Abstract
ATP7B is a copper-transporting P-type ATPase present predominantly in liver. In basal copper, hepatic ATP7B is in a post-trans-Golgi network (TGN) compartment where it loads cytoplasmic Cu(I) onto newly synthesized ceruloplasmin. When copper levels rise, the protein redistributes via unique vesicles to the apical periphery where it exports intracellular Cu(I) into bile. We want to understand the mechanisms regulating the copper-sensitive trafficking of ATP7B. Earlier, our laboratory reported the presence of apical targeting/TGN retention information within residues 1–63 of human ATP7B; deletion of these residues resulted in a mutant protein that was not efficiently retained in the post-TGN in low copper and constitutively trafficked to the basolateral membrane of polarized, hepatic WIF-B cells with and without copper (13). In this study, we used mutagenesis and adenovirus infection of WIF-B cells followed by confocal immunofluorescence microscopy analysis to identify the precise retention/targeting sequences in the context of full-length ATP7B. We also analyzed the expression of selected mutants in livers of copper-deficient and -loaded mice. Our combined results clearly demonstrate that nine amino acids, F37AFDNVGYE45, comprise an essential apical targeting determinant for ATP7B in elevated copper and participate in the TGN retention of the protein under low-copper conditions. The signal is novel, does not require phosphorylation, and is highly conserved in ∼24 species of ATP7B. Furthermore, N41S, which is part of the signal we identified, is the first and only Wilson disease-causing missense mutation in residues 1–63 of ATP7B. Expression of N41S-ATP7B in WIF-B cells severely disabled the targeting and retention of the protein. We present a working model of how this physiologically relevant signal might work.
Keywords: Wilson protein, WIF-B cells, mutagenesis, in vivo, trans-Golgi network
atp7b is a copper-transporting P-type ATPase that is present predominantly in liver. A second copper-transporting P-type ATPase, ATP7A, is expressed more widely throughout the body. Both proteins play essential roles in copper homeostasis in humans as evidenced by Menkes and Wilson diseases, which are caused by mutations in the genes encoding ATP7A and ATP7B, respectively (23, 32). The ∼165-kDa proteins are ∼60% identical, and each consists of a ∼650-amino acid cytoplasmic NH2 terminus containing six metal-binding domains (MBDs), followed by eight transmembrane domains (TMD) containing two large cytoplasmic loops that harbor the signature motifs of heavy metal P-type ATPases and an 80–90-amino acid cytoplasmic COOH terminus.
The Cu-ATPases transport Cu(I) across biological membranes using the energy from ATP hydrolysis. They carry out this function in different intracellular locations. Both transport copper into the late secretory pathway of cells to metallate newly synthesized apoproteins, for example, haephestin in intestinal cells (by ATP7A) and ceruloplasmin in hepatocytes (by ATP7B) (Refs. 28 and 40, respectively). This activity takes place in a post-trans-Golgi network (TGN) compartment (29, 46; reviewed in Ref. 38). Both proteins also export copper using mechanisms that involve their exit from the TGN in vesicles and movement to the cell periphery (reviewed in Refs. 19 and 22).
The human diseases point to an essential difference between the two Cu-ATPases (reviewed in Ref. 21). In Menkes disease, dietary copper accumulates in the small intestine but fails to be released into the circulation, leading to copper deficiency throughout the body. This phenotype indicates that the export function of ATP7A occurs at/near the basolateral plasma membrane of polarized intestinal cells. In contrast, Wilson disease is characterized by accumulation of copper in the liver hepatocytes and failure to excrete the excess copper into bile, leading to copper toxicity. This phenotype places the export activity of ATP7B at/near the hepatocyte apical plasma membrane. Thus the vesicular carriers of each Cu-ATPase move to opposite poles of intestinal and hepatic epithelial cells.
The signals on each Cu-ATPase that regulate its movements are just being uncovered (reviewed in Refs. 19 and 22). In our initial study, we reported the presence of apical targeting information in the NH2 terminus of ATP7B; the first 63 amino acids plus MBDs 5 and 6 were sufficient to direct ATP7B to the apical region in a copper-dependent manner (13). Human ATP7A lacks the 63-amino acid sequence. In the present study, we have further dissected this signal in the context of full-length ATP7B using a mutagenesis approach. Our results demonstrate that nine amino acids, F37AFDNVGYE45, comprise an essential apical targeting determinant for ATP7B in elevated copper conditions and participate in the TGN retention of the protein under low copper conditions.
MATERIALS AND METHODS
Generation of ATP7B Mutations
The ATP7B constructs used in this study are listed in the Supplementary Table S1. (Supplemental data for this article is available online at the American Journal of Physiology Gastrointestinal and Liver Physiology website.) Generation of full-length green fluorescent protein wild-type (GFP-wt)ATP7B (cataloging designation, pYG7), which contains GFP at the NH2 terminus, was described previously (13). The QuickChange II XL Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA) was used with pYG7 as a template to create plasmids designated pYGs 63, 70, 75, 76, 83, 84, 87, 91, and 92 as well as those designated pLBs 1033, 1037, 1039, 1041, 1044, and 1052. Untagged ATP7B (pYG80) and N41S ATP7B (pYG82) were generated from pYG7 and pYG70, respectively, by digesting the plasmid DNAs first with SphI then with BsrGI (partial digest), filling in and religating the cDNAs. Untagged Δ32-ATP7B (pLB1064) and Δ32Y44A-ATP7B (pLB1067) were generated from pYG80 and pLB1064, respectively, using QuickChange as above.
All primers were from Integrated DNA Technologies (Coralville, IA); their sequences are available upon request. Sequences of all mutated regions in each construct were verified. Selected constructs (pYG7, pYG91, pLB1033, pLB1039, and pLB1064) were sequenced in their entirety by the Johns Hopkins University DNA Sequencing Facility. All constructs were packaged into adenoviruses and purified as described (5).
Cell Culture
Immortalized derivatives of SV40-transformed Menkes-null (MNK y/-) fibroblasts (13) were generated by puromycin selection (0.5 μg/ml) following transfection of pBabePuro/hTERT vector (14), which constitutively expresses human telomerase. The cell line was designated YST.
WIF-B cells were seeded at 2 × 104 cells/cm2 on glass coverslips (22 × 22 mm), cultured as described (7, 18), and used ∼11–12 days later when maximal polarity had been achieved.
Adenoviral Infection and Copper Treatments
In vitro.
WIF-B cells were infected (1 h with ∼1–3 × 108 virus particles/coverslip) and then cultured ∼16 h in basal medium [0.02 μM Cu(II)] containing 10 μM bathocuproine disulfonic acid (BCS) (Sigma, St. Louis, MO) to chelate copper. In most experiments, infected cells were treated with basal medium plus 10 μM CuCl2 for 4 h before fixation. In some experiments, the cells were incubated in copper ranging from 1–100 μM for 4 h before fixation. Each construct was tested at least twice.
In vivo.
