Abstract
Oral exposure to dietary fat results in an early initial spike, followed by a prolonged elevation, of serum triglycerides in humans. The physiological and pathophysiological implications remain unknown. This study sought to determine the incidence of the effect, the required fat exposure duration, and its reliability. Thirty-four healthy adults participated in four to six response-driven trials held at least a week apart. They reported to the laboratory after an overnight fast, a catheter was placed in an antecubital vein, and a blood sample was obtained. Participants then ingested 50 g of safflower oil in capsules with 500 ml of water within 15 min to mimic a high fat meal but without oral fat exposure. Blood was collected 0, 10, 20, 30, 40, 50, 60, 120, 240, 360, and 480 min after capsule ingestion with different forms (full fat, nonfat, none) and durations of oral fat exposures (10 s, 5 min, 20 min, and/or 2 h). A triglyceride response (increase of triglyceride >10 mg/dl within 30 min) was observed in 88.2%, 70.5%, and 50% of participants with full-fat, nonfat, and no oral exposure, respectively. Test-retest reliability was 75% with full-fat exposure but only 45.4% with nonfat exposure. Full-fat and nonfat exposures led to comparable significant elevations of triglyceride over no oral stimulation with 10-s exposures, but full fat led to a greater rise than nonfat with 20 min of exposure. These data indicate that nutritionally relevant oral fat exposures reliably elevate serum triglyceride concentrations in most people.
Keywords: taste, lipid, cardiovascular disease, chemosensory, cephalic phase
aside from contributing to the sensory appeal of foods, oral exposure to dietary fat elicits physiological responses involved with nutrient processing in the gut. Oral exposure to linoleic acid elicits pancreatic exocrine secretion in mice (7), and linoleic, linolenic, and oleic acids have the same effect in rats (9, 14). Oral fat exposure in rats also slows (27) and aids regulation of gastric emptying (12) and prolongs the postprandial elevation of serum triacylglycerol (TAG) (27). Similarly, in humans, oral fat exposure elicits a range of effects including modulation of gut peptide secretion (8), appetitive sensations (8, 30), preferred fat levels in foods (16), energy intake (4) and postprandial lipemia (18–20, 25). The latter effect is reported to be biphasic. The first phase is, in part, due to mobilization of lipid stored, presumably in the enterocyte, from the previous meal (5, 20, 25, 29). The second phase reflects sensory exposure effects on absorption of the lipid providing the initial oral stimulation, de novo synthesis and secretion of lipid particles, and/or clearance of lipid from the blood. To date, work on oral fat exposure and postprandial TAG concentrations in humans has involved exposures that exceed those likely to be experienced under free-living conditions. The intent of this study was to test a concept rather than establish the physiological relevance of the effect. However, there is evidence that exposure to fat for only 3 min is sufficient to elicit a cephalic-phase pancreatic polypeptide response (4), indicative of vagal activation, the presumed basis for the first-phase TAG response. Thus it may be hypothesized that only brief oral exposure to fat may be sufficient to alter postprandial lipemia in humans.
The aim of this project was to better characterize the effects of oral fat exposure on serum TAG concentrations. First, TAG responses to realistic exposure times were explored. This entailed monitoring serum TAG concentrations after a single 10-s oral exposure, 5 min of exposure to simulate a snack, 20 min of exposure to mimic a meal, and 2 h of exposure to replicate our earlier trials. To be considered physiological, responses to exposures consistent with typical dietary snack and meal times were expected. Secondly, the effects of these different levels of oral exposure on the first and second phases of lipid absorption were monitored. The first phase likely reflects the sensory-mediated mobilization of lipid stored in enterocytes from the previous meal, whereas the second stems from absorption of the lipid from the present meal (25, 30). The function of the first-phase response is largely unknown and remains to be fully characterized. Also, the reliability of the response was evaluated by retesting individuals who responded to the initial exposure after an interval of at least 2 wk. Thirdly, response specificity was examined by contrasting serum TAG concentrations after oral exposure to a high-fat food, a nonfat version of the same food, and no oral stimulation. Finally, the possibility that there are responders and nonresponders was explored because this has been proposed on the basis of fatty-acid sensory-detection data (11, 23) and cephalic-phase responses to other stimuli (32).
