Abstract
Prostanoids, produced endogenously via cyclooxygenases (COXs), have been implicated in the sustained contraction of different smooth muscles. The two major types of COXs are COX-1 and COX-2. The COX subtype involved in the basal state of the internal anal sphincter (IAS) smooth muscle tone is not known. To identify the COX subtype, we examined the effect of COX-1- and COX-2-selective inhibitors, SC-560 and rofecoxib, respectively, on basal tone in the rat IAS. We also determined the effect of selective deletion of COX-1 and COX-2 genes (COX-1−/− and COX-2−/− mice) on basal tone in murine IAS. Our data show that SC-560 causes significantly more efficacious and potent concentration-dependent decreases in IAS tone than rofecoxib. In support of these data, significantly higher levels of COX-1 than COX-2 mRNA were found in the IAS. In addition, higher levels of COX-1 mRNA and protein were expressed in rat IAS than rectal smooth muscle. In wild-type mice, IAS tone was decreased 41.4 ± 3.4% (mean ± SE) by SC-560 (1 × 10−5 M) and 5.4 ± 2.2% by rofecoxib (P < 0.05, n = 5). Basal tone was 0.172 ± 0.021 mN//mg in the IAS from wild-type mice and significantly less (0.080 ± 0.015 mN/mg) in the IAS from COX-1−/− mice (P < 0.05, n = 5). However, basal tone in COX-2−/− mice was not significantly different from that in wild-type mice. We conclude that COX-1-related products contribute significantly to IAS tone.
Keywords: smooth muscle tone, cyclooxygenase
cyclooxygenases (COXs) catalyze the conversion of arachidonic acid (AA) to PGH2, which is the common precursor of PGE2, PGF2α, PGI2, and thromboxane A2 (TxA2), collectively named prostanoids (35). Such prostanoids, produced via COX activation, play crucial and diverse roles in gastrointestinal (GI) cytoprotection (39) and in the pathophysiology of gut inflammation and ulcerative and neoplastic disorders (17). In addition, prostanoids play a significant role in the regulation of myenteric neurons and smooth muscle function of the GI tract (36, 40).
There are two primary isoforms of COX, COX-1 and COX-2. Although both isoforms catalyze the same reaction, they differ in terms of their expression patterns. The constitutive isoform COX-1 is expressed in most tissues and is involved in a number of cellular housekeeping functions (3, 34). The inducible isoform, COX-2, is expressed, both rapidly and transiently, in response to inflammatory or mitogenic stimuli (20, 21). However, there are certain exceptions when COX-2 may be constitutively expressed (10, 17, 18).
COXs can be chemically inhibited by synthetic compounds. Indomethacin is one of the first COX inhibitors introduced for therapy of inflammation and pain management. Because of its ulcerogenic activity in the GI tract, indomethacin has been replaced by more selective inhibitors of COX. SC-560 is a potent and selective inhibitor of COX-1, whereas rofecoxib inhibits COX-2 (8, 33). Both of these inhibitors are efficacious and have been extensively used in a number of clinical settings.
The spontaneously tonic smooth muscle of the internal anal sphincter (IAS) plays a significant role in anorectal continence and in the pathophysiology of numerous GI disorders, such as anal fissures, constipation, hemorrhoids, and Hirschsprung's disease (6, 23, 25, 26, 29, 32). The cellular signals that trigger the myogenic tone in the IAS are not known. Recent studies have suggested that the renin-angiotensin system (RAS) may be partly responsible for IAS tone. It has been suggested that, in the tonic smooth muscle of the lower esophageal sphincter (LES), PGF2α and thromboxanes, released from AA via the COX pathway, play a significant role in basal tone (5, 9). Earlier studies from our laboratory showed that IAS smooth muscle has the ability to produce AA via conversion of membrane phospholipids by group I secreted phospholipase A2 (sPLA2) (16). These observations provide the basis for the role of the COX pathway in basal tone in the IAS. However, the specific COX species, COX-1 or COX-2, and their relative contribution to IAS tone have not been determined.
Therefore, the focus of the present studies is on determination of the expression levels (at the transcriptional and translational levels) and the function of COX-1 vs. COX-2 in rat IAS. Functions of COX isoforms were evaluated by the use of isoform-selective inhibitors and specific knockout mice with selective deletion of COX-1 or COX-2 (COX-1−/− and COX-2−/− mice).
MATERIALS AND METHODS
Tissue preparation.