We modified a published rat protocol (33) to obtain copper-deficient mice. Pregnant dams (Charles River Breeding Labs, Wilmington, MA) were fed a copper-deficient diet (no. TD.80388; Harlan Tekland, Madison, WI) from P15 until weaning (∼21 days postparturition). The weanlings were kept on the same diet until they were used. Copper loading was achieved by gastric intubation of freshly prepared 4 mM CuSO4 diluted in 10 mM HCl (0.1 ml/10 g animal weight) twice daily. Typically, mice were given two doses of copper before and two to three doses after adenoviral infection.
Copper-deprived or -loaded mice were infected via retro-orbital or tail vein injection (1–5 × 1010 virus particles) and then maintained on the same copper regimen for 16–72 h, when they were lightly anesthetized and decapitated. Animal handling and experimental procedures were approved by the Institutional Animal Care and Use Committee.
Antibodies and Immunofluorescence
Primary antibodies were obtained from the following sources: rabbit anti-GFP (Molecular Probes, Eugene, OR); mouse anti-TGN38 (BD Biosciences, San Jose, CA); and rabbit anti-TGN-38 (B. Eipper, U. Connecticut Health Center, Farmington, CT). Rabbit anti-aminopeptidase N (APN, #1637) was described previously (3). Guinea pig anti-dipeptidyl peptidase 4 (DPP4, #200) and anti-CE9 (#3) were generated against rat liver plasma membrane DPP4 and CE9, respectively, which were purified by immunoaffinity chromatography using mouse monoclonal antibodies (15) coupled to Sepharose, followed by elution at low pH, neutralization, application to wheat germ agglutinin-Sepharose, and elution in N-acetyl glucosamine. Rabbit anti-ATP7B (no. 3985) was generated by Covance Research, PA, against the NH2-terminal 653 residues of human ATP7B expressed as a GST-fusion protein in pGEX-6P-2. PreScission Protease (Amersham Pharmacia Biotech, Piscataway, NJ) was used to cleave GST from the recombinant peptide before immunization. Its specificity is documented in Supplemental Fig. S1. Secondary antibodies (used at 1.5–4 μg/ml) conjugated to Cy3 or Cy5 were from Jackson ImmunoResearch Laboratories (West Grove, PA). Hoechst-33258 dye was from Sigma-Aldrich.
Cells.
Infected cells were fixed and further processed for indirect immunofluorescence according to previously published methods (17). Labeled cells were analyzed using a 63× PLAN-APO, 1.4 NA oil immersion objective on an LSM 510 META confocal microscope (Zeiss, Germany). We focused on cells that expressed low levels of exogenous protein (∼3× higher than endogenous ATP7B, as determined in Ref. 13) and then assessed the distribution of the GFP protein relative to those of the two organelle markers, TGN38 and APN. Experiments were repeated two or more times and images of 8–20 cells evaluated independently by at least two experienced individuals.
Tissue.
Livers were excised, rinsed in cold 0.9% saline, cut into 5 × 20 mm blocks, and fixed by immersion (1 h, room temperature) in freshly prepared 2% paraformaldehyde-lysine-periodate (26). Subsequent processing of the tissue and sections has been described (29). Labeled sections were analyzed by confocal microscopy (see above).
Additional Methods
Tyrosinase activation assay.
Each ATP7B construct was evaluated for Cu(I) transport activity in Menkes-null fibroblasts as described (13). For the present study, the YST cell line was transfected with pcTYR encoding human tyrosinase in pcDNA 3.1 A (-) myc/His (from Dr. D. Hebert, University of Massachusetts). G418 (200 μg/ml) and puromycin- (0.5 μg/ml) resistant cells (designated YSTT) grown on glass coverslips were further cotransfected with 1 μg each pcTYR and an ATP7B construct using Lipofectamine (Invitrogen, Carlsbad, CA). Eighteen to twenty-four hours later, cells were fixed and incubated in 0.2–0.4 mg/ml levo-3,4-dihydroxy-L-phenylalanine as described (41) and then evaluated by phase and fluorescence microscopy on a Zeiss Axiovert.
Western blotting.
Each ATP7B construct was evaluated for protein expression. YSTT or WIF-B cells were infected with recombinant adenovirus and solubilized 2 days later as described (13), and samples were analyzed by SDS-PAGE and immunoblotting with anti-ATP7B and anti-GFP (13).
RESULTS
Copper Transport Activity of ATP7B Mutant Proteins in MNKy/− Fibroblasts and Their Expression in WIF-B Cells
Our goal was to identify the sequence in the NH2 terminus of ATP7B that targeted the protein to its correct destination in a copper-dependent fashion. To accomplish this, we generated 18 human ATP7B constructs having mutations of selected residues in the first 63 amino acids (Supplemental Table S1). Each construct was assayed for its ability to metallate apotyrosinase in MNK y/- fibroblasts, which lack ATP7A and ATP7B (13). All mutant proteins were able to load Cu(I) onto apotyrosinase (Supplemental Table S1).
We also assessed the protein expression of each ATP7B construct using SDS-PAGE and Western blot analysis of lysates from infected YSTT cells. All GFP proteins with single point mutations migrated at the same mobility as GFP-wtATP7B, indicating that they were intact (data not shown; see Ref. 13). The four untagged ATP7B constructs migrated at their expected sizes.
GFP-wtATP7B Traffics in Response to Physiological Copper Levels
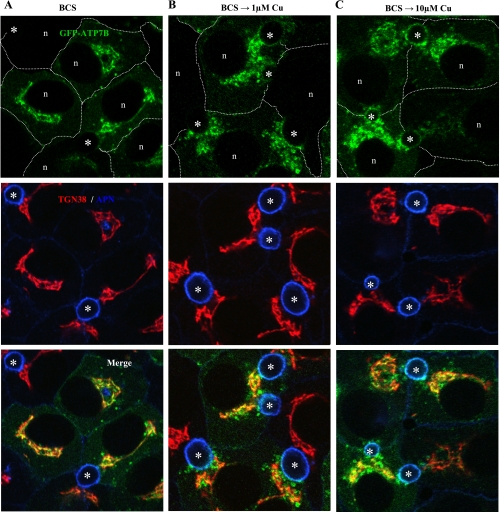
In our previous report, we chose conditions of copper depletion (200 μM BCS) and loading (200 μM CuCl2) that were designed to obtain maximal responses of ATP7B and had been used by others (e.g., Refs. 27, 37). However, human serum copper levels are much lower (<25 μM; 6), prompting us to reassess the behavior of exogenous ATP7B under physiological copper conditions. We first cultured GFP-wtATP7B-infected cells in 10 μM BCS overnight to deplete intracellular copper levels, a condition that staged the protein in the TGN, as evidenced by its close association with a trans-Golgi marker, TGN38 (Fig. 1A). After the cells were thoroughly rinsed, we incubated cells with 1, 10, 20, 50, and 100 μM CuCl2 for 4 h. By immunofluorescence, GFP-wtATP7B showed redistribution into small vesicles in 1 μM copper (Fig. 1B). At this copper concentration, there was moderate overlap with the apical resident protein, APN (Fig. 1B). Exposure of infected cells to higher copper levels (e.g., 10 μM for 4 h) resulted in considerable overlap of GFP-ATP7B with APN (Fig. 1C). We chose to use 10 μM copper in all subsequent WIF-B trafficking assays because it is within the physiological range and the apical labeling pattern1 gave a clear measure of wild-type targeting by ATP7B constructs.