MATERIALS AND METHODS
Participants.
Eligibility criteria included the following: healthy males and females with a body mass index (BMI) between 18 and 30 kg/m2; those not initiating or terminating the use of medications reported to affect appetite or body weight during the proposed study period [oral contraceptive agents (n = 5), antidepressants (n = 2), antihistamine (n = 1), thyroid supplement (n = 1)]; a stable activity level (no deviation >1 time/wk for 30 min/session); a resting serum TAG <250 mg/dl; rate test stimuli as acceptable (i.e., above neutral on a hedonic generally labeled magnitude scale); and agreement to abstain from alcohol consumption and vigorous physical activity for 48 h before study days.
General protocol.
On the basis of responses to an online questionnaire eliciting demographic, health, and lifestyle information, interested individuals completed a screening session to confirm their eligibility. During this session, their inherent taste sensitivity to the bitterness of propylthiouracil (PROP taster status) (26) was determined by a three-solution method since this has been related to fat-taste responsiveness (11). They also rated the test foods for acceptability, and a blood sample was collected from an arm vein to evaluate their resting (overnight fasted) TAG concentration.
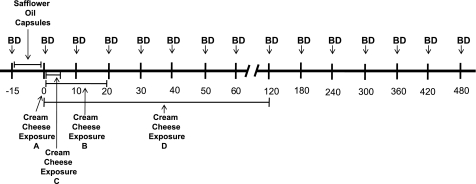
Eligible individuals choosing to participate consumed 50 g of Reese's Pieces (provided by the researchers) as the last eating event of the day before reporting to the laboratory after a 10-h (overnight) fast. This ensured the last eating event of the day before testing was high in fat. The load contained ∼245 kcal and was comprised of about 43% fat, 10% protein, and 49% carbohydrate. Testing was conducted on four to six occasions, depending on participant performance, at roughly weekly intervals. A timeline of session activities is presented in Fig. 1. At each session, a blood sample was collected from an in-dwelling catheter, and participants ingested 50 g of safflower oil (Nature's Plus, Melville, NY) in capsules with 500 ml of water in 15 min. Such a load is comparable to a high-fat meal and is ecologically reasonable. Another blood sample was then collected. No additional foods or fluids were ingested over the subsequent 8 h. The testing sequence is outlined in Fig. 2. On the first two test days, participants then masticated and expectorated a single test-food sample for 10 s (a single exposure). This was either full-fat or nonfat cream cheese, presented on a spoon in random order. Expectorated samples were collected, dried, and weighed to verify that none was consumed. Additional blood was collected at 10, 20, 30, 40, 50, and 60 min as well as 2, 4, 6, and 8 h. If the serum TAG response over the first 30 min after oral stimulation with the full-fat cream cheese was more than 10 mg/dl greater than each individual's baseline TAG concentration, then the two treatments were replicated at two additional test sessions to establish the reliability of the response. Twenty individuals exhibited the requisite rise so were retested for reliability. These individuals were also asked to return for a fifth visit where they received no oral stimulation, but all other aspects of the testing regime were identical. If no response was observed after the first two trials, the study was repeated with 10-s oral exposures to samples at 30-s intervals for 10 min and 1-min intervals for an additional 10 min (simulation of a 20-min meal). Again, full-fat and nonfat samples were presented in random order with a minimum of 1 wk between tests. If a TAG response was observed with the full-fat stimulus at the 20-min exposure period, testing with full-fat and nonfat samples was repeated with oral stimulation provided at 30-s intervals for 5 min (simulation of a snack). If no difference was observed with the 20-min exposure, participants were tested on two additional days with 30-s exposures at 5-min intervals for 1 h, then every 15 min with 30-s exposures for an additional 1 h. Therefore, participants showing a TAG response to the single stimulation were tested on four or five occasions, and those failing to show a response on the first two test sessions were tested on six occasions. This response-driven testing protocol, rather than a random presentation approach, was used because the amount of blood drawn at each trial precluded more than six trials per participant and 11 sessions would have been required to test all individuals under all conditions. The protocol was approved by the Perdue University Institutional Review Board. Participants were recruited by public announcements, provided written consent, and received financial remuneration.
