Abstract
The SCN5A-encoded Nav1.5 Na+ channel is expressed in interstitial cells of Cajal and smooth muscle in the circular layer of the human intestine. Patients with mutations in SCN5A are more likely to report gastrointestinal symptoms, especially abdominal pain. Twin and family studies of irritable bowel syndrome (IBS) suggest a genetic basis for IBS, but no genes have been identified to date. Therefore, our aims were to evaluate SCN5A as a candidate gene involved in the pathogenesis of IBS and to determine physiological consequences of identified mutations. Mutational analysis was performed on genomic DNA obtained from 49 subjects diagnosed with IBS who reported at least moderately severe abdominal pain. One patient hosted a loss-of-function missense mutation, G298S, that was not observed in >3,000 reference alleles derived from 1,500 healthy control subjects. Na+ currents were recorded from the four common human SCN5A transcripts in transfected HEK-293 cells. Comparing Nav1.5 with G298S-SCN5A versus wild type in HEK cells, Na+ current density was significantly less by 49–77%, and channel activation time was delayed in backgrounds that also contained the common H558R polymorphism. Single-channel measurements showed no change in Nav1.5 conductance. Mechanosensitivity was reduced in the H558/Q1077del transcript but not in the other three backgrounds. In conclusion, the G298S-SCN5A missense mutation caused a marked reduction of whole cell Na+ current and loss of function of Nav1.5, suggesting SCN5A as a candidate gene in the pathophysiology of IBS.
Keywords: SCN5A, Nav1.5, current density, mechanosensitivity
the scn5a-encoded Nav1.5 sodium channel is expressed in cardiac myocytes, and loss-of-function or gain-of-function mutations in this gene are known to result in cardiac channelopathies such as Brugada syndrome and type 3 long-QT (LQT) syndrome, respectively (15). Recent data show that Nav1.5 expression is not limited to the heart but that this sodium channel is also expressed in human intestinal interstitial cells of Cajal (25) and smooth muscle (18). Modulation of the mechanosensitive sodium channel results in changes in human smooth muscle membrane potential and slow wave upstroke, suggesting a role of intestinal Nav1.5 in the regulation of gastrointestinal function. In response to a gastrointestinal symptom questionnaire sent to families genotyped for mutations in SCN5A, individuals with SCN5A mutations reported more gastrointestinal symptoms, especially abdominal pain, compared with patients in the same families without mutations, with an odds ratio of 5.7 (95% CI: 1.3–24.4) (11). This finding appeared to be specific to SCN5A, because no association was seen with KCNH2, a potassium channel-encoding gene responsible for type 2 LQT syndrome that is expressed in both heart and gastrointestinal tract.
Characterized by chronic or recurrent abdominal pain or discomfort, irritable bowel syndrome (IBS) is a very common disorder affecting 10–20% of Americans and accompanied closely by diarrhea- or constipation-type symptoms (20). Several studies have demonstrated that IBS aggregates in families (9, 12, 22, 23), lending support for the idea that either shared environmental factor(s) or genetic factor(s) (or both) may subserve the familial clustering. Furthermore, twin studies support both an environmental and a genetic basis for IBS (3, 10, 16). Thus IBS appears to be a complex, heterogeneous multifactorial disorder; both environmental and genetic interactions may result in symptom development, and this perhaps explains the clinical heterogeneity inherent to IBS. However, specific IBS-predisposing genetic defects have yet to be identified.
Given the association between genetic defects in SCN5A and gastrointestinal symptoms (11), the aims of this study were to evaluate SCN5A as a candidate gene involved in the pathogenesis of IBS and to determine the role of any identified mutation on Nav1.5 current density, kinetics, mechanosensitivity, and single-channel conductance.
METHODS
This study was approved by the Mayo Clinic Institutional Review Board.
Subjects
Cases.
DNA samples were collected from 49 patients participating in an ongoing genetic IBS study in which gastrointestinal symptom data were collected from cases. Subjects were outpatients with IBS, ages 18–69 yr, seen in the Division of Gastroenterology of the Mayo Clinic, who were recruited between February 2004 and July 2005. All subjects were then asked to complete a self-reported bowel disease questionnaire (26) and donate 20 ml of blood. For the present study, those IBS cases who met Rome II criteria (27) for IBS and reported abdominal pain at least 2 yr in duration and rated as at least moderately severe (not mild) were selected. Lymphocyte DNA was extracted with a Gentra Autopure automated DNA extractor, and DNA quantification was performed with a spectrophotometer (SPECTRAmax PLUS 384).
Control subjects/reference group.