Male Sprague-Dawley rats (300–350 g body wt) were killed by decapitation, and IAS smooth muscle strips were prepared as described elsewhere (13). Briefly, IAS strips (∼1 × 7 mm) from the circular smooth muscle layer were prepared in oxygenated Krebs physiological solution (KPS). The composition of KPS was as follows (in mM): 118.07 NaCl, 4.69 KCl, 2.52 CaCl2, 1.16 MgSO4, 1.01 NaH2PO4, 25 NaHCO3, and 11.10 glucose. The experimental protocol of the study was approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University and was in accordance with the recommendations of the American Association for the Accreditation of Laboratory Animal Care.
Measurement of isometric tension.
The smooth muscle strips were transferred to 2-ml muscle baths containing oxygenated KPS at 37°C. One end of the smooth muscle strips was anchored at the bottom of the muscle bath, and the other end was connected to a force transducer (model FT03, Grass Instruments, Quincy, MA). Isometric tension was measured by the PowerLab/8SP data acquisition system using Chart 4.1.2 (ADInstruments). Each smooth muscle strip was initially stretched to a tension of 1.0 g and then allowed to equilibrate for 60 min. During this equilibration period, the muscle bath was replenished with fresh KPS at 20-min intervals. Only the strips that developed spontaneous tone and relaxed to electrical field stimulation (10 Hz, 20 V, 0.5-ms pulse duration, and 4-s train duration) delivered from a stimulator (model S88, Grass Instruments) were used.
Drug responses.
First, we determined the effects of cumulative concentrations of indomethacin (0.1–300 μM) on basal tone of the IAS. Next, we examined the effects of selective inhibitors of COX-1 and COX-2, SC-560 and rofecoxib, respectively (both at 0.01–10 μM). These experiments were repeated in the presence of PGF2α and TxA2 receptor antagonists, AL-8810 and SQ-29598 (both 0.1–10 μM), respectively. The decreases in IAS tone induced by the inhibitors were measured and normalized to the maximal decrease in tone induced by 50 mM EDTA, as previously described (14).
Western blot analysis.
Western blot studies were performed to determine the relative distribution of COX-1 and COX-2 following the previously described method from our laboratory (14). Briefly, after their isolation, IAS and rectum smooth muscle (RSM), which was used as an internal control, were subjected to homogenization and protein extraction. Proteins were determined by the method of Lowry et al. (24) and then separated by gel electrophoresis and transferred onto a nitrocellulose membrane (NCM) at 4°C.
Nonspecific binding on the NCM was blocked with nonfat milk (5%) in Tris-buffered saline-Tween 20 [20 mM Tris (pH 7.6), 137 mM NaCl, and 0.1% Tween 20] overnight at 4°C. Then the NCM was incubated with the specific primary antibody (rabbit anti-COX-1 and goat anti-COX-2 at 1:1,000 dilution) for 2 h at room temperature. After it was washed with Tris-buffered saline-Tween 20, the NCM was incubated with horseradish peroxidase-labeled secondary antibody (1:10,000 dilution) for 1 h at room temperature. The corresponding bands were visualized with enhanced chemiluminescence substrate using SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL) and Hyperfilm MP (Amersham Bioscience).
The NCM was stripped of antibodies by exposure to Restore Western blot stripping buffer (Pierce) for 10 min at room temperature and then reprobed for α-actin using the specific primary [mouse IgG (1:10,000 dilution) for α-actin] and secondary (1:10,000 dilution) antibodies. Bands corresponding to different proteins were scanned (SnapScan 310, Agfa, Ridgefield Park, NJ), and the integrated optical density (IOD) was determined using Image J (National Institutes of Health, Bethesda, MD). The relative densities were calculated by normalization of the IOD of each blot to that of α-actin.
RT-PCR.
Total RNA was isolated and purified from different tissues by the acid guanidine-phenol-chloroform method (11) and quantified by measurement of absorbance at 260 nm in a spectrophotometer. Total RNA (2.0 μg) was subjected to first-strand cDNA synthesis using oligo(dT) primers (Promega, Madison, WI) and the Omniscript RT kit (Qiagen, Germantown, MD) in a final volume of 20 μl at 42°C for 60 min. PCR primers specific for COX-1, COX-2, and β-actin cDNA were designed as shown in Table 1. PCR was performed in a Promega 2× Master Mix in a final volume of 25 μl using a Perkin-Elmer thermal cycler (PerkinElmer Life and Analytical Sciences). The PCR conditions were as follows: 94°C for 2 min followed by 35 cycles of 94°C for 30 s (denaturation), 59°C for 30 s (annealing), and 72°C for 1 min (extension) and a final extension at 72°C for 7 min. The PCR products were separated on a 1.5% (wt/vol) agarose gel containing ethidium bromide and visualized with UV light. The relative densities of COX-1 and COX-2 were calculated by normalization of the IOD of each blot to that of β-actin.
Table 1.