Fig. 1.
Time line of test session activities. Each exposure equals 2 trials one with full-fat and one with nonfat cream cheese, in random order. BD, blood draw; A, first treatment, 10-s exposures; B, second treatment, 20-min exposures to nonresponders to A; C, third treatment, 5-min exposures to responders to B; D, third treatment, 2-h exposures to nonresponders to B. Responder, triacylglycerol rise >10 mg/dl over baseline within 30 min.
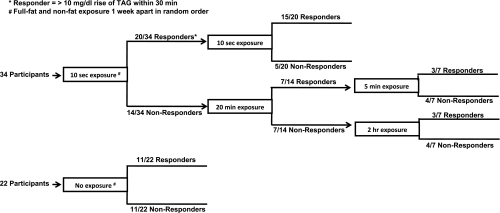
Fig. 2.
Participant testing protocol. All participants initially received 10-s exposures to full-fat and nonfat stimuli on separate days held about 1 wk apart. Those exhibiting a triacylglycerol (TAG) increase of >10 mg/dl within 30 min of pill ingestion received these exposures again to assess response reliability. Individuals who did not exhibit a TAG rise of this magnitude next received 20-min exposures to the 2 stimuli. Those exhibiting a TAG rise next received 5-min exposures, and those failing to exhibit a TAG rise received 2 h of exposure to the full-fat and nonfat stimuli. The identical protocol was conducted for 22 individuals receiving no oral stimulation as a control for time effects.
Study samples.
Oral stimuli consisted of 5.2-g samples of cream cheese and nonfat cream cheese (Philadelphia Brand; Kraft Foods, Glenview, IL). Expectorated samples were collected quantitatively, dried for 6 h at 55°C, and reweighed individually. The percent recovery was calculated relative to the dry weight of unmasticated samples. Lipid loads were comprised of safflower oil in commercially available capsules (Soft Gel Technologies, Los Angeles, CA).
Hematology.
Four milliliters of blood were collected in a red-top vacutainer from a catheter in an antecubital vein at each sampling time. Samples were centrifuged and the serum aliquoted and frozen at −70°C for later analyses. TAG concentrations were determined by an enzymatic procedure with the use of a Cobas Integra 400 Analyzer (Roche Diagnostics, Summerville, NJ). The precision of the assay was 2%.
Statistical analyses.
Serum TAG changes were evaluated by repeated-measures ANOVA using treatment [i.e., high fat, low fat, and, in a subset of participants (N = 22), no oral stimulation] and time as within-subject factors. Changes from baseline were assessed by one-sample t-tests. Correlation analysis was used to assess test-retest reliability. Although both phases of the TAG response to oral fat stimulation were evaluated, the first phase was the primary focus. Thus a TAG response was defined as an increase of serum TAG >10 mg/dl within the first 30 min after the initiation of oral stimulation relative to the resting TAG concentration for each individual on each test day. This represented an increase of about 10% over the resting value of the study population and approximated the value of the 95% confidence interval for population categories recently used to explore the association between TAG and cardiovascular disease (CVD) risk (24). Statistical analyses were performed with the use of the Statistical Package for the Social Sciences (SPSS), version 15.0 (SPSS, Chicago, IL). The criterion level for statistical significance was P < 0.05, two-tailed. All data are expressed as means ± SE.
