Abstract
The route of absorption of ingested compounds is a determinant of their distribution and metabolism. Portal vein absorption results in direct transport to the liver, where metabolism may take place before extrahepatic delivery. Lymphatic absorption can result in delivery of parent compound to nonhepatic tissues. Understanding the fate of an ingested compound requires determination of the importance of each of these routes. Portal vein absorption can be estimated from the difference in concentrations of an ingested compound between the portal vein and peripheral vessel blood. To make these estimations, one must make assumptions on the basis of estimates of flow rate and dilution. We report here methodology that allows a direct measurement of portal vein absorption that is independent of these assumptions. Mesenteric lymph was diverted from rats by cannulation. Portal blood was sampled after duodenal infusion of a bolus of compound of interest along with a portal absorption marker, 3-O-methylglucose. Since lymph was diverted, the appearance in portal blood was solely the result of portal absorption. Absorption was quantified by the areas under the curve for the compound and marker. Portal absorption was a function of the octanol/water partition coefficients for four organochlorine compounds: hexachlorobenzene, pentachlorophenol, DDT, and its metabolite 1,1,1-trichloro-2,2-bischlorophenylethylene.
Keywords: mesenteric lymph duct; DDT; 1,1,1-trichloro-2,2-bischlorophenylethylene; hexachlorobenzene; pentachlorophenol; 3-O-methylglucose
within the last century, compounds with multiple carbon-halogen bonds have been introduced into the biosphere both as functional chemicals and as industrial byproducts. Many of these compounds are classified as toxins and carcinogens, and the high stability of their carbon-halogen bonds has resulted in their persistent presence in the environment. They are present in many species of flora and fauna, and measurements indicate that they are present in all humans (1, 9). They are found in breast milk (10), and they are known to have an effect in neurological development (15). Although the “no-effect” levels of these compounds in humans are not known, it is generally assumed that many potentially present a risk to health.
Despite the knowledge that the principal route of their entry into the body is in the diet, there have been relatively few investigations of the processes involved in their absorption from the intestine. It has been assumed that organochlorine compounds (OCs) are absorbed with fat in the diet and therefore accompany the fat in chylomicrons into lymph. Lymphatic absorption has been reported for DDT (2) and hexachlorobenzene (HCB) (8). There is also evidence that some DDT is absorbed in part via the portal vein (2). However, the study of the route of delivery of OCs has generally been ignored.
Since the route of delivery can be extremely important to the fate of a compound that is absorbed from the diet, we have developed methodology that allows the simultaneous measurement of entry by the lymphatic system and portal vein in the rat. The method involves the periodic sampling of portal blood from an animal with a mesenteric lymph duct fistula. To provide a quantitative estimate of the appearance in portal blood, a marker (3-O-methyl glucose) that is absorbed via the portal vein is given along with the OC of interest (23). To characterize the absorbed OC further, we have determined the distribution of the OC among red cells and lipoproteins in the portal blood plasma.
OCs that are available for absorption enter the intestine with diets as well as part of the enterohepatic circulation in bile, sloughed enterocytes, and possibly direct exudates from the enterocytes. These participants in enterohepatic circulation can include metabolites and parent compounds. Unlike aromatic hydrocarbons that enter the enterocyte (13), there is no evidence for metabolism of OCs in the enterocyte.
We have applied the method of simultaneous portal and lymphatic measurement to two OCs, HCB, and DDT. Since the OCs can be metabolized to various metabolites, which are also absorbed via the enterohepatic circulation, portal and lymphatic measurements of the principal metabolites of HCB and DDT, 1,1,1-trichloro-2,2-bischlorophenylethylene (DDE) and pentachlorophenol (PCP), respectively, were also measured. These compounds span a range of lipophilicity as defined by their octanol/water partition coefficients given in Table 1.
Table 1.
Octanol/water partition coefficients of compounds studied
| Compound | Log10P(octanol/water partition coefficient) | Reference |
|---|---|---|
| HCB | 5.7 | Fisk, 1999 |
| DDT | 6.91 | deBruijn, 1989 |
| DDE | 6.96 | deBruijn, 1989 |
| PCP | 3.5 at pH 7.0 | Klaus, 1982 |
HCB, hexachlorobenzene; DDE, 1,1,1-trichloro-2,2-bischlorophenylethylene; PCP, pentachlorophenol.
We report here the results of the application of this method to quantitatively estimate the contribution of the portal vein to the absorption of these compounds. The method is clearly applicable to other OCs and also to other materials of interest including pharmaceuticals.
MATERIALS AND METHODS
Materials
HCB-UL-14C ([14C]HCB); 4,4′-DDT-Ring-UL-14C; 1,1,1-Trichloro-2,2-bis[4-chlorophenyl]ethane ([14C]DDT); 4,4′-DDE-Ring-UL-14C; 1,1,1-Trichloro-2,2-bis[4-chlorophenyl]ethylene ([14C]DDE); PCP-UL-14C ([14C]PCP); and 3-O-(3H-methyl)-d-glucose ([3H]OMG) were obtained from Sigma-Aldrich (St. Louis, MO). Triolein [9,10-3H(N)] was purchased from Perkin Elmer (Waltham, MA). Specific activity of the HCB, DDT, and DDE was 10–30 mC/mmol and of PCP, 1–15 mCi/mmol. The specific activity of the triolein and OMG was 60–90 Ci/mmol. Doses of 5–10 μCi of HCB, DDT, DDE, PCP, and triolein were dissolved in olive oil and given by duodenal infusion of a bolus of 100 μl of the oil. A similar amount of radioactivity as [3H]OMG was dissolved in 1.0 ml of water and delivered by duodenal infusion as a bolus dose immediately after the infusion of the oil. Aliquots of the dosing solution were taken during the procedure to assay the delivered dose. The gavage was immediately followed by a bolus of 0.5 ml of saline solution. In a preliminary study of tissue deposition in lymph-cannulated rats, the material was delivered by infusion pump over the 6 h of the lymph collection period.
