Abstract
Monocarboxylate transporter (MCT1) plays an important role in the absorption of short-chain fatty acids (SCFA) such as butyrate in the human colon. Previous studies from our laboratory have demonstrated that phorbol ester, PMA (1 μM, 24 h), upregulates butyrate transport and MCT1 protein expression in human intestinal Caco-2 cells. However, the molecular mechanisms involved in the transcriptional regulation of MCT1 gene expression by PMA in the intestine are not known. In the present study, we showed that PMA (0.1 μM, 24 h) increased the MCT1 promoter activity (−871/+91) by approximately fourfold. A corresponding increase in MCT1 mRNA abundance in response to PMA was also observed. PMA-induced stimulation of MCT1 promoter activity was observed as early as 1 h and persisted until 24 h, suggesting that the effects of PMA are attributable to initial PKC activation. Kinase inhibitor and phosphorylation studies indicated that these effects may be mediated through activation of the atypical PKC-ζ isoform. 5′-deletion studies demonstrated that the MCT1 core promoter region (−229/+91) is the PMA-responsive region. Site-directed mutagenesis studies showed the predominant involvement of potential activator protein 2 (AP2) binding site in the activation of MCT1 promoter activity by PMA. In addition, overexpression of AP2 in Caco-2 cells significantly increased MCT1 promoter activity in a dose-dependent manner. These findings showing the regulation of MCT1 promoter by PKC and AP2 are of significant importance for an understanding of the molecular regulation of SCFA absorption in the human intestine.
Keywords: short-chain fatty acid absorption, transcriptional regulation, human intestine, protein kinase C-ζ, activator protein 2
monocarboxylate transporter 1 (MCT1) is a member of the solute carrier family1 of MCTs known to mediate the transport of monocarboxylates such as lactate and pyruvate (21, 46) and a wide range of short-chain fatty acids (SCFAs), e.g., acetate, propionate, and butyrate, across the plasma membrane of a variety of cell types (17, 22, 32). In humans, at least 14 MCT isoforms have been identified, but only MCT1-MCT4 have been structurally and functionally well characterized (21). Evidence suggests that SCFAs, particularly butyrate, serve as the principal metabolic fuel for colonic epithelial cells and exert a variety of effects fundamental to the health of normal colonic mucosa (47). For example, butyrate plays an essential role in suppressing mucosal inflammation (26, 34) and exhibits antitumorigenic effects such as induction of cell cycle arrest, differentiation, and apoptosis (20, 23, 40, 57). Butyrate is also known to stimulate colonic electroneutral NaCl absorption and to inhibit Cl− secretion (2, 45).
Although much is known with respect to the functional aspects of MCT1, very little is known regarding the mechanisms controlling MCT1 gene expression. In this regard, we and others have recently reported the cloning of the promoter region of the human MCT1 gene (11, 18, 19). The cloned fragment of MCT1 promoter (−871/+91) was found to be highly active in Caco-2 cells (18) and colonic AA/C1 cells (10). The promoter has been shown to lack the TATA and CCAAT boxes and to have a long GC- rich area at its 3′ end. Moreover, the core promoter region (−229/+91) harbors a number of potential binding sites for various transcription factors such as activator protein 2 (AP2), SP1, and upstream stimulatory factor (USF). Previous studies on MCT1 gene regulation have demonstrated butyrate-induced upregulation of MCT1 expression and function in colonic AA/C1 cells, indicating involvement of both transcriptional and posttranscriptional mechanisms (10). Recent studies have shown also the role of insulin-like growth factor receptor type I in the stimulation of MCT1 protein expression in hepatocarcinoma cells (29) and the upregulation of MCT1 and MCT4 mRNA by testosterone in rat skeletal muscle (15). MCT4, but not MCT1, promoter activity has been shown to be upregulated by hypoxia through a hypoxia-inducible factor-1α-dependent mechanism (54). To date, however, there is no information available on the transcriptional regulation of MCT1 by protein kinases.
We previously showed that the well-known PKC agonist PMA (phorbol ester, 24 h) significantly increased apical butyrate uptake and MCT1 protein expression in Caco-2 cells (1). However, the detailed effects of PMA on MCT1 transcriptional regulation were not examined. Therefore, to elucidate the mechanisms involved in the transcriptional regulation of the human MCT1 gene by PMA and the potential role of PKC, studies were designed to investigate in detail the potential cis element(s), transcription factors, and signaling mechanism(s) involved in the PKC-induced regulation of MCT1 promoter. Our studies demonstrated that PMA enhanced MCT1 promoter activity in Caco-2 cells via the activation of transcription factor AP2. Furthermore, pharmacological inhibitor studies indicated that PKC-ζ might be involved in mediating the stimulatory effects of PMA on MCT1 promoter activity. These findings are important in providing significant insights into the mechanisms involved in the regulation of MCT1 gene expression in the human colon.
MATERIALS AND METHODS
Materials.
PMA and 4α-PMA (inactive analog) were obtained from Biomol (Plymouth Meeting, PA), and bisindolylmaleimide I (BIM) was obtained from Calbiochem (San Diego, CA). All restriction endonucleases and other modifying enzymes were procured from New England BioLabs (Beverly, MA), Invitrogen (Gaithersburg, MD), or Promega (Madison, WI). Luciferase assay system was procured from Promega. β-Galactosidase assay kit was obtained from BD Biosciences Clontech (Palo Alto, CA).
RNA extraction and real-time PCR analysis.
Total RNA was prepared from Caco-2 cells treated with 100 nM 4α-PMA or PMA for 24 h using Absolutely RNA RT-PCR Miniprep Kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. Equal amounts of RNA from both treated and control samples were reverse transcribed and amplified in a one-step reaction using Brilliant SYBR Green QRT-PCR Master Mix kit (Stratagene). Real-time PCR was performed using Mxp3000 (Stratagene). MCT1 was amplified with gene-specific primers (sense primer, 5′-TCTGTGTCTATGCGGGATTCTT-3′; antisense primer, 5′-TTGAGCCGACCTAAAAGTGGT-3′). Histone was amplified as an internal control using gene-specific primers (sense primer, 5′-ACCGACCTTCGTTTCCAGAG-3′; antisense primer, 5′-CTTGGCGTGAATAGCACAGA-3′). Because the amplification efficiencies for both MCT1 and histone were shown to be approximately equal, the quantitation was expressed as a ratio, 2ΔCt−MCT1/2ΔCt−histone, where ΔCt−MCT1 and ΔCt−histone represent the differences between the threshold cycles (Ct) of amplification of treated and control RNA for MCT1 and histone, respectively.
Reporter plasmid construction.