Fig. 1.
Exogenous green fluorescent protein wild-type (GFP-wt)ATP7B redistributes in response to physiological levels of CuCl2. Polarized WIF-B cells were infected with the GFP-wtATP7B, cultured overnight in 10 μM bathocuproine disulfonic acid (BCS), then kept in BCS (A) or incubated in 1 μM (B) or 10 μM CuCl2 (C) for 4 h, fixed, stained with antibodies to the indicated organelle markers, and imaged by confocal microscopy. The basolateral membranes of several cells are approximated by dashed white lines. Note that the extent of the exit of GFP-ATP7B from the trans-Golgi network (TGN) was variable among cells at each copper concentration. TGN38, trans-Golgi marker; APN: aminopeptidase N, apical marker; n, nucleus; asterisks, apical lumen.
Single Substitutions Within a Nine-Amino Acid Stretch Disable the Copper-Sensitive Apical Targeting of GFP-ATP7B and Its Retention in the TGN
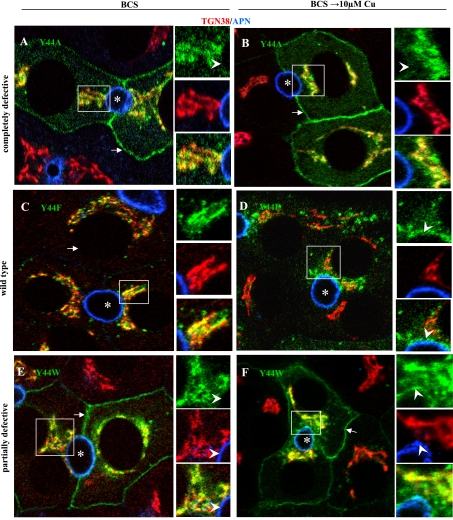
In our previous study, we had determined that the first 63 amino acids of human ATP7B contained copper-sensitive, apical-targeting information. Sequence alignment of this region from five species revealed considerable conservation (Table 1). We focused on a nine-amino acid stretch between F37 and E45 because it contained an NXXY motif (see below). Eight of the nine residues were individually substituted with alanine in the background of full-length GFP-ATP7B and the trafficking behavior of each mutant protein assessed in polarized WIF-B cells. (Note: alanine 38 was not changed.) After infection, the cells were cultured ∼16 h in basal medium containing 10 μM BCS and then left in BCS or incubated with 10 μM CuCl2 for 4 h before fixation. Table 2 summarizes our results, which showed that mutation of each of the eight residues to alanine affected ATP7B trafficking. The example of Y44A is presented in Fig. 2, A and B. This mutant protein was localized at the basolateral membrane and the TGN region under either depleted- or high-copper conditions, a behavior we termed “completely defective” (Table 2). Five of the other seven alanine mutants behaved similarly. Furthermore, there were virtually no vesicles containing a mutant GFP protein in any of these infected cells. That is, the ATP7B proteins were localized in the TGN and the basolateral membrane but nowhere else. Additionally, the proteins were conspicuously absent from the apical region/membrane. Interestingly, F37A and N41A exhibited intermediate trafficking behaviors, as did N41S and Y44W (see below).
Table 1.
Alignment of the NH2 termini of 5 mammalian ATP7B sequences
| human-----------MPEQERQITAREGASRKILSKLSLPTRAWEPAMKKSFAFDNVGYEGGLDGLGPSSQVATSTVR | 63 |
| rat-----------MPEQERKVTAKE-ASRKILSKLALPTRPWGQSMKQSFAFD NVGYEGGLDSTC-SSSTTTGVVS | 62 |
| mouseMDPRKNLASVGTMPEQERQVTAKE-ASRKILSKLALPGRPWEQSMKQSFAFD NVGYEGGLDSTS-SSPAATDVVN | 74 |
| dog----------------------------------------MKQSFAFD NVGYEGGLDSVC-PPQTATSTIS | 31 |
| sheep----------MKPEEERPIIDREKASRRILSKLFQP------AMKQSFAFD NNGYEDDLDGVC-PSQTAAGTIS | 58 |
| ★★★★★★★★ ★★★★★★★★★★★★★★★★★★★★★★ |
Summary of mutagenesis/trafficking results of residues 37–45 in NH2 terminus of ATP7B. Residues that are underlined were chosen for mutagenesis.
Table 2.
Results of trafficking studies of mutant ATP7B proteins in WIF-B cells
|
Copper-Induced Targeting of Mutant ATP7B Proteins | ||
|---|---|---|
| Wild-type |
Defective |
|
| Partially | Completely | |
| F37Y, F37W | F37A | |
| F39Y | F39A, F39W | |
| D40A | ||
| N41S, N41A | ||
| V42I | V42A | |
| G43A | ||
| Y44F | Y44W | Y44A |
| E45A | ||
| Δ32 | ||
| Δ32-Y44A (BCS) | Δ32-Y44A (Cu) | |
Summary of mutagenesis/trafficking results of residues 37–45 in NH2 terminus of ATP7B. Each protein was classified on the basis of its intracellular location in infected WIF-B cells cultured in 10 μ.M bathocuproine disulfonic acid (BCS) or 10 μ.M copper (4 h). Wild-type refers to trans-Golgi network (TGN) in BCS and apical/vesicles in copper (see Fig. 1, Fig. 2, C and D, and Fig. 4, A and B); partially defective refers to TGN plus basolateral membrane in BCS and apical/vesicles (variably) in copper (see Fig. 2, E and F, Fig. 3, and Fig. 4, C and D); and completely defective refers to TGN plus basolateral membrane in BCS and copper (see Fig. 2, A and B).
Fig. 2.
Y44 mutants must be aromatic but need not be phosphorylated to correctly target ATP7B into the apical region of polarized WIF-B cells. Cells were infected with the indicated GFP-ATP7B mutant, cultured overnight in 10 μM BCS, then kept in BCS or incubated in 10 μM Cu(II) for 4 h, fixed, stained with antibodies to the indicated organelle markers, and imaged by confocal microscopy. Arrows, basolateral membrane; arrowheads, apical membrane; asterisks, apical lumen. Boxed regions are enlarged at the right of each panel.
We noted that the sequence N41-Y44 resembled an NXXY-like motif that had been identified as a possible apical targeting signal for the endocytic receptor megalin (39). Such motifs can be recognized by proteins containing phosphotyrosine-binding (PTB) domains (reviewed in Refs. 25, 35). Therefore, we further probed the nature of Y44 in the targeting signal by generating GFP-tagged ATP7B proteins containing single substitutions of Y44F and Y44W. These mutants were capable of activating tyrosinase in the copper transport assay, and they were expressed as full-length proteins (Supplemental Table S1). When the Y44F mutant was expressed in polarized WIF-B cells, it exhibited wild-type behavior (Fig. 2, C and D). That is, in copper-depleted cells, Y44F was restricted to the TGN region (Fig. 2C), and, when copper levels were raised, it dispersed into vesicles (Fig. 2D). The vesicles accumulated between the TGN and the apical membrane but were also found elsewhere in the cell. In contrast, Y44W showed a partially defective trafficking pattern (Fig. 2, E and F). In BCS, the mutant protein exhibited a pattern typical of other defective mutant constructs. That is, it was present in both the TGN and the basolateral membrane (Fig. 2E). However, in 10 μM copper, the protein was still seen in the TGN and basolateral membrane (as other defective mutants) but was also dispersed into small vesicles and present at/near the apical plasma membrane, a behavior similar to that of wild-type ATP7B (Fig. 2F) (see Footnote 1).