RESULTS
The study sample was comprised of 18 male and 16 female adults (mean age = 25.0 ± 1.2 yr) with a mean BMI of 24.4 ± 0.53 kg/m2. Twenty-seven participants were Caucasian, five were African American, and there was one Asian and one Hispanic. Their mean resting TAG concentration was 99.9 ± 10.1 mg/dl. Upon initial exposures, their mean hedonic ratings of the full-fat and nonfat cream cheese samples were 3.8 ± 0.4 and 3.5 ± 0.5, respectively on a 9-cm visual analog scale (0 = extremely palatable; 9 = extremely unpalatable). The ratings were 3.4 ± 0.5 and 3.2 ± 0.5 with repeat exposures. These values do not differ from each other, and the correlations between them were all significant (Pearson coefficients ranged from 0.62 to 0.81). The mean percent recovery of oral cream cheese samples was 107 ± 2% and 103 ± 2% for the 10-s exposures with full-fat and nonfat stimuli, respectively, and 105 ± 1% for all samples tested in the trial. The high percent recovery indicates little sample was ingested but also that drying may not have been complete.
Incidence of response.
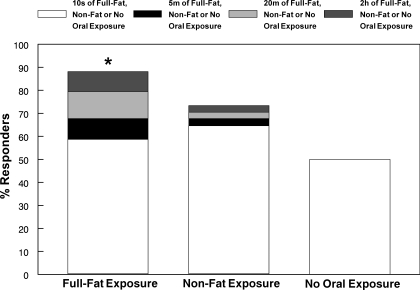
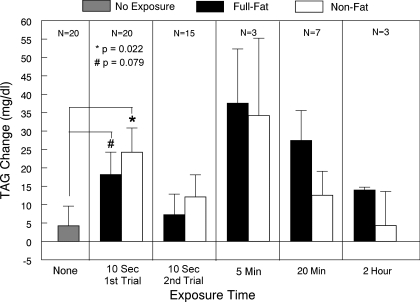
The incidence of responses to orosensory stimulation with dietary fat is depicted in Fig. 3. A single 10-s oral exposure elicited a TAG response within 30 min in 58.8% (20/34) of participants. Among the nonresponders to the single exposure, 50% (7/14) responded to oral exposure over a 20-min period. 42.9% (3/7) of those responding to the 20-min period of exposure also responded after only 5 min of oral fat exposure. For those not responding to 20 min of oral exposure, 42.9% (3/7) responded to exposure over a 2-h period. Collectively, 88.2% (30/34) responded to oral fat exposure. When participants received oral exposure with the nonfat cream cheese, 64.7% (22/34) responded to the initial 10-s exposure. Among the nonresponders, 16.7% (2/12) responded to 20-min exposure. One of these twelve individuals (8.3%) responded to 5-min exposure. Among the participants not responding to 20-min exposure, 8.3% (1/12) responded to 2 h of exposure. Thus a total of 73.5% (25/34) of participants responded to oral exposure with the nonfat cream cheese. Fifty percent (11/22) of participants receiving no oral exposure experienced a TAG rise. This group was comprised of the 20 individuals responding to the initial fat exposure who underwent retesting, as well as two individuals who only completed the initial testing; hence N = 22. The proportion of responders to full-fat cream cheese was significantly greater than chance (P = 0.001; sign test), whereas this was not the case for the proportion of responders to the nonfat cream cheese.
Fig. 3.
Distribution of responders (i.e., TAG increase of >10 mg/dl within 30 min of pill ingestion) with oral exposure to full-fat or nonfat cream cheese for 10 s, 5 min, 20 min, or 2 h or after no oral stimulation during the first-phase TAG response; *number of responders greater than chance (P < 0.05); N = 22.
Characterization of the first-phase response.
