Abstract
Lubiprostone, a bicyclic fatty acid chloride channel activator, is efficacious in treatment of chronic constipation and constipation-predominant irritable bowel syndrome. The study aim was to compare effects of lubiprostone and placebo on colonic sensory and motor functions in humans. In double-blind, randomized fashion, 60 healthy adults received three oral doses of placebo or 24 μg lubiprostone per day in a parallel-group, placebo-controlled trial. A barostat-manometry tube was placed in the left colon by flexible sigmoidoscopy and fluoroscopy. We measured treatment effects on colonic sensation and motility with validated methods, with the following end points: colonic compliance, fasting and postprandial tone and motility indexes, pain thresholds, and sensory ratings to distensions. Among participants receiving lubiprostone or placebo, 26 of 30 and 28 of 30, respectively, completed the study. There were no overall effects of lubiprostone on compliance, fasting tone, motility indexes, or sensation. However, there was a treatment-by-sex interaction effect for compliance (P = 0.02), with lubiprostone inducing decreased fasting compliance in women (P = 0.06) and an overall decreased colonic tone contraction after a standard meal relative to fasting tone (P = 0.014), with greater effect in women (P < 0.01). Numerical differences of first sensation and pain thresholds (P = 0.11 in women) in the two groups were not significant. We concluded that oral lubiprostone 24 μg does not increase colonic motor function. The findings of decreased colonic compliance and decreased postprandial colonic tone in women suggest that motor effects are unlikely to cause accelerated colonic transit with lubiprostone, although they may facilitate laxation. Effects of lubiprostone on sensitivity deserve further study.
Keywords: tone, compliance, threshold, trial, constipation, irritable bowel
lubiprostone, a bicyclic fatty acid, is a chloride channel type 2 (ClC-2) activator that accelerates small intestinal and colonic transit (6) in healthy humans. The acceleration in gastrointestinal transit is attributed to increased chloride and water secretion by activation of ClC-2 on intestinal and colonic epithelial cells (9). ClC-2 channels are members of the nine member Cl− channel family that are widely distributed in nature (18). ClC-2 channels appear to be uniquely capable to promote anion secretion with little anion reabsorption. On the other hand, the cystic fibrosis transmembrane conductance regulator (CFTR) could promote either reabsorption or secretion depending on the anion driving forces (2).
In addition to being located on intestinal and colonic epithelial cells, ClC-2 channels have been shown to modulate afferent function in the ear and laryngeal mucosa and in proprioceptive afferents (7, 13, 22). Therefore, lubiprostone may reduce visceral sensitivity by altering the function of visceral afferents.
There is evidence that lubiprostone may affect smooth muscle cell function. Lubiprostone is a prostone, which is derived from a metabolite of prostaglandin E1. However, unlike prostaglandins, prostones have little or no effect on prostaglandin E or F receptors and do not stimulate smooth muscle contraction (23). Cuppoletti et al. (8) have shown that lubiprostone causes smooth muscle membrane hyperpolarization independent of prostaglandin E receptor activation. This membrane hyperpolarization may be mediated through lubiprostone's highly selective activity on ClC-2 located on smooth muscle cells. Since lubiprostone is efficacious in the treatment of chronic constipation (16, 17) and constipation-predominant irritable bowel syndrome (IBS; C-IBS) (15) and relieves symptoms such as pain and bloating (6), it is relevant to explore whether this chloride channel activator alters human colonic neuromuscular or neurosensory function. Understanding effects on these functions may provide insights into the effects of this class of compounds on visceral hypersensitivity and motor dysfunctions that are associated with IBS.
From a mechanistic perspective, lubiprostone was shown to have a direct effect in smooth muscle hyperpolarization and on laryngeal sensory nerves. Therefore, the study hypothesis was that the chloride channel activator, lubiprostone, modulates colonic motility and sensation. The specific aims were to compare the effects of placebo and lubiprostone on colonic sensory function and motility in healthy volunteers: specifically, pain and gas sensation ratings in response to phasic distension, thresholds for colonic sensation of first sensation, gas and pain in response to distension, fasting colonic compliance, and tone and postprandial tonic response to standard meal ingestion.