Primers used in RT-PCRs for amplification of mRNA encoding COX-1, COX-2, and β-actin
| Accession No. | Sequence (5′–3′) | |
|---|---|---|
| COX-1 | S67721 | |
| Forward | CTCACCAGTCATTCCCTGTTGTTAC | |
| Reverse | CTCCATCCAGCACCTGGTACTTAA | |
| COX-2 | S67722 | |
| Forward | ACCGTGGTGAATGTATGAGCATAGGA | |
| Reverse | TCAGGTGTTGCACGTAGTCTTCGAT | |
| β-Actin | NM_007393 | |
| Forward | TGTTTGAGACCTTCAACACCCC | |
| Reverse | ACGTCACACTCCATGATGGAA |
COX, cyclooxygenase.
Breeding and genotyping of COX-1−/− and COX-2−/− mice.
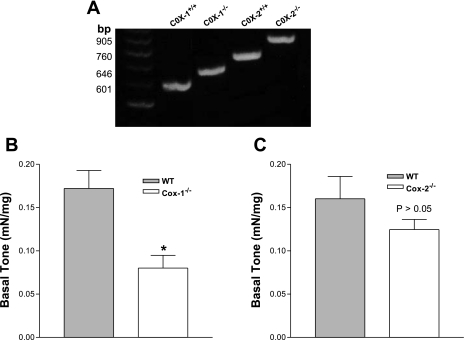
Breeding pairs of mice with targeted disruption of genes encoding COX-1 or COX-2 with a 129/Ola C57BL/6 genetic background, as originally described (22), were kindly donated by Dr. Robert Langenbach (Laboratory of Experimental Carcinogenesis and Mutagenesis, National Institute of Environmental Health Sciences, Research Triangle Park, NC). The mice were housed at the Animal Facility of Thomas Jefferson University and genotyped as described previously (12). Briefly, mouse tail biopsies were collected and identified, and genomic DNA was isolated by precipitation in 70% ethanol, as described previously. The genomic DNA was characterized for the COX-1 genotype by the COX-1−/− forward primer sequence 5′-GCA GCC TCT GTT CCA CAT ACA C-′ the COX-1 wild-type forward primer 5′-AGG AGA TGG CTG CTG AGT TGG-3′, and the reverse primer 5′-AAT CTG ACT TTC TGA GTT GCC-′. DNA was characterized for the COX-2 genotype by the COX-2−/− forward primer 5′-ACG CGT CAC CTT AAT ATG CG-′, the COX-2 wild-type forward primer 5′-ACA CAC TCT ATC ACT GGC ACC-′, and the reverse primer 5′-ATC CCT TCA CTA AAT GCC CTC-′. The thermal cycler was programmed for 1 cycle at 94°C for 1 min and 30 cycles at 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s and was then held at 4°C. The COX-1 wild-type band was 601 bp. The COX-1−/− band was 646 bp. The COX-2 wild-type band was 760 bp. The COX-2−/− band was 905 bp.
Comparison of basal tone in IAS from knockout mice.
To further evaluate the role of COX isoforms in IAS tone, isometric force measurements were obtained from IAS strips isolated from the COX-1−/− and COX-2−/− mice and their wild-type counterparts. Data were collected as described above before and after cumulative concentrations of SC-560 or rofecoxib (both at 0.01–10 μM).
Drugs and antibodies.
Rofecoxib was obtained from Fisher Scientific (Pittsburg, PA), indomethacin and SC-560 from Sigma-Aldrich (St. Louis, MO), and AL-8810 and SQ-29598 from Cayman Chemical (Ann Arbor, MI). The antibody for α-actin was obtained from Sigma (St. Louis, MO), and all other antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Data analysis.
Values are means ± SE. Concentration-response curves were analyzed using a nonlinear interactive fitting program (Prism 3.0, Graph Pad Software). Inhibitor potencies and maximum inhibition are expressed as pIC50 (the negative logarithm of the molar concentration of inhibitor producing 50% of the maximum inhibition) and Imax (maximum inhibition elicited by the inhibitor), respectively. Statistical significance was tested by one-way ANOVA followed by Dunnett's post hoc test when three or more different groups were compared. Student's t-test was used to compare only two different groups. P < 0.05 was considered statistically significant.
RESULTS
Effects of indomethacin on basal tone in the IAS.
The nonselective COX inhibitor indomethacin produced a concentration-dependent decrease in basal tone in the IAS, with Imax of 71.5 ± 5.2% and pIC50 of 5.2 ± 0.1 (n = 9). The vehicle (Na2CO3) solution did not produce a significant (P > 0.05) effect (Fig. 1A). An actual trace of the effect of indomethacin on the basal tone in the IAS is shown in Fig. 1B. Data suggest a significant contribution by COX to IAS tone.