Previously, genomic DNA from 829 apparently healthy anonymized subjects [319 African Americans (blacks), 295 Caucasians of European ancestry (whites), 112 Asians, and 103 Hispanics], obtained either from the Human Genetic Cell Repository sponsored by the National Institute of General Medical Sciences and the Coriell Institute for Medical Research (Camden, NJ) or from volunteer blood donors, underwent comprehensive SCN5A open reading frame/splice site genetic analysis (2). Ongoing SCN5A analysis now exceeds 3,000 reference alleles derived from at least 1,500 healthy volunteers. Volunteer donors were apparently healthy at the time of collection and self-reported their ethnicity. To be included in a specific ethnic group, each subject represented that all four grandparents were from the same group.
Genetic Analysis for SCN5A Mutations
With previously published primers (29), SCN5A mutational analysis was performed with polymerase chain reaction, denaturing high-performance liquid chromatography (dHPLC, WAVE, Transgenomic, San Jose, CA), and dye terminator cycle sequencing (ABI Prism 377, PE Biosystems, Foster City, CA) as previously described (1). Only nonsynonymous polymorphisms (amino acid-altering variants) absent in control subjects were considered potentially pathogenic. Synonymous polymorphisms (silent nucleotide substitutions that did not alter the primary amino acid sequence) were excluded from this analysis.
Expression Vector Construction and HEK-293 Transfection
To assess the physiological significance of the observed mutation(s) in the IBS cases, wild-type and mutated SCN5A were transfected with Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) in HEK-293 cells (American Type Culture Collection, Manassas, VA) together with green fluorescent protein (GFP) pEGFP-C1 (Clontech, Palo Alto, CA). The expression vectors containing the human jejunum Na+ channel SCN5A-encoded α-subunit were described previously (13, 19). HEK cells expressing SCN5A were cultured for 1 day after transfection. Transfected cells were identified by fluorescent microscopy and patch-clamped to record whole cell currents, and on-cell patches were obtained to study single-channel currents.
To accurately depict the functional consequence of any putative SCN5A mutation, it is important to express putative Na+ channel mutations in their proper context or genetic background. All humans express two abundant SCN5A transcripts (1 lacking the codon for Q1077 and 1 encoding Q1077) resulting from alternative splicing. Thus two different Nav1.5 channels, designated Q1077del-SCN5A and Q1077-SCN5A, are expressed at all times in all humans and may demonstrate unique phenotypes when coexpressed with additional variants (13). Additionally, a common polymorphism, H558R (histidine 558 arginine), present with a 35% heterozygous frequency among white subjects, has been shown to alter the phenotypic expression of some type 3 LQT (LQT3)/Brugada syndrome SCN5A mutations (13). The patient identified with the G298S-SCN5A mutation (see below) was heterozygous for H558R-SCN5A; therefore, her cardiac and intestinal cells presumably express all four SCN5A transcripts with relative abundances as follows: H558/Q1077del = R558/Q1077del > H558/Q1077 = R558/Q1077. Since tissue was not available to extract mRNA needed to determine whether G298S resided on the H558-encoded allele or the R558-encoded allele, patch-clamp records were obtained from cells with and without the missense mutation G298S in the context of all four Na+ channel genetic backgrounds.
Patch-Clamp Recordings
Whole patch-clamp recordings were obtained at 22°C with standard whole cell and on-cell techniques (6, 7). Microelectrodes were pulled from Kimble KG-12 glass for the whole cell records and Schott 8250 for the single-channel records on a P-97 puller (Sutter Instruments, Novato, CA). Electrodes were coated with R6101 (Dow Corning, Midland, MI) and fire-polished to a final resistance of 3–5 MΩ for the whole cell records and 10–15 MΩ for the single-channel records. Currents were amplified, digitized, and processed with an Axopatch 200B amplifier, Digidata 1322A, and pCLAMP 9 software (Molecular Devices, Union City, CA). Whole cell records were sampled at 10 kHz and filtered at 4 kHz with an eight-pole Bessel filter. Cells were held at a holding potential of −100, −90, −80, −70, or −60 mV and then stepped from −80 to +35 mV in 5-mV intervals for 50 ms each. An 80–95% series resistance compensation with a lag of 10–60 μs was applied during each recording. Resistance after access was established was 5–10 MΩ. Single-channel records were sampled at 10 kHz and filtered at 5.1 kHz. Cells were held at transmembrane voltages ranging from −70 to −120 mV, briefly stepped to −50 mV for 100 μs to open the channel, and then ramped from −60 to +60 mV.
