier dysfunction and dramatically increased susceptibility to colitis (10). Furthermore, overexpression of MMP-2 in cultured intestinal epithelial cells demonstrated increased barrier protection measured by decreased translocation of fluorescently labeled dextran as well as transepithelial resistance (unpublished data). Conversely, MMP-2−/− mice exhibited decreased barrier function as measured by translocation of fluorescently labeled dextran. Together these data suggested that MMP-2 expression during colitis served to protect from the development of inflammatory response (10) likely through its effect on the epithelial barrier. In contrast, we and others have previously demonstrated that the absence of MMP-9 attenuated the development of inflammatory response to colitic agents (4, 32, 43). We demonstrated that overexpression of MMP-9 impaired wound healing in cultured intestinal epithelial cells in vitro (4) and modulated colonic epithelial differentiation (10), suggesting that MMP-9 mediates inflammatory response and/or tissue damage. Our study with MMP-2−/−/MMP-9−/− dKO mice with chemical/and bacterial models of colitis showed that unlike MMP-2−/− mice, MMP-2−/−/MMP-9−/− dKO mice mimic MMP-9−/− mice during colitis. Our data implicate that the tissue damaging effect of MMP-9 overrides the barrier protective role of MMP-2 during colitis.
Recently it has been found that there are a number of nonmatrix MMP substrates that potently influence cellular functions (30), such as cytokines (16), growth factor receptors (25), and chemokines (31). It is well known that MMP-2 and MMP-9 have similar substrate specificities for matrix protein (28, 41). So the opposite roles of MMP-9 and MMP-2 during colitis or the dominating role of MMP-9 over MMP-2 during colitis may be caused by the differences of nonmatrix substrates. For example, MMP-9 cannot degrade monocyte chemoattractant protein-3 (31) and fibroblast growth factor receptor-1 (25). This hypothesis is also supported by the fact that MMP-9 has an additional collagen-V like domain that is highly glycosylated and alters the substrate specificity and confers resistance to degradation (53).
Various studies have examined the effect of MMP-9 and MMP-2 deletion in the development of inflammation in a variety of organs, and for the most part these studies have observed that the effects of MMP-2 and MMP-9 were negated in the dKO mice in that the responses to injury in the dKO were similar to those of WT mice. For example, Itoh et al. (18) have observed that their MMP-2−/−/MMP-9−/− dKO mice showed no significant difference from the WT mice in an arthritis model. In another study, Corry et al. (6) have shown the overlapping and independent roles of MMP-9 and MMP-2 to lung allergic inflammatory cell egression. They observed that allergic lung phenotype of MMP-9−/− mice were similar to WT and didn't alter by concomitant deletion of MMP-2 gene. In another study, done by Zeisberg et al. (57), observed that ablation of both MMP-9 and MMP-2 did not display significant abnormalities in the kidney. In contrast to these studies, Lambert et al. (23) have shown the synergistic effect of MMP-2 and MMP-9 in promoting the choroidal neovascularization. Their study showed that both MMP-9 and MMP-2 cooperate in the course of experimental choroidal neovascularization. Indeed, choroidal pathological angiogenesis was almost completely prevented in MMP-2−/−/MMP-9−/− dKO mice (23). Our data contrast these studies in demonstrating a nonredundant role for MMP-9 in inflammation and tissue damage.
Synthetic MMP inhibitors containing reactive zinc-chelating groups such as thiol or hydroxamate (51) that inhibit the active enzymatic site common to all MMP have been demonstrated to reduce tissue injury and inflammation in some animal models of IBD (7, 33, 34, 37, 50). For example, an orally active MMP inhibitor, ONO-4817, reduces DSS-induced colitis in mice (37). However, lack of efficacy and/or significant side effects associated with general MMP inhibition observed in clinical trials for cancer have precluded their use in human trials (42). Recently, attempts have been made to synthesize specific gelatinase inhibitors (21). However, because of the structural similarity of the gelatinases, targeted inhibition of a specific gelatinase has not been possible and the gelatinase inhibitors developed thus far do not differentiate between MMP-9 and MMP-2 (21). One potential problem of the nonspecificity of inhibitors will be the inhibition of MMP-2. For example, MMP-2 is required for wound healing in different organs (24, 27, 29, 38, 49, 52, 56). Targeted synthetic peptides also cannot solve the problem completely because of their instability (13, 21, 44). Alternatively, tissue inhibitors of metalloproteinases (TIMP)-1 could be used to inhibit MMP-9 given its ability to form 1:1 specific stoichiometry complex with pro-MMP-9 and inhibit MMP-9 activity (35). However, it also can form such complexes with other nonmembrane-bound active MMPs and inhibits their proteolytic activity, e.g., MMP-3, MT-MMPs, and MMP-19 (15, 22, 48). In addition, independent of its action on MMPs, TIMP-1 has been recently recognized to have other biological activities including cell growth, migration, and apoptosis (48). Therefore, therapeutic application of TIMPs through gene therapy is still in an early stage (9).