Fig. 1.
A: indomethacin [a nonselective cyclooxygenase (COX) inhibitor] produces a concentration-dependent and significant decrease in internal anal sphincter (IAS) tone in rats; solvent vehicle (Na2CO3) has no significant effect (P > 0.05). Values are means ± SE (n = 9). *P < 0.05. B: an actual tracing of the effect of 10 μM indomethacin.
To examine the specific nature of the COX involved in IAS tone, experiments with selective inhibitors were designed to evaluate the relative contribution of COX-1 and COX-2. COX-1 and COX-2 inhibitors, SC-560 and rofecoxib, respectively, produced significant decreases in basal tone in rat IAS (P < 0.05, n = 5). However, SC-560 was significantly (P < 0.05, n = 5; Fig. 2) more efficacious and potent (Imax = 29.9 ± 5.7% and pIC50 = 6.7 ± 0.1, n = 5) than rofecoxib (Imax = 13.5 ± 5.7% and pIC50 = 5.0 ± 0.1, n = 4). These data suggest that COX-1 is the main isoform responsible for maintenance of basal tone in the IAS.
Fig. 2.
Effects of COX-1 and COX-2 inhibitors (SC-560 and rofecoxib, respectively) on basal tone in rat IAS. Both inhibitors significantly decrease IAS tone (*P < 0.05). However, SC-560 is more potent than rofecoxib (#P < 0.05). Values are means ± SE (n = 5).
RT-PCR.
We compared the relative levels of COX-1 and COX-2 in RNA extracts from rat IAS and RSM. The IAS expressed higher levels of COX-1 and COX-2 than the RSM (P < 0.05, n = 5; Fig. 3, A and B).
Fig. 3.
A and B: RT-PCR shows significantly higher levels of COX-1 and COX-2 in IAS than in rectal smooth muscle (RSM) from rat. Data were normalized to β-actin levels. C and D: Western blot analyses show higher expression of COX-1 in IAS than RSM. Data were normalized to α-actin levels. Values are means ± SE. *P < 0.05.
Western blots.
We also evaluated the presence of COX-1 and COX-2 in the protein extracts obtained from IAS and RSM samples. On the basis of calculations normalized to α-actin levels, significantly higher levels of COX-1 were expressed in the IAS than in the RSM (P < 0.05, n = 5; Fig. 3C). However, differences in COX-2 levels between the IAS and RSM, normalized to α-actin expression, were not statistically significant (P > 0.05, n = 5; Fig. 3D).
Comparison of basal tone in the IAS from knockout mice.
COX-1−/− and COX-2−/− mice were identified by genotyping (Fig. 4A), and differences in tone development by IAS smooth muscle strips isolated from COX-1−/− and COX-2−/− mice was compared with that from their wild-type counterparts. Data show significantly less spontaneous tone developed by IAS smooth muscle from COX-1−/− mice than by the control group (0.08 ± 0.02 vs. 0.17 ± 0.02 mN/mg, P < 0.05, n = 5; Fig. 4B). Conversely, COX-2 gene deletion did not significantly affect basal tone (P > 0.05, n = 5; Fig. 4C).
Fig. 4.
A: RT-PCR products of genomic DNA from COX-1- and COX-2-knockout (COX-1−/− and COX-2−/−) and wild-type [WT (COX-1+/+ and COX-2+/+)] mice. B: IAS from COX-1−/− mice developed significantly lower basal tone than IAS from WT mice. C: there were no significant differences in IAS tone between WT and COX-2−/− mice (P > 0.05). Values are means ± SE (n = 5). *P < 0.05.
Effects of selective inhibitors of COX-1 (SC-560) and COX-2 (rofecoxib) on basal tone in the IAS of wild-type vs. COX-1−/− and COX-2−/− mice.
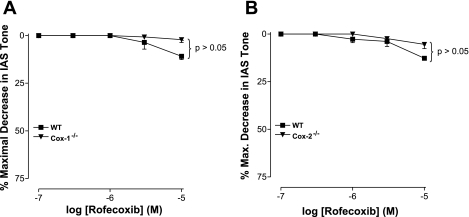
The purpose of these experiments was to compare the effects of COX-1- and COX-2-selective inhibitors and to cross-examine the effect of selective deletions of COX-1 and COX-2 genes in the mice on basal tone in the IAS. SC-560 and rofecoxib data from the wild-type mice confirm the significantly higher contribution of COX-1 than COX-2 to tone in murine IAS. SC-560 was significantly more potent than rofecoxib in decreasing IAS tone (P < 0.05, n = 4; Figs. 5 and 6).
