Abstract
Impaired gastric accommodation and gastric dysrhythmia are common in gastroparesis and functional dyspepsia. Recent studies have shown that synchronized gastric electrical stimulation (SGES) accelerates gastric emptying and enhances antral contractions in dogs. The aim of this study was to investigate the effects and mechanism of SGES on gastric accommodation and slow waves impaired by vagotomy in dogs. Gastric tone, compliance, and accommodation as well as slow waves with and without SGES were assessed in seven female regular dogs and seven dogs with bilateral truncal vagotomy, chronically implanted with gastric serosal electrodes and a gastric cannula. We found that 1) vagotomy impaired gastric accommodation that was normalized by SGES. The postprandial increase in gastric volume was 283.5 ± 50.6 ml in the controlled dogs, 155.2 ± 49.2 ml in the vagotomized dogs, and 304.0 ± 57.8 ml in the vagotomized dogs with SGES. The ameliorating effect of SGES was no longer observed after application of Nω-nitro-l-arginine (l-NNA); 2) vagotomy did not alter gastric compliance whereas SGES improved gastric compliance in the vagotomized dogs, and the improvement was also blocked by l-NNA; and 3) vagotomy impaired antral slow wave rhythmicity in both fasting and fed states. SGES at the proximal stomach enhanced the postprandial rhythmicity and amplitude (dominant power) of the gastric slow waves in the antrum. In conclusion, SGES with appropriate parameters restores gastric accommodation and improves gastric slow waves impaired by vagotomy. The improvement in gastric accommodation with SGES is mediated via the nitrergic pathway. Combined with previously reported findings (enhanced antral contractions and accelerated gastric emptying) and findings in this study (improved gastric accommodation and slow waves), SGES may be a viable therapy for gastroparesis.
Keywords: gastric accommodation, gastric electrical stimulation, gastric slow waves, gastric tone, gastroparesis, functional dyspepsia, gastrointestinal motility
gastroparesis, with objective evidence of delayed gastric emptying of solids in the absence of mechanical obstruction, is one of the major health problems in clinical gastroenterology. The true prevalence of gastroparesis is unknown. Gastroparesis affects 27–58% of type 1 diabetes (22, 60) and up to 30% of patients with type 2 diabetes (21). Patients with gastroparesis commonly complain of nausea, vomiting, early satiety, postprandial fullness or abdominal bloating, and frequent hospitalizations. The pathogenesis of gastroparesis is complex and may involve impaired gastric accommodation (25, 52), visceral hypersensitivity (28), attenuated antral contractions, and delayed gastric empty (36) as well as impaired pacemaking functions such as loss of interstitial cells of Cajal and slow wave dysrhythmia (10, 16, 17, 23, 39). A recent report estimated the inpatient cost for a patient with severe gastroparesis of approximately $7,000/mo (14).
Currently available therapies for gastroparesis are limited and unsatisfactory. These include dietary or nonmedical measures (6), prokinetic therapy (35, 42, 44), antiemetic medication therapy (45), endoscopic treatment (8, 27), surgical management, and nutritional support (18). Surgical procedures are performed less often, and their benefits are not well documented. The medical therapies are mainly focused on prokinetic agents. Although a number of prokinetic agents are able to accelerate gastric motility and improve antral contractions, their therapeutic benefits are limited, especially in relieving symptoms of gastroparesis or functional dyspepsia. This is because gastroparetic/dyspeptic symptoms are often attributed to impaired gastric accommodation, whereas the prokinetic agents do not improve but often impair gastric accommodation (25, 52).
Recent basic and clinical researches have suggested therapeutic potentials of gastric electrical stimulation (GES) for gastroparesis and functional dyspepsia (5, 15, 20, 30–34, 43). Long-pulse GES has been shown to consistently normalize dysrhythmia in patients with gastroparesis (34). Short-pulse GES has been reported to improve nausea and vomiting in gastroparetic patients with limited or no effects on gastric motility or slow-wave stabilization (1, 3, 31). Dual-pulse GES, combined with both short pulses and long pulses, has advantages of both long-pulse GES and short-pulse GES and is capable of improving dysrhythmia and nausea and vomiting (48). Recently, a number of studies have shown that synchronized GES (or SGES, electrical stimuli are delivered at the occurrence of intrinsic slow waves) has a prokinetic effect: increasing antral contractions and accelerating gastric empty (47, 57, 59). However, it is unknown whether SGES is able to improve or impair (similar to prokinetics) gastric accommodation, and this is crucial if SGES is to be used for treating gastroparesis and functional dyspepsia, since impaired accommodation is common in these diseases.
Therefore, the primary aim of this study was to investigate the effects and mechanisms of SGES on gastric tone, compliance, and accommodation in a canine model of impaired accommodation induced by vagotomy.
MATERIALS AND METHODS
Animal preparation.
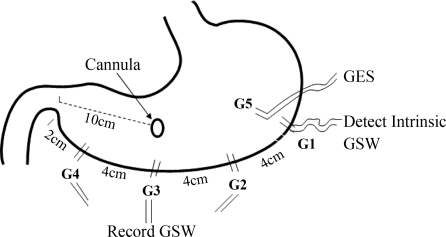
A total of 14 healthy female hound dogs (20–26 kg) were used in this study. After an overnight fast, the dogs were anesthetized with an initial intravenous infusion of sodium thiopental (5 mg/kg; Abbott Laboratories, North Chicago, IL) and maintained on IsoFlo (1.5% isoflurane, inhalation anesthesia; Abbott) in oxygen-nitrous oxide (1:1) carrier gases delivered from a ventilator following endotracheal intubation. Midline laparotomy was performed. Four pairs of 28-gauge stainless steel cardiac pacing wires (A&E Medical, Farmingdale, NJ) were implanted on the serosal surface along the greater curvature at an interval of 4 cm with the distal pair 2 cm proximal to the pylorus (see Fig. 1). The proximal pair of electrodes G1 was used to detect intrinsic slow waves that were used to synchronize electrical stimuli. Electrode pairs G2, G3, and G4 were used to record gastric slow waves that were used to assess the effects of SGES on gastric slow waves. Electrode pair G5 was implanted on the serosal surface 3 cm away circumferentially from G1 and used for electrical stimulation. The two electrodes in a pair were arranged circumferentially with a distance of ∼0.5–1.0 cm (Fig. 1). The electrodes were penetrated in the subserosal layer and affixed to the serosa by nonabsorbable sutures. The connecting wires of the electrodes were tunneled through the anterior abdominal wall subcutaneously along the right side of the trunk and placed outside the skin around the right hypochondrium for the attachment to the recording or stimulation equipment. A gastric cannula was placed in the anterior wall of the stomach, 10 cm above the pylorus.