Animals
Young, adult, male, Sprague-Dawley rats weighing ∼350 g were used in all studies. They were purchased from Harlan (Indianapolis, IN) and were maintained in our vivarium on rat chow in a room with light cycling (12-h light:12-h dark) for ∼2 wk before experimentation. After 2 wk, the portal vein was cannulated followed by cannulation of the mesenteric lymph duct. The two surgeries were separated by three days. The description of the surgeries is given below. Lymph was collected for 2 h in the study of HCB and for 6 h in the studies of the other compounds. At the conclusion of the lymph collection, the animals were euthanized with pentobarbital sodium overdose, and tissue samples were taken. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati.
Portal Vein Cannulation
The animals were fasted overnight. The animals were anesthetized with isoflurane [2-chloro-2-(difluoromethoxy)-1,1,1-trifluoro-ethane] and surgically prepared (clipped and scrubbed). A ventral midline incision was made to expose the abdominal viscera to allow the placement of a tube in the portal vein via the ileocolic vein. This placement did not occlude the flow of blood. The cannula used was Micro-Renathane type MRE-033 (OD 0.033 inches, ID 0.014 inches) manufactured by Braintree Scientific (Braintree, MA). The incision was closed by first closing the muscle layer followed by the skin layer. The portal vein cannula was heparinized, closed with a knot, and placed inside the peritoneal cavity. It was exteriorized at the time of lymphatic surgery to allow blood withdrawal. At that time the animals were in restraining cages that prevented their access to this cannula.
Analgesic (Buprenex) was given to alleviate pain associated with surgery. The animals were observed for 3 days and allowed access to chow. Only animals with body weight that returned to the approximate level of the presurgical weight, with no apparent blood loss attributable to portal vein cannulation were used for lymphatic cannulation. Animals not meeting this recovery criterion were euthanized. Portal blood flow was not interrupted by periodic sampling of samples of 100 μl of blood taken by a syringe inserted into the cannula. A total of six blood samples was taken from each animal.
Lymphatic Cannulation
The animals were fasted overnight before lymph duct cannulation surgery to facilitate the viewing of the lymphatic duct. Preoperative analgesic (Buprenex) was administered, and the animals were anesthetized and surgically prepared (clipped and scrubbed). The major lymphatic duct was cannulated according to the procedure as described by Bollman, Cain, and Grindlay (22) with slight modification. Instead of suture, a drop of tissue glue was used to secure the lymphatic cannula. A soft silicone tube was introduced about 1 cm down the duodenum through the fundus of the stomach. The duodenal infusion tube was secured in the duodenum through a transmural suture, and the fundal incision was closed. Following surgery, the animals were infused via the duodenal tube with a saline solution containing 5% glucose. The animals were allowed to recover overnight in restraining cages (Bollman cages) that were kept warm in a chamber (∼30°C). Although the animals were restrained, they had considerable freedom to move backwards, forwards, and sideways. Analgesics were provided to alleviate pain. Isotope-labeled test substances/markers were administered via the duodenal tube. Lymph was collected for 4 h in the study of HCB and DDT and for 6 h in the studies of PCP and DDE. The animals were then euthanized. In the preliminary study of indirect measurement of portal absorption, only lymphatic cannulation was performed.
Analysis of Tissues
Tissues and red blood cells (RBCs) were burned at 900°C in an oxidizer (Harvey Biological Oxidizer OX700; R.J. Harvey, Hillsdale, NJ), and [14C]CO2 and [3H]H2O were collected directly in scintillation fluid. Plasma and lymph samples were mixed directly in scintillation fluid for assay. The radioactivity was assayed by scintillation counting.
Lipoprotein Separation
Portal vein pooled plasma samples were separated by ultracentrifugation with serial sequences of KBr solutions of densities 1.021 and 1.063 g/ml (40 h; 49,000 revolution/min, 50.3 Ti rotor) A third spin for 72 h at density 1.210 g/ml separated HDL from the albumin-rich fraction.
Statistical Analyses
Statistical analyses (analysis of variance, Tukey) and areas under the curve (AUCs) were calculated with Sigmaplot. Significance was accepted with P < 0.05. All errors are presented as standard errors.
RESULTS
Preliminary Study of HCB in Tissues and RBCs from Rats with Lymphatic Cannulation
Duodenal and intragastric infusion comparison.
Preliminary comparison of duodenal and intragastric infusion of HCB on the appearance of HCB in the lymph of rats showed the delivery modes to be equivalent. In each case, the percent of the HCB dose in lymph after 24 h was markedly less than that of triolein, which was given simultaneously. Intragastric infusion resulted in HCB absorption of 26.3% of that of triolein (54% of its dose), and intraduodenal infusion resulted in HCB absorption equal to 26.8% of the triolein (74% of its dose). In both cases, the dose of HCB and triolein remaining in the stomach and intestinal contents was ∼1% of the infused dose when the animals were euthanized at the conclusion of the lymph collection. Subsequent studies utilized intraduodenal infusion.
HCB in RBCs.
Six rats were given a bolus of 0.5 ml of olive oil containing [14C]HCB. Three animals were euthanized after 24 h and 3 animals, after 48 h. Samples of blood were taken from the heart. The distribution of 14C between plasma and RBCs was measured, and 85 ± 3.1% was found in the red cells in the 24-h sample and 87 ± 2.6%, after 48 h. This affinity of HCB for RBC membranes is consistent with the report of Gomez-Catalan and coworkers (4).
Preliminary study of the appearance of HCB in tissues of lymph-diverted rats.
Two additional rats were fitted with a mesenteric lymph cannula (to provide chylomicrons to recipient animals) as reported previously (8) and gastrically intubated with 1 ml of olive oil containing 10 μCi of [14C]HCB and 50 μCi of [3H]triolein. The animals were euthanized at the completion of the lymphatic collection, and tissues were analyzed for 14C and 3H. We found markedly higher concentrations of HCB relative to that of triolein in the tissues of both animals. The concentration of HCB was 4–11 times higher in the liver and 60–200 times higher in the fat pad. The appearance of HCB was also markedly greater than that of triolein in the heart, spleen, muscle, and abdominal fat (data not shown).
Simultaneous Measurement of OCs in Lymph and Portal Blood
HCB.