Drugs and Solutions
For whole cell experiments, the intracellular solution contained (in mM) 130 Cs+, 125 methanesulfonate, 20 Cl−, 5 Na+, 5 Mg+, 5 HEPES, 2 EGTA, 2.5 ATP, and 0.1 GTP. The extracellular solution before, during, and after perfusion was normal Ringer solution (in mM): 149.2 Na+, 159 Cl−, 4.74 K+, 2.54 Ca2+, 5 HEPES, and 5 glucose. Intra- and extracellular solutions were equilibrated to pH 7.0 and pH 7.35 with CsOH or NaOH, respectively. Osmolality of either solution was 300 mosmol/kgH2O. The flow rate of solution during perfusion to test for mechanosensitivity was 10 ml/min, and this flow rate was applied for 60 s. For single-channel experiments, the pipette and bath solutions contained (in mM) 149.2 Na+, 159 Cl−, 4.74 K+, 2.54 Ca2+, and 5 HEPES, equilibrated to 7.35 with NaOH.
Data Analysis
For the genotyping portion of the study, demographic comparisons used t-tests and χ2-tests for continuous and categorical data, respectively. Allele and genotype frequencies (%) were calculated for each polymorphism among the IBS cases and the reference group. Comparison of these frequencies between IBS cases and the reference group was performed with χ2-analysis (or Fisher's exact tests) and Armitage trend tests with SAS statistical software (SAS Institute, Cary, NC).
For the functional studies of sodium channel properties, data were analyzed with Clampfit (Molecular Devices), Excel (Microsoft, Redmont, WA), GraphPad InStat (GraphPad Software, San Diego, CA), and SigmaPlot (SPSS, Chicago, IL). For current density-voltage graphs, currents (in pA) were expressed as a fraction of each whole cell capacitance (pF). For steady-state kinetics, the peak inward current of a single control trace was normalized to 1 with the equation |INORM| = 100× (IV/IMAX), where INORM is normalized current, IV is measured current at a given voltage, and IMAX is maximum peak inward current of the control trace. Thus peaks of all other traces per cell were expressed as a fraction of 100. For perfusion experiments, data are expressed as values normalized to the amount of baseline current measured before perfusion (100%). Statistical comparisons were made with a paired, two-tailed Student's t-test or one-way ANOVA. Statistical significance was accepted when P values were <0.05. Data are expressed as means ± SE. Single-channel conductances were determined from the difference of the slopes of the open and closed channel traces.
RESULTS
Characteristics of Subjects
The median age of the 49 patients with IBS was 33 (range 18–49) yr. Of this sample, 43 (88%) were female and 6 (12%) were male. The racial distribution by self-report was as follows: 47 (96%) reported only Caucasian backgrounds; 1 (2%) reported Caucasian, black or African American, and Hispanic or Latino backgrounds; and 1 (2%) reported Caucasian and Hispanic or Latino backgrounds. The usual bowel habit in the last year was reported to be diarrhea by 25 (51%), constipation by 15 (31%), mixed by 8 (16%), and normal by 1 (2%).
SCN5A Genetic Polymorphisms
H558R polymorphism.
The genotype and allele frequencies for the known H558R polymorphism are shown in Table 1. No difference in genotype frequency was observed between IBS cases and the reference group. Although the frequency of the R allele was numerically greater than in the reference group (28% vs. 22%), this was not statistically significant (P = 0.18).
Table 1.
Genotype and allele frequencies of H558R common polymorphism in irritable bowel syndrome cases and control reference group
|
Genotypes |
Alleles
|
||||
|---|---|---|---|---|---|
| HH | HR | RR | H allele | R allele | |
| Cases | 27 (55) | 17 (35) | 5 (10) | 72% | 28% |
| Control | 1,227 (60) | 701 (35) | 103 (5) | 78% | 22% |
Genotype frequency values are numbers of subjects (%).
G298S missense mutation.
One of the 49 patients (2%) hosted a G298S missense mutation. She was also heterozygous for the H558R common polymorphism. This patient was a 43-yr-old woman with diarrhea of ∼6-mo duration and a 3-yr history of diffuse lower abdominal cramps. Accompanying symptoms included mild bloating and distension. No symptoms of malabsorption such as steatorrhea or weight loss were reported. Extensive work-up had been negative, including thyroid function testing, stool pathogens, upper endoscopy with small bowel biopsy and aspirates, colonoscopy with biopsy, and neuroendocrine hormone levels. In her questionnaire, she reported that her mother, two of four brothers, and one sister also experienced diarrhea-type symptoms. Blood was not available from these relatives for testing. An ECG was normal, with no evidence of either a spontaneous Brugada ECG pattern (i.e., incomplete right bundle branch block and ST segment elevation in the right precordial leads) or a prolonged heart rate-corrected QT interval (QTc = 439 ms).
Effects of G298S mutation on Na+ current density, kinetics, and mechanosensitivity.