Fig. 1.
A line diagram of the implantation of electrodes in the stomach. GSW, gastric slow waves.
In addition, in 7 of the 14 dogs, the bilateral truncal vagotomy was performed at the level of hernia of the diaphragm. A segment (1 cm) of ventral and dorsal trunks of the vagus innervating the stomach was excised to prevent the regeneration of these nerves. The ramifications to the stomach were also served to ensure that all these vagal nerves innervating the stomach were denervated.
The dogs were transferred to a recovery cage after receiving medications for postoperative pain control. The study was initiated after the dogs were completely recovered from the surgery (usually 2 wk after the surgery). The study was approved by the Institutional Animal Care and Use Committees of the University of Texas Medical Branch at Galveston.
Experimental protocol.
The study was composed of four experiments. Gastric tone, compliance, and accommodation were assessed. Gastric tone was defined as the gastric fundic volume at a constant distension pressure. An increase in fundic volume is indicative of relaxation of the stomach, whereas a decrease in volume reflects increased tone. Gastric compliance represents the pressure-volume relationship that reflects the elasticity of the stomach. It was calculated by averaging the balloon volume over the middle of 2 min distention. Gastric accommodation was defined as the difference in average gastric volume between the 30-min postprandial period and the 30-min fasting period.
Experiment A was designed to assess the effects of vagotomy on gastric accommodation, tone, compliance, and gastric slow waves. It was performed in the regular dogs and the vagotomized dogs in two randomized sessions on different days. Both sessions were initiated after an overnight fast. Session one was for the assessment of gastric tone and accommodation and composed of a 30-min baseline recording of gastric fundic tone and a 30-min postprandial recording after a test meal of 237 ml of Boost (240 cal, Nutritional; Mead Johnson, Evansville, IN). Gastric slow waves were record continuously during the entire session. Session two was designed for the measurement of gastric compliance (fundic volumes under different pressures). The pressure of the intragastric balloon was increased in a piecewise fashion at an increment of 2 mmHg from 2 to 16 mmHg. Each pressure was maintained for 2 min, and there was 1 min of deflation between two consecutive pressure settings.
Experiment B was designed to investigate the effect and possible nitrergic mechanism of synchronized GES or SGES on fundic tone in the fasting state in the vagotomized dogs. It was performed in two sessions on different days in the seven vagotomized dogs. Session one was composed of a 30-min baseline recording, a 30-min recording with the SGES, and a 30-min recovery period. Session two was to study the involvement of nitric oxide and was composed of a 30-min baseline recording, a 30-min recording with intravenous infusion of l-NNA (5 mg·kg−1·h−1), a blocker of the synthesis of nitric oxide, and another 30-min recording with the SGES. Fundic tone was measured during the entire experimental periods using an intragastric balloon and a barostat system.
Experiment C was designed to study the effect and possible mechanism of SGES on fundic compliance in the vagotomized dogs. The experiment was performed in the fasting state in four randomized sessions on four different days at an interval of 2–7 days [control, SGES, Nω-nitro-l-arginine (l-NNA) only, and SGES plus l-NNA]. The protocol of the control session was the same as that of session two in experiment A. The protocol of other sessions was the same except that SGES was performed during the entire session for the SGES session; l-NNA was infused continuously intravenously for 30 min before the recording in the l-NNA session. Both SGES and l-NNA were given in the SGES plus l-NNA session.
Experiment D was conducted to study the effect of SGES on fundic accommodation and its possible nitrergic mechanism in the vagotomized dogs. The experiment consisted of four sessions on four separate days at an interval of 2–7 days in a randomized order: session one without SGES as the control; session two with SGES applied during the entire session (a 30-min fasting state recording of gastric fundic tone and a 30-min postprandial recording). In this session, gastric slow waves were simultaneously recorded. Session three was without SGES, but l-NNA (5 mg·kg−1·h−1) was infused continuously for 30 min intravenously before the recording. Session four was with a combination of SGES and l-NNA infusion. In each session, gastric volume was measured via the intragastric balloon and the barostat system for 30 min in the fasting state and another 30 min after the same test meal of 237 ml of Boost.
Measurements and analysis of gastric tone and gastric accommodation.
Gastric tone was assessed by the measurement of gastric volume at a constant pressure using the established standard method of barostat described as follows: a polyethylene bag (CT-BP800; H&A Mui Enterprise, Mississauga, Ontario, Canada) was positioned in the proximal stomach via the chronically implanted gastric cannula. The bag was noncompliant, has a maximal volume of 700 ml, and was attached to the distal end of a double-lumen catheter. The intubation procedure was tested during surgery when the abdomen was open to ensure accurate placement. The direction of the catheter heading, the depth of the catheter inserted, and the placement of the bag had been carefully verified before the initiation of the study. The catheter was connected to a computer-controlled electrical barostat device (Distender Series IIR; G & J Electronics, Willowdale, Ontario, Canada) (23). When the fundic pressure reduces, the computerized device pumps more air in the balloon and vice versa. Accordingly, the variation in the volume of the balloon reflects fundic tone. An increase in fundic volume is indicative of relaxation of the stomach, whereas a decrease in volume reflects increased tone. After its placement in the fundus, the balloon was briefly inflated with 300 ml air to fully unfold and deflated completely thereafter. After a brief rest period (5 min), the minimal distending pressure was determined by inflating the bag in 1-mmHg increments until a pressure at which evident respiratory excursions were recorded and the bag volume was equal to or larger than 30 ml (38). The gastric volume was then recorded at an operating pressure of 2 mmHg higher than the minimal distending pressure during the entire experimental period.
Gastric accommodation was defined as the difference in average gastric volume between the 30-min postprandial period and the 30-min fasting period.
Measurement and analysis of gastric compliance.
Gastric compliance represents the pressure-volume relationship that reflects the elasticity of the stomach. It was tested by isobaric phasic distention using the intragastric balloon, with a stepwise increment of 2 mmHg in the balloon pressure, starting from 2 mmHg up to 16 mmHg. Each distention was maintained for 2 min with 1 min deflation in between.