Plasmids used for functional analysis of the MCT1 promoter activity were generated using pGL2 basic vector (Promega) that contains a promoterless luciferase reporter gene. Three 5′-deletion constructs of p-625/+91, p-378/+91, and p-229/+91 were generated by the PCR amplification method as previously described by our laboratory (18). Three different forward primers contained an internal site for Xho1 restriction enzyme and their sequences are primer-1, 5′-GACTCGAGTGGCTCTATGGTGGCAAGTTGCAT-3′; primer-2, 5′-ATCTCGAGATGCTGCCCATTCCCCGCTCCTCAGT-3′; and primer-3, 5′-GACTCGAGAGATTGCCTAGAGCTCGTCAGACA-3′. The sequence of the reverse primer contained site for HindIII enzyme and is 5′-ATCAAGCTTAGTACCCACGCAGCTAGCCAGTCACGTCGCA-3′. The amplifications were performed using proof reading Elongase enzyme mix (Invitrogen) according to the manufacturer's instructions. PCR products were then digested with Xho1 and HindIII enzymes and subcloned into luciferase reporter gene vector, pGL-2 basic (Promega). The fidelity of the constructs were then confirmed by sequencing, and plasmids were prepared for transfection using a kit from Qiagen (Valencia, CA).
Site-directed mutagenesis.
Site-directed mutations corresponding to the critical potential binding sites for transcription factors AP2 (−94 to −83), SP1 (−22 to −13), and overlapping USF/SP1 (−118 to −106) were made in the background of p-871/+91 using the QuickChange Site-Directed Mutagenesis kit (Stratagene) according to the manufacturer's instructions and confirmed by sequencing. The bold letters indicate the mutations. The mutant oligonucleotides used are: AP2 mutant: 5′-CGCCGGCGCGAACGGGAAAGCTAGAGGG-3′; SP1 mutant: 5′-GGATGTCTGTGTAACGGAAAGGGGGCGGCGG-3′; USF/SP1 mutant: 5′-GACGCCGGTCACAAAACGGGGAGGGGGCG-3′.
Transient transfection and luciferase assays.
For transfection studies, Caco-2 cells (1.5 × 105) were seeded into 24-well plates and transfected while still in suspension with one of the MCT1 promoter-luciferase constructs using Lipofectamine 2000 reagent (Invitrogen). A total of 2 μg DNA per well at a ratio of 1:2 for DNA vs. Lipofectamine 2000 was used for each transfection. Twenty-four hours posttransfection, cells were treated with 4α-PMA or PMA (100 nM) in serum-reduced media (1% FBS) for 10 min or 1, 2, 4, or 24 h. For studies with the specific PKC inhibitor BIM, transfected cells were treated for 1 h with the inhibitor and then treated with PMA (100 nM) in the presence of the inhibitor for an additional 4 or 24 h. After 48 h, cells were washed with 1× PBS and cell lysates prepared in reporter lysis buffer (Promega). Luciferase activity was measured by luminometer according to the manufacturer's instructions using the kit obtained from Promega. Relative light unit (RLU) values obtained from the assay were normalized to the amount of protein used as determined by the Bradford method (3) and promoter activity expressed as RLU/mg protein.
AP2 overexpression studies.
For these experiments, transient transfections were performed via electroporation at 0.22 kV and 0.95 μF (Bio-Rad, Hercules, CA) in 5 × 106 cells with 10 μg of p-871/+91 or p-229/+91 construct, 4 μg of pCMV-β, (β-galactosidase mammalian expression vector; BD Biosciences Clontech), and increasing concentrations of AP2 expression vector, 5, 10, 15, 25, and 40 μg. pCMV-β vector served as an internal control for transfection efficiency. A total of 1.25 μg of p-871/+91 or p-229/+91 construct, 0.5 μg of pCMV-β, and increasing concentrations of AP2 expression vector, ∼0.6, 1.3, 2.1, 3.1, or 5.0 μg per well were used. The total amount of DNA per well was adjusted in these experiments with different amounts of the vector not expressing AP2 protein. In some experiments, Caco-2 cells were transfected using an Amaxa Nucleofector System according to the manufacturer's instructions. Briefly, ∼2 × 106 cells were harvested and then electroplated in 100 μl of solution T (supplied by Amaxa) along with 6 μg of MCT1 promoter (p-871/+91) or AP2 mutant, 3 μg of AP2 expression vector, and 0.6 μg of pCMV-β. Cells were transferred to full media and plated on eight wells of a 24-well plate. Forty-eight hours posttransfection, cells were lysed in reporter lysis buffer (Promega), and the activities of both firefly luciferase and β-galactosidase were measured by luminometer according to the manufacturer's instructions using kits from Promega and Clontech, respectively. RLU values obtained were normalized to β-galactosidase activity.
Statistical analysis.
Results are expressed as means ± SE. Student's t-test was used in statistical analysis. P < 0.05 or less was considered statistically significant.
RESULTS
PMA increases MCT1 mRNA.
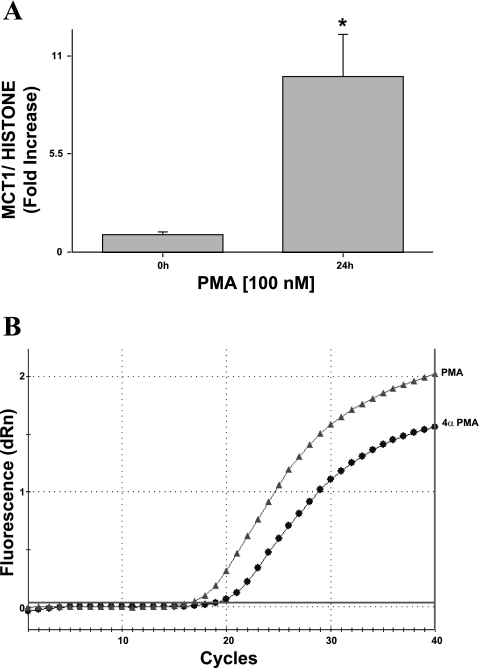
Our previous studies demonstrated the role of PMA in the upregulation of butyrate transport and MCT1 protein expression in Caco-2 cells (1). To determine whether the effects of PMA are also observed at the transcriptional level, we examined MCT1 mRNA levels utilizing real-time PCR. Caco-2 cells were treated with 100 nM of 4α-PMA (control) or PMA for 24 h in serum-starved medium. Real-time PCR was carried out using total RNA extracted from control and PMA-treated Caco-2 cells and MCT1-specific primers. As shown in Fig. 1, PMA (100 nM, 24 h) significantly increased the relative abundance of MCT1 mRNA by approximately ninefold compared with control.
Fig. 1.
Effect of PMA on human monocarboxylate transporter 1 (MCT1) expression in Caco-2 cells. Postconfluent Caco-2 cells were treated with 100 nM of PMA or 4α-PMA (control) for 24 h in media containing 1% FBS. Total RNA was then extracted from the cells, and 100 ng was amplified with MCT1 or histone gene-specific primers using one-step RT-PCR mix containing SYBR Green fluorescence dye for real-time PCR quantitation. A: relative abundance of MCT1 mRNA normalized to the level of histone mRNA was calculated as described in materials and methods and represents means ± SE of 6 separate experiments performed in triplicate. *P < 0.05 compared with control. B: representative traces of the amplification plots of MCT1 in the presence and absence of PMA are shown.
PMA induces MCT1 promoter activity in Caco-2 cells.