Fig. 5.
A: SC-560 causes significantly more efficacious, potent, and concentration-dependent decrease in IAS tone in WT than COX-1−/− mice. B: SC-560 causes significant and concentration-dependent decreases in IAS tone not only in the WT, but also in COX-2−/−, mice. Values are means ± SE (n = 4). *P < 0.05.
Fig. 6.
COX-2-selective inhibitor rofecoxib causes no significant (P > 0.05, n = 4) decrease in IAS tone of COX-1−/− (A), COX-2−/− (B), or their respective WT mice. Higher concentrations of rofecoxib (not shown) may, however, cause a modest decrease in IAS tone. Potency comparisons reveal that the COX-2 inhibitor is significantly less efficacious and potent than the COX-1 inhibitor (see Table 2). Values are means ± SE (n = 4).
In the wild-type mice for COX-1, the COX-1 inhibitor SC-560 (1 × 10−5 M) produced a significant decrease in IAS tone (41.4 ± 3.4%, P < 0.05, n = 4; Fig. 5A). In COX-1−/− mice, the same concentration of SC-560 produced no significant decrease (5.5 ± 3.9%, P > 0.05; Fig. 5A). These data show that SC-560 was significantly less efficacious and potent (P < 0.05) in the COX-1−/− than in the wild-type mice. These findings further authenticate the selective deletion of the COX-1 gene in these mice. Interestingly, the SC-560-mediated decrease in IAS tone was similar and significant in the COX-2−/− mice, as well as in their wild-type counterparts (P < 0.05, n = 4; Fig. 5B).
In contrast to the prominent effects of the COX-1 inhibitor, the COX-2 inhibitor rofecoxib produced insignificant effects in IAS tone of COX-1−/− and COX-2−/− mice, as well as their wild-type counterparts (P > 0.05, n = 4; Fig. 6). Quantitative data in Table 2 show lower potency of the COX-2 than the COX-1 inhibitor in COX-1−/− and COX-2−/− mice and their wild-type counterparts. These data further demonstrate the distinct importance of COX-1 vs. COX-2 in basal tone in the IAS.
Table 2.
Effects of the COX-1 inhibitor SC-560 and COX-2 inhibitor rofecoxib on basal tone of IAS from WT, COX-1−/−, and COX-2−/− mice
| pIC50 | |
|---|---|
| SC-560 | |
| WT | 6.2±0.1 |
| COX-1−/− | 3.5±0.2* |
| WT | 6.4±0.1 |
| COX-2−/− | 5.6±0.1* |
| Rofecoxib | |
| WT | 4.9±0.03 |
| COX-1−/− | 4.5±0.02* |
| WT | 4.9±0.03 |
| COX-2−/− | 4.3±0.04* |
Values are means ± SE (n = 4). WT, wild-type mice; COX-1−/− and COX-2−/−, COX-1- and COX-2-knockout mice; pIC50, negative logarithm of concentration of inhibitor that causes 50% of maximal decrease in internal anal sphincter (IAS) tone.
P < 0.05 vs. group-specific WT (by unpaired Student's t-test).
Effects of selective inhibitor of PGF2α (AL-8810) and TxA2 (SQ-29598) receptors on IAS tone.
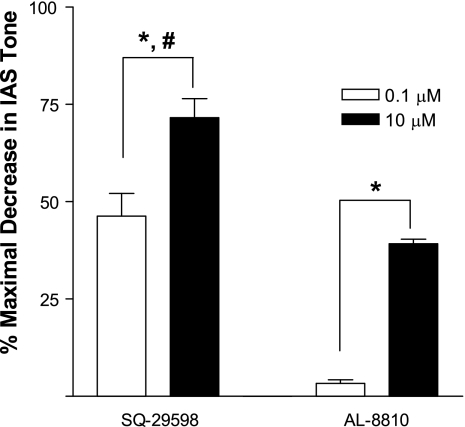
The PGF2α and TxA2 receptor-selective inhibitors AL-8810 and SQ-29598, respectively, produced a significant concentration-dependent decrease in IAS tone (P < 0.05, n = 4). SQ-29598 (71.6 ± 9.8%, n = 4) was significantly more efficacious (P < 0.05) than AL-8810 (39.2 ± 2.3%, n = 4; Fig. 7). These data suggest a more important role for TxA2 than PGF2α in the IAS.
Fig. 7.
Inhibitors of thromboxane A2 and PGF2α receptors, SQ-29598 and AL-8810, respectively, produce significant concentration-dependent decrease in basal tone in rat IAS (*P < 0.05). SQ-29598 is more potent than AL-8810 (#P < 0.05). Values are means ± SE (n = 4).