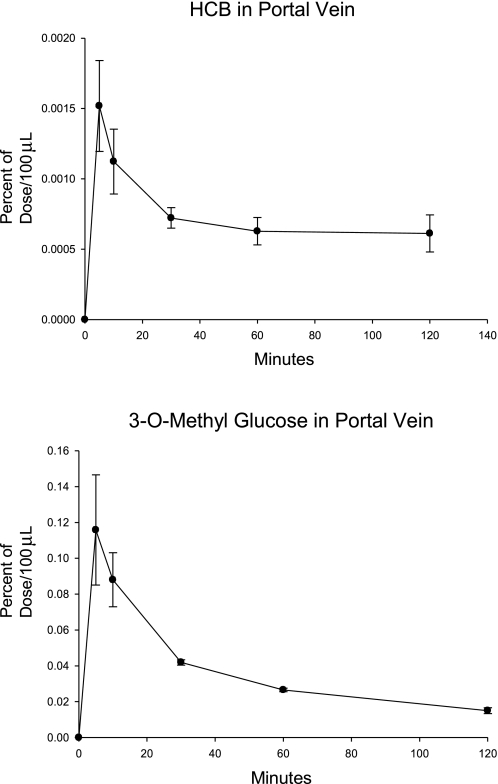
Five rats were fitted with cannulas in the portal vein, and after their recovery each underwent cannulation of the mesenteric lymph duct to divert lymph from the animals. Duodenal infusion cannulas were also surgically inserted. They received an intraduodenal gavage with 100 μl of olive oil, containing [14C]HCB. Within 30 s of the oil gavage, they received an aqueous infusion of [3H]OMG in 100 μl of water. This compound was reported to be absorbed completely by the portal vein (23). Periodic samples of portal vein blood were obtained for 120 min, and 14C and 3H were measured. The [14C]HCB and [3H]OMG in portal vein plasma are shown in Fig. 1. The HCB appearance was ∼2% of that of the [3H]OMG on the basis of AUCs of the portal vein plasma.
Fig. 1.
[14C]hexachlorobenzene (HCB) and [3H]3-O-methylglucose (OMG) in portal vein plasma. Rats fitted with both mesenteric lymph and portal vein cannulas were gavaged with a bolus of the isotope-labeled compounds. Periodic samples of 100 μl of blood were analyzed (n = 4; means ± SE shown).
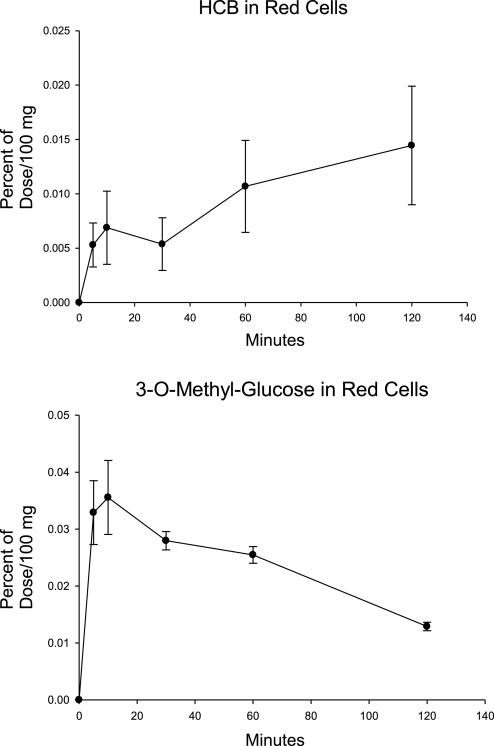
Preliminary measurements of HCB in blood from rats given an oral bolus of [14C]HCB indicated that >85% of the HCB was located in the RBCs (data not shown). We therefore measured HCB and OMG in the RBCs from the portal vein blood. The RBC levels of [14C]HCB are given in Fig. 2.
Fig. 2.
The appearance of [14C]HCB and [3H]OMG in red blood cells (RBCs). RBCs from the portal vein blood from the study described in Fig. 1 were analyzed.
On the basis of the high concentration in RBCs relative to that in plasma, it is evident that the [14C]HCB carried in the RBCs accounted for most of the radioactivity in the blood. On the basis of the AUCs combining both plasma and RBCs during the first 30 min, the HCB recovery was 11.0 ± 4.2% (mean ± SE) of that of the OMG.
DDT.
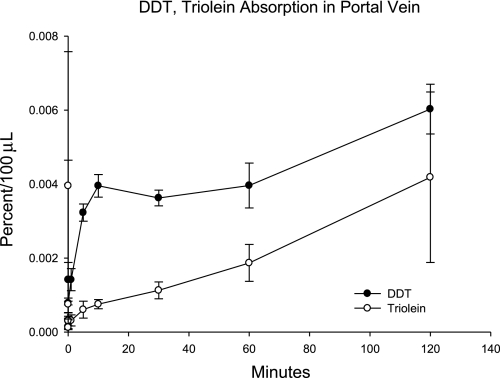
Four rats with cannulated mesenteric lymph ducts and portal veins were given a bolus dose of [14C] DT and [3H]triolein in olive oil into the duodenum. Radioactivity in lymph, plasma, RBCs, and tissues was measured. One animal had only traces of 14C in lymph, and the measurements from its lymph and tissues were not included in the analyses. The appearance of 14C and 3H in portal blood plasma is shown in Fig. 3.
Fig. 3.
The appearance of [14C]DDT and [3H]triolein in portal blood plasma after duodenal infusion. The study was carried out as described in Fig. 1 (n = 4; means ± SE shown), except that [3H]triolein, which is normally entirely absorbed lymphatically, was used as a marker instead of [3H]OMG.
DDT in the 4-h lymph collection accounted for 29.4 ± 6.8% of the dose, which was not different (P = 0.08) from that of the lymphatic triolein, 50.9 ± 8.1. However, the lymphatic appearance of DDT in the subsequent study with the OMG marker described below was 31.0 ± 8.0, and the pooled values from the two studies gave a mean of 30.0 ± 4.7, which was less than that of the triolein (P < 0.05). Although this result is consistent with incomplete lymphatic absorption for DDT, it should be noted that the value is for 4 h of collection, and absorption of lipids is generally not complete for at least 24 h. The radioactivity remaining in the lumen of the intestine at the end of the study was also not different between the two groups, with 16.0 ± 5.6 and 8.4 ± 3.15% of the dose found for the DDT and triolein groups, respectively. The concentration of DDT in RBCs was approximately equal to 20% of that in plasma, which was greater than that of triolein.