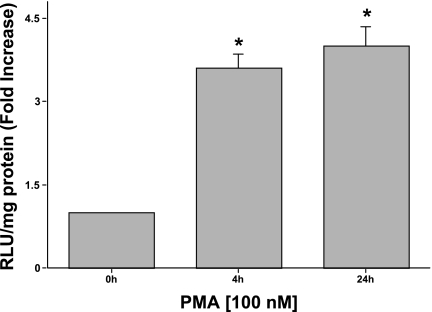
Since the effects of PMA were at the mRNA level, it was therefore considered to be of interest to examine MCT1 promoter activity in response to PMA. Caco-2 cells were transiently transfected with MCT1 promoter-reporter construct, p-871/+91 (962 bp fragment), in which the MCT1 promoter region was fused to the promoterless luciferase gene in pGL2-basic. Twenty-four hours posttransfection, cells were treated with 100 nM PMA for 4 h or 24 h, and luciferase assays were performed 48 h posttransfection. PMA significantly increased MCT1 promoter activity by approximately fourfold compared with control (Fig. 2). These results provided further evidence that PMA regulates MCT1 at the transcriptional level.
Fig. 2.
Effect of PMA on MCT1 promoter activity. Caco-2 cells were transiently transfected with the MCT1 luciferase promoter construct (p-871/+91). Twenty-four hours posttransfection, cells were then treated with 100 nM of PMA or 4α-PMA (control) for an additional 4 or 24 h in media containing 1% FBS. Cells were then harvested 48 h posttransfection, and the promoter activity was assessed by measuring luciferase activity and expressed as relative light units (RLU)/mg of protein as described in materials and methods. Results represent means ± SE of 3 separate experiments performed in triplicate and are expressed as fold increase comparing transfected cells treated with PMA with cells treated with 4α-PMA (control). *P < 0.05 compared with control.
PMA induces MCT1 promoter activity via initial PKC activation.
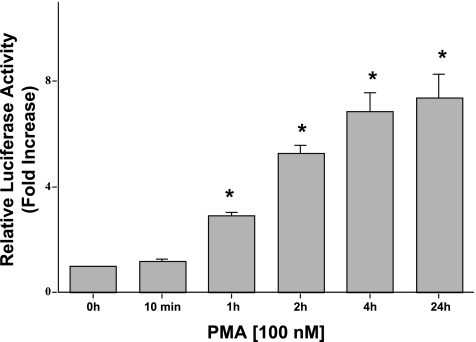
Since long-term treatment of PMA (24 h) is associated with downregulation of PKC (31), we next examined whether the effects of PMA on MCT1 promoter activity are due to initial PKC activation or downregulation of PKC. To achieve this, a time course of PMA treatment was carried out by wash-out experiments. Twenty-four hours posttransfection, Caco-2 cells were treated with PMA (100 nM) at various time points ranging from 10 min to 24 h; after which the cells were washed with 1% serum medium (1% FBS), and reporter gene assays were performed 48 h posttransfection. As shown in Fig. 3, the observed increase in MCT1 promoter activity in response to PMA was seen as early as 1 h and persisted until the 24-h time point. However, PMA treatment for only 10 min showed no change in MCT1 promoter activity, indicating complete removal of PMA in the wash-out experiments. On the basis of our results, it appears that the increase in MCT1 promoter activity by PMA at 24 h is secondary to initial PKC activation and not attributable to downregulation of PKC.
Fig. 3.
PMA-induced stimulation of MCT1 promoter activity is due to initial PKC activation. Caco-2 cells were transfected with the MCT1 luciferase promoter construct (p-871/+91). Twenty-four hours posttransfection, Caco-2 cells were treated with 100 nM of PMA or 4α-PMA (control) for 10 min, 1 h, 2 h, 4 h, and 24 h after which the cells were washed with media containing 1% FBS. Cells were then harvested 48 h posttransfection, and the promoter activity was assessed. Results represent means ± SE of 6 separate experiments performed in triplicate and are expressed as fold increase comparing transfected cells treated with PMA with cells treated with 4α-PMA (control). *P < 0.05 compared with control.
PKC is involved in mediating the stimulatory effects of PMA on MCT1 promoter activity.
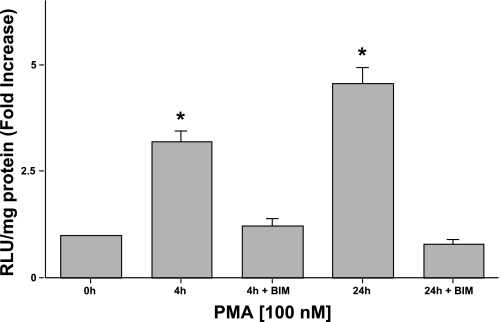
To further confirm the involvement of PKC in PMA effects on MCT1 promoter activity, the specific PKC inhibitor BIM at varying concentrations (0.25, 0.5, 1, and 5 μM) was used. Previous studies have shown that BIM at 0.25–1.0 μM concentrations is sufficient to inhibit most of the PKC isoforms except for the PKC-ζ isoform (IC50 = 5.8 μM) (38). Transiently transfected Caco-2 cells were treated with BIM for 1 h before the addition of PMA (100 nM), followed by coincubation at 4 or 24 h. BIM at 0.25, 0.5, 1, or 2 μM concentrations failed to block the stimulatory effects of PMA (4 h) on MCT1 promoter activity (Table 1), ruling out the possibility of both conventional (diacylglycerol- and Ca2+-dependent) and novel (diacylglycerol- and Ca2+-independent) PKC isoforms. However, BIM at 5 μM significantly blocked the PMA-induced effects (Fig. 4), indicating that the PMA effects could be mediated through the activation of atypical PKC-ζ isoform. Other specific PKC isoform inhibitors were also used, such as Go6976, Ro318820, and rottlerin, which have been shown to differentially inhibit conventional and novel PKCs, namely PKC-α, -ε, and -δ isoforms. Go6976 (5 nM), Ro318820 (100 nM), and rottlerin (10 μM) failed to abolish the stimulatory effects of PMA (4 h) on MCT1 promoter activity (Table 2). These results indicate a possible role of PKC-ζ in mediating the effects of PMA on MCT1 promoter activity.
Table 1.
Effect of varying concentrations of BIM on PMA-mediated stimulation of MCT1 promoter activity in Caco-2 cells
| Treatment | MCT1 Promoter Activity, Fold Increase Versus Control |
|---|---|
| PMA | 4.21±0.07* |
| PMA + BIM (0.25 μM) | 3.82±0.19 |
| PMA + BIM (0.5 μM) | 3.24±0.56 |
| PMA + BIM (1.0 μM) | 3.54±0.72 |
Values represent means ± SE of 4 separate experiments performed in triplicate and are expressed as fold increase comparing transfected cells treated with PMA with cells treated with 4α-PMA (control). Caco-2 cells were transfected with the monocarboxylate transporter 1 (MCT1) luciferase promoter construct (p-871/+91). Twenty-four hours posttransfection, Caco-2 cells were pretreated with the specific PKC inhibitor bisindolylmaleimide (BIM) for 60 min before the addition of 100 nM PMA or 4α-PMA (control) for another 4 h. Cells were then harvested 48 h posttransfection, and the promoter activity was assessed.