Gastric compliance was calculated by averaging the balloon volume over the middle of 2 min distention. A power exponential model was used to fit the nonlinear compliance curve, with parameter β representing the overall shape of the curve and κ essentially representing the change in volume as a function of 1/P (P stands for pressure) at any given point (7). The estimated κ and β for each animal were used to calculate the pressure corresponding to half-maximal volume on the pressure-volume curve (P1/2). A shift of the compliance curve is appropriately reflected in a change in P1/2, and the values of P1/2 are considered to be the primary endpoint for compliance.
SGES.
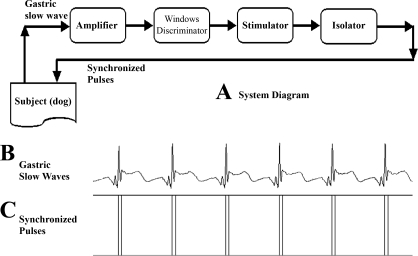
Electrical stimulation synchronized with intrinsic slow waves was previously shown to enhance antral and intestinal contractions and accelerate gastric emptying and intestinal transit in dogs (47, 57, 59). From another study (11, 38), it was known that GES with short pulses but not long pulses improved animal behaviors suggestive of nausea and vomiting. Accordingly, in this study, we used SGES of dual pulses (each stimulus was composed of a long pulses followed with a few short pulses). The concept of SGES is shown in Fig. 2. This SGES system is composed of an amplifier, a windows discriminator, a stimulator, and an isolator. The amplifier used in this system is a specially designed amplifier with a frequency range of 0.012–5 Hz, which is suitable for amplifying gastric slow waves (Fig. 2B). After amplification, the recorded gastric slow waves are sent to the windows discriminator. The windows discriminator detects each peak of the recorded signal to determine whether there is a normal occurrence of a gastric slow wave. Once a wave is detected, a synchronizing signal is generated by the discriminator and sent to the stimulator. Upon receipt of the synchronizing signal, dual pulses (a long pulse followed with short pulses with their amplitude and width predetermined by the user) are generated by the stimulator. This electrical stimulus synchronized with the peak of the gastric slow wave is then sent to the subject via an isolator and the stimulation electrodes (in this study, the most proximal electrode pair was used for stimulation) (Fig. 2C).
Fig. 2.
Synchronized (S) gastric electrical stimulation (GES) system. A: system configuration. B: recorded intrinsic GSW. C: synchronized electrical stimuli (the component of short pulses is not visible because of the narrowness of the pulses). In this system, two pairs of electrodes are used, one for the detection of the intrinsic GSW and the other for GES.
Each stimulus was composed of a long pulse with a width 300 ms and amplitude of 4 mA followed (after a 100-ms delay) with five short pulses (frequency of 100 Hz, width of 0.33 ms, and amplitude of 4 mA). The rising edge of the long pulse was synchronized with the detected peak of each slow wave. The selection of these parameters was based on our previous studies in which long-pulse GES with similar parameters was shown to reduce gastric tone (56), short-pulse GES was able to reduce vomiting induced by vasopressin (11), and SGES was reported to enhance antral contractions and gastric emptying (59). The method of GES use in this study, synchronized dual-pulse GES, was a combination of these previous three methods (long-pulse GES, short-pulse GES, and SGES).
Measurement and analysis of intrinsic gastric slow waves.
In session one of the experiment A (control data in fasting and postprandial states) and session two of the experiment D (SGES in fasting and postprandial states), gastric slow waves were recorded from four pairs of electrodes using a multichannel recorder (Acknowledge III, EOG 100A; Biopac Systems, Santa Barbara, CA) in both the vagotomized and control dogs. All signals were displayed on a computer monitor and saved on the computer's hard disk. The low and high cutoff frequencies of the amplifiers were 0.05 and 35 Hz, respectively. The signals were initially sampled at a frequency of 100 Hz, and then, for the analysis of gastric slow waves, the signals were downsampled to 2 Hz after low pass filtering with a cut off frequency of 1 Hz.
The slow wave recording from the most distal pair of electrodes G4 was subjected to spectral analyses to derive the following parameters (11).
Dominant frequency and power of the slow wave.
The frequency at which the power spectrum of an entire recording had a peak power was defined as the dominant frequency. The power at the dominant frequency in the power spectrum was defined as the electrogastrography dominant power. These two parameters were calculated by using the smooth-power spectral analysis method. Decibel units were used to represent the power of the gastric slow wave.
Percentage of normal gastric slow waves.
The percentage of normal gastric slow waves was defined as the percentage of time during which regular 4 to 6 cycles/min (cpm) slow waves were present over the entire recording period. It was computed by using the adaptive spectral analysis method. In this method, each recording was divided into blocks of 1 min without overlap. The power spectrum of each 1-min recording was calculated and examined to see if the peak power was within the range of 4–6 cpm. The 1-min recording was categorized as normal if the peak power was within the 4- to 6-cpm range; otherwise, it was categorized as dysrhythmia. The definition of the normal frequency range of the 4–6 cpm in the stomach was based on our previous study (11).
Statistical analysis.
Data are reported as means ± SE. Paired Student's t-test was used to investigate the differences between any two periods or sessions if the ANOVA revealed a significant difference among the three or more sessions/periods in the vagotomized dogs. Unpaired Student's t-test was used to analyze the differences between the vagotomized dogs and the regular control dogs.
RESULTS
Effects of vagotomy and SGES on gastric accommodation.
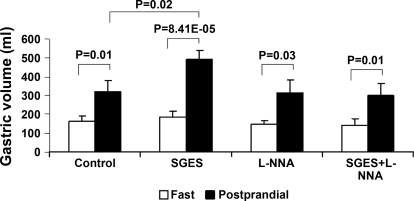
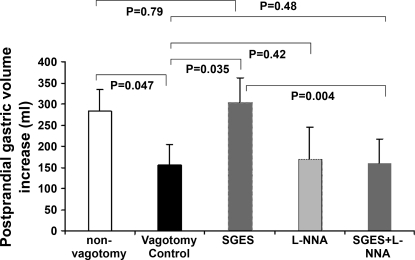
The vagotomized dogs showed impaired gastric accommodation, and the impaired accommodation was normalized by SGES compared with gastric accommodation in the regular dogs. Gastric volume was increased significantly after the test meal compared with that in the fasting state in all sessions (Fig. 3). However, there were significant differences of the postprandial gastric volume increase among different sessions. As shown in Fig. 4, the postprandial gastric volume increase was 155.2 ± 49.2 ml in the vagotomized dogs, which was significantly lower than that in regular dogs 283.5 ± 50.6 ml (P < 0.05). SGES increased the postprandial increase in gastric volume to 304.0 ± 57.8 ml in the vagotomized dogs, which was comparable to but not better than that in the regular dogs (P > 0.05 vs. 283.5 ± 50.6 ml). SGES was also applied in three regular dogs (intact vagus), and no obvious improvement in gastric accommodation was noted in any of these dogs.