MATERIALS AND METHODS
Study Population
The study was conducted between September 2007 and July 2008. Healthy volunteers between 18 and 65 yr of age with a body mass index between 18 and 35 kg/m2 were recruited from the local community by public advertisement. The exclusion criteria included clinical evidence (including physical exam and ECG) of significant cardiovascular, respiratory, renal, hepatic, gastrointestinal, hematological, neurological, psychiatric, or other disease that would interfere with the objectives of the study; gastrointestinal structural or metabolic diseases, or functional gastrointestinal disorders as defined by a Bowel Disease Questionnaire (24); use of drugs or agents within the past 2 wk that alter gastrointestinal transit including laxatives, magnesium or aluminum-containing antacids, prokinetics, erythromycin, narcotics, anticholinergics, tricyclic antidepressants, selective serotonin reuptake inhibitor, and newer antidepressants; use of central nervous system depressants within the past 2 wk; pregnancy or breast feeding; significant affective or anxiety disorder as defined by the Hospital Anxiety and Depression Scale (27) used for screening purposes; symptoms of a significant clinical illness in the preceding 2 wk; alcoholism not in remission or known substance abuse; and participation in another clinical study within the past 30 days. The study was approved by the Mayo Clinic Institutional Review Board, and written, informed consent was obtained prior to enrollment in the study.
Subject Discontinuation
Participation in the study was strictly voluntary. A participant had the right to withdraw from the study at any time and for any reason.
Study Design
This was a randomized, double-blind, placebo-controlled, single-center, parallel-group, pharmacodynamic study. The screening period was within 2 wk of the treatment period. The subjects were allocated to the treatment groups by use of a preestablished randomization schedule in chronological order of enrollment. Each subject received three doses (one per day) of either lubiprostone 24 μg or an identical-appearing placebo. On days 1 and 2, participants took the study medication with their breakfast meal and recorded the time. On day 2, starting at 4:00 PM, participants started a polyethylene glycol-based bowel preparation to cleanse the colon. After overnight bowel preparation, participants reported fasting to the study center at 7:00 AM on day 3. Colonic sensorimotor functions were assessed by an endoscopically placed barostat-manometric assembly.
After 30 min of rest following tube placement, fasting colonic tone, colonic compliance, and colonic sensation were tested. The last dose of medication was ingested and 1 h later the same colonic functions, as well as colonic response to a standardized meal of a 1,000-kcal chocolate milkshake were assessed. The patient was able to leave the study center in the afternoon, after a meal had been ingested (if desired).
Study Procedures
The methods, procedure, data and statistical analyses for measurement of colonic motor and sensory functions have been published extensively elsewhere (4, 14, 25).
Colonic tube placement.
After an overnight bowel preparation with oral colonic lavage solution (NuLytely; Braintree Laboratories, Braintree, MA), participants reported fasting to the study center at 7:00 AM on day 3. A flexible sigmoidoscopy was performed without sedation to evaluate the left side of the colon and to place a Teflon-coated guide wire (Microvasive, Hobbs Medical Stafford Springs, CT) beyond the splenic flexure. The colon was deflated as the sigmoidoscope was removed and a barostat catheter (constructed at Mayo Clinic, Rochester, MN) with six manometric point sensors and a polyethylene balloon (MUI Scientific, Mississauga, Canada) was introduced into the colon over the guide wire. The barostat catheter was positioned in the middescending or upper sigmoid with the aid of fluoroscopy. The final position of the barostatically controlled balloon was confirmed by fluoroscopy. In the placebo group, the balloon was positioned in the descending (n = 20) or proximal sigmoid (n = 8) colon. In the lubiprostone group, the balloon was placed in the descending (n = 17) or proximal sigmoid (n = 9) colon.
Colonic motor and sensory assessment.
After colonic tube placement, participants returned to a regular hospital room and were allowed to rest for 30 min. The catheter was connected to a rigid piston barostat machine (Mayo Clinic, Rochester, MN) and colonic tone was assessed by noting the changes in the balloon volume in the presence of a constant operating pressure in the balloon. After transient inflation of the barostat bag to a volume of 75 ml to ensure it was unfolded, it was deflated. Thereafter, it was inflated in 2-mmHg increments to baseline operating pressure (BOP), which was defined as 2 mmHg above the minimal distension pressure at which respiratory excursions were clearly recorded by the barostat tracing. Previous studies have shown that an initial “conditioning” distension to 20 mmHg renders subsequent assessments of compliance and perception more reproducible (14). Therefore, a conditioning distention from 0 to 20 mmHg in increments of 2 mmHg for 30 s was performed.
After an equilibration period of 5 min at 0 mmHg, a visual analog scale (VAS) was used to determine the level of anxiety and tiredness, which are known to be significant covariates in the assessment of visceral sensation scores. Colonic compliance and sensory thresholds for first sensation, gas, and pain were then measured by stepwise inflation in increments of 4 mmHg at 60-s intervals up to a maximum pressure of 64 mmHg. During this assessment, participants were asked to report when they had the first perception of gas and pain. We recorded the threshold pressure at which the participants reported these sensations. The inflations were stopped when participants reported pain. After a 10-min equilibration period at BOP, the participants recorded their intensity of gas and pain on a 100-mm VAS. Subsequently, randomized-order phasic distensions at 8, 16, 24, 32, and 36 mmHg above baseline operating pressure were applied and the participants recorded their intensity of gas and pain sensation on a 100-mm VAS during each distention. After another 10 min rest at BOP, colonic tone was assessed for 30 min at BOP.