P < 0.05 compared with control.
Fig. 4.
PMA-induced stimulation of MCT1 promoter activity is PKC dependent. Caco-2 cells were transfected with the MCT1 luciferase promoter construct (p-871/+91). Twenty-four hours posttransfection, Caco-2 cells were pretreated with the specific PKC inhibitor bisindolylmaleimide (BIM) (5 μM) for 60 min before the addition of 100 nM PMA or 4α-PMA (control) for another 4 or 24 h. Cells were then harvested 48 h posttransfection, and the promoter activity was assessed. Results represent means ± SE of 3 separate experiments performed in triplicate and are expressed as fold increase comparing transfected cells treated with PMA with cells treated with 4α-PMA (control). *P < 0.05 compared with control.
Table 2.
Conventional and novel PKC isoforms are not involved in PMA-mediated effects
| Treatment | MCT1 Promoter Activity, Fold Increase Versus Control |
|---|---|
| PMA | 4.56±0.23* |
| PMA + Go6976 (5 nM) | 4.02±0.36 |
| PMA + Ro318820 (100 nM) | 3.86±0.76 |
| PMA + Rottlerin (10 μM) | 4.22±0.12 |
Values represent means ± SE of 3 separate experiments performed in triplicate and are expressed as fold increase comparing transfected cells treated with PMA with cells treated with 4α-PMA (control). Caco-2 cells were transfected with the MCT1 luciferase promoter construct (p-871/+91). Twenty-four hours posttransfection, Caco-2 cells were pretreated with the specific PKC isoform inhibitors for 60 min before the addition of 100 nM PMA or 4α-PMA (control) for another 4 h. Cells were then harvested 48 h posttransfection, and the promoter activity was assessed.
P < 0.05 compared with control.
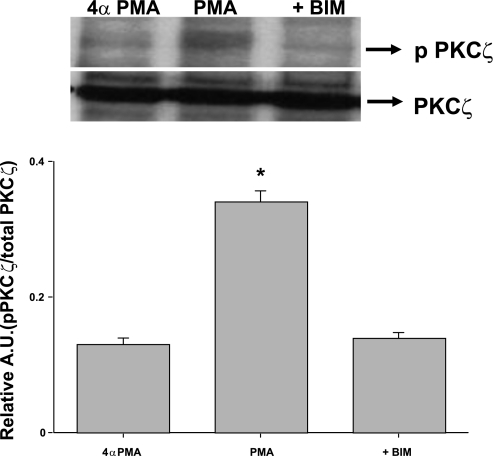
PMA induces PKC-ζ phosphorylation in Caco-2 cells.
With regards to the activation of PKC-ζ by PMA, previous published results show conflicting results. For example, PMA did not activate PKC-ζ in rat colonic epithelial cells (52), adipocytes (16), cardiomyocytes (44), and C6 glioma cells (5); however, on the contrary, studies in different cell types including epithelial rat thyroid cells (39), mouse epidermal cells (42), human leukemia cells (49), and rat adipocytes (50) have demonstrated that PMA could activate PKC-ζ. Therefore, it was considered important to examine the potential stimulation of PKC-ζ by PMA in Caco-2 cells under the conditions used in our study. Our results showed increased phosphorylation of PKC-ζ in Caco-2 cells after 1 h of PMA treatment. However, in the presence of BIM (5 μM), the increased PKC-ζ phosphorylation was significantly reduced (Fig. 5). These findings confirm that PMA activates PKC-ζ in Caco-2 cells, which is blocked by BIM and therefore suggest that PKC-ζ may be involved in mediating the stimulatory effects of PMA on MCT1 promoter activity.
Fig. 5.
PMA induces PKC-ζ phosphorylation in Caco-2 cells. Caco-2 cells were incubated with PMA or 4α-PMA (control) (100 nM) in serum-starved cell culture medium for 60 min. Cells were also pretreated with the PKC inhibitor BIM (5 μM) for 60 min and then coincubated with PMA or 4α-PMA (control) (100 nM) for another 60 min. Cells were washed with 1× PBS, lysed, and immunoprecipitated with anti-PKC-ζ antibody. Immunoprecipitates were analyzed by 8% SDS-polyacrylamide gel electrophoresis, followed by transfer of proteins to nitrocellulose and probed with anti-PKC serine/threonine pan antibody (p-PKC-ζ). PMA-induced phosphorylation of PKC-ζ at 60 min was blocked by PKC inhibitor BIM. The blots were stripped and reprobed with the anti-PKC-ζ antibody (PKC-ζ) to indicate equal loading of protein in each lane. The data were quantified by densitometric analysis and expressed as arbitrary units (A.U.) and represent means ± SE of 3 determinations. *P < 0.05 compared with 4α-PMA (control).
PKA is not involved.
Previous studies have shown that BIM at 2 μM concentration may also inhibit PKA (53). Since our results showed that BIM (5 μM) attenuated the stimulatory effects of PMA on MCT1 promoter activity, the possible role of PKA cannot be ruled out. Therefore, it was important to determine whether PKA has any role in mediating the stimulatory effects of PMA on MCT1 promoter activity. However, the specific PKA inhibitor RpcAMP (25 μM) showed no effect on the PMA-induced stimulation of MCT1 promoter activity [fold increase vs. control: 2.27 ± 0.46 (PMA) vs. 2.34 ± 0.15 (PMA + RpcAMP)]. These results suggest that PKA is not involved in mediating the stimulatory effects of PMA.
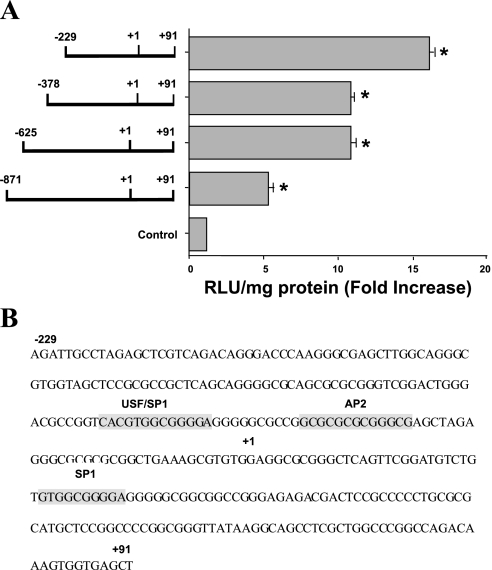
Identification of the PMA-responsive region in MCT1 promoter.