In summary, we demonstrate that MMP-9 is indispensible for inflammatory response and tissue damage. Together with our previous work, the data here underscore the overriding role of MMP-9 over protective effect of MMP-2 in mediating tissue damage and the potential therapeutic efficacy of MMP-9 inhibition. Data presented herein suggest that concomitant inhibition of MMP-2 and MMP-9 suppresses inflammation underscoring the potential therapeutic efficacy of gelatinase inhibition in the treatment of IBD.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK 06411 (S. V. Sitaraman) and DK 02831 (D. Merlin) and by Digestive Disease Research Center Grant 5R24DK064399. P. Garg is a recipient of Research Fellowship from Crohn's & Colitis Foundation of America.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bailey CJ, Hembry RM, Alexander A, Irving MH, Grant ME, Shuttleworth CA. Distribution of the matrix metalloproteinases stromelysin, gelatinases A and B, and collagenase in Crohn's disease and normal intestine. J Clin Pathol 47: 113–116, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barthel M, Hapfelmeier S, Quintanilla-Martinez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Russmann H, Hardt WD. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun 71: 2839–2858, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol 3: 207–214, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Castaneda FE, Walia B, Vijay-Kumar M, Patel NR, Roser S, Kolachala VL, Rojas M, Wang L, Oprea G, Garg P, Gewirtz AT, Roman J, Merlin D, Sitaraman SV. Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: central role of epithelial-derived MMP. Gastroenterology 129: 1991–2008, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 69: 238–249, 1993. [PubMed] [Google Scholar]
- 6.Corry DB, Kiss A, Song LZ, Song L, Xu J, Lee SH, Werb Z, Kheradmand F. Overlapping and independent contributions of MMP2 and MMP9 to lung allergic inflammatory cell egression through decreased CC chemokines. FASEB J 18: 995–997, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Sebastiano P, di Mola FF, Artese L, Rossi C, Mascetta G, Pernthaler H, Innocenti P. Beneficial effects of Batimastat (BB-94), a matrix metalloproteinase inhibitor, in rat experimental colitis. Digestion 63: 234–239, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Dubois B, Opdenakker G, Carton H. Gelatinase B in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neurol Belg 99: 53–56, 1999. [PubMed] [Google Scholar]
- 9.Ganea E, Trifan M, Laslo AC, Putina G, Cristescu C. Matrix metalloproteinases: useful and deleterious. Biochem Soc Trans 35: 689–691, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Garg PRM, Ravi A, Bockbrader K, Epstein S, Vijay-Kumar M, Gewirtz AT, Merlin D, Sitaraman SV. Selective ablation of matrix metalloproteinase-2 exacerbates experimental colitis: contrasting role of gelatinases in the pathogenesis of colitis. J Immunol 177: 4103–4112, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Gewirtz AT, Collier-Hyams LS, Young AN, Kucharzik T, Guilford WJ, Parkinson JF, Williams IR, Neish AS, Madara JL. Lipoxin a4 analogs attenuate induction of intestinal epithelial proinflammatory gene expression and reduce the severity of dextran sodium sulfate-induced colitis. J Immunol 168: 5260–5267, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Gewirtz AT, Simon PO Jr, Schmitt CK, Taylor LJ, Hagedorn CH, O'Brien AD, Neish AS, Madara JL. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J Clin Invest 107: 99–109, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higashi S, Miyazaki K. Identification of a region of beta-amyloid precursor protein essential for its gelatinase A inhibitory activity. J Biol Chem 278: 14020–14028, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Ishida K, Takai S, Murano M, Nishikawa T, Inoue T, Murano N, Inoue N, Jin D, Umegaki E, Higuchi K, Miyazaki M. Role of chymase-dependent matrix metalloproteinase-9 activation in mice with dextran sodium sulfate-induced colitis. J Pharmacol Exp Ther 324: 422–426, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Islekel H, Oktay G, Terzi C, Canda AE, Fuzun M, Kupelioglu A. Matrix metalloproteinase-9,-3 and tissue inhibitor of matrix metalloproteinase-1 in colorectal cancer: relationship to clinicopathological variables. Cell Biochem Funct 25: 433–441, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Ito A, Mukaiyama A, Itoh Y, Nagase H, Thogersen IB, Enghild JJ, Sasaguri Y, Mori Y. Degradation of interleukin 1beta by matrix metalloproteinases. J Biol Chem 271: 14657–14660, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Itoh T, Ikeda T, Gomi H, Nakao S, Suzuki T, Itohara S. Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J Biol Chem 272: 22389–22392, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Itoh T, Matsuda H, Tanioka M, Kuwabara K, Itohara S, Suzuki R. The role of matrix metalloproteinase-2 and matrix metalloproteinase-9 in antibody-induced arthritis. J Immunol 169: 2643–2647, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Kawabata K, Murakami A, Ohigashi H. Auraptene decreases the activity of matrix metalloproteinases in dextran sulfate sodium-induced ulcerative colitis in ICR mice. Biosci Biotechnol Biochem 70: 3062–3065, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Kirkegaard T, Pedersen G, Saermark T, Brynskov J. Tumour necrosis factor-alpha converting enzyme (TACE) activity in human colonic epithelial cells. Clin Exp Immunol 135: 146–153, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koivunen E, Arap W, Valtanen H, Rainisalo A, Medina OP, Heikkila P, Kantor C, Gahmberg CG, Salo T, Konttinen YT, Sorsa T, Ruoslahti E, Pasqualini R. Tumor targeting with a selective gelatinase inhibitor. Nat Biotechnol 17: 768–774, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Lambert E, Dasse E, Haye B, Petitfrere E. TIMPs as multifacial proteins. Crit Rev Oncol Hematol 49: 187–198, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Lambert V, Wielockx B, Munaut C, Galopin C, Jost M, Itoh T, Werb Z, Baker A, Libert C, Krell HW, Foidart JM, Noel A, Rakic JM. MMP-2 and MMP-9 synergize in promoting choroidal neovascularization. FASEB J 17: 2290–2292, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Lechapt-Zalcman E, Pruliere-Escabasse V, Advenier D, Galiacy S, Charriere-Bertrand C, Coste A, Harf A, d'Ortho MP, Escudier E. Transforming growth factor-β1 increases airway wound repair via MMP-2 upregulation: a new pathway for epithelial wound repair? Am J Physiol Lung Cell Mol Physiol 290: L1277–L1282, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Levi E, Fridman R, Miao HQ, Ma YS, Yayon A, Vlodavsky I. Matrix metalloproteinase 2 releases active soluble ectodomain of fibroblast growth factor receptor 1. Proc Natl Acad Sci USA 93: 7069–7074, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Z, Shipley JM, Vu TH, Zhou X, Diaz LA, Werb Z, Senior RM. Gelatinase B-deficient mice are resistant to experimental bullous pemphigoid. J Exp Med 188: 475–482, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makela M, Larjava H, Pirila E, Maisi P, Salo T, Sorsa T, Uitto VJ. Matrix metalloproteinase 2 (gelatinase A) is related to migration of keratinocytes. Exp Cell Res 251: 67–78, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Matrisian LM The matrix-degrading metalloproteinases. Bioessays 14: 455–463, 1992. [DOI] [PubMed] [Google Scholar]
- 29.Matsubara M, Zieske JD, Fini ME. Mechanism of basement membrane dissolution preceding corneal ulceration. Invest Ophthalmol Vis Sci 32: 3221–3237, 1991. [PubMed] [Google Scholar]
- 30.McCawley LJ, Matrisian LM. Matrix metalloproteinases: they're not just for matrix anymore! Curr Opin Cell Biol 13: 534–540, 2001. [DOI] [PubMed] [Google Scholar]
- 31.McQuibban GA, Gong JH, Tam EM, McCulloch CA, Clark-Lewis I, Overall CM. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science 289: 1202–1206, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Medina C, Santana A, Paz MC, Diaz-Gonzalez F, Farre E, Salas A, Radomski MW, Quintero E. Matrix metalloproteinase-9 modulates intestinal injury in rats with transmural colitis. J Leukoc Biol 79: 954–962, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Medina C, Videla S, Radomski A, Radomski M, Antolin M, Guarner F, Vilaseca J, Salas A, Malagelada JR. Therapeutic effect of phenantroline in two rat models of inflammatory bowel disease. Scand J Gastroenterol 36: 1314–1319, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Medina C, Videla S, Radomski A, Radomski MW, Antolin M, Guarner F, Vilaseca J, Salas A, Malagelada JR. Increased activity and expression of matrix metalloproteinase-9 in a rat model of distal colitis. Am J Physiol Gastrointest Liver Physiol 284: G116–G122, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Mook OR, Frederiks WM, Van Noorden CJ. The role of gelatinases in colorectal cancer progression and metastasis. Biochim Biophys Acta 1705: 69–89, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol 16: 558–564, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naito Y, Takagi T, Kuroda M, Katada K, Ichikawa H, Kokura S, Yoshida N, Okanoue T, Yoshikawa T. An orally active matrix metalloproteinase inhibitor, ONO-4817, reduces dextran sulfate