Fig. 3.
Gastric volume in the fasting and postprandial states in different experimental sessions in the vagotomized dogs. The legends in the x-axis indicate the name of the session. A postprandial increase in gastric volume was noted in all sessions (P < 0.02, postprandial vs. fasting state in 4 sessions).
Fig. 4.
Gatric accommodation (postprandial increase in gastric volume) under various conditions: 1) vagotomy reduced gastric accommodation (P = 0.047, “normal control” vs. “vagotomy control”); 2) SGES improved vagotomy-induced impairment in gastric accommodation (P = 0.035, “SGES” vs. vagotomy control); 3) Nω-nitro-l-arginine (l-NNA) did not alter gastric accommodation (P > 0.05, “l-NNA” vs. vagotomy control) but blocked the effect of SGES (P > 0.05, “SGES + l-NNA” vs. vagotomy control).
l-NNA blocked the ameliorating effect of SGES on gastric accommodation in the vagotomized dogs. As shown in Fig. 4, the postprandial increase in gastric volume in the vagotomized dogs was not altered by the application of l-NNA (panel 4) and remained unchanged even during SGES (panel 5, 158.5 ± 57.4 ml, P = 0.48, vs. vagotmy control), suggesting the ameliorating effect of SGES on gastric accommodation was mediated via the nitric oxide pathway.
Effects of vagotomy and SGES on basal fundic tone.
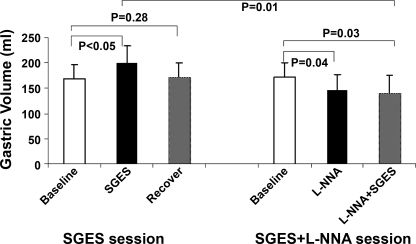
The basal (fasting) fundic tone was 164.7 ± 25.3 ml in the vagotomized dogs, not significantly different from that in the regular normal dogs, 133.9 ± 16.3 ml (P = 0.16). However, the basal fundic tone in the vagotomized dogs was increased significantly during SGES. As shown in Fig. 5, in the fasting state, the average intragastric balloon volume was 168.1 ± 28.7 ml at baseline and significantly increased to 200.5 ± 32.2 ml during SGES (P < 0.05) and recovered to 172.4 ± 28.0 ml, which was comparable to the baseline of 168.1 ± 28.7 ml (P = 0.28). After intravenous infusion of l-NNA, the gastric volume was decreased to 146.2 ± 31.1 ml (P < 0.04 vs. baseline of 171.9 ± 29.3 ml) and remained unchanged during SGES (140.9 ± 35.5 with SGES). These data indicated that l-NNA blocked the inhibitory effect of SGES on gastric tone in the fasting state.
Fig. 5.
In the fasting state, the gastric volume of vagotomized dogs was significantly increased after applying the SGES, and this relaxation effect was no longer observed after iv infusion l-NNA.
Effects of vagotomy and SGES on fundic compliance.
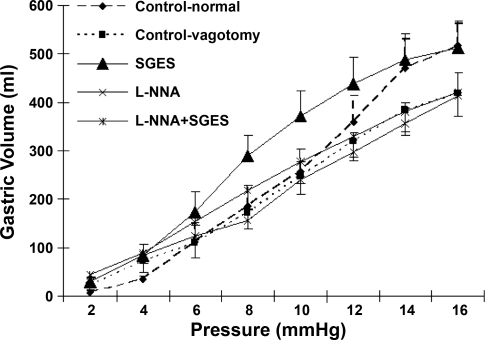
Vagotomy did not seem to alter gastric compliance in the fasting state. SGES enhanced gastric compliance in the vagotomized dogs, and the effect was mediated via the nitrergic pathway. The volume-pressure curves are shown in Fig. 5, and the parameters of gastric compliance are presented in Table 1. As shown in Fig. 5, the slope of the volume-pressure curve was not altered by vagotomy and nor was any of the gastric compliance parameters (see Table 1). These data suggested that mechanisms other than the vagal pathway might be involved in the regulation of gastric compliance in the fasting state.
Table 1.
Comparison of gastric compliance variables among the five sessions
| Sessions | κ | β | P1/2, mmHg |
|---|---|---|---|
| Normal control | 13.8±1.7 | 1.1±0.2 | 8.6±0.7 |
| Vagotomy control | 11.6±1.8 | 1.0±0.1 | 8.0±0.6 |
| SGES | 8.9±1.1* | 1.1±0.04 | 6.8±0.5* |
| l-NNA | 11.3±1.7 | 0.7±0.1* | 8.3±0.6 |
| SGES + l-NNA | 9.3±1.2 | 0.9±0.1 | 7.4±0.6 |
Data are means ± SE. SGES, synchronized gastric electrical stimulation; l-NNA, Nω-nitro-l-arginine; κ, change in volume as a function of 1/pressure at any given point; β, overall shape of the curve; P1/2, pressure corresponding to half-maximal volume on the pressure-volume curve.
P < 0.03 vs. vagotomy control.
SGES enhanced gastric compliance. From Fig. 6, increases in gastric volume were noted at almost all pressures in the vagotomized dogs. The compliance of the stomach in the vagotomized dogs with SGES was even higher than the control animals without vagotomy. From Table 1, it is seen that SGES significantly reduced the κ value (P < 0.03) and P1/2 (P < 0.03) compared with the control values. A lowered κ value (κ representing the instantaneous slope of the curve) and P1/2 value were indicative of increased compliance (Fig. 6) (7). The relaxation effect of SGES was no longer present after intravenous infusion of l-NNA. As shown in Table 1, there were no significant differences in parameter κ or P1/2 between the SGES plus l-NNA session and the vagotomy control session (P > 0.05).
Fig. 6.
Gastric compliance in five sessions. The slope of the curve was increased significantly after applying SGES compared with that in the control session (P < 0.03), and the relaxation effect of SGES was no longer exhibited after iv infusion l-NNA (vs. control P = 0.15).
Effects of vagotomy and SGES on gastric slow waves.