To determine which region of MCT1 promoter is responsible for PMA-induced stimulation of MCT1 promoter activity, a series of 5′-truncated MCT1-reporter constructs containing progressive deletions from the 5′-end of the full length MCT1 promoter (18) were analyzed in transiently transfected Caco-2 cells. Figure 6A depicts the promoter activity of 5′-deletion constructs in response to PMA. The full-length promoter construct p-871/+91 exhibited approximately fivefold activation in promoter activity after PMA treatment compared with 4α-PMA (control). Deletion constructs p-625/+91 and p-378/+91 showed ∼11-fold stimulation, whereas p-229/+91 showed maximal stimulation (∼16-fold) in promoter activity in response to PMA. These results suggest that a PMA-responsive element(s) is located within the shortest construct, p-229/+91. Previous studies from our laboratory identified p-229/+91 as the core promoter region required for basal regulation of MCT1 promoter. Further sequence analysis of this core promoter region (p-229/+91) indicated potential binding sites for various transcription factors, AP2, USF, and SP1 (18). The nucleotide sequence of the minimal promoter region of MCT1 promoter from −229 to +91 is shown in Fig. 6B, and the location of the potential cis-elements are indicated. Our results also indicated that the PMA-response element(s) is located within this region (p-229/+91) and that these transcription factors, AP2, USF, and SP1, might be involved in mediating the effects of PMA on MCT1 promoter activity.
Fig. 6.
Functional analysis of various deletion constructs in response to PMA. A: Caco-2 cells were transiently transfected with different 5′-deletion constructs of MCT1 promoter as described in Fig. 2. Twenty-four hours posttransfection, cells were then treated with 100 nM of PMA or 4α-PMA (control) for another 4 h in media containing 1% FBS. Cells were then harvested 48 h posttransfection, and the promoter activity was assessed. Results represent means ± SE of 4 separate experiments performed in triplicate and are expressed as fold increase comparing transfected cells treated with PMA with cells treated with 4α-PMA (control). *P < 0.05 compared with respective control. B: nucleotide sequence of the human MCT1 promoter region from −229 to +91 bp is shown, and the locations of the potential cis-elements are highlighted and labeled. AP2, activator protein 2; USF, upstream stimulatory factor.
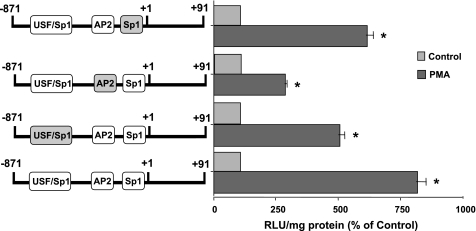
Potential AP2 site is essential for PMA-induced MCT1 promoter activity.
To map the exact location of the PMA response element in the minimal reporter region, we generated site-directed mutants of the potential binding sites for the various transcription factors in the minimal promoter region. When the potential binding regions that harbor overlapping binding sites for USF/SP1 (−118 to −106) and SP1 (−22 to −13) were mutated, stimulation of MCT1 promoter activity by PMA was decreased only by ∼37 and 25%, respectively. However, when the potential AP2 binding site (−94 to −83) was mutated, stimulation of MCT1 promoter activity in response to PMA was attenuated by 65% (Fig. 7). These results suggest the involvement of the potential AP2 site as the major contributor in mediating the stimulatory effects of PMA on MCT1 promoter activity.
Fig. 7.
Potential AP2 site is the PMA-response motif. Caco-2 cells were transiently transfected with MCT1 promoter construct (−871/+91) or AP2 or SP1 or USF/SP1 mutant (mutated sites are shown as grey boxes). Twenty-four hours posttransfection, cells were then treated with 100 nM of PMA or 4α-PMA (control) for another 4 h in media containing 1% FBS. Cells were then harvested 48 h posttransfection, and the promoter activity was assessed. Results represent means ± SE of 3 separate experiments performed in triplicate and are expressed as % of control comparing transfected cells treated with PMA with cells treated with 4α-PMA (control). *P < 0.05 compared with control.
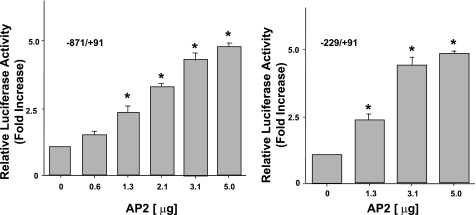
AP2 overexpression induces transactivation of MCT1 promoter activity.
To assess directly whether AP2 could activate MCT1 promoter expression in vivo, cotransfection experiments were performed in Caco-2 cells. Cotransfection of increasing concentrations of AP2 expression vector (phAP2-α) (0.6, 1.3, 2.1, 3.1, and 5.0 μg) with either MCT1 promoter-reporter constructs, p-871/+91 or p-229/+91, stimulated the MCT1 promoter activity in a dose-dependent manner (approximately two- to fourfold increase) (Fig. 8). These observations, therefore, demonstrate that upregulation of MCT1 promoter activity in response to overexpression of AP2 may be predominantly due to the AP2 binding site at position −94 to −83, leading to transcriptional activation of MCT1 promoter in both constructs.
Fig. 8.
Overexpression of AP2 stimulates MCT1 promoter activity. Caco-2 cells were transiently cotransfected with MCT1 luciferase promoter constructs p-871/+91 or p-229/+91 and increasing concentrations of AP2 expression vector (pAC-hAP2-α) along with pCMV-β vector (served as an internal control for transfection efficiency). Cells were then harvested 48 h posttransfection, and the promoter activity was assessed by measuring luciferase and β-galactosidase activities and expressed as RLU/β-galactosidase activity as described in materials and methods. Results represent means ± SE of 5 separate experiments performed in triplicate and are expressed relative to the activity (fold increase) of p-871/+91 or p-229/+91 cotransfected with the empty vector. *P < 0.05 compared with p-871/+91 or p-229/+91 (cotransfected with the empty vector).
AP2 mutant construct attenuates AP2-induced transactivation of MCT1 promoter activity.
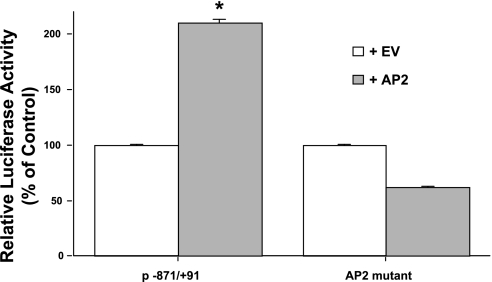
To confirm that the potential AP2 binding site regulates the transcription of MCT1 promoter in Caco-2 cells, we carried out cotransfection studies with the full-length MCT1 promoter construct (p-871/+91) and AP2 expression vector (phAP2-α) or empty vector (not expressing AP2) along with the pCMV-β-gal vector (internal control) in Caco-2 cells. Similar studies were also performed with the AP2 mutant MCT1 promoter construct (described in Fig. 7) and AP2 expression vector. The results showed that cotransfection of MCT1 promoter construct (p-871/+91) with an AP2 expression vector significantly increased MCT1 promoter activity by approximately twofold. However, when the mutated MCT1 promoter construct AP2 mutant was transfected in cells with AP2 expression vector, there was no increase in promoter activity (Fig. 9). These studies further support the fact that AP2 through interaction with the potential AP2 binding site activates MCT1 expression.
Fig. 9.