We made similar point mutations in the other aromatic residues within the F37-E45 sequence of ATP7B and found that position 37 tolerated F, Y, or W, giving wild-type patterns in each case, whereas position 39 tolerated only F or Y (Table 2). These results clearly showed that positions 39 and 44 had to be aromatic amino acids and of a precise size since the larger residue tryptophan could not substitute for phenylalanine or tyrosine. Moreover, phosphorylation of Y44 was not required for the targeting signal to function.
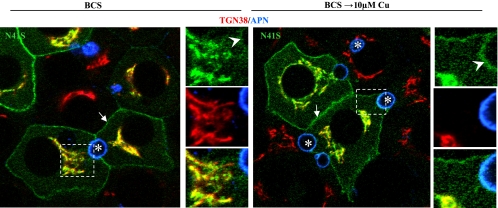
In searching various databases for sequence information on ATP7B, we made two interesting findings. First, we discovered that the equivalent of human ATP7B position 42 in many species was isoleucine rather than valine (see Supplemental Table S2). We made this mutant and found that the V42I-ATP7B protein behaved in a wild-type manner (Table 2). Second, we discovered that N41S had been newly entered as a patient mutation in the Wilson disease mutation database (http://www.medicalgenetics.med.ualberta.ca/wilson/index.php). This is the first and only disease-causing missense mutation within residues 1–63 of human Wilson protein. The N41A-ATP7B mutant we had already generated gave a partially defective trafficking pattern, which bordered on being completely defective. We subsequently made N41S-ATP7B with or without a GFP tag and expressed each mutant in WIF-B cells. As shown in Fig. 3, the disease-causing mutant protein was almost completely defective in that it was present at the basolateral membrane and in the TGN in BCS and copper in all cells but was variably present in the apical membrane of some copper-loaded cells. Apical vesicles were not detected. These results further reinforced the importance of the nine-amino acid signal in the TGN retention and apical targeting of ATP7B.
Fig. 3.
Trafficking of N41S, a disease-causing mutation in ATP7B, borders on being completely defective. Polarized WIF-B cells were infected with GFP-N41S-ATP7B, cultured overnight in 10 μM BCS, then kept in BCS or incubated in 10 μM Cu(II) for 4 h, fixed, stained with antibodies to the indicated organelle markers, and imaged by confocal microscopy. Arrows, basolateral membrane; arrowheads, apical membrane; asterisks, apical lumen. Boxed regions are enlarged at the right of each panel.
A Shorter Form of Human ATP7B Behaves Like the Full-Length Protein
Of the five ATP7B NH2 termini in Table 1, dog ATP7B is reported to begin at residue 33 of the human ATP7B (10). To determine the protein phenotype of this variant, we generated two human ATP7B constructs, Δ32 and Δ32Y44A. We wanted to avoid possible stearic hindrance by GFP with the putative apical targeting signal, so neither construct was epitope tagged. Expression of Δ32 in polarized WIF-B cells that were maintained in 10 μM BCS, fixed, and immunolabeled revealed a tight TGN labeling pattern (Fig. 4A) comparable to that of full-length ATP7B. When infected cells were incubated for 4 h in 10 μM copper, Δ32-ATP7B showed typical wild-type behavior. That is, it dispersed from the TGN into vesicles that congregated around and overlapped with the apical membrane (Fig. 4B). Given the phenotype of Δ32-ATP7B, we expected Δ32-Y44A to be completely defective as was Y44A in the full-length ATP7B background (Fig. 2, A and B). Surprisingly, this was not the case. In BCS, all infected cells showed strong expression of the mutant protein in the TGN region, but only ∼1/2 showed additional light basolateral membrane staining (Fig. 4C). However, in 10 μM copper, the pattern was similar in all cells, with strong TGN and basolateral membrane staining (Fig. 4D). The combined results of these two mutants, which lack the variable upstream sequences seen in several species (Table 1), behaved in elevated copper as their full-length human protein counterparts (wild-type and Y44A), providing convincing evidence that the F37-E45 sequence we identified is essential in the copper-sensitive apical targeting of ATP7B.
Fig. 4.
The first 32 residues of ATP7B are dispensable for wild-type trafficking but influence the defective behavior of a Y44A substitution in the shorter form. Cells were infected with the indicated ATP7B mutant, cultured overnight in 10 μM BCS, then kept in BCS or incubated in 10 μM Cu(II) for 4 h, fixed, stained with rabbit anti-human ATP7B and TGN38, and imaged by confocal microscopy. Note that this anti-ATP7B (85–3) does not detect the endogenous Cu-ATPase by immunofluorescence. Arrows, basolateral membrane; arrowheads, apical membrane; asterisks, apical lumen. Boxed regions are enlarged at the right of each panel.
The F37-E45 Signal in ATP7B is Also Required In Vivo
The ultimate test of the NH2-terminal ATP7B signal we had identified using polarized WIF-B cells was to express selected GFP constructs in vivo. Therefore, we chose two mutant ATP7B proteins, F37W, which targeted apically in WIF-B cells like wild-type ATP7B, and F39A, which exhibited defective trafficking to the basolateral membrane (Table 2). Mice were either copper-depleted or -loaded, infected with a recombinant adenovirus, and euthanized 26–48 h later, their livers excised and fixed sections processed for immunofluorescence detection of organelle markers and GFP. An unfixed portion of each infected liver was solubilized and then subjected to SDS-PAGE and immunoblot analysis to determine the size of the expressed GFP proteins. In all livers where expression was obtained, the GFP proteins migrated with similar mobilities to that of the GFP-wtATP7B (data not shown).