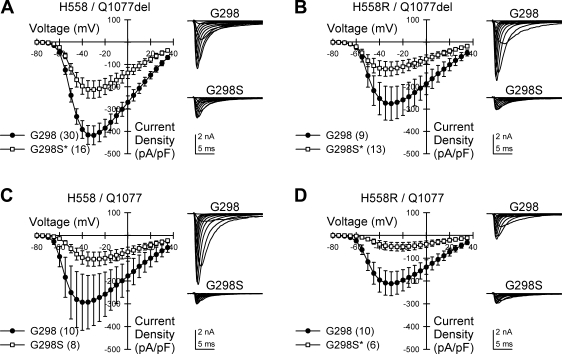
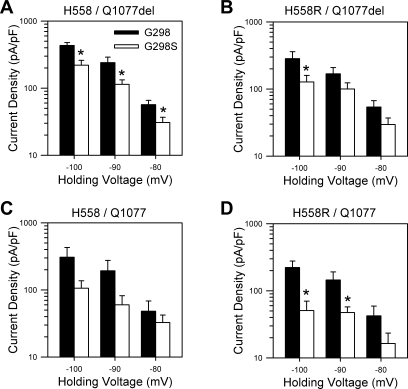
To test for an electrophysiological significance of the mutation in each of the four common Na+ channel transcripts expressed by the patient, we transfected HEK-293 cells with each background, with or without the G298S mutation. At a holding voltage of −100 mV, the G298S mutation significantly reduced peak inward Na+ current density in H558/Q1077del by 49% [−433 ± 45 pA/pF (n = 30) to −220 ± 40 pA/pF (n = 16); P < 0.05], in H558R/Q1077del by 55% [−283 ± 77 pA/pF (n = 9) to −127 ± 32 pA/pF (n = 13); P < 0.05], and in H558R/Q1077 by 77% [−222 ± 56 pA/pF (n = 10) to −51 ± 19 pA/pF (n = 6); P < 0.05] (Fig. 1). The current decreased by 65% in the G298S fourth variant, H558/Q1077, but this did not reach significance [−307 ± 122 pA/pF (n = 10) to −106 ± 31 pA/pF (n = 8); P = 0.17, Fig. 1C], likely because of the large variance in current density in the control cells. Significant decreases in current density were also observed at more positive holding voltages in two backgrounds (Fig. 2, A and D). We found no difference in the average voltage at peak current between the four variants [H558/Q1077del/G298 −32.3 ± 0.8 mV (n = 30); H558R/Q1077del/G298 −28.5 ± 1.7 mV (n = 10); H558/Q1077/G298 −30.5 ± 3.4 mV (n = 10); H558R/Q1077del/G298 −27.0 ± 2.7 mV (n = 10); P > 0.05].
Fig. 1.
G298S mutation reduces Na+ current density from SCN5A transfected in HEK cells. Current-voltage relationships of Na+ current expressed in HEK cells from SCN5A transcript backgrounds H558/Q1077del (A), H558R/Q1077del (B), H558/Q1077 (C), or H558R/Q1077 (D) with the G298 wild-type allele or G298S missense mutation are shown. Insets: representative Na+ currents recorded from channels with G298 or G298S. *P < 0.05; n values are indicated in parentheses.
Fig. 2.
G298S mutation reduces Na+ current density at multiple holding voltages. Peak Na+ current densities sorted by holding voltage and recorded from HEK cells expressing SCN5A transcript backgrounds H558/Q1077del (A, n = 14–30), H558R/Q1077del (B, n = 9–17), H558/Q1077 (C, n = 5–10), or H558R/Q1077 (D, n = 5–10) with the G298 wild-type allele or the G298S missense mutation are shown. *P < 0.05.
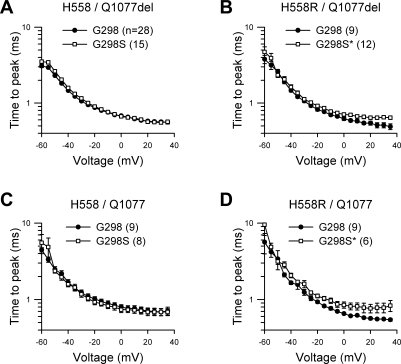
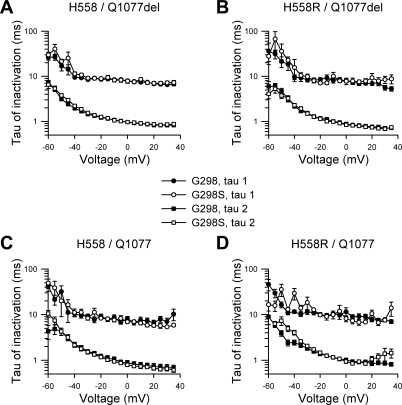
Kinetic analysis showed that the time to peak current was significantly longer from step potentials 0 through −35 mV with G298S present in both alternatively spliced transcripts containing the common polymorphism H558R (H558R/Q1077del/G298S and H558R/Q1077/G298S; Fig. 3, B and D). The fast and slow time constants (τ) of inactivation remained unchanged in all four backgrounds (Fig. 4). The net result of a slower time to peak and no change in inactivation would be a reduction in Na+ current density.