The appearances of DDT and triolein in the epididymal fat pad, liver, and brain are presented in Table 2. In each tissue the concentration of 14C was significantly greater than that of the concentration of 3H in terms of percent of dose per gram of tissue.
Table 2.
Tissue levels of DDT and triolein in rats with mesenteric lymph duct cannulation
| Tissue | DDT, % of dose/g | Triolein, % of dose/g |
|---|---|---|
| Fat Pad | 0.231±0.0.040 | 0.014±0.003 |
| Liver | 0.704±0.057 | 0.0716±0.017 |
| Brain | 0.096±0.016 | 0.030±0.007 |
Values are means ± SE.
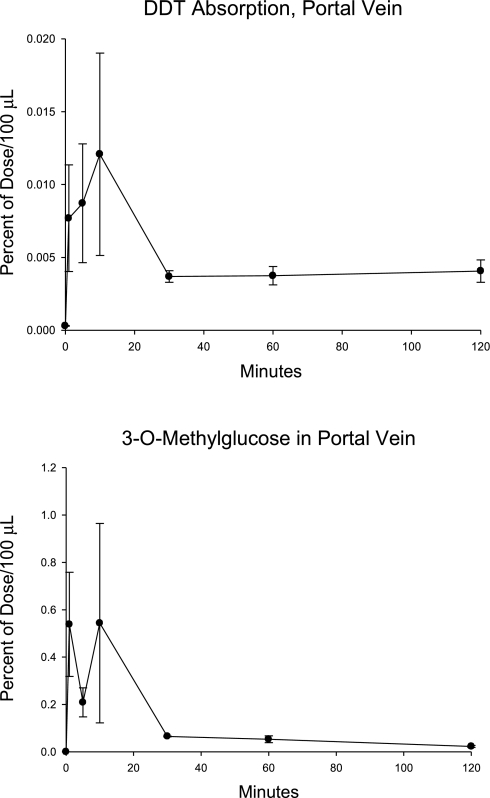
[14C]DDT was also administered to four animals with lymphatic and portal vein cannulas, which then immediately received a bolus of an aqueous solution of [3H]OMG. The appearances of isotopes in the portal blood, lymph, and tissues were measured. In this study, [14C]DDT was present at a mean level corresponding to 3.00 ± 0.43% of that of the [3H]OMG. As noted above, the lymphatic appearance of [14C]DDT was similar to that observed when triolein was simultaneously given. The radioactivity in the portal vein is shown in Fig. 4. The appearances of [14C]DDT and [3H]OMG in the fat pad, liver, and brain of the animals are given in Table 3.
Fig. 4.
The appearance of [14C]DDT and [3H]OMG in portal vein plasma after intraduodenal administration. The study was carried out as described in Fig. 1 (n = 4; means ± SE shown).
Table 3.
Tissue levels of DDT and OMG in rats with mesenteric lymph duct cannulation
| Tissue | DDT, % of dose/g | OMG, % of dose/g |
|---|---|---|
| Fat Pad | 0.231±0.0.053 | 0.095±0.028 |
| Liver | 0.707±0.028 | 0.1169±0.020 |
| Brain | 0.083±0.011 | 0.077±0.040 |
Values are means ± SE. OMG, 3-O-methylglucose.
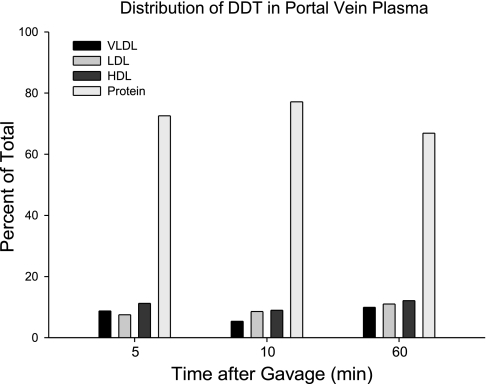
The distribution of 14C among the lipoprotein fractions is presented in Fig. 5. The majority of the radioactivity was carried in the fraction of proteins with density greater than that of HDL.
Fig. 5.
The relative distribution of [14C]DDT among plasma lipoproteins sampled from the portal vein at times after gavage. Plasma from the animals in the study described in Fig. 4 was pooled, separated by ultracentrifugation with solutions of KBr, and assayed for radioactivity.
PCP.
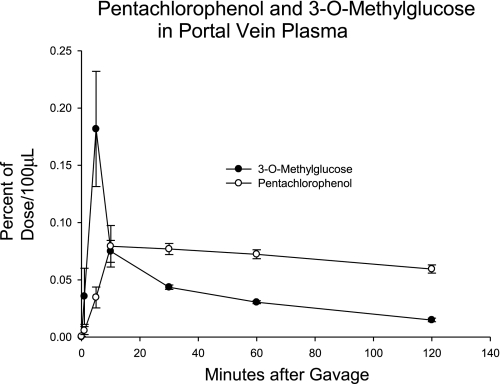
Seven animals were fitted with lymphatic and portal vein cannulation as described above and were gavaged with [14C]PCP and then with [3H]OMG. The appearance in the portal plasma is presented in Fig. 6. In 6 h of lymph collection, the PCP was 2.0 ± 0.4% of dose, less than that of the OMG, 5.5 ± 0.1%. On the basis of the AUCs for 30 min, the amount of PCP was 90.8 ± 15.2% of that of the OMG.
Fig. 6.
The appearance of [14C]pentachlorophenol (PCP) and [3H]OMG in portal vein plasma. The study was carried out as described in Fig. 1 (n = 7; means ± SE shown).
Low levels of PCP (0.235 ± 0.027% dose/g) were found in the fat pad, but these were significantly greater than those of the OMG (0.034 ± 0.008%). Similarly the low level observed in the brain (0.090 ± 0.013% dose/g) were greater than that of OMG (0.029 ± 0.002%). PCP concentration in the liver (0.86 ± 0.07% of dose/g) was significantly greater than that of OMG (0.07 ± 0.006%).