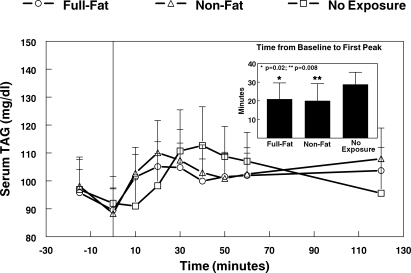
The TAG response within the first 30 min following capsule ingestion is depicted in Fig. 5. All conditions led to an early, transient rise of serum TAG. Among the 22 participants tested with and without oral exposure, only when the oral stimulus was the full-fat cream cheese did a peak occur within 30 min at better than chance levels of probability 16/22 (72.7%) (P = 0.026, binomial distribution). The proportions of first-phase responses with oral exposure to nonfat cream cheese and no oral exposure were 13/22 (59.1%) and 10/22 (45.4%), respectively. The time to peak from baseline was significantly shorter for the two oral exposure conditions compared with the no-exposure condition (P = 0.02, full-fat cream cheese; P = 0.008 nonfat cream cheese; Fig. 4, inset). The peak magnitudes were not significantly different.
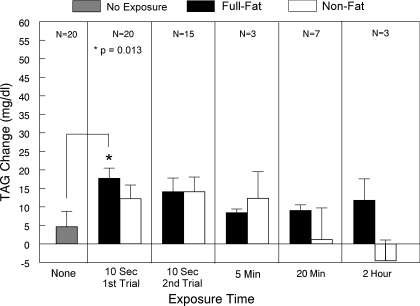
Fig. 5.
Mean ± SE change of serum TAG concentration from times 0–30 min for responders (TAG rise >10 mg/dl) following oral stimulation to full-fat and nonfat cream cheese for 10 s, 5 min, 20 min, or 2 h, as well as after no oral stimulation. Because of limited sample sizes, statistical analyses were only conducted for comparisons with 20 participants.
Fig. 4.
First phase change of serum TAG following oral exposure to full-fat or nonfat cream cheese for 10 s or no oral exposure. Inset: time to peak serum TAG over the first 30 min after capsule ingestion. Time −15 min represents TAG concentration just before capsule ingestion, and time 0 represents immediately after capsule ingestion before first oral exposure (N = 22).
Because of the exposure testing strategy, all participants were not tested under each exposure condition (i.e., it was not a complete crossover design), and different individuals were tested under more than one condition (i.e., the groups were not independent). Most importantly, only those participants not responding to a short period of stimulation were exposed to the longer exposures, so there was some sorting of participants according to responsiveness. Thus, it is not appropriate to directly compare TAG response magnitudes of all those receiving each exposure. To provide a common basis for comparisons, Fig. 5 presents the mean response magnitude only of responders to each level of exposure. Paired comparisons for conditions with N ≥ 15 indicate the mean TAG response magnitude during the initial 30 min after capsule ingestion, and only 10 s of exposure was greater with fat compared with no oral exposure (P = 0.013). The difference in TAG response to the nonfat cream cheese did not differ significantly from the response to either full-fat cream cheese or no oral exposure.
Although the sample size is small, the magnitude of response after full-fat exposure is relatively stable across the range of stimulation times tested. However, there was a decline in response to longer stimulation with the nonfat cream cheese. This pattern held for maximal peak responses as well.
Characterization of the second-phase response.
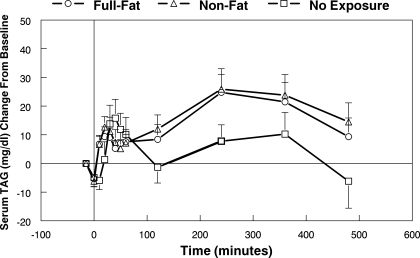
The serum TAG concentration over the 8 h after capsule ingestion followed by a single 10-s oral exposure to full-fat or nonfat cream cheese or no oral exposure is shown in Fig. 6. In the time interval between 120 and 480 min, there was a significant treatment effect [F(2,42) = 4.34, P = 0.019] and a treatment by time interaction [F(20,420) = 1.66, P = 0.038]. The mean rise with full-fat cream cheese exposure was 13.9 mg/dl, and the value after nonfat cream cheese exposure was 15.5 mg/dl. In contrast, the mean rise with no oral exposure was only 4.8 mg/dl. Both stimulus exposures were greater than the no exposure treatment (P = 0.01 for full-fat cream cheese and P = 0.028 for the nonfat cream cheese).