Fig. 3.
G298S mutation slows activation kinetics. Time to peak of Na+ current expressed in HEK cells from transcript backgrounds H558/Q1077del (A), H558R/Q1077del (B), H558/Q1077 (C), and H558R/Q1077 (D) with the G298 wild-type allele or the G298S mutation is shown. *P < 0.05 from 0 to 35 mV; n values are indicated in parentheses.
Fig. 4.
G298S mutation does not alter inactivation kinetics. Fast (τ2) and slow (τ1) time constants of inactivation of Na+ current from SCN5A with G298 wild-type allele or G298S mutation are shown. No change in inactivation kinetics was noted at any voltage for transcript backgrounds H558/Q1077del (A, n = 16–28), H558R/Q1077del (B, n = 9–12), H558/Q1077 (C, n = 8 or 9), or H558R/Q1077 (D, n = 6–9).
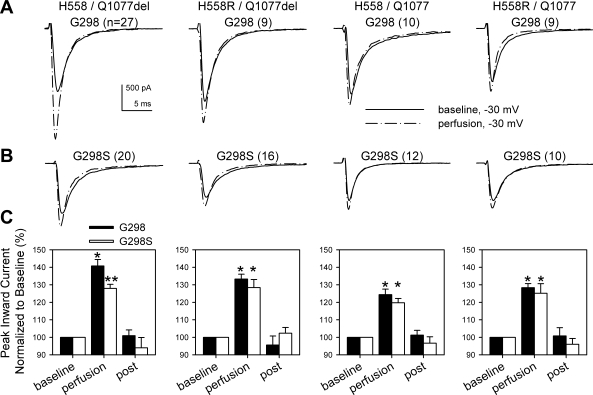
To test whether G298S could alter mechanosensitivity of Nav1.5, HEK cells with one of four SCN5A transcripts with or without the rare missense mutation were perfused with Ringer solution to create shear stress and mechanically activate the channel. Similar to human jejunum cells (24, 25), wild-type (G298) Na+ channels expressed in HEK cells responded to perfusion with a robust mechanosensitive increase in current measured at peak voltage (−30 mV, Fig. 5A; n = 9–27, P < 0.01 baseline to perfusion). Na+ channels from all backgrounds with G298S exhibited a similar significant increase in current (Fig. 5B; n = 10–20, P < 0.01 baseline to perfusion). Compared with backgrounds with G298, the increase in mechanosensitive Na+ current for cells with G298S was similar in H558R/Q1077del [G298 33 ± 3% increase (n = 9), G298S 28 ± 5% increase (n = 16); P < 0.01 baseline to perfusion, P > 0.05 between groups], H558/Q1077 [G298 24 ± 3% increase (n = 10), G298S 20 ± 2% increase (n = 12); P < 0.01 baseline to perfusion, P > 0.05 between groups], and H558R/Q1077 [G298 28 ± 2% increase (n = 9), G298S 25 ± 6% increase (n = 10); P < 0.01 baseline to perfusion, P > 0.05 between groups] (Fig. 5). However, in the background H558/Q1077del, perfusion increased Na+ current from G298 controls by 41 ± 4% increase (n = 27, P < 0.01 baseline to perfusion) but by only 28 ± 2% from G298S (n = 20, P < 0.01 baseline to perfusion, P < 0.01 between groups; Fig. 5).
Fig. 5.
G298S mutation reduces mechanosensitivity in the H558/Q1077del variant. A and B: representative Na+ currents from cells stepped from −100 mV to −30 mV before (solid line) or during (dashed line) mechanical stimulation by perfusion with NaCl Ringer solution. Perfusion experiments were conducted on cells expressing channels from each of the 4 common SCN5A transcripts without (A) or with (B) the G298S mutation; n values are indicated in parentheses. C: mean increases in peak Na+ current induced by perfusion from G298 wild type or G298S mutation. *P < 0.01, baseline to perfusion; **P < 0.01, baseline to perfusion and G298 to G298S.
To determine whether the changes noted in the whole cell current density were due to changes in single-channel conductance, we measured single-channel conductance in each of the four backgrounds with and without the G298S mutation (data not shown). G298S did not yield a significant change in single-channel conductance in any of the four SCN5A transcripts.