Participants received the medication to which they were randomized and, after 1 h, colonic compliance and sensation were reassessed as described above. Thereafter, colonic tone was measured before (30 min) and after (60 min) a standard liquid, high-fat 1,000-kcal meal (10).
When the recording was finished, the assembly was removed by gentle traction of the tube. The participants were assessed, offered a general meal, and dismissed from the study.
Data Analysis
Colonic compliance.
Colonic compliance was analyzed by a linear interpolation method recently described and validated in our laboratory (20). The pressure at half maximum volume serves as a summary of colonic compliance.
Colonic motor function.
Colonic tone was assessed operationally as in the prior literature as the intracolonic balloon volume measured at the operating pressure. Tone was calculated by the baseline colonic volumes measured throughout the period of interest during fasting or after the meal. Changes in colonic tone were calculated as absolute volume changes during fasting in response to the study medication and as the symmetric percent change in volume postprandially. This relative change in tone (%) is calculated as 100 × loge (average baseline volume in first 30 min postprandially/average baseline volume in fasting 30 min premeal).
Colonic manometry.
The same computer program was used to measure the postprandial phasic motor activity in the proximal and distal three manometric sensors. Because of variation in the location of the barostat balloon in the upper or lower descending colon, the phasic activity was summarized in each individual for the three sensors that were located 5, 10, and 15 cm distal to the barostat balloon. Data were compared for the fasting period of 30 min vs. the two 30-min periods after the 1,000-kcal meal was ingested. Colonic phasic pressure activity was summarized as a motility index (MI) where MI = loge [(sum of amplitudes × number of contractions) + 1].
High-amplitude propagated contractions (HAPCs) were defined as contractions of at least 75 mmHg, propagated rapidly over at least 10 cm [3 adjacent manometric sensors (19)].
The effect of treatment on the number of HAPCs was assessed by first dichotomizing the counts (0 vs. >0) for each response period (separately, fasting and postprandial) and then comparing treatment groups using a χ2 test for proportions (counts >0).
Statistical Analysis
The analyses included all randomized subjects based on an intention-to-treat principle. The primary investigator(s) remained blinded to the treatment assignments until all response data had been edited and documented in a SAS database developed in the Mayo Clinic Section of Biostatistics.
The primary goal of this study was to compare the responses (colonic sensation, motility) among the two treatment groups (placebo and lubiprostone). A descriptive summary of subject characteristics (e.g., age, sex) at baseline was compiled by treatment group.
Colonic sensation.
The assessment of the gas and pain scores on the VAS during the phasic distentions were based on repeated-measures analysis of covariance. A compound-symmetry variance-covariance matrix for the five repeated values (score at 8, 16, 24, 32, and 36 mmHg) was used. Gas and pain scores were analyzed by separate models. The covariates in this analysis included age, sex, body mass index (BMI), and the individual subject mean baseline score (overdistension levels) predrug. The pressure thresholds at which participants reported first sensation, gas, and pain during the assessment of colonic compliance (ramp distention) were assessed by using a proportional hazards regression model to account for potential censoring of threshold levels (i.e., the sensation was not experienced by the completion of the pressure distension using the ascending method of limits).
Colonic motor function.
The analysis of primary motility end points [fasting colonic tone, postprandial change in colonic tone, and colonic compliance summarized as pressure at half-maximum volume (Pr 1/2)] was also based on an analysis of covariance with sex, BMI, and the predrug Pr 1/2 values for postdrug colonic compliance, and the fasting (predrug) colonic tone in the analysis of the postdrug fasting and postprandial changes in colonic tone as covariates.
Each variable of interest corresponds to different physiological and/or biological parameters of interest; lubiprostone may have independent effects on any of these parameters and, thus, no adjustment in the α level (two-sided level of 0.05) for multiple types of response end points (e.g., different types of VAS scores) was used. Additional analyses and summaries of the response data (by treatment group) focused on those subjects with complete data.
Primary and Secondary End Points
The primary end points included colonic compliance, fasting colonic tone, postprandial colonic tone, and pain sensation during colonic distensions. The secondary end points were gas sensation during colonic distensions and thresholds for colonic first gas and pain sensation.
In keeping with the intention-to-treat paradigm, randomized subjects with missing data were assigned an appropriate value for the missing data, consistent with the null hypothesis for these analyses. For example, the overall (both treatment groups) mean value for a continuous response (e.g., postdrug fasting colonic tone) was used to impute missing values, and a corresponding adjustment in the error degrees of freedom in the analysis of covariance was made (subtracting one degree of freedom for each value imputed).