Mutations in potential AP2 site abrogates AP2-induced stimulation of MCT1 promoter activity. Caco-2 cells were transiently cotransfected with MCT1 luciferase promoter construct p-871/+91 or AP2 mutant and AP2 expression vector along with pCMV-β vector (served as an internal control for transfection efficiency). Cells were then harvested 48 h posttransfection, and the promoter activity was assessed by measuring luciferase and β-galactosidase activities and expressed as RLU/β-galactosidase activity as described in materials and methods. Results represent means ± SE of 3 separate experiments performed in triplicate and are expressed relative to the activity (% of control) of p-871/+91 or AP2 mutant cotransfected with the empty vector (EV). *P < 0.05 compared with p-871/+91 or AP2 mutant (cotransfected with the empty vector).
DISCUSSION
To better understand the mechanisms involved in the regulation of the human MCT1 gene expression, we examined the effects of the well-known diacylglycerol analog, PMA (phorbol ester) on MCT1 mRNA and promoter activity. Our results show that PMA caused a significant increase in endogenous MCT1 mRNA expression and promoter activity in human intestinal epithelial Caco-2 cells. In addition, we provide evidence that the effects of PMA on MCT1 gene expression are mediated through a PKC-dependent pathway and involve AP2 transcription factor.
In general, PMA is well known to show biphasic effects on PKC activity. Under short-term conditions PMA elicits its effects via the activation of PKC that influence the expression of various target genes (31). On the other hand, under long-term conditions, PMA is known to downregulate PKC (31). Interestingly, our wash-out experiments showed that PMA at all time points (1, 2, 4, and 24 h) significantly increased MCT1 promoter activity. However, PMA failed to show any effect at 10 min, suggesting complete removal of PMA in the wash-out experiments. These findings clearly demonstrated that the stimulatory effects of PMA on MCT1 promoter activity were mainly attributable to initial PKC activation observed at earlier time points (1 h), and this stimulation in MCT1 promoter activity persisted until 24 h. Furthermore, our inhibitor and phosphorylation studies suggested a possible role of the atypical PKC-ζ isoform in the PMA-mediated effects. Consistent with our findings, previous studies have also shown that, in addition to the conventional (PKC-α, -β, and -γ) and novel (PKC-ε and -δ) isoforms, PMA could also activate the atypical PKC-ζ isoform (9, 33, 39, 42, 49, 50, 56).
Deletion studies demonstrated that PMA significantly stimulated the activity of the 5′ progressive deletion constructs but with different magnitudes compared with the full-length MCT1 promoter construct, p-871/+91 (approximately fivefold increase). The activity of both p-625/+91 and p-378/+91 constructs were induced by ∼11-fold, whereas p-229/+91 construct was stimulated by ∼16-fold. The difference in the magnitude of fold increase observed in the full length MCT1 promoter construct (−871/+91) compared with the deletion constructs (p625/+91, p-378/+91, and p-229/+91) could be due to the presence of potential inhibitory elements between −871 to −625 bp region. These findings indicated the presence of potential PMA response elements in the shortest construct (p-229/+91) of MCT1 promoter. We have previously identified p-229/+91 as the core promoter region necessary for the basal regulation of MCT1 promoter (18). Also sequence analysis of the core promoter region revealed the presence of a number of potential cis-elements for various transcription factors such as AP2, SP1, and USF.
Since previous studies have shown that the effects of PMA on gene expression could be mediated through AP2, SP1, and USF (6–8, 24, 25, 28, 43, 51, 62), we further investigated their roles in mediating the effects of PMA on MCT1 promoter activity. Our studies showed that mutations in the potential AP2 (−94 to −83) site attenuated the activation of MCT1 promoter in response to PMA by ∼65%. However, mutations in the potential SP1 (−22 to −13) and overlapping USF/SP1 (−118 to −106) sites only modestly decreased the PMA stimulatory response by 25 and 37%, respectively. These findings suggested that the potential AP2 site might play a major role in the observed modulation of MCT1 gene expression by PMA. AP2 is a DNA-specific binding protein that serves as a transcription factor regulating the expression of a number of target genes involved in growth and differentiation (30, 48, 63). It has been reported that AP2 may function as an activator (12, 28) or as a repressor (27, 61) of gene transcription. AP2 activity is regulated in a cell type-specific manner (41) and is induced by phorbol esters, retinoic acid, and cAMP (41, 58, 59). Our results are similar to the previous findings demonstrating that AP2 also plays an important role in the activation of the mouse Na+/H+ exchanger (NHE)1 gene expression (12) and human NHE3 promoter (36). However, our recent studies (37) showed that PMA-induced stimulation of NHE3 promoter activity was not dependent on PKC, as also shown by several other studies (4, 13, 14, 55).
The direct involvement of AP2 in MCT1 transcriptional regulation was further confirmed by cotransfection studies with AP2 expression vector. We demonstrated that AP2 caused a dose-dependent activation of MCT1 promoter activity in transfected Caco-2 cells. Interestingly, the stimulatory effect of AP2 on MCT1 promoter activity was not observed when AP2 expression vector was cotransfected with a construct harboring a mutated AP2 binding site. Taken together, these observations indicate the importance of the AP2 motif in the regulation of MCT1 promoter by PMA. Since AP2 is involved in a number of cellular events including growth and differentiation (30, 48, 63), we hypothesize that AP2 may be involved in the induction of MCT1 expression during differentiation because previous studies have shown increased MCT1 protein expression in surface epithelial cells compared with crypt cells of the human colon (35). Since our present studies showed that mutations in the overlapping USF/SP1 and SP1 sites slightly decreased the stimulatory response of PMA on MCT1 promoter activity, we cannot completely rule out the involvement of USF/SP1 and SP1 sites in partly mediating the stimulatory effects of PMA along with AP2. Also recent studies from our laboratory and others have shown that AP2 and SP1 together are essential for the basal regulation of the human NHE3 promoter in intestinal epithelial cells (36) and mouse ganglioside GM3 promoter in neuroblastoma cells (60).
In summary, our data demonstrated that intestinal MCT1 gene expression in response to PMA is regulated at the transcriptional level and is dependent on PKC activation. We also demonstrated the role of specific transcription factor AP2 in the PMA-induced upregulation of MCT1 promoter. Our findings suggest that PKC-ζ and AP2 may be involved in the upregulation of MCT1 gene expression in human intestinal epithelial cells and are therefore of significant importance for a better understanding of the molecular regulation of SCFA absorption in the human intestine.
GRANTS
These studies were supported by the Department of Veterans Affairs and the NIDDK grants DK 54016 (to P. Dudeja), DK 33349 (to K. Ramaswamy), DK 71596 (to W. Alrefai), and P01 DK 067887 (to P. Dudeja, K. Ramaswamy, and J. Malakooti).