DISCUSSION
The main findings of this report are that the G298S-SCN5A missense mutation results in loss of whole cell current density and a delay in channel activation kinetics without a change in single-channel conductance. Also, the G298S mutation resulted in reduced mechanosensitivity in the setting of a common SCN5A transcript, H558/Q1077del. Furthermore, we found this marked loss-of-function sodium channel mutation in a patient with IBS, raising the possibility that ion channel mutations may underlie a subset of patients with this heterogeneous and poorly understood disorder. To date, there are over a hundred rare mutations in SCN5A that have been associated with either LQT3 syndrome, type 1 Brugada syndrome, inherited cardiac conduction defects, sudden unexpected nocturnal death syndrome (SUNDS), or sudden infant death syndrome (SIDS) (15). None has been reported in gastrointestinal diseases. Several studies suggest that there may be an underlying genetic basis to IBS. While several candidate gene association studies have been performed evaluating neurotransmitter processing proteins and inflammatory mediators (21), no genetic abnormalities in genes encoding intestinal channel proteins have been evaluated thus far. In light of previous data from our group suggesting that congenital LQT syndrome and Brugada syndrome patients with rare mutations in the SCN5A gene have more gastrointestinal symptoms (11), this study aimed to determine whether genetic variants in SCN5A might be associated with IBS. Thus sequence analysis of this gene in 49 patients with IBS and moderate to severe pain was performed. In 1 of the 49 patients studied, all of whom fulfilled the Rome II criteria for IBS, we found a mutation in SCN5A (G298S). This G to A substitution at nucleotide 892 on exon 7 results in a glycine (G) to serine (S) missense mutation involving residue 298 (G298S) that localizes to the domain I/S5-S6 loop (pore) region of the sodium channel. Notably, this specific loss-of-function mutation was reported previously in two siblings with an atrioventricular conduction defect (28). Given that this report predated our association of gastrointestinal symptoms in cardiac patients with SCN5A mutations, it is not surprising that there was no mention of gastrointestinal symptomatology. G298S was not observed in our reference group, nor has it been reported in over 3,000 reference alleles worldwide. Interestingly, the patient in the present IBS study had no evidence of a cardiac conduction defect and a completely normal ECG despite the marked changes in Na+ whole cell current density. The patient has declined a provocation study with procainamide to see whether a Brugada ECG pattern is inducible.
Nav1.5 is present in the population as several distinct backgrounds. The most common are H558/Q1077del, H558R/Q1077del, H558/Q1077, and H558R/Q1077, which are estimated to be present in the population with frequencies of 45%, 20%, 25%, and 10%, respectively (13). These genetic backgrounds are functionally distinct, with the H558R/Q1077 minor transcript associated with reduced sodium current compared with the three other main transcripts (13). This patient was heterozygous for the common H558R polymorphism and therefore expressed all four transcripts. Since tissue was not available to extract mRNA needed to determine whether the G298S missense mutation resided on the H558-encoded allele or the R558-encoded allele, all eight possible SCN5A transcripts were engineered (i.e., with and without G298S).
We found that the mutation was functionally significant and that the functional effects of the mutation were in fact dependent on the channel background. The effect of the G298S mutation on whole cell Na+ channel current density was characterized previously by Wang et al. (28) in the context of the hH1 Na+ channel clone (H558/Q1077). While we saw a 65% decrease in current in H558/Q1077, this did not reach statistical significance in our study, likely because of the high variability in the control subjects. In this study we also extend the finding to H558/Q1077del (49% decrease), H558R/Q1077del (55% decrease), and H558R/Q1077 (77% decrease). These data fit with the observed changes in activation kinetics. Time to peak was increased by the G298S missense mutation in both H558R-containing backgrounds, which also showed a decrease in peak current. The combination of slower activation and unchanged inactivation kinetics would result in a decrease in peak Na+ current density. However, the decrease in peak current density in G298S/H558/Q1077del-SCN5A suggests that the delay in activation kinetics is not the sole cause of the smaller current. A smaller current can be due to a change in channel kinetics, a change in open probability, or a change in the number of channels available. We did not see a change in single-channel conductance, suggesting that the number of available channels was decreased. Whether this is due to defective trafficking or a change in the number of channels available to open was not determined in this study.
There is increasing evidence that Nav1.5 is a mechanosensitive channel. This was first shown in whole cell current experiments (24, 25). Mechanosensitivity is dependent on an intact cytoskeleton and is abolished when the link is severed between the PDZ domain of syntrophin γ and the COOH terminus of the SCN5A-encoded α-subunit (19). Recently, mechanosensitivity has been shown at the level of single sodium channels (17). Mechanosensitivity was reduced when the G298S mutation was expressed in the most abundant sodium channel transcript, H558/Q1077del. Reduced mechanosensitivity in the gut, together with the observed changes in peak current density, may also contribute to the development of an observable phenotype. Of note, the degree of mechanosensitivity in all four G298S-containing transcripts was approximately the same; the relative difference observed in the context of the H558/Q1077del transcript was predominantly due to the greater mechanosensitivity in the common wild-type sodium channel, G298/H558/Q1077del. We therefore tripled the n value of our observations to determine whether this was a statistical anomaly. Even with this increased number of observations, the same result was obtained, suggesting that G298S in the context of H558/Q1077del-SCN5A does indeed reduce mechanosensitivity.