sodium-induced colitis in mice. Inflamm Res 53: 462–468, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Okada A, Tomasetto C, Lutz Y, Bellocq JP, Rio MC, Basset P. Expression of matrix metalloproteinases during rat skin wound healing: evidence that membrane type-1 matrix metalloproteinase is a stromal activator of pro-gelatinase A. J Cell Biol 137: 67–77, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Overall CM Molecular determinants of metalloproteinase substrate specificity: matrix metalloproteinase substrate binding domains, modules, and exosites. Mol Biotechnol 22: 51–86, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Perez SE, Cano DA, Dao-Pick T, Rougier JP, Werb Z, Hebrok M. Matrix metalloproteinases 2 and 9 are dispensable for pancreatic islet formation and function in vivo. Diabetes 54: 694–701, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ravi A, Garg P, Sitaraman SV. Matrix metalloproteinases in inflammatory bowel disease: boon or a bane? Inflamm Bowel Dis 13: 97–107, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Rudek MA, Venitz J, Figg WD. Matrix metalloproteinase inhibitors: do they have a place in anticancer therapy? Pharmacotherapy 22: 705–720, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Santana A, Medina C, Paz-Cabrera MC, Diaz-Gonzalez F, Farre E, Salas A, Radomski MW, Quintero E. Attenuation of dextran sodium sulphate induced colitis in matrix metalloproteinase-9 deficient mice. World J Gastroenterol 12: 6464–6472, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulz R Intracellular targets of matrix metalloproteinase-2 in cardiac disease: rationale and therapeutic approaches. Annu Rev Pharmacol Toxicol 47: 211–242, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Sitaraman SV, Merlin D, Wang L, Wong M, Gewirtz AT, Si-Tahar M, Madara JL. Neutrophil-epithelial crosstalk at the intestinal lumenal surface mediated by reciprocal secretion of adenosine and IL-6. J Clin Invest 107: 861–869, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stallmach A, Chan CC, Ecker KW, Feifel G, Herbst H, Schuppan D, Zeitz M. Comparable expression of matrix metalloproteinases 1 and 2 in pouchitis and ulcerative colitis. Gut 47: 415–422, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17: 463–516, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stetler-Stevenson WG Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal 1: re6, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stetler-Stevenson WG, Krutzsch HC, Wacher MP, Margulies IM, Liotta LA. The activation of human type IV collagenase proenzyme. Sequence identification of the major conversion product following organomercurial activation. J Biol Chem 264: 1353–1356, 1989. [PubMed] [Google Scholar]
- 50.Sykes AP, Bhogal R, Brampton C, Chander C, Whelan C, Parsons ME, Bird J. The effect of an inhibitor of matrix metalloproteinases on colonic inflammation in a trinitrobenzenesulphonic acid rat model of inflammatory bowel disease. Aliment Pharmacol Ther 13: 1535–1542, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Talbot DC, Brown PD. Experimental and clinical studies on the use of matrix metalloproteinase inhibitors for the treatment of cancer. Eur J Cancer 32A: 2528–2533, 1996. [DOI] [PubMed]
- 52.Turck J, Pollock AS, Lee LK, Marti HP, Lovett DH. Matrix metalloproteinase 2 (gelatinase A) regulates glomerular mesangial cell proliferation and differentiation. J Biol Chem 271: 15074–15083, 1996. [DOI] [PubMed] [Google Scholar]
- 53.Van den Steen PE, Opdenakker G, Wormald MR, Dwek RA, Rudd PM. Matrix remodelling enzymes, the protease cascade and glycosylation. Biochim Biophys Acta 1528: 61–73, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Vermaelen KY, Cataldo D, Tournoy K, Maes T, Dhulst A, Louis R, Foidart JM, Noel A, Pauwels R. Matrix metalloproteinase-9-mediated dendritic cell recruitment into the airways is a critical step in a mouse model of asthma. J Immunol 171: 1016–1022, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Von Lampe B, Barthel B, Coupland SE, Riecken EO, Rosewicz S. Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut 47: 63–73, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xue M, Le NT, Jackson CJ. Targeting matrix metalloproteases to improve cutaneous wound healing. Expert Opin Ther Targets 10: 143–155, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Zeisberg M, Khurana M, Rao VH, Cosgrove D, Rougier JP, Werner MC, Shield CF 3rd, Werb Z, Kalluri R. Stage-specific action of matrix metalloproteinases influences progressive hereditary kidney disease. PLoS Med 3: e100, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]