DISCUSSION
Previous studies from our laboratory showed that sPLA2, which converts membrane phospholipids to AA within the IAS, contracts the smooth muscle cells of the IAS, resulting in tone development (16). In the present studies, we show that COX inhibition significantly decreases IAS tone. Using COX-1−/− and COX-2−/− mice combined with the application of selective inhibitors, we further demonstrate, for the first time, that COX-1 contributes significantly to basal tone in the IAS.
The present studies were carried out in rats and mice. The IAS of rats and mice has characteristics similar to the IAS of humans: it develops spontaneous tone and relaxes in response to nonadrenergic noncholinergic nerve stimulation (7, 29, 37, 38). Our earlier studies suggested that RhoA/ROCK provides the molecular basis for the bulk of the tone in the IAS (28, 30). However, the external trigger(s) that excites the molecular machinery is not exactly known. In earlier studies, we provided evidence for the involvement of a local RAS in the partial (∼25%) maintenance of basal tone in rat IAS in vitro (13, 14) and in vivo (15). These data suggest the involvement of other mechanisms.
Using multipronged approaches of functional, molecular biology and genetically modified animals, we evaluated the relative contribution of COXs to basal tone in the IAS. We have shown that nonselective inhibition of COXs by indomethacin and selective inhibition by the COX-1 inhibitor SC-560 cause a significant decrease in basal tone in rat IAS. On the other hand, the selective inhibitor of COX-2 does not significantly decrease basal tone, except at the higher concentrations. Similar data obtained from study of wild-type mice show that SC-560 significantly decreases IAS tone.
Data obtained from COX-1−/− mice support the above-stated hypothesis that COX-1, rather than COX-2, plays a major role in regulation of basal tone in the IAS. IAS smooth muscle from COX-1−/− mice developed significantly less basal tone than IAS smooth muscle from wild-type mice: 0.17 ± 0.02 vs. 0.08 ± 0.01 mN/mg, which represents a 53% decrease in basal tone in the COX-1−/− mice. This decrease is similar to that produced by the COX-1-selective inhibitor in the wild-type mice. On the contrary, the changes in the basal tone of the mice were not significantly different from controls. In addition, rofecoxib has no significant effect on IAS tone in the COX-2−/− or wild-type mice.
In rat IAS, the maximal inhibitory effects of the dual COX inhibitor indomethacin (71.4 ± 5.1%) were significantly greater than those of the COX-1-selective inhibitor alone (30 ± 5.6%). One explanation for this finding might be a shared, nonselective inhibitory effect of indomethacin and rofecoxib on basal tone (1); another might be lower efficacy and potency of the COX-1-selective inhibitor SC-560 in rat IAS.
The maximal inhibitory effect of indomethacin observed in the present studies in rat IAS is comparable to that induced by the sPLA2-selective inhibitor MJ-33 (66.7%) (16). These data support the notion that basal IAS tone is primarily controlled by a cascade of reactions that modulate the conversion of membrane phospholipids to contractile molecules via sPLA2 and COX-1 activities, respectively.
RT-PCR and Western blot data confirmed the presence of COX-1, as well as COX-2, in the anorectal region. Translational and transcriptional studies reveal the presence of higher levels of COX-1 in the IAS than RSM. On the basis of functional data, the significance of higher levels of COX-1 in the IAS is self-explanatory.
Collectively, these data suggest that the COX-1 pathway (by the production of smooth muscle contractile prostanoids, such as PGF2α and thromboxanes) contributes significantly to basal tone in the IAS. This hypothesis was further validated by the effects of the PGF2α and TxA2 receptor-selective antagonists AL-8810 and SQ-29598, respectively, on IAS tone. These antagonists caused a significant concentration-dependent decrease in IAS tone. SQ-29598 is more potent than AL-8810 (71.6 ± 9.8% vs. 39.2 ± 2.3%, n = 4, P < 0.05), suggesting a more important role of TxA2 than PGF2α in the IAS. Earlier studies showed that PGF2α and thromboxanes significantly increase basal tone in LES smooth muscle (5, 9, 31). Consistent with these findings, the COX-1-selective inhibitors and COX-1 small interfering RNA significantly decrease the tone of the LES and gallbladder (4, 5, 9). In the present studies, we did not measure the local levels of PGF2α, TxA2, or other prostanoids in the IAS. The existing evidence, however, supports our hypothesis that these prostanoids, produced via COX-1, provide an important external trigger for basal tone in the IAS.