Sample Size Assessment
The proposed sample size (N = 30 per treatment group) was selected because it was expected to provide 80% power to detect the effect sizes listed below between two groups based on a simple two-sample t-test (Table 1). The analysis of covariance was expected to provide similar power for somewhat smaller (overall) differences by incorporating relevant covariates.
Table 1.
Effect size detectable for main sensory and motor end points (based on proposed sample of n = 30 per treatment group)
| Response | CV, % | Effect size detectable, 30 vs. 30 |
|---|---|---|
| Pain score with 32 mmHg distension | 67 | 49% |
| Gas score with 32 mmHg distension | 54 | 40% |
| Colonic meal response (tone) | 51 | 38% |
| Fasting colonic tone | 32 | 24% |
| Colonic compliance (Pr 1/2) | 17 | 12% |
The coefficients of variation (CV) are based on previous data and studies performed in our laboratory, and effect size is the difference between groups as a percentage of the overall (groups) mean.
The effect sizes detectable were deemed to have clinical significance since they were in the range of changes observed in disease states and in response to medications, such as the effect of a 5-HT3 antagonist on postprandial colonic tone (26).
Safety Measurements
We monitored clinical parameters including complete physical examination, weight, heart rate, and systolic and diastolic blood pressure measured in supine and in standing position at screening visit and noted any change in health status at each visit. Adverse events were recorded. A urine pregnancy test was done within 48 h of study day for women of childbearing potential. All subjects received a follow-up phone call within 48–72 h after completion of the study to monitor for adverse effects.
RESULTS
Participants and Disposition
Thirty healthy subjects were assigned to each group (Table 2); of those receiving lubiprostone or placebo, 26 and 28, respectively, completed the study. The reasons for discontinuation were as follows: one withdrew before randomization to medication and four withdrew after treatment but before the colonic tube placement. None of the participants withdrew because of serious adverse events or pregnancy. The investigator terminated two studies, one because of a difficult tube placement and the other because of a leak in the barostat catheter.
Table 2.
Participant demographics and state of anxiety prior to sensation studies
|
Lubiprostone |
Placebo
|
|||
|---|---|---|---|---|
| N | Mean ± SE | N | Mean ± SE | |
| Sex, n (%) female | 16 (53%) | 15 (50%) | ||
| Age, yr | 30 | 35.1±2.3 | 30 | 33.2±2.2 |
| BMI, kg/m2 | 30 | 25.3±0.5 | 30 | 25.5±0.7 |
| Pretired | 26 | 33.2±3.2 | 28 | 39.0±3.3 |
| Prepeace | 26 | 29.5±2.9 | 28 | 31.6±4.1 |
| Preworried | 26 | 73.5±2.5 | 28 | 74.5±3.4 |
| Preactive | 26 | 65.2±3.1 | 28 | 55.0±3.2 |
BMI, body mass index.
Effects on Compliance and Fasting Tone
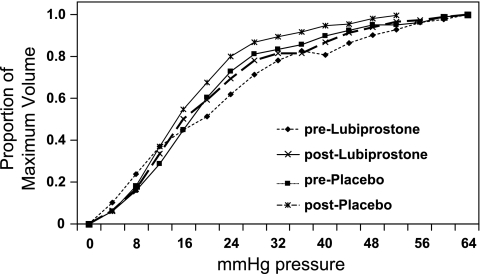
In an analysis involving all participants (male and female sex), lubiprostone had no effect on colonic compliance (P = 0.78, Fig. 1) or fasting tone (P = 0.087, Fig. 2) compared with placebo group. However, there was a significant treatment-by-sex interaction (P = 0.021) in the effect on colonic compliance, suggesting lower colonic compliance with lubiprostone in women (P = 0.06, unadjusted, Figs. 3 and 4). The significance of a difference in Pr 1/2 of the magnitude observed is unclear.
Fig. 1.
Effects of lubiprostone and placebo on colonic compliance: overall (male and female) data.
Fig. 2.
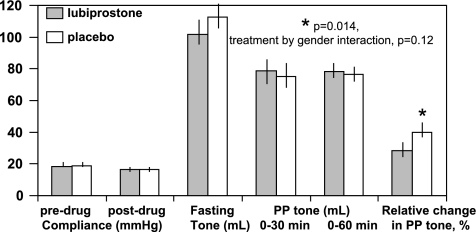
Effects of lubiprostone and placebo on colonic fasting tone, postprandial (PP) tone over 60 min, and relative change in colonic tone during first 30 min after meal compared with fasting. Results obtained in all participants. Note the significantly lower colonic tone contractile response to standard meal ingestion relative to the fasting colonic tone. There is a trend to a higher fasting colonic tone (lower volume) with lubiprostone treatment. Relative change in tone is calculated as 100 × loge (average baseline volume in first 30 min postprandially/average baseline volume in fasting 30 min premeal).