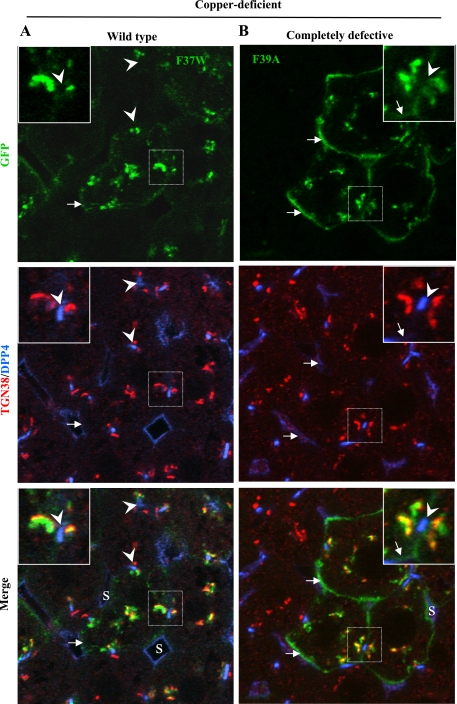
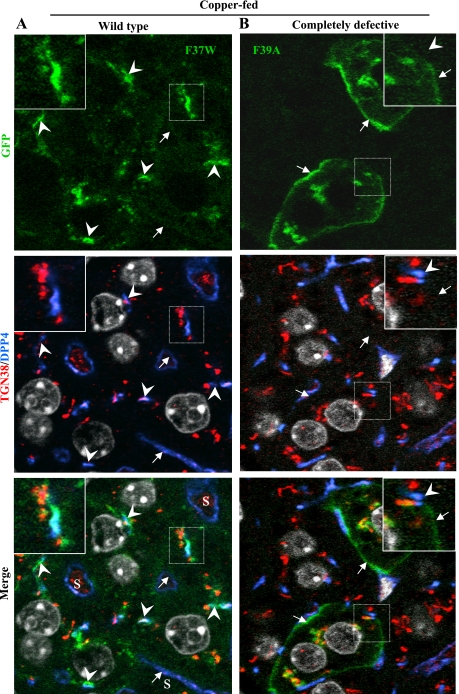
We found that each recombinant protein was expressed predominantly in hepatocytes of the liver, not in Kupffer or endothelial cells lining the sinusoids (Figs. 5 and 6). Since the levels of exogenous protein varied within liver cords, we focused on cells expressing modest amounts, such as those shown in Fig. 5 and 6, where it is clear that the two mutant proteins behaved very differently from one another. In the livers from copper-deficient animals (Fig. 5A), the F37W protein (wild-type in WIF-B) was confined to the Golgi region, as evidenced by its close association/overlap with TGN38, whereas F39A (completely defective in WIF-B) was present both in the Golgi region and the basolateral plasma membrane (Fig. 5B). In the livers from copper-fed animals (Fig. 6), only the distribution of F37W changed (Fig. 6A). The mutant protein was present in Golgi regions and near/at the apical plasma membrane. It was absent from the basolateral membrane (Supplemental Fig. S3). In contrast, the distribution of F39A was unchanged from that seen in livers of copper-deficient mice, and there was no indication that this mutant protein had moved near the apical plasma membrane (compare Figs. 5B and 6B). In summary, the two ATP7B constructs behaved in vivo as predicted from the results we obtained in WIF-B cells; that is, F37W was copper responsive and wild-type, whereas F39A was completely defective. These results confirm that the nine-amino acid sequence is required for copper-dependent ATP7B trafficking in vivo.
Fig. 5.
In vivo localization of mutant ATP7B proteins in low copper is similar to that in WIF-B cells. GFP-ATP7B mutants [wild-type F37W (A) and completely defective F39A (B)] were intravenously injected into mice fed a Cu-deficient diet. 24–36 h later, livers were excised and fixed; 10-μm sections were stained with antibodies to markers of the TGN (TGN38) and apical membrane (dipeptidyl peptidase 4, DPP4) and imaged by confocal microscopy. Note that DPP4 is also present in the plasma membrane of endothelial cells lining the sinusoids (S). Its labeling pattern is more irregular and surrounds a larger space than that of DPP4 in the apical membrane of hepatocytes. Boxed regions are enlarged in the insets. Arrows, basolateral membrane; arrowheads, apical membrane.
Fig. 6.
In vivo trafficking of mutant ATP7B proteins in elevated copper is similar to that in WIF-B cells. GFP-ATP7B mutants [wild-type F37W (A) and completely defective F39A (B)] were intravenously injected into mice that were administered CuSO4 via gastric intubation. 24–36 h later, livers were excised, fixed; then 10-μm sections were stained with antibodies as in Fig. 4 and Hoechst dye and then imaged. Boxed regions are enlarged in the insets. Arrows, basolateral membrane; arrowheads, apical membrane. See Fig. 4 for comments regarding DPP4.
DISCUSSION
We have identified a novel, copper-responsive targeting signal in the NH2 terminus of human ATP7B. When copper levels rise, this nine-amino acid sequence, F37AFDNVGYE45, is required to target the Cu-ATPase from a post-TGN compartment to the apical region/membrane of both WIF-B cells and hepatocytes in vivo. The same signal is part of a mechanism that retains ATP7B in the post-TGN under low copper conditions since in BCS many mutant proteins were found in the TGN and at the basolateral membrane but not in the apical region/membrane. These results raise two interesting questions: 1) what features of the signal are important for its two functions? and 2) how might one signal carry out such different functions? Although we have no definitive answers at present, in the remaining sections we offer several insights and present a working model for the two functions of the signal.
The Signal is Stringent and Highly Conserved
We found several interesting features of the signal from the results of our mutagenesis experiments. First, the signal is quite stringent since single alanine substitutions of six of eight residues rendered it completely nonfunctional in low or high copper. The remaining two alanine substitutions, F37A and N41A, and two additional point mutations, N41S and Y44W, gave partially functional phenotypes. That is, in BCS, these mutant proteins constitutively trafficked to the basolateral membrane instead of staying in the TGN, but, in 10 μM copper, F37A and Y44W displayed a wild-type trafficking pattern, moving near/at the apical membrane and residing in vesicles. This partially defective phenotype suggests to us that residues 37 and 44 are not required for apical targeting but are important in Golgi retention/retrieval. We also determined that aromatic residues 37, 39, and 44 tolerate substitution by other aromatic amino acids to varying degrees and that Y44 appears not to require phosphorylation for signal function. Thus the sequence necessary for correct ATP7B trafficking, including the tolerated changes, is (F/Y/W)37A(F/Y)39DN(V/I)42G(Y/F)44E45.
Only one Wilson disease-causing mutation within the first 63 amino acids of ATP7B, N41S, has been identified to date (11), and N41 is contained within the nine-amino acid retention/targeting signal we identified. Thus our finding that both N41A and N41S-ATP7B proteins bordered on being completely defective when expressed in polarized WIF-B cells further reinforces the functional importance of this signal in the export of Cu(I) from hepatocytes. Moreover, this finding shows very clearly that ATP7B can be active (see Supplemental Table S1, tyrosinase activation results) yet traffics abnormally. The sole patient with this mutation presented at 24 years of age with liver symptoms (11). He or she was compound heterozygote with the second ATP7B allele, 3402delC, causing a frame shift terminating the protein shortly after P1134, which is in the ATP-binding domain of ATP7B. Although the stability of the prematurely truncated product is unknown, it cannot be functional, leaving the N41S mutant protein as the only expressed form of ATP7B. The puzzle is why copper would accumulate in hepatocytes since the mutant protein is present at the basolateral, where it presumably could transport excess free copper back into the circulation. It will be interesting to study the copper export activity of this missense protein in the future.