Gastric slow waves in the distal antrum were impaired after bilateral vagotomy both in the fasting and postprandial states. The percentage of normal slow waves was 93.4 ± 3.6% in the control dogs and decreased to 80.2 ± 4.6% in the vagotomized dogs in the fasting state (P < 0.02). In the postprandial state, the percentage of normal slow waves was 97.0 ± 1.6% in the control dogs and 77.4 ± 6.2 in the vagotomized dogs (P < 0.01).
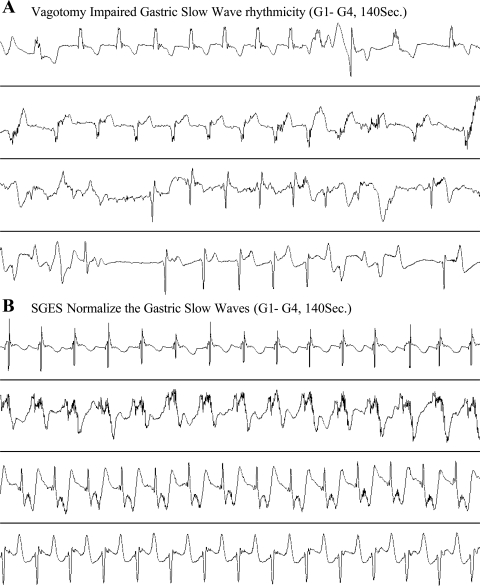
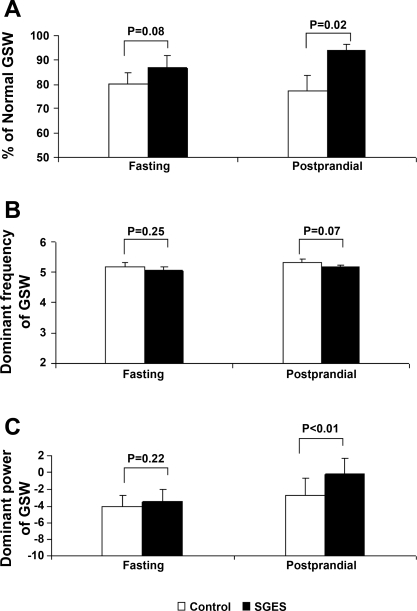
SGES delivered at the proximal stomach improved gastric slow waves in the distal antrum in the vagotomized dogs (Fig. 7). The effects of SGES on gastric slow waves are shown in Fig. 8. SGES increased the percentage of normal gastric slow waves (Fig. 8A); however, only under the postprandial state was the increases significant compared with the control session. The percentage of normal gastric slow waves was 77.4 ± 6.2% in the control session and increased to 94.0 ± 2.4% with SGES (P = 0.02). The dominant frequency of gastric slow wave was not altered by SGES (Fig. 8B). In addition, SGES increased the dominant power of gastric slow waves in the postprandial state. The postprandial dominant power was increased from its control value of −2.7 ± 2.1 to −0.24 ± 1.9 dB with SGES (P < 0.01, Fig. 8C).
Fig. 7.
Representative tracings showing impaired slow waves (A) and improved slow waves with SGES (B). Top to bottom: slow waves recorded from electrode pairs G1 to G4.
Fig. 8.
Effects of SGES at the proximal stomach on parameters of slow waves measured from the distal antrum. A: percentages of normal GSW in the control (no SGES) and SGES sessions. B: dominant frequency of GSW. C: dominant power of GSW.
DISCUSSION
In the present study, we found that both the vagal pathway and the inhibitory nitrergic pathway were involved in the regulation of gastric fundic relaxation. Vagotomy significantly impaired gastric accommodation and slow waves but did not induce changes in basal gastric tone or gastric compliance. GES synchronized with intrinsic gastric slow waves was able to not only restore the impaired gastric accommodation and improve gastric slow waves but also reduce basal gastric tone and increase gastric compliance. The mechanisms of these ameliorating effects of SGES were mediated via the nitrergic pathway.
Attenuated antral contractions, delayed gastric emptying, and impaired fundic accommodation are common in patients with gastroparesis and functional dyspepsia (25, 52). Although the prokinetic effects of SGES on antral contractions and gastric emptying were previously reported, the objective of this study was to investigate the effects and mechanisms of SGES on impaired gastric accommodation. Vagotomy was used in this study to induce impaired gastric accommodation. Previously, Mochiki et al. (37) studied the function of the pylorus in normal dogs and dogs with truncal vagotomy and reported a disappearance of pyloric relaxation with vagotomy during phase III of the interdigestive migrating motor complex. In our previous studies, delayed gastric empty, impaired gastric fundic accommodation to a meal, and slower small intestinal transit were observed in vagotomized dogs (38, 55, 58). In clinical practice, vagotomy is known to exert extensive effects on gastrointestinal motility. Delayed gastric solid emptying was noted after truncal vagotomy (24, 41). Impaired gastric accommodation is frequently presented in postvagotomy patients, reflected as an elevated gastric tone and/or absent or diminished postprandial gastric relaxation (53). These previous findings demonstrate that vagotomy is a good model for studying impaired gastric accommodation associated with gastroparesis or functional dyspepsia. In this study, gastric accommodation was significantly impaired in vagotomized dogs, and this was in agreement with the findings in postvagotomy patients.
The accommodation response is mediated via a vagovagal reflex pathway involving intrinsic nitrergic neurons in the gastric wall (4). Delayed gastric solid emptying was presented after truncal vagotomy and returned to normal ∼1 mo later in one-half of patients after truncal vagotomy, suggesting that neural pathways other than the truncal vagus compensated impaired motility functions (24). To assure the sustained effect of vagotomy, all experiments in the vagotomized dogs in this study were completed within 1 mo after vagotomy before the function of the vagus could be potentially compensated by other mechanisms. Interestingly, as reported in previous human studies, gastric accommodation in vagotomized dogs was only partially impaired (a 45% reduction compared with that in the normal dogs). This indicates that other pathways are also involved in the regulation of gastric accommodation reflex. Accordingly, it is possible to restore the impaired relaxation function in vagotomized dogs by enhancing the compensatory pathways.
In clinical practice, therapies for gastroparesis and functional dyspepsia are limited and unsatisfactory. Prokinetic agents, such as metoclopramide, erythromycin, and domperidone, are the mainstays of treatment. Although certain prokinetics, such as erythromycin, are able to improve gastric motility and emptying, their ameliorating effects on symptoms of dyspepsia are often limited. The major reason is that the prokinetics do not improve or even impair gastric accommodation (19, 51). On the other hand, more and more recent studies have revealed impaired gastric accommodation as one of the major pathogeneses of gastroparesis and functional dyspepsia (13, 25, 50, 52).