Fig. 3.
Effects of lubiprostone and placebo on colonic compliance in women only, showing decreased colonic compliance (higher Pr 1/2) in women.
Fig. 4.
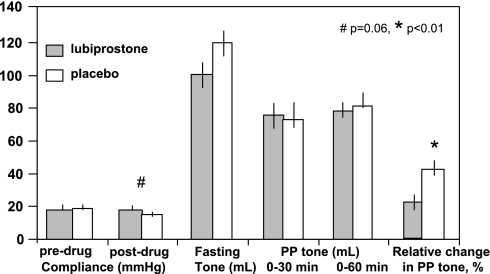
Effects of lubiprostone and placebo on colonic compliance, fasting tone, postprandial tone over 60 min, and relative change in colonic tone during first 30 min after meal compared with fasting. Results obtained only in women show a borderline significant effect on compliance in women (P = 0.06) and a significant effect on the relative change in postprandial tone (P < 0.01).
Effects on Postprandial Tone and Motility Indexes
There was no significant overall effect of lubiprostone on postprandial tone over the first 60 min after the meal (P = 0.83, Fig. 2). However, the relative change in the colonic tone during the first 30 min postprandially, relative to fasting tone, which reflects the initial contractile response of colonic tone to standard meal ingestion was significantly lower with lubiprostone (P = 0.014), and this effect was most prominent in women (Fig. 4, P < 0.01, unadjusted). The trend (P = 0.087) to a significant difference in fasting tone (higher tone or lower volume at baseline pressure) with lubiprostone appears to influence the estimated relative change in tone after the meal, since the actual postprandial balloon volumes or tone were not different with lubiprostone or placebo (Figs. 2 and 4). Effects on motility index (Table 3) were not significant. There was no difference in the number of HAPCs observed during fasting (0.12 ± 0.066 lubiprostone vs. 0.107 ± 0.079 placebo) or postprandially (0.48 ± 0.232 vs. 0.5 ± 0.244, respectively), χ2 P > 0.5.
Table 3.
Motility indexes, MI = loge [(sum of amplitudes × number of contractions) +1]
| Lubiprostone, N =26 | Placebo, N =28 | |
|---|---|---|
| Fasting, predrug | 11.67±0.57 | 12.59±0.3 |
| Fasting, postdrug | 11.49±0.57 | 12.06±0.47 |
| Postdrug, premeal 30 min | 12.07±0.42 | 12.5±0.3 |
| First 30 min postmeal | 12.67±0.41 | 13.1±0.31 |
| Second minute postmeal | 12.5±0.41 | 12.63±0.38 |
| Relative change in postprandial MI | 5.09±1.33 | 4.34±1.45 |
Values are means ± SE. Relative change in postprandial motility index (MI) = 100 × log(postmeal 30 min MI/30 min premeal MI).
Effects on Sensation Thresholds and Ratings
The numerical differences of lubiprostone vs. placebo observed for first sensation and pain threshold were not significant (Fig. 5). There were no significant sex-by-treatment interactions detected; however, pain sensation thresholds appeared to be higher in women with lubiprostone treatment compared with placebo (P = 0.11, unadjusted).
Fig. 5.
Effects of lubiprostone and placebo on sensory thresholds. Note that, although the curves for first sensation and pain are shifted to the right with lubiprostone (by an average of 4 mmHg for the pressure when 50% participants declared those sensations), this reduction in pain sensation with lubiprostone was not statistically significant.
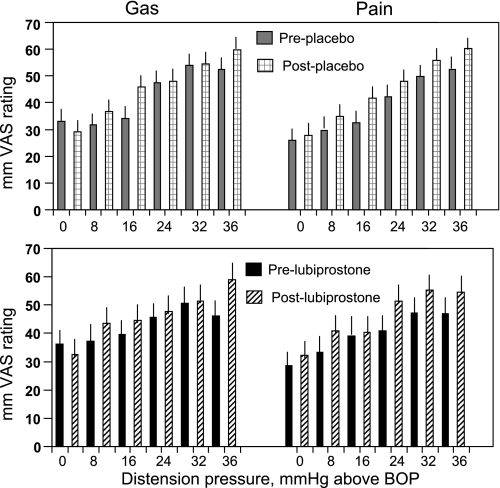
There were no differences in sensory ratings for pain or gas sensation (Fig. 6).
Fig. 6.