In silico analysis revealed additional interesting features of the targeting/retention sequence in ATP7B. First, it is novel since only other ATP7B sequences were obtained when the NCBI Protein Database was queried with the signal. Second, it is highly conserved in 24 species, from platypus to elephant (Supplemental Table S2). In 10 species, the nine amino acids are 100% identical to that of human ATP7B, and in eight only a single residue is changed, often from V42 in the human sequence to isoleucine. Expression of the V42I variant in WIF-B cells confirmed it to be wild-type in its behavior. Third, there is considerable variation among species in the number of residues NH2 terminal to the nine-residue sequence, with the spectrum ranging from 4 (dog) to >100 [horse and red jungle fowl (Supplemental Table S2)]. Because the dog ATP7B sequence reportedly starts only four amino acids before the signal we identified, we generated and tested two constructs, Δ32 and Δ32-Y44A, in the context of the human ATP7B in WIF-B cells. Δ32 was wild-type in every respect. However, the Δ32-Y44A, which we classified as partially defective, was different from the other four mutants classified similarly. In many cells, Δ32-Y44A showed wild-type behavior in BCS; that is, it was found predominantly in the post-TGN region. However, in 10 μM copper all expressing cells exhibited a strong basolateral membrane signal, with total exclusion from the apical region/membrane. This puzzling result implies that upstream sequence might modulate the TGN retention function of the signal. A fourth interesting feature of the NH2 termini of ATP7B in all species is the similar length of the sequences between the end of the signal we identified (E45 in human) and the copper-binding motif of the first MBD (Supplemental Table S2). Thirteen out of twenty-four species have nineteen residues, with an additional eight being only one or two residues shorter or longer. Such a narrow window of “allowable” lengths hints at the possible existence of a conformational constraint and/or requirement in this linker region.
Finally, with the use of residues 1–63 in several algorithms (e.g., Rosetta, www.predictprotein.org), FAFDNVGYE is predicted to fold into a short a-helix, which is connected to the rest of the protein by flexible linkers (Fig. 7). Perhaps the shape/size of this helix is critical for recognition by other proteins and/or domains of ATP7B, such that any change introduced by mutations is not tolerated.
Fig. 7.
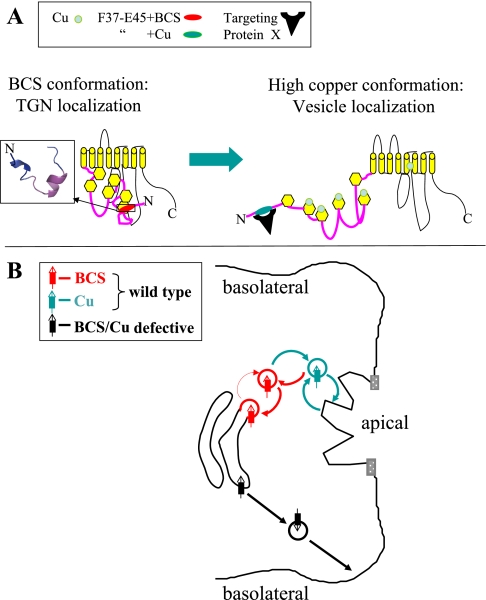
Working models for the copper-sensitive signal function and trafficking of ATP7B in polarized hepatic cells. A: possible dispositions of F37AFDNVGYE45 in the context of full-length ATP7B in low and high copper. In low copper, F37-E45 contributes to TGN retention through interaction with other domains of ATP7B and is unavailable to Protein X and the apical sorting/trafficking machinery. In high copper, F37-E45 becomes exposed and binds to Protein X, which initiates vesicle formation and subsequent apical trafficking. B: possible steady-state distributions of ATP7B under low (red) and high (blue-green) copper conditions. In low copper (red), ATP7B remains largely in the last TGN compartment, occasionally being released but rapidly retrieved (thin and thick red arrows, respectively). When copper levels are raised (blue-green), formation of ATP7B-positive vesicles increases (thick blue arrow), vesicles move into the apical region, and release their contents intermittently by fusion with the apical plasma membrane (see Footnote 1).
Working Models for the Dual-Function Signal and Copper-Sensitive Trafficking of ATP7B
Figure 7A presents a molecular model for how this newly identified signal might function in the copper-sensitive trafficking of ATP7B. In BCS, we hypothesize that the signal is interacting with another domain of ATP7B. In support of this possibility, Tsivkovskii et al. (43) used protein copurification and demonstrated a tight interaction between the entire NH2-terminal domain, consisting of 650+ amino acids, and the ATP-binding domain when N-ATP7B was in apoform. The interaction became weaker when the NH2-terminal domain was in a copper-bound form. It remains to be tested whether the NH2-terminal 1–63 alone can interact with another ATP7B cytoplasmic domain under these conditions. With the use of nuclear magnetic resonance, others have shown that individual and groups of the six MBDs also undergo copper-dependent conformational changes (e.g., Refs. 1, 2, 45). Thus there is precedence for the NH2 terminus of ATP7B assuming different conformations under varying copper conditions.
In elevated copper, we hypothesize that the signal becomes available for binding to a trafficking molecule, which we call “Protein X” (Fig. 7A). At present, we don't know whether another domain of ATP7B might also be required for this binding, nor do we know the identity of Protein X. Nonetheless, we speculate that, once the ATP7B-Protein X complex forms, it is recognized by additional trafficking machinery (e.g., rabs, adaptors, coats), resulting in the formation and movement of a vesicle containing ATP7B to the apical region/membrane, perhaps along microtubules (20).
Given the presence of an NXXY-like motif in our nine-residue signal, it is tempting to speculate that Protein X is a PTB domain-containing protein. PTB proteins contain one or more autonomously folding sequences of ∼100–150 amino acids, which bind short sequences containing NPXY or NXXY in other proteins. This large protein family is classified into three subgroups (phosphotyrosine-dependent Shc-like, phosphotyrosine-dependent IRS-like, and phosphotyrosine-independent Dab-like PTBs; reviewed in Ref. 44). Since the Y44F-ATP7B mutant exhibited wild-type behavior, it is possible that a Dab-like protein might be the putative ATP7B binding partner. Although members so far identified are predominantly involved in endocytic trafficking, several have been reported to serve as monomeric adaptors in exocytic trafficking (36; reviewed in Ref. 42).
Figure 7B presents a cellular model of the trafficking of ATP7B between the post-TGN compartment under low-copper conditions and the apical region/membrane when copper levels are elevated. First, consider the BCS or low-copper condition. The steady-state localization of a membrane protein in the Golgi is viewed as a balance between the retention of the protein within the organelle and its retrieval from a downstream compartment (reviewed in Ref. 24). For ATP7B, we have depicted this balance as residence of the protein in a budding region of the TGN and in a “released” vesicle (ATP7B protein and membranes shown in red, Fig. 7B). The release process may involve transient copper loading of ATP7B or transient exposure of the targeting signal with its consequent binding to Protein X. The retrieval process under low to no Cu(I) conditions would presumably occur upon completion of a single catalytic cycle of the enzyme and/or release of Protein X. In earlier work, others reported that TMD3 of ATP7A could target a reporter molecule to the Golgi complex (12). Could the TMD3 of ATP7B be playing a similar role? Comparison of the two human Cu-ATPases reveals that their TMD3s are remarkably similar, with 17/21 residues identical and 20/21 conserved (Supplemental Fig. S3). Given that the TMD3 of ATP7B is involved, we argue that our nine-residue signal must also contribute to Golgi retention/retrieval since multiple ATP7B signal mutants in the present study, all with intact TMD3, failed to be retained in the Golgi and ended up at the basolateral membrane. Thus TMD3 cannot be sufficient to target/retain full-length ATP7B in its post-TGN compartment. Finally, results of mutagenesis experiments have indicated that di- and trileucine motifs present in the COOH termini of ATP7A and ATP7B, respectively, are required to retrieve their respective proteins back to the TGN under low-copper conditions (9, 30). The question now is when, where, and how these three putative “retention/retrieval signals” work, whether cooperatively, sequentially, or independently.