The mutation in the patient with IBS therefore appears to be physiologically significant. It is at present unclear what contribution this mutation made to the patient's symptoms. Our present understanding is that Nav1.5 in the human gut is expressed in smooth muscle cells and interstitial cells of Cajal. Expression of Nav1.5 in enteric nerves is not known. Given the role that smooth muscle and interstitial cells of Cajal play in regulation of gastrointestinal motility, attribution of motility-like symptoms such as diarrhea and constipation to changes in ion channels expressed in these cell types is plausible. In this regard a preliminary report (5) from a second institution has also linked mutations in SCN5A (in patients with Brugada syndrome) with gastrointestinal symptoms. The finding of a predominance of abdominal pain in our previous study (11) and again in this patient with abdominal pain, however, suggests that additional mechanisms aside from changes in motility are also operative. Interstitial cells of Cajal are associated closely with vagal afferents and may be involved in transduction of afferent signals (4, 8). Also, the significant role that central processing plays in the expression of symptoms in functional bowel diseases such as IBS may result in pain as the dominant symptom in some patients while not in others, as a result of differential central processing of peripheral motor signals (14). It is also possible that the expression of Nav1.5 in the gut is more widespread than currently thought.
If our novel discovery of a rare, functionally significant missense mutation in a patient with idiopathic IBS is a real pathogenic association, this represents a major breakthrough in helping to understand possible mechanisms underlying IBS, at least in a subset of patients with this heterogeneous disorder. This patient underwent an extensive work-up ruling out other causes for her IBS symptoms, making a misdiagnosis of IBS unlikely. However, this mutation was the only mutation discovered in SCN5A and was only found in 1 of 49 IBS patients tested. IBS is quite variable in presentation, suggesting that there may be underlying mechanistic heterogeneity (i.e., multiple genes and environmental risk factors) responsible for this common disorder that affects 12–20% of the general population. Our study suggests that there are other disease susceptibility loci for IBS that remain to be discovered.
In summary, these data suggest that a rare missense mutation in SCN5A (G298S) results in loss of whole cell current density, delayed activation kinetics, and, in the setting of the most abundant alternatively spliced transcript, H558/Q1077del-SCN5A, an attenuation in mechanosensitivity. This mutation was found in a patient with IBS and suggests that perturbations in SCN5A may confer susceptibility to the development of IBS. Additional studies are needed to determine the potential role of this mutation specifically and the prevalence of rare SCN5A mutations generally in a larger sample of patients with IBS. The concept of an intestinal channelopathy resulting in IBS or IBS-like symptoms deserves further investigation.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants DK-52766 and DK-57061 (G. Farrugia), the American College of Gastroenterology and NIH Grant DK-066271 (Y. A. Saito), RO1-HL-71092 (J. C. Makielski), UO1-DK-65713 (N. J. Talley), and the Mayo Clinic Windland Smith Rice Comprehensive Sudden Cardiac Death Program (M. J. Ackerman).
Acknowledgments
We thank Kristy Zodrow and Lori Anderson for secretarial assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ackerman MJ, Siu BL, Sturner WQ, Tester DJ, Valdivia CR, Makielski JC, Towbin JA. Postmortem molecular analysis of SCN5A defects in sudden infant death syndrome. JAMA 286: 2264–2269, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Ackerman MJ, Splawski I, Makielski JC, Tester DJ, Will ML, Timothy KW, Keating MT, Jones G, Chadha M, Burrow CR, Stephens JC, Xu C, Judson R, Curran ME. Spectrum and prevalence of cardiac sodium channel variants among black, white, Asian, and Hispanic individuals: implications for arrhythmogenic susceptibility and Brugada/long QT syndrome genetic testing. Heart Rhythm 1: 600–607, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Bengtson MB, Ronning T, Vatn MH, Harris JR. Irritable bowel syndrome in twins: genes and environment. Gut 55: 1754–1759, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berthoud HR, Powley TL. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J Comp Neurol 319: 261–276, 1992. [DOI] [PubMed] [Google Scholar]
- 5.Braak B, Klooker TK, Scholvinck S, Hofman N, Wilde A, Boeckxstaens GE. Abdominal symptoms in patients with long QT syndrome and a “gain of function” mutation in the NaV1.5 sodium channel (Abstract). Gastroenterology 134: W1337, 2008. [Google Scholar]
- 6.Farrugia G, Rae JL, Sarr MG, Szurszewski JH. Potassium current in circular smooth muscle of human jejunum activated by fenamates. Am J Physiol Gastrointest Liver Physiol 265: G873–G879, 1993. [DOI] [PubMed] [Google Scholar]