Effects of lubiprostone and placebo on sensory ratings. Data are shown pre- and posttreatment and show the absence of any significant change in sensory ratings with treatment. VAS, visual analog scale; BOP, baseline operating pressure.
DISCUSSION
This study of human subjects treated with oral doses of lubiprostone, 24 μg (administered on 3 successive days), provides novel insights into the potential motor and sensory effects of this medication, which has relevance to understanding its efficacy in the treatment of chronic constipation and C-IBS.
The first relevant finding is that, at the dose tested, lubiprostone does not produce motor effects associated with accelerated transit. This information confirms the recently published data that, in contrast to prostaglandins, this prostone does not evoke contraction of human smooth muscle, as shown in human uterine muscle in which lubiprostone leads to smooth muscle membrane hyperpolarization and, hence, relaxation in vitro (8). The decreased colonic compliance (in women) and decreased colonic contraction in response to a meal in both sexes (with a predominant effect in women) might argue against a prokinetic motor effect of lubiprostone. These effects are unlikely to account for the acceleration of colonic transit with lubiprostone.
The finding that lubiprostone is associated with reduced colonic tone response to meal ingestion and with no increase in phasic motility suggests that motor effects are not likely to be the mechanism for the acceleration of transit or the relief of constipation. It is conceivable that the reduced tone response may actually facilitate transport of intraluminal content since the colon is not contracted to induce resistance to flow. This concept was first proposed by Misiewicz et al. (20), because patients with diarrhea had lower contractile activity of the proximal and, particularly, the distal colon after a meal, relative to healthy controls. Our observations in human colonic tone in vivo are supported in part by the recent report (3) that lubiprostone reduced electrically stimulated neuronal contractions in rat and human colon circular muscle preparations, an effect mediated prejunctionally, and reduced by the prostaglandin E4 receptor antagonist, but not by prostaglandin E1 or E3 receptor antagonists. On the other hand, we did not observe any change in phasic pressure activity in the colons of humans with lubiprostone. Moreover, lubiprostone also induced contractions in isolated human stomach muscle strips (3).
The mechanism for the significant sex interaction in the effects of lubiprostone is unclear. There are no current data pertaining to ClC-2 channels; however, there are data indicating effects of sex or sex steroids on other chloride channels. Thus it is intriguing that sex hormones rapidly and reversibly inhibit wild-type ClC-1 channels expressed in Xenopus oocytes by causing a prominent rightward shift in the voltage dependence of their open probability. However, the effect is observed with both testosterone and progesterone (12). On the other hand, the estrogen sex steroid 17β-estradiol rapidly inhibits secretagogue-stimulated cAMP-dependent Cl− secretion in the female rat distal colonic crypt by the inhibition of basolateral K+ channels; this process involves the inhibition of KCNQ1 channel activity via PKCδ- and PKA-dependent signaling pathways (21). This suggests a novel sex-specific mechanism of regulation of an ion channel by estrogen. Further studies are needed to assess the effect of sex-related mechanisms on ClC-2 channels.
The next finding of import is the numerical differences in the sensation thresholds for first sensation and pain. Although these were not statistically significant, there was a numerical tendency for the pain sensation thresholds to be higher in women treated with lubiprostone compared with placebo. These changes in threshold with lubiprostone are of a magnitude similar to those observed with the 5-HT3 antagonist medication, alosetron. Thus, in the study by Delvaux et al. (10), the median distension pressure needed to induce the first abdominal sensation was 26 mmHg during dosing with placebo (range 12 ± 36 mmHg) compared with 24 mmHg (range 20 ± 32 mmHg) and 28 mmHg (range 16 ± 40 mmHg) with alosetron, 0.25 and 4 mg bid, respectively.
Although the differences between alosetron and placebo treatment groups were not significant, subsequent studies went on to show that alosetron reduced abdominal pain in nonconstipated IBS patients (1). Interestingly, the effects of lubiprostone on pain sensation threshold in women (P = 0.11, unadjusted) appears to show a similar trend to alosetron, since a recent therapeutic trial with lubiprostone in IBS with constipation showed relief of pain and discomfort, and 90% of the participants in the clinical trial were female (15).