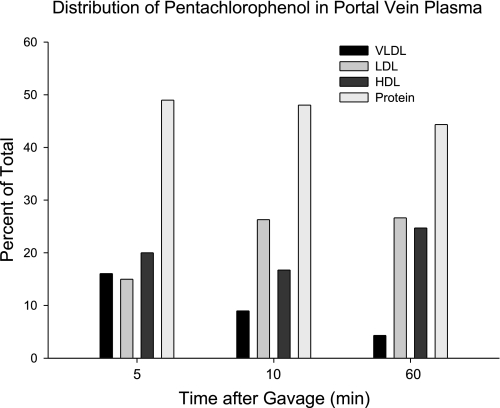
Carriers of PCP were estimated by ultracentrifugation. The results are presented in Fig. 7. Most was carried in the albumin-rich fraction.
Fig. 7.
The relative distribution of [14C]PCP among plasma lipoproteins sampled from the portal vein at times after gavage. Plasma from the animals in the study described in Fig. 5 was pooled, separated by ultracentrifugation with solutions of KBr, and assayed for radioactivity.
DDE.
Six rats with cannulated mesenteric lymph ducts and portal veins were given intraduodenally a bolus dose of [14C]DDE in olive oil followed immediately by an aqueous bolus dose of [3H]OMG. Radioactivity in lymph, plasma, RBCs, and tissues was measured.
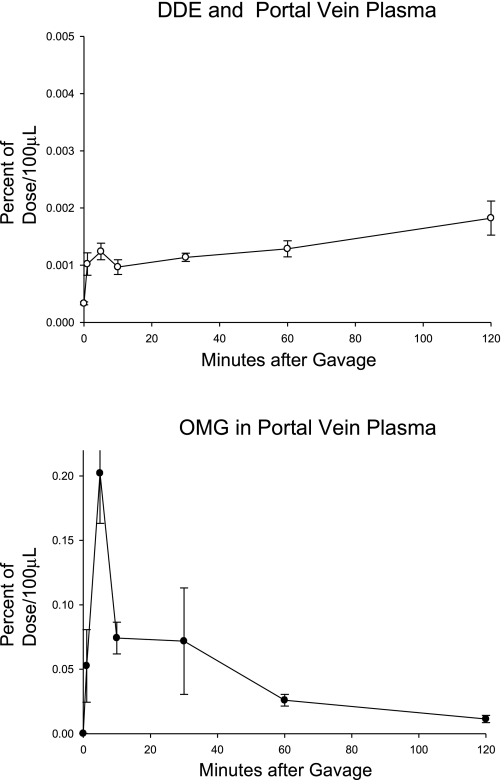
The appearance of 14C and 3H in portal blood plasma is shown in Fig. 8. During the initial 30 min after dosing, the AUC for DDE was 1.57 ± 0.17% of that of the OMG.
Fig. 8.
The appearance of [14C]1,1,1-trichloro-2,2-bischlorophenylethylene (DDE) and [3H]OMG in portal vein plasma. The study was carried out as described in Fig. 1 (n = 6; means ± SE shown).
DDE in the 6-h collection of lymph accounted for 29.5 ± 2.0% of the dose, significantly greater than that of OMG (2.8 ± 0.4%). The radioactivity remaining in the lumen of the intestine at the end of the study was small in both groups (3.3 ± 0.4% and 2.4 ± 0.7% of the dose for the DDE and OMG, respectively), consistent with absorption of both compounds from the lumen. There was not a measurable concentration of DDE in RBCs.
Only trace amounts of OMG were found in the fat pad, brain, and liver. The DDE in the fat pad, brain, and liver accounted for 1.47 ± 0.10, 0.22 ± 0.01, and 3.00 ± 0.17% of the dose, respectively.
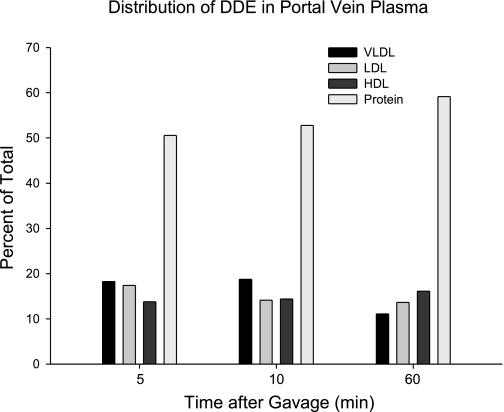
The distribution of 14C among the lipoprotein fractions is presented in Fig. 9. The majority of the radioactivity was carried in the fraction of proteins with density greater than that of HDL.
Fig. 9.
The relative distribution of [14C]PCP among plasma lipoproteins sampled from the portal vein at times after gavage. Plasma from the animals in the study described in Fig. 5 was pooled, separated by ultracentrifugation with solutions of KBr, and assayed for radioactivity.
Portal absorption.
Quantitative estimates of portal absorption were made from the AUCs for OMG and the lipophilic compound during the first 30 min of portal blood collection. Absorption was based on the combined concentration of organochlorine and OMG in plasma and in RBCs. A summary of the percent portal absorption is given in Table 4.
Table 4.
Percent absorption by portal vein on the basis of areas under curves for 30 min for OMG and organochlorine compound
| Compound (number of animals) | log10P | Percent Portal Absorption |
|---|---|---|
| HCB (5) | 5.7 | 11.0±4.2 |
| PCP (7) | 3.5 | 82.8±12.6 |
| DDT (4) | 6.91 | 4.6±2.0 |
| DDE (6) | 6.96 | 2.7±0.50 |
Applicable values are means ± SE. Absorption of PCP was significantly greater than that of the other compounds on the basis of comparison of the 4 groups. HCB absorption was greater than that of DDT on the basis of comparing HCB, DDT, and DDE by ANOVA.
DISCUSSION
The understanding of the entry, packaging, and transport of lipophilic compounds in the enterocyte continues to evolve. The present view of the events in the lumen of the intestine that lead up to entry of a lipophile into the enterocyte is driven by the physical chemistry of the system. These events are the result of the lipophilicity of the specific compound and the surface active properties of endogenous compounds in the intestinal milieu.
The formation of mixed micelles of bile salts, phospholipids, and digestion products from triacylglycerols and phospholipids as discovered by Hofmann is required for the absorption of lipophilic substances (6). The formation of a phase of vesicles can also occur along with a micellar phase, and vesicular components can exchange with micelles (20). Contact with the enterocyte membrane requires the transport by micelles through a hydrophilic barrier that consists of an unstirred water phase and glycocalyx of polymeric carbohydrates (26). Micelles that are compatible with an aqueous phase can move through this barrier to deliver lipophilic compounds to the membrane.