Turning to the dynamics of ATP7B when copper levels are raised, in Fig. 7B we envision an increase in the rate of formation/movement of ATP7B-positive vesicles into the apical region/membrane. This could occur via multiple conformational changes in ATP7B, driven by occupation of key intramembranous site(s) with Cu(I), and activation of the ATP catalytic cycle, particularly formation of the acylphosphate intermediate. Regarding this latter possibility, others have reported that alanine mutagenesis of phosphatase consensus sites on both ATP7B (8) and ATP7A (31) results in dispersion of the mutant proteins from the TGN. In the acylphosphate intermediate state, we speculate that the nine-amino acid signal becomes exposed and binds to Protein X with subsequent recruitment of additional trafficking machinery, which results in formation of ATP7B-positive vesicles and their movement further into the apical region of the cell (ATP7B protein and vesicles shown in blue-green, Fig. 7B).
There are many remaining questions, particularly about the fates of defective ATP7B mutant proteins, which end up in the basolateral membrane rather than in apical vesicles (ATP7B protein and vesicles shown in black, Fig. 7B). How do they get to the basolateral membrane? One possibility is that disabling the nine-residue signal could divert these proteins into the constitutive, basolaterally directed pathway taken by secreted proteins (5) and two classes of newly synthesized apical membrane proteins in hepatocytes (4, 34). Why is there continued presence of defective proteins in the Golgi? There are two possible explanations: 1) continuous recycling between the basolateral membrane and Golgi; and/or 2) the transient presence of newly synthesized molecules in this compartment before their diversion. At present, the WIF-B cells show extreme sensitivity to the protein synthesis inhibitor cycloheximide, prohibiting a meaningful test of the second possibility. Finally, do the partially defective mutant proteins move to both apical and basolateral regions in high copper? Future studies using different approaches will be needed to answer these questions.
Concluding Remarks
In this study, we have identified a novel signal in ATP7B that is bifunctional, acting in TGN retention and apical targeting. Our finding that selected mutants of this signal, which we dissected in a cell model, behaved similarly in vivo means that the signal is physiologically relevant. This is the first such demonstration in animals. The additional fact that a human Wilson disease-causing mutation, N41S, occurs within the signal we identified reinforces its importance in the Cu(I) export function of ATP7B in hepatocytes. The goal now is to understand how the signal works.
GRANTS
This work was supported by NIH (DK072084, DK063096 and DK064388).
Supplementary Material
Acknowledgments
Contributions by the three co-first authors were as follows: Author 1 (L. Braiterman) suggested generation of the Δ32 and Δ32-Y44A-ATP7B constructs, made and packaged 8 of the 20 ATP7B constructs, then purified 19 of the 20 viruses used in this study, supervised/performed WIF-B culture/propagation, titered viruses, infected polarized WIF-B cells, evaluated confocal images, and participated in data evaluation and manuscript preparation. Author 2 (L. Nyasae) performed WIF-B culture, propagation, mutant ATP7B protein expression experiments, and immunoblotting, antibody stained WIF-B cells infected and treated by Author 1, performed subsequent confocal microscopy, participated in animal experiments, including tissue processing, antibody staining, and confocal microscopy, participated in data evaluation, and prepared publication figures containing confocal images. Author 3 (Y. Guo) identified NXXY as a possible apical targeting motif, found the N41S mutation in the Wilson disease database, made, packaged, and was the first to purify and test 12 of the 20 ATP7B constructs reported in this study by infecting WIF-B cells, and analyzed their trafficking by confocal microscopy. We thank M. Donowitz and F. Leves for constructive comments on an early draft of the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The extent to which Cu-ATPase-positive vesicles fuse with the plasma membrane is still controversial and not yet resolved as is whether the proteins transport cytoplasmic Cu(I) directly across the plasma membrane or into the vesicles, which then fuse intermittently with the plasma membrane and release their contents. It is our contention that results obtained using in vitro cell models should be viewed as a guide to the in vivo situation, rather than definitive proof of a particular destination or mechanism. See Ref. 16 for more discussion on this issue.
REFERENCES
- 1.Achila D, Banci L, Bertini I, Bunce J, Ciofi-Baffoni S, Huffman DL. Structure of human Wilson protein domains 5 and 6 and their interplay with domain 4 and the copper chaperone HAH1 in copper uptake. Proc Natl Acad Sci USA 103: 5729–5734, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banci L, Bertini I, Cantini F, Rosenzweig AC, Yatsunyk LA. Metal binding domains 3 and 4 of the Wilson disease protein: solution structure and interaction with the copper(I) chaperone HAH1. Biochemistry 47: 7423–7429, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barr VA, Hubbard AL. Newly synthesized hepatocyte plasma membrane proteins are transported in transcytotic vesicles in the bile duct-ligated rat. Gastroenterology 105: 554–571, 1993. [DOI] [PubMed] [Google Scholar]
- 4.Bartles JR, Feracci HM, Stieger B, Hubbard AL. Biogenesis of the rat hepatocyte plasma membrane in vivo: comparison of the pathways taken by apical and basolateral proteins using subcellular fractionation. J Cell Biol 105: 1241–1251, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastaki M, Braiterman LT, Johns DC, Chen YH, Hubbard AL. Absence of direct delivery for single transmembrane apical proteins or their “Secretory” forms in polarized hepatic cells. Mol Biol Cell 13: 225–237, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belbraouet S, Biaudet H, Tebi A, Chau N, Gray-Donald K, Debry G. Serum zinc and copper status in hospitalized vs. healthy elderly subjects. J Am Coll Nutr 26: 650–654, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Cassio D, Hamon-Benais C, Guerin M, Lecoq O. Hybrid cell lines constitute a potential reservoir of polarized cells: isolation and study of highly differentiated hepatoma-derived hybrid cells able to form functional bile canaliculi in vitro. J Cell Biol 115: 1397–1408, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cater MA, La Fontaine S, Mercer JF. Copper binding to the N-terminal metal-binding sites or the CPC motif is not essential for copper-induced trafficking of the human Wilson protein (ATP7B). Biochem J 401: 143–153, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cater MA, La Fontaine S, Shield K, Deal Y, Mercer JF. ATP7B mediates vesicular sequestration of copper: insight into biliary copper excretion. Gastroenterology 130: 493–506, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Coronado VA, O'Neill B, Nanji M, Cox DW. Polymorphisms in canine ATP7B: candidate modifier of copper toxicosis in the Bedlington terrier. Vet J 177: 293–296, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Deguti MM, Genschel J, Cancado EL, Barbosa ER, Bochow B, Mucenic M, Porta G, Lochs H, Carrilho FJ, Schmidt HH. Wilson disease: novel mutations in the ATP7B gene and clinical correlation in Brazilian