With 30 subjects per treatment, there was better than 80% power to detect a clinically important difference in mean pain thresholds of 12 mmHg (e.g., 40 vs. ≥52 mmHg) based on a two-sample t-test (α = 0.05) assuming the standard deviation (15.4) observed in the predrug sensation threshold assessments. Differences in rectal sensory thresholds for pain differ among studies in the literature. For example, Bouin et al. (5) reported a difference of pain thresholds in IBS patients (30.4 ± 6.7 mmHg) compared with controls (44.5 ± 5 mmHg), and Dorn et al. (11) recorded a median difference of 12 mmHg in IBS and controls (median: 28 vs. 40 mmHg). The observed medians (accounting for censoring) in the overall treatment groups (44 mmHg on lubiprostone vs. 40 mmHg on placebo) and 48 vs. 40 mmHg, respectively, in women, were not statistically significant. Thus, although the magnitude of difference in the two treatment groups did not reach the level that differentiates health from IBS, the observed treatment-associated difference in threshold may be indicative of an effect on pain sensation in response to treatment that may be clinically important, as observed for alosetron and discussed above (1, 10).
The decreased colonic compliance observed in women does not explain the possible effect of lubiprostone on sensation to distension. Since the distensions with the barostat were based on pressure increments, we perceive that the change in compliance is not likely to be contributing to the trend to a difference in sensation threshold. The sex difference in effect of lubiprostone on colonic compliance is also unlikely to be clinically important. To date, there are insufficient clinical trial data to suggest any sex-related difference in the clinical efficacy of lubiprostone. For example, the largest IBS trial of lubiprostone to date (15) included only 10% men in the whole study cohort.
Since the ClC family comprises a group of integral membrane proteins whose major action is to translocate chloride (Cl−) ions across the cell membranes, it is conceivable that they affect afferent function, as has been demonstrated in the ear and laryngeal mucosa and in proprioceptive afferents (7, 13, 22). The observations in our study show only a trend to increase in sensation threshold. Thus the effects of this ClC-2 activator on sensation are unclear and require further study.
Our study provides initial understanding of the effect of lubiprostone on colonic motility. Overall, the results show a small decrease of colonic compliance in women that is of unclear clinical significance and a somewhat more impressive overall reduction in tonic response to a meal in women. In the setting of the increased fluid and electrolyte secretion, the reduced tone may provide a mechanistic explanation for the effect of lubiprostone in relieving constipation.
A potential criticism of our study is the dose of lubiprostone used. The 24-μg dose was selected because this study was being conducted in healthy subjects and the 24-μg dose was expected to be sufficient for demonstration of the mechanisms modulated by lubiprostone. Further supporting this decision was the fact that the maximum dose approved for use in humans with chronic constipation is 24 μg bid (16, 17), whereas the dose approved for use in C-IBS (15) is 8 μg bid. Moreover, it has been reported that the single 24-μg dose is effective since 56.7% of patients with chronic constipation experienced a spontaneous bowel movement within 24 h after the first dose of 24 μg (16). The strengths of the study include the use of well-established measurements, and the sample size selected to detect an effect size that was clinically relevant.
Conclusion
Lubiprostone has pharmacological effects in women, in addition to inducing chloride and water secretion, by decreasing compliance and decreasing the relative change in postprandial tone in the colon. These may contribute to its therapeutic benefit. Effects on pain thresholds were not significant (P = 0.11 in women) in this study. Further studies of the effects of lubiprostone on visceral sensory and motor functions are warranted.
GRANTS
The study was supported by a research grant from Takeda-Sucampo. M. Camilleri is supported in part by Grants RO1-DK54681 and K24-DK02638 from the National Institute of Diabetes and Digestive and Kidney Diseases. The study was enabled by the Gastrointestinal Imaging and Physiology Core and the Nursing Core of the Mayo Clinic Center for Translational Science Activities grant (NIH RR0024150).