It had been assumed that passive diffusion across the enterocyte membrane occurs for lipophiles that are delivered in micelles. This view has been altered by studies of cholesterol absorption. It is now clear that the absorption of cholesterol into the cell is dependent on transporters. Scavenger receptor B1 (SRB1) and Niemann Pick C1-like protein 1 facilitate enterocyte uptake and transport, and ABCG5/8 limits the amount that is retained (25). Whether or not other lipophiles interact with the cholesterol transporting system is not known although a recent report suggests that SRB1 is involved in lycopene uptake (16). Consequently, the understanding of the relative importance of passive and receptor-mediated transport is still incomplete.
The absorption and transport of OCs can differ from these processes for dietary lipids. One consideration when studying the absorption and transport of OCs is their relatively small mass and low concentration in the intestinal lumen and subsequently in the enterocyte. For comparison, cholesterol entry into the lumen from diet and bile is ∼1 g/day. This amount is possibly five to six orders of magnitude greater than that of organochlorine xenobiotics that enter from the diet (19). These relatively low levels of xenobiotics can lead to many possible consequences, such as enhanced solubility in lipophilic regions or binding by carrier proteins that accommodate small masses. Since many OCs undergo negligible metabolism in the enterocyte, they retain their lipophilicity in the absorption processes from the lumen to the lymph or portal blood. In our studies we intentionally used trace masses of OCs to approximate typical human exposure. Further studies will help predict whether or not higher masses of materials will affect the route of absorption.
In the lumen, OCs can be carried with lipids in intestinal mixed micelles and transported to the enterocyte membrane where they likely diffuse through the enterocyte membrane. Because of the high lipophilicity of many OCs, movement from the membrane to the cytosol requires affinity to a lipophilic domain unless energy is expended to “pump” the compound into the cytosol. Two lipophilic domains may attract these compounds. First, fatty acid binding proteins are present in abundance in the enterocyte, and their lipophilic cavities can bind lipophilic xenobiotics and presumably account for part of their distribution in the cytosol (21).
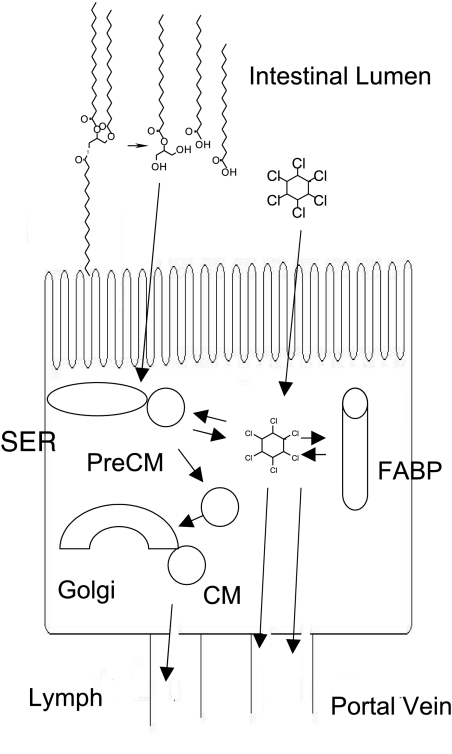
Second, the triacylglycerol that has been synthesized from absorbed digestion products of dietary triacylglycerol in the smooth endoplasmic reticulum of the enterocytes could be a lipophilic domain. Once in the cells, the lipids, including xenobiotics, are packaged in vesicles (prechylomicrons) and transported to the Golgi where chylomicrons are formed with the addition of apolipoprotein A-I. Both prechylomicron vesicles and chylomicrons contain triacylglycerol into which lipophilic xenobiotics can partition. The movement of a lipophile through the enterocyte is illustrated in Fig. 10 with a molecule of HCB in dynamic distribution between lipid-rich particles, fatty acid binding protein, and cytoplasm before entry into lymph and portal vein plasma.
Fig. 10.
Distribution of lipophiles within the enterocyte. Triacylglycerols are resynthesized from fat digestion products to form prechylomicrons (PreCM). Lipophiles such as HCB can partition into lipid-rich prechylomicrons, chylomicrons (CM), and fatty acid binding proteins (FABP) during movement from the enterocyte into lymph or portal blood. SER, smooth endoplasmic reticulum.
The common measure of lipophilicity of a compound is the partition coefficient defined by the ratio of its solubility in octanol to that in water. We may assume that octanol approximates the lipid phase of triacylglycerol in prechylomicrons and chylomicron. In addition, there is evidence that drugs with lipophilic character defined in this manner interact with fatty acid binding proteins and cause their upregulation (21). An estimate of the distribution in an enterocyte between the aqueous phase and lipophilic domains (triacylglycerol in prechylomicrons and chylomicrons; fatty acid binding proteins) depends on the relative masses of the aqueous and lipophilic phases and on the relative binding affinities for proteins and lipids.
We have found that PCP, with a partition coefficient of ∼3.5 at physiological pH (11), is transported predominantly by the portal vein. Although the partition coefficient reflects an affinity for lipid phase relative to aqueous phase of more than 3,000-fold, on the basis of concentrations in the two phases, the aqueous volume relative to the volume of the lipid domains apparently results in the distribution of virtually all of the PCP mass into the aqueous phase.
The results that we report here are consistent with the estimate by Charman and Stella (2) that log10 P of at least 5 directs transport into the lymphatic system. It is clear that there is a significant shift toward lymphatic absorption as log10 P increases, with HCB (log10 P of 5.5) showing measurable absorption by both routes.
In addition to measuring the appearance of compounds in lymph and portal blood, we determined the level of isotopes in tissues of the animals with lymphatic diversion. If diversion of lymph flow is complete and there is no absorption via the portal vein, then the appearance of a lipophile in tissues would not be expected. We interpreted the appearance of HCB in adipose tissue and liver of lymph-diverted animals as indirect evidence of portal vein absorption. However, direct portal vein measurements showed that, although DDT and DDE were minimally absorbed by the portal vein, these compounds were found in measurable levels in liver and fat. Thus we concluded that tissue levels alone in animals with lymphatic diversion may not be sufficient indicators of absorption via the portal vein. A possible explanation for this apparent disparity between minimal appearance in portal vein blood and measurable appearance in tissues is a small level of absorption via the accessory lymphatic duct (22). Although the mesenteric lymph duct, which was utilized in our studies, accounts for essentially all intestinal absorption, there may be some absorption of lipophiles via the accessory duct.