Furthermore, it is possible that COX-1 products, along with ANG II, provide a bulk of the signal for IAS tone. We speculate that COX-1 and RAS products may work in concert by activation of RhoA/ROCK and PKC, in definite proportions, to account for the molecular mechanisms for the spontaneous basal tone. RhoA/ROCK and PKC have been suggested to play a major role in the sustained contraction and basal tone of different smooth muscle tissues (2, 19, 27, 30). However, the specific role of RhoA/ROCK and PKC in the COX-1-sustained tone in the IAS remains to be determined.
In summary, the present data suggest that the COX-1 pathway plays a significant role in the maintenance of basal tone in IAS smooth muscle. However, studies of the specific effects of COX-1 products and their specific inhibitors are needed. The present study has important implications for the therapeutic rationale for COX-1-selective inhibitors in the hypertensive IAS associated with anorectal motility disorders.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-35385 and an institutional grant from Thomas Jefferson University.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Andrade EL, Ferreira J, André E, Calixto JB. Contractile mechanism coupled to TRPA1 receptor activation in rat urinary bladder. Biochem Pharmacol 72: 104–114, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Arnt-Ramos LR, O'Brien WE, Vincent SR. Immunohistochemical localization of argininosuccinate synthetase in the rat brain in relation to nitric oxide synthase-containing neurons. Neuroscience 51: 773–789, 1992. [DOI] [PubMed] [Google Scholar]
- 3.Brannon TS, North AJ, Wells LB, Shaul PW. Prostacyclin synthesis in ovine pulmonary artery is developmentally regulated by changes in cyclooxygenase-1 gene expression. J Clin Invest 93: 2230–2235, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao W, Chen Q, Sohn UD, Kim N, Kirber MT, Harnett KM, Behar J, Biancani P. Ca2+-induced contraction of cat esophageal circular smooth muscle cells. Am J Physiol Cell Physiol 280: C980–C992, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Cao W, Harnett KM, Behar J, Biancani P. PGF2α-induced contraction of cat esophageal and lower esophageal sphincter circular smooth muscle. Am J Physiol Gastrointest Liver Physiol 283: G282–G291, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Casselbrant C, Edebo A, Wennerblom J, Lönroth H, Helander HF, Veith M, Lundell L, Fändriks L. Actions by angiotensin II on esophageal contractility in humans. Gastroenterology 132: 249–260, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Chakder S, McHugh KM, Rattan S. Inhibitory neurotransmission in lethal spotted mutant mice: a model for Hirschsprung's disease. Gastroenterology 112: 1575–1585, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Chan CC, Boyce C, Brideau S, Charleson W, et al. Rofecoxib [Vioxx, MK-0966; 4-(4′-methylsulfonylphenyl)-3-phenyl-2-(5H)-furanone]: a potent and orally active cyclooxygenase-2 inhibitor. J Pharmacol Exp Ther 290: 551–560, 1999. [PubMed] [Google Scholar]
- 9.Cheng L, Cao W, Behar J, Biancani P, Harnett KM. Inflammation-induced changes in arachidonic acid metabolism in cat LES circular muscle. Am J Physiol Gastrointest Liver Physiol 288: G787–G797, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Cheng Y Role of prostacyclin in the cardiovascular response to thromboxane. Science 296: 539–542, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987. [DOI] [PubMed] [Google Scholar]
- 12.Darling RL, Romero JJ, Dial EJ, Akunda JK, Langenbach R, Lichtenberger LM. The effects of aspirin on gastric mucosal integrity, surface hydrophobicity, and prostaglandin metabolism in cyclooxygenase knockout mice. Gastroenterology 127: 94–104, 2004. [DOI] [PubMed] [Google Scholar]
- 13.De Godoy MAF, Dunn SR, Rattan S. Evidence for the role of angiotensin II biosynthesis in the rat internal anal sphincter tone. Gastroenterology 127: 127–138, 2004. [DOI] [PubMed] [Google Scholar]
- 14.De Godoy MAF, Rattan S. Autocrine regulation of internal anal sphincter tone by renin-angiotensin system: comparison with phasic smooth muscle. Am J Physiol Gastrointest Liver Physiol 289: G1164–G1175, 2005. [DOI] [PubMed] [Google Scholar]
- 15.De Godoy MAF, Rattan S. Angiotensin-converting enzyme and angiotensin II receptor subtype 1 inhibitors restitute hypertensive internal anal sphincter in the spontaneously hypertensive rats. J Pharmacol Exp Ther 318: 725–734, 2006. [DOI] [PubMed] [Google Scholar]