Acknowledgments
We thank Cindy Stanislav for excellent secretarial assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Andresen V, Montori VM, Keller J, West CP, Layer P, Camilleri M. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in non-constipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol 6: 545–555, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao HF, Liu L, Self J, Duke BJ, Ueno R, Eaton DC. A synthetic prostone activates apical chloride channels in A6 epithelial cells. Am J Physiol Gastrointest Liver Physiol 295: G234–G251, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassil AK, Borman RA, Jarvie EM, McArthur-Wilson RJ, Thangiah R, Sung EZ, Lee K, Sanger GJ. Activation of prostaglandin EP receptors by lubiprostone in rat and human stomach and colon. Br J Pharmacol 154: 126–135, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharucha AE, Camilleri M, Haydock S, Ferber I, Burton D, Cooper S, Tompson D, Fitzpatrick K, Higgins R, Zinsmeister AR. Effects of a serotonin 5-HT4 receptor antagonist SB-207266 on gastrointestinal motor and sensory function in humans. Gut 4: 667–674, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouin M, Plourde V, Boivin M, Riberdy M, Lupien F, Laganière M, Verrier P, Poitras P. Rectal distention testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology 122: 1771–1777, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Camilleri M, Bharucha AE, Ueno R, Burton D, Thomforde GM, Baxter K, McKinzie S, Zinsmeister AR. Effect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory, and motor functions in healthy volunteers. Am J Physiol Gastrointest Liver Physiol 290: G942–G947, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Cattaert D, el Manira A, Clarac F. Chloride conductance produces both presynaptic inhibition and antidromic spikes in primary afferents. Brain Res 666: 109–112, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Cuppoletti J, Malinowska DH, Chakrabarti J, Ueno R. Effects of lubiprostone on human uterine smooth muscle cells. Prostaglandins Other Lipid Mediat 86: 56–60, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Cuppoletti J, Malinowska DH, Tewari KP, Li QJ, Sherry AM, Patchen ML, Ueno R. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol Cell Physiol 287: C1173–C1183, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Delvaux M, Louvel D, Mamet JP, Campos-Oriola R, Frexinos J. Effect of alosetron on responses to colonic distension in patients with irritable bowel syndrome. Aliment Pharmacol Ther 2: 849–855, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Dorn SD, Palsson OS, Thiwan SI, Kanazawa M, Clark WC, van Tilburg MA, Drossman DA, Scarlett Y, Levy RL, Ringel Y, Crowell MD, Olden KW, Whitehead WE. Increased colonic pain sensitivity in irritable bowel syndrome is the result of an increased tendency to report pain rather than increased neurosensory sensitivity. Gut 56: 1202–1209, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fialho D, Kullmann DM, Hanna MG, Schorge S. Non-genomic effects of sex hormones on ClC-1 may contribute to gender differences in myotonia congenita. Neuromuscul Disord 18: 869–872, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh TK, Van Scott MR, Mathew OP. Activation of water-responsive laryngeal afferents: role of epithelial ion transport. Respir Physiol 105: 163–169, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Hammer HF, Phillips SF, Camilleri M, Hanson RB. Rectal tone, distensibility, and perception: reproducibility and response to different distensions. Am J Physiol Gastrointest Liver Physiol 274: G584–G590, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Johanson JF, Drossman DA, Panas R, Wahle A, Ueno R. Clinical trial: phase 2 study of lubiprostone for irritable bowel syndrome with constipation. Aliment Pharmacol Ther 27: 685–696, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Johanson JF, Morton D, Geenen J, Ueno R. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of lubiprostone, a locally-acting type-2 chloride channel activator, in patients with chronic constipation. Am J Gastroenterol 103: 170–177, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Johanson JF, Ueno R. Lubiprostone, a locally acting chloride channel activator, in adult patients with chronic constipation: a double-blind, placebo-controlled, dose-ranging study to evaluate efficacy and safety. Aliment Pharmacol Ther 25: 1351–1361, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Lipecka J, Bali M, Thomas A, Fanen P, Edelman A, Fritsch J. Distribution of ClC-2 chloride channel in rat and human epithelial tissues. Am J Physiol Cell Physiol 282: C805–C816, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Malcolm A, Camilleri M. Coloanal motor coordination in association with high-amplitude colonic contractions after pharmacological stimulation. Am J Gastroenterol 95: 715–719, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Misiewicz JJ, Connell AM, Pontes FA. Comparison of the effect of meals and prostigmine on the proximal and distal colon in patients with and without diarrhoea. Gut 7: 468–473, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Mahony F, Alzamora R, Betts V, LaPaix F, Carter D, Irnaten M, Harvey BJ. Female gender-specific inhibition of KCNQ1 channels and chloride secretion by 17beta-estradiol in rat distal colonic crypts. J Biol Chem 282: 24563–24573, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Pantoja AM, Holt JC, Guth PS. A role for chloride in the suppressive effect of acetylcholine on afferent vestibular activity. Hear Res 112: 21–32, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Perentesis GP, Crawford DF, Engelke KJ, Osama H, Ueno R. Effects of lubiprostone, a novel GI chloride channel activator, on isolated smooth muscle. Neurogastroenterol Motil 17: 625, 2005. [Google Scholar]
- 24.Talley NJ, Phillips SF, Wiltgen CM, Zinsmeister AR, Melton LJ 3rd. Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc 65: 1456–1479, 1990. [DOI] [PubMed] [Google Scholar]
- 25.Viramontes BE, Malcolm A, Camilleri M, Szarka LA, McKinzie S, Burton DD, Zinsmeister AR. Effects of an α2-adrenergic agonist on gastrointestinal transit, colonic motility, and sensation in humans. Am J Physiol Gastrointest Liver Physiol 281: G1468–G1476, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Von der Ohe MR, Hanson RB, Camilleri M. Serotonergic mediation of postprandial colonic tonic and phasic responses in humans. Gut 35: 536–541, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 67: 361–370, 1983. [DOI] [PubMed] [Google Scholar]