As noted above, we and others have found that HCB is carried predominantly in RBCs. In portal blood the concentration in RBCs (percent of dose/mg) was ∼5–25 times that of that in plasma (percent of dose/100 μl). The maximum concentration in plasma was attained in 5 min, whereas the first maximum in RBCs appeared at 10 min. The immediate high concentration in RBCs is consistent with very fast uptake by these cells.
Consideration of the mass balance of HCB in our study raises important questions. The appearance of HCB in lymph over 24 h was 26% of that of triolein, which is absorbed mostly by the lymphatic route. We calculated that 11% of the HCB was absorbed via portal blood. We also found only small quantities of HCB remaining in the intestinal contents, so there was no evidence of incomplete uptake. One explanation for these observations is suggested by the study of HCB distribution in rats by Iatropoulus and coworkers (7). They found an accumulation of HCB in mesenteric lymph nodes that peaked at 6 h after gastric gavage of the HCB and was maintained for 48 h. Since the mesenteric lymph that we collected was postnodal lymph, it is entirely possible that appearance in mesenteric lymph may underestimate lymphatic absorption. On the basis of the relative levels of HCB in lymph ducts and liver of the rats, Iatropoulus et al. (7) concluded that lymphatic absorption was the primary route of absorption of HCB, consistent with the findings we report here. The potential retention of lipophilic compounds by lymph nodes is an area that is poorly understood and certainly merits further study.
The application of similar mass-balance considerations to our results with DDT also suggests incomplete appearance in lymph; however, since lymph was collected for only 6 h, the study was not designed to measure complete lymphatic absorption. Other studies have found as much as 63% absorption of DDT in lymph collected for 72 h (18) and 67% (24) when collected for 48 h. In addition, Palin et al. (17) showed that the appearance of DDT in plasma of rats with tail-clip samples was abolished by lymphatic cannulation. Consistent with our findings, these workers concluded that the major, if not the only, route for DDT absorption was by the lymphatic system.
It is not possible to predict the universal applicability of this method to the determination of the extent of portal absorption for all compounds. We reproducibly observed a rapid absorption of the OMG marker, and it is possible that some compounds may exit the enterocyte more slowly than OMG, so that the use of the AUCs at 30 min would lead to an underestimation of the portal absorption for such compounds. Compounds that are more lipophilic would be expected to exit slowly into the portal vein, but more likely they would enter the lymphatic system. Compounds like HCB, with an apparent affinity for RBC, and presumably other membranes might be slowed by residence in the basolateral membrane.
To determine the appropriateness of our 30-min sample, the comparison of the calculations at 30 and 120 min was made and is presented in Table 5. The value calculated for HCB at 120 min did not differ from that at 30 min. The calculated absorption for PCP at 120 min suggests that PCP from circulating blood has entered the portal blood and that the PCP has not been taken up by hepatic or peripheral tissues as fast as the OMG marker. Additionally, we found label presumably associated with triolein in the portal blood appearing after 30 min (Fig. 3). Likewise, Kristensen and coworkers (12) reported the appearance of triacylglycerol in the portal blood of pigs fed diacylglycerol 50–100 min after bolus dosing. Finally, Hoffman and coworkers (5) found the portal concentration of verapamil (log10P of 3.8) and of a less lipophilic drug (A-79035; reported log10D, oil/water distribution coefficient of 1.71) returned to the systemic level within 30 min. These observations suggest that the use of 30 min for the AUC calculations minimizes the effects of the addition of newly absorbed compound in systemic blood to portal blood.
Table 5.
Percent absorption by portal vein on the basis of the areas under curves for 30 and 120 min for OMG and organochlorine compound
| Compound (number of animals) | Percent Portal Absorption (30 min) | Percent Portal Absorption (120 min) |
|---|---|---|
| HCB (5) | 11.0±4.2 | 17.2±5.9 |
| PCP (7) | 82.8±12.6 | 137.6±11.8 |
| DDT (4) | 4.6±2.0 | 5.0±1.7 |
| DDE (6) | 2.7±0.50 | 4.8±0.74 |
Values are means ± SE. The absorption calculated at the two times was significantly different for PCP and for DDE (P < 0.05).
To summarize our rationale for the use of specific time points, although there may be retardation of entry into the portal vein for some compounds relative to OMG, we propose that the comparison of AUCs at 30 min provides a meaningful estimate of portal absorption, on the basis of the following reasons: 1) Other workers report complete portal absorption of moderately lipophilic compounds in 30 min. 2) We calculate similar absorption values for AUCs at 30 and 120 min. 3) After 30 min, there is the possibility of mixing with systemic blood that may contain lymphatically absorbed compounds. We recognize, however, that compounds such as HCB, with affinity for membranes, may enter more slowly, and the method could underestimate portal absorption for compounds with this behavior.
The combined portal and lymphatic cannulation methodology also provides lymphatic concentrations that can complement the portal concentrations. In the compounds that we studied, lymphatic concentrations reflected their lipophilicity, with only traces of PCP in lymph and significant appearance of DDT and DDE. As noted in results of our preliminary studies with HCB, we observed 26% of the HCB dose in lymph in 24 h, consistent with absorption via both portal and lymphatic routes.
To summarize, we have introduced new methodology into the study of the route of absorption. Previous measurements of absorption via the portal and lymphatic routes have used methods that separately determine the involvement of each of the two routes. Portal vein measurements have used the difference between concentrations in portal vein and systemic blood to estimate the relative contribution of the portal vein. Assumptions of flow rate and volume are needed to estimate portal vein absorption with this methodology. Portal blood flow rate can be measured by methods with ultrasound or electromagnetic flowmeter (3, 14). This measurement is not within the scope of our studies; however, it is not necessary for our calculations. Estimates of portal vein absorption in animals with lymphatic cannulation have been inferred by difference, i.e., the absorbed amount not appearing in lymph is used to calculate portal vein absorption. However, the absence of appearance in lymph is an indirect assay that may ignore missing compound attributable to other reasons related to experimental technique and method. It is now possible to directly measure the appearance of compounds in the portal vein in animals with lymphatic diversion by using an internal marker that is completely absorbed by the portal vein. The use of this marker corrects for the flow rate of the blood and its dilution, and does not require the assumptions about missing material. This method can be used to the study of many xenobiotics with lipophilic properties.