Various methods of GES have been proposed for the treatment of gastroparesis, and different effects have been reported related to each of these different methods of GES. In this study, a comprehensive method of GES was used: dual-pulse GES synchronized with gastric slow waves (called SGES in short). It possessed advantages of various methods of GES and was well suited for treating gastroparesis because of the following: 1) the short-pulse component is known to have antiemetic effects (3, 34); 2) the long-pulse component was reported to normalize slow wave dysrhythmia (34); and 3) the stimulation-synchronized gastric slow waves have recently been reported to enhance antral contractions and accelerate gastric emptying (59). However, it was unknown whether this prokinetic-like SGES method would have detrimental effects on gastric accommodation similar to some of the prokinetic agents. In a number of previous studies, the conventional method of GES with long pulses (not synchronized with slow waves) was reported to reduce gastric tone in normal dogs via the nitrergic mechanism (49, 55). Because the SGES method proposed in this study also contained the component of long pulses, it was hypothesized that the SGES method was able to improve gastric accommodation in vagotomized dogs.
The exact detailed mechanisms by which the gastric wall maintains the tone and relaxes to a meal or distension have not been clearly elucidated, although the interactions between cholinergic vagal input and inhibitory neurons are known to play an important role (29, 40). In this study, bilateral truncal vagotomy showed no effects on basal gastric tone or gastric compliance, and this suggested the involvement of local vagus or intrinsic neurons in the maintenance of gastric tone and compliance. Although not altered by vagotomy, gastric tone and compliance in the fasting state were both improved with SGES. The fasting gastric fundic tone was reduced by SGES via the nitrergic pathway. In parallel, fasting gastric compliance was also improved in the vagotomized dogs with SGES via the same nitrergic pathway.
Gastric accommodation is a consistent physiological response that allows the stomach to accommodate the ingested food without increasing intragastric pressure or inducing discomfort. The regulation of accommodation reflex relies on neural pathways, especially the vagal nerve, as shown by the findings of this study: the interruption of this pathway by truncal vagotomy substantially reduced the extent of accommodation. As hypothesized, SGES normalized vagotomy-induced impairment in gastric accommodation. The postprandial increase in gastric volume at the constant operating pressure in the vagotomized dogs with SGES was comparable with that in the control dogs without SGES. Furthermore, the ameliorating effect of SGES on gastric accommodation was completely blocked by l-NNA, indicative of the nitrergic pathway. Because the vagus was cut bilaterally, local vagal and/or intrinsic neural pathways are believed to be involved in SGES. However, it should be pointed out that SGES did not seem to be able to improve gastric accommodation in normal dogs with intact vagus, and this indicates that testing fundal accommodation as an essential prerequisite is necessary before SGES is considered as a therapeutic option in humans.
In addition to the restoration of gastric accommodation, SGES was also found to improve gastric slow waves. A reduced percentage of normal slow waves was noted in both fasting and fed states in the vagotomized dogs, and this was consistent with previous findings (26, 54). It was interesting to note that SGES delivered in the proximal stomach was able to normalize slow waves in the distal antrum, especially in the postprandial state. In addition, SGES also increased the dominant power of the slow waves. The dominant power of the gastric slow wave was previously reported to be positively correlated with gastric contractility (12). Accordingly, the finding of the increased postprandial slow wave power with SGES seemed to suggest enhanced antral contractions, which was reported in a previous study (59). Reduced percentage of normal slow waves and reduced postprandial slow wave power have been frequently reported in patients with gastroparesis and functional dyspepsia (9, 46) and were reported to be correlated and predictive of delayed gastric emptying (10). Accordingly, the improvement in gastric slow waves with SGES would bring additional benefits to patients with gastroparesis or functional dyspepsia.
The clinical relevance and significance of this study are obvious. As stated earlier, pathogeneses of gastroparesis and functional dyspepsia include impaired gastric accommodation, abnormal interstitial cells of Cajal and gastric slow waves, antral hypomotility, and delayed gastric emptying. The findings of this study demonstrated the ameliorating effects of SGES on gastric accommodation and gastric slow waves. Previous studies have shown the prokinetic effects of SGES: enhancement of antral contractions and acceleration of gastric emptying. Taking all these findings together, the proposed method of SGES is expected to be a viable therapy for gastroparesis. The implementation of this therapy is minimally invasive. It requires implantation of two pairs of electrodes (one for the detection of gastric slow waves and the other for stimulation) and a subcutaneous stimulator. This can be achieved by a laparoscopic procedure. The safety of this procedure has already been proven by the Enterra therapy (short-pulse GES) in >1,000 patients (2). However, the performance of the proposed SGES is expected to be much better than the Enterra therapy that primarily reduces nausea and vomiting. Testing fundal accommodation as an essential prerequisite before considering SGES as a therapeutic option is suggested, since insufficient preliminary studies did not show increases in accommodation in regular control dogs. Further clinical studies are needed to prove the clinical efficacy of SGES.
In a previous study, SGES was found to significantly accelerate glucagon-induced delayed gastric emptying, and the effect was noted to be mediated via the cholinergic pathway (59). Accordingly, an acceleration in gastric emptying would not be expected in the vagotomized dogs used in this study. In other words, SGES may not be a good option for accelerating gastric empty in patients with severe autonomic neuronpathy.