Acknowledgments
We thank Dr. Reinhard Buettner (Institute of Pathology, University of Bonn Medical School, Bonn, Germany) for providing the human AP2-α expression vector.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alrefai WA, Tyagi S, Gill R, Saksena S, Hadjiagapiou C, Mansour F, Ramaswamy K, Dudeja PK. Regulation of butyrate uptake in Caco-2 cells by phorbol 12-myristate 13-acetate. Am J Physiol Gastrointest Liver Physiol 286: G197–G203, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Binder HJ, Mehta P. Short-chain fatty acids stimulate active Na and Cl absorption in vitro in the rat distal colon. Gastroenterology 96: 989–996, 1989. [DOI] [PubMed] [Google Scholar]
- 3.Bradford M A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 4.Caloca MJ, Garcia-Bermejo ML, Blumberg PM, Lewin NE, Kremmer E, Mischak H, Wang S, Nacro K, Bienfait B, Marquez VE, Kazanietz MG. Beta2-chimaerin is a novel target for diacylglycerol: binding properties and changes in subcellular localization mediated by ligand binding to its C1 domain. Proc Natl Acad Sci USA 96: 11854–11859, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen CC Protein kinase C alpha, delta, epsilon and zeta in C6 glioma cells. TPA induces translocation and down-regulation of conventional and new PKC isoforms but not atypical PKC zeta. FEBS Lett 332: 169–173, 1993. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Malcolm T, Estable MC, Roeder RG, Sadowski I. TFII-I regulates induction of chromosomally integrated human immunodeficiency virus type 1 long terminal repeat in cooperation with USF. J Virol 79: 4396–4406, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou SF, Chen HL, Lu SC. Sp1 and Sp3 are involved in up-regulation of human deoxyribonuclease II transcription during differentiation of HL-60 cells. Eur J Biochem 270: 1855–1862, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Clark JH, Haridasse V, Glazer RI. Modulation of the human protein kinase C alpha gene promoter by activator protein-2. Biochemistry 41: 11847–11856, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Crabos M, Fabbro D, Stabel S, Erne P. Effect of tumour-promoting phorbol ester, thrombin and vasopressin on translocation of three distinct protein kinase C isoforms in human platelets and regulation by calcium. Biochem J 288: 891–896, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuff MA, Lambert DW, and Shirazi-Beechey SP. Substrate-induced regulation of the human colonic monocarboxylate transporter, MCT. J Physiol 539: 361–371, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuff MA, and Shirazi-Beechey SP. The human monocarboxylate transporter, MCT1: genomic organization and promoter analysis. Biochem Biophys Res Commun 292: 1048–1056, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Dyck JR, Silva NL, Fliegel L. Activation of the Na+/H+ exchanger gene by the transcription factor AP-2. J Biol Chem 270: 1375–1381, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Ebinu JO, Bottorff DA, Chan EY, Stang SL, Dunn RJ, Stone JC. RasGRP, a Ras guanyl nucleotide-releasing protein with calcium- and diacylglycerol-binding motifs. Science 280: 1082–1086, 1998. [DOI] [PubMed] [Google Scholar]
- 14.El-Shemerly MY, Besser D, Nagasawa M, Nagamine Y. 12-O-Tetradecanoylphorbol-13-acetate activates the Ras/extracellular signal-regulated kinase (ERK) signaling pathway upstream of SOS involving serine phosphorylation of Shc in NIH3T3 cells. J Biol Chem 272: 30599–30602, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Enoki T, Yoshida Y, Lally J, Hatta H, Bonen A. Testosterone increases lactate transport, monocarboxylate transporter (MCT) 1 and MCT4 in rat skeletal muscle. J Physiol 577: 433–443, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farese RV, Standaert ML, Francois AJ, Ways K, Arnold TP, Hernandez H, Cooper DR. Effects of insulin and phorbol esters on subcellular distribution of protein kinase C isoforms in rat adipocytes. Biochem J 288: 319–323, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia CK, Li X, Luna J, Francke U. cDNA cloning of the human monocarboxylate transporter 1 and chromosomal localization of the SLC16A1 locus to 1p13.2-p12. Genomics 23: 500–503, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Hadjiagapiou C, Borthakur A, Dahdal RY, Gill RK, Malakooti J, Ramaswamy K, Dudeja PK. Role of USF1 and USF2 as potential repressor proteins for human intestinal monocarboxylate transporter 1 promoter. Am J Physiol Gastrointest Liver Physiol 288: G1118–G1126, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Hadjiagapiou C, Dahlal R, Ramaswamy K, Dudeja PK. Molecular cloning of the human monocarboxylate transporter 1 (MCT1) promoter (Abstract). Gastroenterology 122: W945, 2002. [Google Scholar]
- 20.Hague A, Elder DJ, Hicks DJ, Paraskeva C. Apoptosis in colorectal tumour cells: induction by the short chain fatty acids butyrate, propionate and acetate and by the bile salt deoxycholate. Int J Cancer 60: 400–406, 1995. [DOI] [PubMed] [Google Scholar]
- 21.Halestrap AP, Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflügers Arch 447: 619–628, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J 343: 281–299, 1999. [PMC free article] [PubMed] [Google Scholar]
- 23.Heerdt BG, Houston MA, Augenlicht LH. Potentiation by specific short-chain fatty acids of differentiation and apoptosis in human colonic carcinoma cell lines. Cancer Res 54: 3288–3293, 1994. [PubMed] [Google Scholar]
- 24.Hyman SE, Comb M, Pearlberg J, Goodman HM. An AP-2 element acts synergistically with the cyclic AMP- and phorbol ester-inducible enhancer of the human proenkephalin gene. Mol Cell Biol 9: 321–324, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imagawa M, Chiu R, Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell 51: 251–260, 1987. [DOI] [PubMed] [Google Scholar]
- 26.Inan MS, Rasoulpour RJ, Yin L, Hubbard AK, Rosenberg DW, Giardina C. The luminal short-chain fatty acid butyrate modulates NF-kappaB activity in a human colonic epithelial cell line. Gastroenterology 118: 724–734, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Jiang JG, DeFrances MC, Machen J, Johnson C, Zarnegar R. The repressive function of AP2 transcription factor on the hepatocyte growth factor gene promoter. Biochem Biophys Res Commun 272: 882–886, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Johnson AC Activation of epidermal growth factor receptor gene transcription by phorbol 12-myristate 13-acetate is mediated by activator protein 2. J Biol Chem 271: 3033–3038, 1996. [PubMed] [Google Scholar]