GRANTS
This work was funded by ESO14464-A1 (NIH/ESO).
Acknowledgments
We greatly appreciate the work of Katie Burke in the ultracentrifugation separations.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Centers for Disease Control and Prevention. Third National Report on Human Exposure to Environmental Chemicals. Atlanta, GA: Department of Health and Human Services, 2005.
- 2.Charman WNA, Stella VJ. Effects of lipid class and vehicle on the intestinal lymphatic transport of DDT. Int J Pharm 33: 165–172, 1986. [Google Scholar]
- 3.D'Almeida MS, Cailmail S, Lebrec D. Validation of transit-time ultrasound flow probes to directly measure portal blood flow in conscious rats. Am J Physiol Heart Circ Physiol 271: H2701–H2709, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Gomez-Catalan J, To-Figueras J, Rodamilans M, Corbella J. Transport of organochlorine residues in the rat and human blood. Arch Environ Contam Toxicol 20: 61–66, 1991. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman DJ, Seifert T, Borre A, Nellans HN. Method to estimate the rate and extent of intestinal absorption in conscious rats using an absorption probe and portal blood sampling. Pharm Res 12: 889–894, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann AF, Borgstrom B. Physico-chemical state of lipid in intestinal contents during their digestion and absorption. Fed Proc 21: 43–50, 1962. [PubMed] [Google Scholar]
- 7.Iatropoulus MA, Milling A, Muller WF, Nohyhk G, Rozman K, Coulston F, Korte F. Absorption, transport and organotropism of dichlorobiphenyl (DCB), dieldrin, and hexachlorobenzene (HCB) in rats. Environ Res 10: 384–389, 1975. [DOI] [PubMed] [Google Scholar]
- 8.Jandacek RJ, Zheng S, Tso P. Rapid clearance of hexachlorobenzene from chylomicrons. Lipids 39: 993–995, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Jandacek RJ, Tso P. Factors affecting the storage and excretion of toxic lipophilic xenobiotics. Lipids 36: 1289–1305, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Jensen AA Chemical contaminants in human milk. Residue Rev 89: 1–128, 1983. [DOI] [PubMed] [Google Scholar]
- 11.Kaiser LE, Valdmanis I. Apparent octanol/water partition coefficients of pentachlorophenol as a function of pH. Can J Chem 60: 2104–2106, 1982. [Google Scholar]
- 12.Kristensen JB, Jorgensen H, Mu H. Diacylglycerol oil does not affect portal vein transport of nonesterified fatty acids but decreases the postprandial plasma lipid response in catheterized pigs. J Nutr 136: 1800–1805, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Laher JM, Rigler MW, Vetter RD, Barrowman JA, Patton JS. Similar bioavailability and lymphatic transport of benzo(a)pyrene when administered to rats in different amounts of dietary fat. J Lipid Res 25: 1337–1342, 1984. [PubMed] [Google Scholar]
- 14.Li X, Benjamin IS, Naftalin R, Alexander B. Location and function of intrahepatic shunts in anaesthetised rats. Gut 52: 1339–1346, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mariussen E, Fonnum F. Neurological targets and behavioral effects of organohalogen compounds: an update. Crit Rev Toxicol 36: 253–289, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Moussa M, Landrier R, Reboul E, Ghiringelli O, Coméra C, Collet X, Fröhlich K, Böhm V, Borel P. Lycopene absorption in human intestinal cells and mice involves scavenger receptor class B type 1 but not niemann pick C1 like1. J Nutr 138: 1432–1436, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Palin KJ, Wilson CG, Davis SS, Philips AJ. The effect of oils on the lymphatic absorption of DDT. J Pharm Pharmacol 34: 707–710, 1982. [DOI] [PubMed] [Google Scholar]
- 18.Pocock DE, Vost A. DDT absorption and chylomicron transport in rat. Lipids 9: 374–381, 1974. [DOI] [PubMed] [Google Scholar]
- 19.Schafer KS, Kegley SE. Persistent toxic chemicals in the US food supply. J Epidemiol Community Health 56: 813–817, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staggers JE, Hernell O, Stafford RJ, Carey MC. Physical-chemical behavior of dietary and biliary lipids during intestinal digestion and absorption. 1. Phase behavior and aggregation states of model lipid systems patterned after aqueous duodenal contents of healthy adult human beings. Biochemistry 29: 2028–2040, 1990. [DOI] [PubMed] [Google Scholar]
- 21.Trevaskis NL, Lo CM, Ma LY, Tso P, Irving HR, Porter CJH, Charman WN. An acute and coincident increase in FABP expression and lymphatic lipid and drug transport occurs during intestinal infusion of lipid-based drug formulations to rats. Pharm Res 23: 1786–1796, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Tso P, Simmonds WJ. The absorption of lipid and lipoprotein synthesis. In: Laboratory and Research Methods in Biology and Medicine, edited by Story JA. New York: Alan R. Liss, 1984, vol. 10, p. 191–216. [PubMed] [Google Scholar]
- 23.Uhing MR, Kimura RE. Active transport of 3-O-methylglucose by the small intestine in chronically catheterized rats. J Clin Invest 95: 2799–2805, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volpenhein RA, Webb MR, Jandacek RJ. The effect of a nonabsorbable lipid sucrose polyester on the absorption of DDT by the rat. J Toxicol Environ Health 6: 669–683, 1980. [Google Scholar]
- 25.Wang DQ Regulation of intestinal cholesterol absorption. Annu Rev Physiol 69: 221–248, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Wilson FA, Sallee VL, Dietschy JM. Unstirred water layers in intestine: rate determinant of fatty acid absorption from micellar solutions. Science 174: 1031–1033, 1971. [DOI] [PubMed] [Google Scholar]