In conclusion, dual-pulse GES synchronized with intrinsic gastric slow waves is able to restore impaired gastric accommodation induced by vagotomy, and this effect is mediated via the nitrergic pathway. In addition, SGES also improves slow wave rhythmicity and amplitude in the postprandial state in vagotomized dogs. Further clinical studies are warranted to prove the clinical efficacy of SGES in treating patients with gastroparesis.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-055437.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abell T, McCallum R, Hocking M, Koch K, Abrahamsson H, Leblanc I, Lindberg G, Konturek J, Nowak T, Quigley EM, Tougas G, Starkebaum W. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology 125: 421–428, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Abell TL, Bernstein RK, Cutts T, Farrugia G, Forster J, Hasler WL, McCallum RW, Olden KW, Parkman HP, Parrish CR, Pasricha PJ, Prather CM, Soffer EE, Twillman R, Vinik AI. Treatment of gastroparesis: a multidisciplinary clinical review. Neurogastroenterol Motil 18: 263–283, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Abell TL, Van Cutsem E, Abrahamsson H, Huizinga JD, Konturek JW, Galmiche JP, VoelIer G, Filez L, Everts B, Waterfall WE, Domschke W, Bruley des Varannes S, Familoni BO, Bourgeois IM, Janssens J, Tougas G. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion 66: 204–212, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Azpiroz F, Malagelada JR. Vagally mediated gastric relaxation induced by intestinal nutrients in the dog. Am J Physiol Gastrointest Liver Physiol 251: G727–G735, 1986. [DOI] [PubMed] [Google Scholar]
- 5.Bellahsene BE, Lind CD, Schirmer BD, Updike OL, McCallum RW. Acceleration of gastric emptying with electrical stimulation in a canine model of gastroparesis. Am J Physiol Gastrointest Liver Physiol 262: G826–G834, 1992. [DOI] [PubMed] [Google Scholar]
- 6.Benini L, Castellani G, Brighenti F, Heaton KW, Brentegani MT, Casiraghi MC, Sembenini C, Pellegrini N, Fioretta A, Minniti G, Porrini M, Testolin G, Vantini I. Gastric emptying of a solid meal is accelerated by the removal of dietary fibre naturally present in food. Gut 36: 825–830, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bharucha AE, Camilleri M, Zinsmeister AR, Hanson RB. Adrenergic modulation of human colonic motor and sensory function. Am J Physiol Gastrointest Liver Physiol 273: G997–G1006, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Bromer MQ, Friedenberg F, Miller LS, Fisher RS, Swartz K, Parkman HP. Endoscopic pyloric injection of botulinum toxin A for the treatment of refractory gastroparesis. Gastrointest Endosc 61: 833–839, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, McCallum RW. Gastric slow wave abnormalities in patients with gastroparesis. Am J Gastroenterol 87: 477–482, 1992. [PubMed] [Google Scholar]
- 10.Chen JD, Lin Z, Pan J, McCallum RW. Abnormal gastric myoelectrical activity and delayed gastric emptying in patients with symptoms suggestive of gastroparesis. Dig Dis Sci 41: 1538–1545, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Chen JD, Qian L, Ouyang H, Yin J. Gastric electrical stimulation with short pulses reduces vomiting but not dysrhythmias in dogs. Gastroenterology 124: 401–409, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Chen JD, Richards RD, McCallum RW. Identification of gastric contractions from the cutaneous electrogastrogram. Am J Gastroenterol 89: 79–85, 1994. [PubMed] [Google Scholar]
- 13.Coulie B, Tack J, Sifrim D, Andrioli A, Janssens J. Role of nitric oxide in fasting gastric fundus tone and in 5-HT1 receptor-mediated relaxation of gastric fundus. Am J Physiol Gastrointest Liver Physiol 276: G373–G377, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Cutts TF, Luo J, Starkebaum W, Rashed H, Abell TL. Is gastric electrical stimulation superior to standard pharmacologic therapy in improving GI symptoms, healthcare resources, and long-term health care benefits? Neurogastroenterol Motil 17: 35–43, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Eagon JC, Kelly KA. Effects of gastric pacing on canine gastric motility and emptying. Am J Physiol Gastrointest Liver Physiol 265: G767–G774, 1993. [DOI] [PubMed] [Google Scholar]
- 16.Ejskjaer NT, Bradley JL, Buxton-Thomas MS, Edmonds ME, Howard ER, Purewal T, Thomas PK, Watkins PJ. Novel surgical treatment and gastric pathology in diabetic gastroparesis. Diabet Med 16: 488–495, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Feldman M, Corbett DB, Ramsey EJ, Walsh JH, Richardson CT. Abnormal gastric function in longstanding, insulin-dependent diabetic patients. Gastroenterology 77: 12–17, 1979. [PubMed] [Google Scholar]
- 18.Forstner-Barthell AW, Murr MM, Nitecki S, Camilleri M, Prather CM, Kelly KA, Sarr MG. Near-total completion gastrectomy for severe postvagotomy gastric stasis: analysis of early and long-term results in 62 patients. J Gastrointest Surg 3: 15–21, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Galligan JJ, Vanner S. Basic and clinical pharmacology of new motility promoting agents. Neurogastroenterol Motil 17: 643–653, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Hocking MP Postoperative gastroparesis and tachygastria–response to electric stimulation and erythromycin. Surgery 114: 538–542, 1993. [PubMed] [Google Scholar]
- 21.Horowitz M, Harding PE, Maddox AF, Wishart JM, Akkermans LM, Chatterton BE, Shearman DJ. Gastric and oesophageal emptying in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 32: 151–159, 1989. [DOI] [PubMed] [Google Scholar]
- 22.Horowitz M, Maddox AF, Wishart JM, Harding PE, Chatterton BE, Shearman DJ. Relationships between oesophageal transit and solid and liquid gastric emptying in diabetes mellitus. Eur J Nucl Med 18: 229–234, 1991. [DOI] [PubMed] [Google Scholar]
- 23.Horvath VJ, Vittal H, Lorincz A, Chen H, Almeida-Porada G, Redelman D, Ordog T. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology 130: 759–770, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Howlett PJ, Ward AS, Duthie HL. Gastric emptying after vagotomy. Proc R Soc Med 67: 836–838, 1974. [PMC free article] [PubMed] [Google Scholar]
- 25.Karamanolis G, Caenepeel P, Arts J, Tack J. Determinants of symptom pattern in idiopathic severely delayed gastric emptying: gastric emptying rate or proximal stomach dysfunction? Gut 56: 29–36, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly KA, Code CF. Effect of transthoracic vagotomy on canine gastric electrical activity. Gastroenterology 57: 51–58, 1969. [PubMed] [Google Scholar]