patients. Hum Mutat 23: 398, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Francis MJ, Jones EE, Levy ER, Ponnambalam S, Chelly J, Monaco AP. A Golgi localization signal identified in the Menkes recombinant protein. Hum Mol Genet 7: 1245–1252, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Guo Y, Nyasae L, Braiterman LT, Hubbard AL. NH2-terminal signals in ATP7B Cu-ATPase mediate its Cu-dependent anterograde traffic in polarized hepatic cells. Am J Physiol Gastrointest Liver Physiol 289: G904–G916, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature 400: 464–468, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Hubbard AL, Bartles JR, Braiterman LT. Identification of rat hepatocyte plasma membrane proteins using monoclonal antibodies. J Cell Biol 100: 1115–1125, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hubbard AL, Braiterman LT. Could ATP7B export Cu(I) at the tight junctions and the apical membrane? Gastroenterology 134: 1255–1257, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Ihrke G, Hubbard AL. Control of vesicle traffic in hepatocytes. Prog Liver Dis 13: 63–99, 1995. [PubMed] [Google Scholar]
- 18.Ihrke G, Neufeld EB, Meads T, Shanks MR, Cassio D, Laurent M, Schroer TA, Pagano RE, Hubbard AL. WIF-B cells: an in vitro model for studies of hepatocyte polarity. J Cell Biol 123: 1761–1775, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.La Fontaine S, Mercer JF. Trafficking of the copper-ATPases, ATP7A and ATP7B: role in copper homeostasis. Arch Biochem Biophys 463: 149–167, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Lim CM, Cater MA, Mercer JF, La Fontaine S. Copper-dependent interaction of dynactin subunit p62 with the N terminus of ATP7B but not ATP7A. J Biol Chem 281: 14006–14014, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. Function and regulation of human copper-transporting ATPases. Physiol Rev 87: 1011–1046, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Lutsenko S, Gupta A, Burkhead JL, Zuzel V. Cellular multitasking: the dual role of human Cu-ATPases in cofactor delivery and intracellular copper balance. Arch Biochem Biophys 476: 22–32, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lutsenko S, Petris MJ. Function and regulation of the mammalian copper-transporting ATPases: insights from biochemical and cell biological approaches. J Membr Biol 191: 1–12, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Luzio JP, Banting G. Eukaryotic membrane traffic: retrieval and retention mechanisms to achieve organelle residence. Trends Biochem Sci 18: 395–398, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Margolis B, Borg JP, Straight S, Meyer D. The function of PTB domain proteins. Kidney Int 56: 1230–1237, 1999. [DOI] [PubMed] [Google Scholar]
- 26.McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem 22: 1077–1083, 1974. [DOI] [PubMed] [Google Scholar]
- 27.Mercer JF, Llanos RM. Molecular and cellular aspects of copper transport in developing mammals. J Nutr 133: 1481S–1484S, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Nittis T, Gitlin JD. Role of copper in the proteosome-mediated degradation of the multicopper oxidase hephaestin. J Biol Chem 279: 25696–25702, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Nyasae L, Bustos R, Braiterman L, Eipper B, Hubbard A. Dynamics of endogenous ATP7A (Menkes protein) in intestinal epithelial cells: copper-dependent redistribution between two intracellular sites. Am J Physiol Gastrointest Liver Physiol 292: G1181–G1194, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Petris MJ, Camakaris J, Greenough M, LaFontaine S, Mercer JF. A C-terminal di-leucine is required for localization of the Menkes protein in the trans-Golgi network. Hum Mol Genet 7: 2063–2071, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Petris MJ, Voskoboinik I, Cater M, Smith K, Kim BE, Llanos RM, Strausak D, Camakaris J, Mercer JF. Copper-regulated trafficking of the Menkes disease copper ATPase is associated with formation of a phosphorylated catalytic intermediate. J Biol Chem 277: 46736–46742, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Scarborough GA Structure and function of the P-type ATPases. Curr Opin Cell Biol 11: 517–522, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Schaefer M, Hopkins RG, Failla ML, Gitlin JD. Hepatocyte-specific localization and copper-dependent trafficking of the Wilson's disease protein in the liver. Am J Physiol Gastrointest Liver Physiol 276: G639–G646, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Schell MJ, Maurice M, Stieger B, Hubbard AL. 5′nucleotidase is sorted to the apical domain of hepatocytes via an indirect route. J Cell Biol 119: 1173–1182, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlessinger J, Lemmon MA. SH2 and PTB domains in tyrosine kinase signaling. Sci STKE 191: RE12, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Shrivastava-Ranjan P, Faundez V, Fang G, Rees H, Lah JJ, Levey AI, Kahn RA. Mint3/X11[gamma] is an ADP-ribosylation factor-dependent adaptor that regulates the traffic of the Alzheimer's precursor protein from the trans-Golgi network. Mol Biol Cell 19: 51–64, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strausak D, La Fontaine S, Hill J, Firth SD, Lockhart PJ, Mercer JF. The role of GMXCXXC metal binding sites in the copper-induced redistribution of the Menkes protein. J Biol Chem 274: 11170–11177, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki M, Gitlin JD. Intracellular localization of the Menkes and Wilson's disease proteins and their role in intracellular copper transport. Pediatr Int 41: 436–442, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Takeda T, Yamazaki H, Farquhar MG. Identification of an apical sorting determinant in the cytoplasmic tail of megalin. Am J Physiol Cell Physiol 284: C1105–C1113, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Terada K, Nakako T, Yang XL, Iida M, Aiba N, Minamiya Y, Nakai M, Sakaki T, Miura N, Sugiyama T. Restoration of holoceruloplasmin synthesis in LEC rat after infusion of recombinant adenovirus bearing WND cDNA. J Biol Chem 273: 1815–1820, 1998. [DOI] [PubMed] [Google Scholar]
- 41.Toyofuku K, Wada I, Hirosaki K, Park JS, Hori Y, Jimbow K. Promotion of tyrosinase folding in COS 7 cells by calnexin. J Biochem (Tokyo) 125: 82–89, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Traub LM Common principles in clathrin-mediated sorting at the Golgi and the plasma membrane. Biochim Biophys Acta 1744: 415–437, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Tsivkovskii R, MacArthur BC, Lutsenko S. The Lys1010-Lys1325 fragment of the Wilson's disease protein binds nucleotides and interacts with the N-terminal domain of this protein in a copper-dependent manner. J Biol Chem 276: 2234–2242, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Uhlik MT, Temple B, Bencharit S, Kimple AJ, Siderovski DP, Johnson GL. Structural and evolutionary division of phosphotyrosine binding (PTB) domains. J Mol Biol 345: 1–20, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Wernimont AK, Huffman DL, Lamb AL, O'Halloran TV, Rosenzweig AC. Structural basis for copper transfer by the metallochaperone for the Menkes/Wilson disease proteins. Nat Struct Biol 7: 766–771, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Yamaguchi Y, Heiny ME, Suzuki M, Gitlin JD. Biochemical characterization and intracellular localization of the Menkes disease protein. Proc Natl Acad Sci USA 93: 14030–14035, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.