- 29.Kang KW, Jin MJ, Han HK. IGF-I receptor gene activation enhanced the expression of monocarboxylic acid transporter 1 in hepatocarcinoma cells. Biochem Biophys Res Commun 342: 1352–1355, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Kannan P, Buettner R, Chiao PJ, Yim SO, Sarkiss M, Tainsky MA. N-ras oncogene causes AP-2 transcriptional self-interference, which leads to transformation. Genes Dev 8: 1258–1269, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Khare S, Bissonnette M, Scaglione-Sewell B, Wali RK, Sitrin MD, Brasitus TA. 1,25-dihydroxyvitamin D3 and TPA activate phospholipase D in Caco-2 cells: role of PKC-α. Am J Physiol Gastrointest Liver Physiol 276: G993–G1004, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Kirat D, Kato S. Monocarboxylate transporter 1 (MCT1) mediates transport of short-chain fatty acids in bovine caecum. Exp Physiol 91: 835–844, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi Y, Bridle KR, Ramm GA, O'Neill R, Britton RS, Bacon BR. Effect of phorbol ester and platelet-derived growth factor on protein kinase C in rat hepatic stellate cells. Liver Int 27: 1066–1075, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Kvale D, Brandtzaeg P. Constitutive and cytokine induced expression of HLA molecules, secretory component, and intercellular adhesion molecule-1 is modulated by butyrate in the colonic epithelial cell line HT-29. Gut 36: 737–742, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambert DW, Wood IS, Ellis A, Shirazi-Beechey SP. Molecular changes in the expression of human colonic nutrient transporters during the transition from normality to malignancy. Br J Cancer 86: 1262–1269, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malakooti J, Memark VC, Dudeja PK, Ramaswamy K. Molecular cloning and functional analysis of the human Na+/H+ exchanger NHE3 promoter. Am J Physiol Gastrointest Liver Physiol 282: G491–G500, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Malakooti J, Sandoval R, Amin MR, Clark J, Dudeja PK, Ramaswamy K. Transcriptional stimulation of the human NHE3 promoter activity by PMA: PKC independence and involvement of the transcription factor EGR-1. Biochem J 396: 327–336, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem 268: 9194–9197, 1993. [PubMed] [Google Scholar]
- 39.Matowe WC, Gupta S, Ginsberg J. Regulation of protein kinase C isoforms in FRTL-5 thyroid cells by TSH and phorbol ester. Thyroid 6: 53–58, 1996. [DOI] [PubMed] [Google Scholar]
- 40.Medina J, Acin A, Prieto J. Molecular cloning and characterization of the human AE2 anion exchanger (SLC4A2) gene. Genomics 39: 74–85, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell PJ, Timmons PM, Hebert JM, Rigby PW, Tjian R. Transcription factor AP-2 is expressed in neural crest cell lineages during mouse embryogenesis. Genes Dev 5: 105–119, 1991. [DOI] [PubMed] [Google Scholar]
- 42.Nishikawa K, Yamamoto S, Nagumo H, Maruyama K, Kato R. The presence of phorbol ester responsive and non-responsive forms of the zeta isozyme of protein kinase C in mouse epidermal cells. Cell Signal 7: 491–504, 1995. [DOI] [PubMed] [Google Scholar]
- 43.Osaki F, Ikeda Y, Suehiro T, Ota K, Tsuzura S, Arii K, Kumon Y, Hashimoto K. Roles of Sp1 and protein kinase C in regulation of human serum paraoxonase 1 (PON1) gene transcription in HepG2 cells. Atherosclerosis 176: 279–287, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Puceat M, Hilal-Dandan R, Strulovici B, Brunton LL, Brown JH. Differential regulation of protein kinase C isoforms in isolated neonatal and adult rat cardiomyocytes. J Biol Chem 269: 16938–16944, 1994. [PubMed] [Google Scholar]
- 45.Resta-Lenert S, Truong F, Barrett KE, Eckmann L. Inhibition of epithelial chloride secretion by butyrate: role of reduced adenylyl cyclase expression and activity. Am J Physiol Cell Physiol 281: C1837–C1849, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Ritzhaupt A, Wood IS, Ellis A, Hosie KB, Shirazi-Beechey SP. Identification and characterization of a monocarboxylate transporter. J Physiol 513: 719–732, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roediger WEW Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology 83: 424–429, 1982. [PubMed] [Google Scholar]
- 48.Schorle H, Meier P, Buchert M, Jaenisch R, Mitchell PJ. Transcription factor AP-2 essential for cranial closure and craniofacial development. Nature 381: 235–238, 1996. [DOI] [PubMed] [Google Scholar]
- 49.Schwende H, Fitzke E, Ambs P, Dieter P. Differences in the state of differentiation of THP-1 cells induced by phorbol ester and 1,25-dihydroxyvitamin D3. J Leukoc Biol 59: 555–561, 1996. [PubMed] [Google Scholar]
- 50.Standaert ML, Cooper DR, Hernandez H, Arnold TP, Farese RV. Differential down-regulation of insulin-sensitive protein kinase-C isoforms by 12-O-tetradecanoylphorbol-13-acetate in rat adipocytes and BC3H-1 myocytes. Endocrinology 132: 689–692, 1993. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka T, Kurabayashi M, Aihara Y, Ohyama Y, Nagai R. Inducible expression of manganese superoxide dismutase by phorbol 12-myristate 13-acetate is mediated by Sp1 in endothelial cells. Arterioscler Thromb Vasc Biol 20: 392–401, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Tepperman BL, Chang Q, Soper BD. Protein kinase C mediates lipopolysaccharide- and phorbol-induced nitric-oxide synthase activity and cellular injury in the rat colon. J Pharmacol Exp Ther 295: 1249–1257, 2000. [PubMed] [Google Scholar]
- 53.Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Duhamel L, Charon D, Kirilovsky J. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem 266: 15771–15781, 1991. [PubMed] [Google Scholar]
- 54.Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem 281: 9030–9037, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Van Lint J, Rykx A, Maeda Y, Vantus T, Sturany S, Malhotra V, Vandenheede JR, Seufferlein T. Protein kinase D: an intracellular traffic regulator on the move. Trends Cell Biol 12: 193–200, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Visnjic D, Batinic D, Lasic Z, Knotek M, Marusic M, Banfic H. Phorbol 12-myristate 13-acetate-mediated signalling in murine bone marrow cells. Biochem J 310: 163–170, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whitehead RH, Young GP, Bhathal PS. Effects of short chain fatty acids on a new human colon carcinoma cell line (LIM1215). Gut 27: 1457–1463, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams T, Admon A, Luscher B, Tjian R. Cloning and expression of AP-2, a cell-type-specific transcription factor that activates inducible enhancer elements. Genes Dev 2: 1557–1569, 1988. [DOI] [PubMed] [Google Scholar]
- 59.Williams T, Tjian R. Analysis of the DNA-binding and activation properties of the human transcription factor AP-2. Genes Dev 5: 670–682, 1991. [DOI] [PubMed] [Google Scholar]
- 60.Xia T, Zeng G, Gao L, Yu RK. Sp1 and AP2 enhance promoter activity of the mouse GM3-synthase gene. Gene 351: 109–118, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Xu Y, Porntadavity S, St. Clair DK. Transcriptional regulation of the human manganese superoxide dismutase gene: the role of specificity protein 1 (Sp1) and activating protein-2 (AP-2). Biochem J 362: 401–412, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang Y, Tesmer VM, Bina M. Regulation of HIV-1 transcription in activated monocyte macrophages. Virology 299: 256–265, 2002. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J, Hagopian-Donaldson S, Serbedzija G, Elsemore J, Plehn-Dujowich D, McMahon AP, Flavell RA, Williams T. Neural tube, skeletal and body wall defects in mice lacking transcription factor AP-2. Nature 381: 238–241, 1996. [DOI] [PubMed] [Google Scholar]