- 27.Kim CH, Nelson DK. Venting percutaneous gastrostomy in the treatment of refractory idiopathic gastroparesis. Gastrointest Endosc 47: 67–70, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Kumar A, Attaluri A, Hashmi S, Schulze KS, Rao SS. Visceral hypersensitivity and impaired accommodation in refractory diabetic gastroparesis. Neurogastroenterol Motil 20: 635–642, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Lidums I, Hebbard GS, Holloway RH. Effect of atropine on proximal gastric motor and sensory function in normal subjects. Gut 47: 30–36, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin Z, Forster J, Sarosiek I, McCallum RW. Effect of high-frequency gastric electrical stimulation on gastric myoelectric activity in gastroparetic patients. Neurogastroenterol Motil 16: 205–212, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care 27: 1071–1076, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Lin Z, Sarosiek I, Forster J, McCallum RW. Symptom responses, long-term outcomes and adverse events beyond 3 years of high-frequency gastric electrical stimulation for gastroparesis. Neurogastroenterol Motil 18: 18–27, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Lin ZY, McCallum RW, Schirmer BD, Chen JD. Effects of pacing parameters on entrainment of gastric slow waves in patients with gastroparesis. Am J Physiol Gastrointest Liver Physiol 274: G186–G191, 1998. [DOI] [PubMed] [Google Scholar]
- 34.McCallum RW, Chen JD, Lin Z, Schirmer BD, Williams RD, Ross RA. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterology 114: 456–461, 1998. [DOI] [PubMed] [Google Scholar]
- 35.McCallum RW, Fink SM, Lerner E, Berkowitz DM. Effects of metoclopramide and bethanechol on delayed gastric emptying present in gastroesophageal reflux patients. Gastroenterology 84: 1573–1577, 1983. [PubMed] [Google Scholar]
- 36.Mearin F, Camilleri M, Malagelada JR. Pyloric dysfunction in diabetics with recurrent nausea and vomiting. Gastroenterology 90: 1919–1925, 1986. [DOI] [PubMed] [Google Scholar]
- 37.Mochiki E, Kuwano H, Nakabayashi T, Garcia M, Haga N, Asao T. Pyloric relaxation regulated via intramural neural pathway of the antrum. Dig Dis Sci 46: 2307–2313, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Ouyang H, Xing J, Chen J. Electroacupuncture restores impaired gastric accommodation in vagotomized dogs. Dig Dis Sci 49: 1418–1424, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Parkman HP, Hasler WL, Barnett JL, Eaker EY. Electrogastrography: a document prepared by the gastric section of the American Motility Society Clinical GI Motility Testing Task Force. Neurogastroenterol Motil 15: 89–102, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Paterson CA, Anvari M, Tougas G, Huizinga JD. Nitrergic and cholinergic vagal pathways involved in the regulation of canine proximal gastric tone: an in vivo study. Neurogastroenterol Motil 12: 301–306, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Petrakis IE, Vrachassotakis N, Vassilakis SJ, Sciacca V, Chalkiadakis G. Erythromycin enhances solid-phase gastric emptying in induced-hyperglycemia in patients with truncal vagotomy and pyloroplasty. Dig Dis Sci 45: 937–945, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Richards RD, Davenport K, McCallum RW. The treatment of idiopathic and diabetic gastroparesis with acute intravenous and chronic oral erythromycin. Am J Gastroenterol 88: 203–207, 1993. [PubMed] [Google Scholar]
- 43.Sarna SK, Daniel EE. Electrical stimulation of gastric electrical control activity. Am J Physiol 225: 125–131, 1973. [DOI] [PubMed] [Google Scholar]
- 44.Sarna SK, Soergel KH, Koch TR, Stone JE, Wood CM, Ryan RP, Arndorfer RC, Cavanaugh JH, Nellans HN, Lee MB. Gastrointestinal motor effects of erythromycin in humans. Gastroenterology 101: 1488–1496, 1991. [DOI] [PubMed] [Google Scholar]
- 45.Sawhney MS, Prakash C, Lustman PJ, Clouse RE. Tricyclic antidepressants for chronic vomiting in diabetic patients. Dig Dis Sci 52: 418–424, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Simonian HP, Panganamamula K, Chen JZ, Fisher RS, Parkman HP. Multichannel electrogastrography (EGG) in symptomatic patients: a single center study. Am J Gastroenterol 99: 478–485, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Song GQ, Chen JD. Synchronized gastric electrical stimulation improves delayed gastric emptying in nonobese mice with diabetic gastroparesis. J Appl Physiol 103: 1560–1564, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Song GQ, Hou X, Yang B, Sun Y, Qian W, Chen JD. A novel method of 2-channel dual-pulse gastric electrical stimulation improves solid gastric emptying in dogs. Surgery 143: 72–78, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun Y, Chen JD. Gastric electrical stimulation inhibits postprandial antral tone partially via nitrergic pathway in conscious dogs. Am J Physiol Regul Integr Comp Physiol 290: R904–R908, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Tack J, Broekaert D, Coulie B, Fischler B, Janssens J. Influence of the selective serotonin re-uptake inhibitor, paroxetine, on gastric sensorimotor function in humans. Aliment Pharmacol Ther 17: 603–608, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Tack J, Caenepeel P, Piessevaux H, Cuomo R, Janssens J. Assessment of meal induced gastric accommodation by a satiety drinking test in health and in severe functional dyspepsia. Gut 52: 1271–1277, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology 115: 1346–1352, 1998. [DOI] [PubMed] [Google Scholar]
- 53.Troncon LE, Thompson DG, Ahluwalia NK, Barlow J, Heggie L. Relations between upper abdominal symptoms and gastric distension abnormalities in dysmotility like functional dyspepsia and after vagotomy. Gut 37: 17–22, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilbur BG, Kelly KA. Effect of proximal gastric, complete gastric, and truncal vagotomy on canine gastric electric activity, motility, and emptying. Ann Surg 178: 295–303, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xing JH, Chen JD. Effects and mechanisms of long-pulse gastric electrical stimulation on canine gastric tone and accommodation. Neurogastroenterol Motil 18: 136–143, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Xing JH, Chen JD. Reproducibility of gastric tone, compliance and gastric accommodation assessed with barostat in conscious dogs. Neurogastroenterol Motil 17: 83–88, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Yin J, Chen J. Excitatory effects of synchronized intestinal electrical stimulation on small intestinal motility in dogs. Am J Physiol Gastrointest Liver Physiol 293: G1190–G1195, 2007. [DOI] [PubMed] [Google Scholar]
- 58.Yin J, Ouyang H, Chen JD. Potential of intestinal electrical stimulation for obesity: a preliminary canine study. Obesity (Silver Spring) 15: 1133–1138, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Zhu H, Sallam H, Chen DD, Chen JD. Therapeutic potential of synchronized gastric electrical stimulation for gastroparesis: enhanced gastric motility in dogs. Am J Physiol Regul Integr Comp Physiol 293: R1875–R1881, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Zitomer BR, Gramm HF, Kozak GP. Gastric neuropathy in diabetes mellitus: clinical and radiologic observations. Metabolism 17: 199–211, 1968. [DOI] [PubMed] [Google Scholar]