- 7.Farrugia G, Rae JL, Szurszewski JH. Characterization of an outward potassium current in canine jejunal circular smooth muscle and its activation by fenamates. J Physiol 468: 297–310, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox EA, Phillips RJ, Byerly MS, Baronowsky EA, Chi MM, Powley TL. Selective loss of vagal intramuscular mechanoreceptors in mice mutant for steel factor, the c-Kit receptor ligand. Anat Embryol (Berl) 205: 325–342, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Kalantar JS, Locke GR 3rd, Zinsmeister AR, Beighley CM, Talley NJ. Familial aggregation of irritable bowel syndrome: a prospective study. Gut 52: 1703–1707, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy RL, Jones KR, Whitehead WE, Feld SI, Talley NJ, Corey LA. Irritable bowel syndrome in twins: heredity and social learning both contribute to etiology. Gastroenterology 121: 799–804, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Locke GR 3rd, Ackerman MJ, Zinsmeister AR, Thapa P, Farrugia G. Gastrointestinal symptoms in families of patients with an SCN5A-encoded cardiac channelopathy: evidence of an intestinal channelopathy. Am J Gastroenterol 101: 1299–1304, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Locke GR 3rd, Zinsmeister AR, Talley NJ, Fett SL, Melton LJ 3rd. Familial association in adults with functional gastrointestinal disorders. Mayo Clin Proc 75: 907–912, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Makielski JC, Ye B, Valdivia CR, Pagel MD, Pu J, Tester DJ, Ackerman MJ. A ubiquitous splice variant and a common polymorphism affect heterologous expression of recombinant human SCN5A heart sodium channels. Circ Res 93: 821–828, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology 131: 1925–1942, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Moric E, Herbert E, Trusz-Gluza M, Filipecki A, Mazurek U, Wilczok T. The implications of genetic mutations in the sodium channel gene (SCN5A). Europace 5: 325–334, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Morris-Yates A, Talley NJ, Boyce PM, Nandurkar S, Andrews G. Evidence of a genetic contribution to functional bowel disorder. Am J Gastroenterol 93: 1311–1317, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Morris CE, Juranka PF. Nav channel mechanosensitivity: activation and inactivation accelerate reversibly with stretch. Biophys J 93: 822–833, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ou Y, Gibbons SJ, Miller SM, Strege PR, Rich A, Distad MA, Ackerman MJ, Rae JL, Szurszewski JH, Farrugia G. SCN5A is expressed in human jejunal circular smooth muscle cells. Neurogastroenterol Motil 14: 477–486, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Ou Y, Strege P, Miller SM, Makielski J, Ackerman M, Gibbons SJ, Farrugia G. Syntrophin gamma 2 regulates SCN5A gating by a PDZ domain-mediated interaction. J Biol Chem 278: 1915–1923, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Saito YA Irritable bowel syndrome. In: GI Epidemiology, edited by Talley NJ, Locke GR 3rd, Saito YR. Oxford: Blackwell, 2007, p. 176–183.
- 21.Saito YA, Petersen GM, Locke GR 3rd, Talley NJ. The genetics of irritable bowel syndrome. Clin Gastroenterol Hepatol 3: 1057–1065, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Saito YA, Talley NJ, Locke GR 3rd, Zimmerman JM, Larson JJ, Fridley BL, de Andrade M, Petersen GM. Evidence for familial aggregation of IBS in a large case-control study (Abstract). Gastroenterology 132: A25, 2007. [Google Scholar]
- 23.Saito YA, Talley NJ, Zimmerman JM, Harmsen WS, de Andrade M, Camilleri M, Locke GR 3rd, Petersen GM. Irritable bowel syndrome aggregates strongly in families: a family-based case control study (Abstract). Gastroenterology 130: A512, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strege PR, Holm AN, Rich A, Miller SM, Ou Y, Sarr MG, Farrugia G. Cytoskeletal modulation of sodium current in human jejunal circular smooth muscle cells. Am J Physiol Cell Physiol 284: C60–C66, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Strege PR, Ou Y, Sha L, Rich A, Gibbons SJ, Szurszewski JH, Sarr MG, Farrugia G. Sodium current in human intestinal interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol 285: G1111–G1121, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Talley NJ, Boyce PM, Owen BK, Newman P, Paterson KJ. Initial validation of a bowel symptom questionnaire and measurement of chronic gastrointestinal symptoms in Australians. Aust NZ J Med 25: 302–308, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Muller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut 45: II43–II47, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang DW, Viswanathan PC, Balser JR, George AL Jr, Benson DW. Clinical, genetic, and biophysical characterization of SCN5A mutations associated with atrioventricular conduction block. Circulation 105: 341–346, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, Moss AJ, Towbin JA, Keating MT. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell 80: 805–811, 1995. [DOI] [PubMed] [Google Scholar]