- 16.De Godoy MAF, Rattan S. Role of phospholipase A2 (group I secreted) in the genesis of basal tone in the internal anal sphincter smooth muscle. Am J Physiol Gastrointest Liver Physiol 293: G979–G986, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Fornai M, Blandizzi C, Colucci L, Antonioli L, Bernardini N, Seganani C, Baragatti B, Barogi S, Berti P, Spisni R, Del Tacca M. Role of cyclooxygenases 1 and 2 in the modulation of neuromuscular functions in the distal colon of humans and mice. Gut 54: 608–616, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gudis K, Sakamoto C. The role of cyclooxygenase in gastric mucosal protection. Dig Dis Sci 50: S16–S23, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Harnett KM, Cao W, Biancani P. Signal-transduction pathways that regulate smooth muscle function. Signal transduction in phasic (esophageal) and tonic (gastroesophageal sphincter) smooth muscles. Am J Physiol Gastrointest Liver Physiol 288: G407–G416, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Jones DA, Carlton DP, McIntyre TM, Zimmerman, GA, Prescott SM. Molecular cloning of human prostaglandins endoperoxide synthase type II and demonstration of expression in response to cytokines. J Biol Chem 12: 9049–9054, 1993. [PubMed] [Google Scholar]
- 21.Kujubu DA, Reddy ST, Fletcher BS, Hershchman HR. Expression of the protein product of the prostaglandin synthase-2/TIS10 gene in mutagen-stimulated Swiss 3T3 cells. J Biol Chem 268: 5425–5430, 1993. [PubMed] [Google Scholar]
- 22.Langenbach R, Morham SG, Tiano HF, Loftin CD, Ghanayem BI, Chulada PC, Mahler JF, Lee CA, Goulding EH, Kluckman KD, Kim HS, Smithies O. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell 83: 483–492, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Lennard-Jones JE Constipation. In: Sleisenger & Fordtran's Gastrointestinal and Liver Disease, edited by Feldman M. Philadelphia, PA: Saunders, 2002, p. 181–210.
- 24.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951. [PubMed] [Google Scholar]
- 25.Madoff RD, Fleshman JW. American Gastroenterological Association technical review on the diagnosis and treatment of hemorrhoids. Gastroenterology 126: 1463–1473, 2004. [DOI] [PubMed] [Google Scholar]
- 26.McCallion K, Gardiner KR. Progress in the understanding and treatment of chronic anal fissure. Postgrad Med J 77: 753–758, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murthy KS Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol 68: 345–374, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Patel CA, Rattan S. Cellular regulation of basal tone in internal anal sphincter smooth muscle by RhoA/ROCK. Am J Physiol Gastrointest Liver Physiol 292: G1747–G1756, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Rattan S The internal anal sphincter: regulation of smooth muscle tone and relaxation. Neurogastroenterol Motil 17: 50–59, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Rattan S, De Godoy MAF, Patel CA. Rho kinase as a novel molecular therapeutic target for hypertensive internal anal sphincter. Gastroenterology 131: 108–116, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Rattan S, Hersh T, Goyal RK. Effect of prostaglandin F2α and gastrin pentapeptide on the lower esophageal sphincter. Proc Soc Exp Biol Med 41: 573–575, 1972. [DOI] [PubMed] [Google Scholar]
- 32.Schiller LR Fecal incontinence. In: Sleisenger & Fordtran's Gastrointestinal and Liver Disease, edited by Feldman M. Philadelphia, PA: Saunders, 2002, p. 164–174.
- 33.Smith CJ, Zhang Y, Koboldt CM, Muhammad J, Zweifel BS, Shaffer A, Talley JJ, Masferrer JL, Seibert K, Isakson PC. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc Natl Acad Sci USA 95: 13313–13318, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith WL The eicosanoids and their biochemical mechanisms of action. Biochem J 259: 315–324, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smythe EM, Burke A, FitzGerald GA. Lipid-derived autacoids: eicosanoids and platelet-activating factor. In: Goodman & Gilman's The Pharmacological Basis of Therapeutics, edited by Brunton LL, Lazo JS, Parker KL. New York: McGraw-Hill, 2005, p. 653–670.
- 36.Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol Sci 24: 96–102, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Terauchi A, Kobayashi D, Mashimo H. Distinct roles of constitutive nitric oxide synthases and interstitial cells of Cajal in rectoanal relaxation. Am J Physiol Gastrointest Liver Physiol 289: G291–G299, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Vinograd I, Hanani M, Hadary A, Merguerian P, Nissan S. Animal model for the study of internal anal sphincter activity. Eur Surg Res 17: 259–263, 1985. [DOI] [PubMed] [Google Scholar]
- 39.Wang D, Mann JR, DuBois RN. The role of prostaglandins and other eicosanoids in the gastrointestinal tract. Gastroenterology 128: 1445–1461, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Warner TD, Giuliano F, Vojnovic I, Bukasa A, Mitchell JA, Vane JR. Nonsteroidal drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci USA 96: 7563–7568, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]