Fig. 6.
Change of serum TAG concentration over 8 h after oral exposure to full-fat or nonfat cream cheese for 10 s or no oral exposure. Time −15 min represents TAG concentration just before capsule ingestion, and time 0 represents immediately after capsule ingestion before first oral exposure (N = 22).
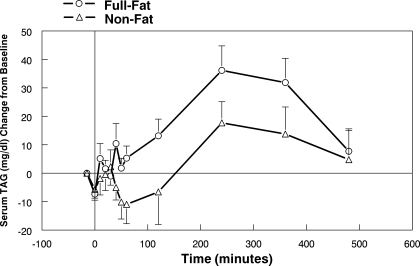
Fourteen participants did not respond to the initial 10-s exposure so were retested with 20 min of exposure. Under these conditions, the serum TAG concentration was significantly [F(1,13) = 4.81, P = 0.047] higher with full-fat cream cheese exposure compared with nonfat cream cheese exposure (Fig. 7). The peak TAG rises were 36.1 ± 8.6 and 17.7 ± 7.4 mg/dl, respectively.
Fig. 7.
Change of serum TAG concentration over 8 h after 20 min of oral exposure to full-fat or nonfat cream cheese. Time −15 min represents TAG concentration just before capsule ingestion, and time 0 represents immediately after capsule ingestion before first oral exposure (N = 14).
Figure 8 presents the mean response magnitude only of responders to each level of exposure. Paired comparisons for conditions with N ≥ 15 indicate the mean TAG response magnitude during 120–480 min after capsule ingestion and only 10 s of exposure, was significantly greater following oral nonfat exposure compared with no oral stimulation (P = 0.022) and tended to be greater with full-fat exposure (P = 0.079). The TAG responses to the full-fat and nonfat cream cheese did not differ significantly from each other.
Fig. 8.
Mean ± SE change of serum TAG concentration from times 120–480 min for responders (TAG rise >10 mg/dl) following each level of stimulation. Because of limited sample sizes, statistical analyses were only conducted for comparisons with 20 participants.
Although the sample size is small, the magnitude of response after full-fat cream cheese exposure remains stronger than the response to nonfat cream cheese with longer stimulation.
Response reliability.
Among the responders to the initial 10-s exposure with full-fat cream cheese, 75% (15/20) responded again upon restimulation 2 wk later. When participants received repeat oral exposure with the nonfat cream cheese, only 45.4% (10/22) responded upon restimulation.
The mean first-phase TAG responses following 10 s of exposure with either full-fat or nonfat cream cheese were similar after duplicate exposures (Fig. 5).
The mean TAG response after the initial oral exposure to fat was significantly correlated with the mean TAG response to the second exposure (r = 0.59, P = 0.021), typically administered 2 wk later. Peak maxima were also correlated (r = 0.60, P = 0.009). The comparable correlations for the repeat nonfat exposures were not significant. No significant correlations were observed between responses to duplicate 10-s exposures for mean TAG magnitude or peak during the second-phase response.
DISCUSSION
There was a twofold rationale for studying the TAG response to oral fat stimulation. Firstly, by serving as a marker for oral fat detection, it may be used to elucidate salient sensory attributes of fats and the mechanisms for their detection. Secondly, the absolute TAG rise itself is of importance because recent evidence documents a direct, independent association between TAG and cardiovascular events (1, 24, 33). The relation holds for resting TAG values but is attenuated with adjustment for other influences (1). In contrast, nonfasting or postprandial TAG concentrations remain strong predictors of myocardial infarction, ischemic heart disease, and death after such adjustments (1, 24). This may be attributable to capturing better predictive information conveyed in the higher variability of nonfasting values (1). Recent evidence indicates that the association between postprandial TAG concentration and ischemic stroke is linear (6), so an increment contributed by oral stimulation could theoretically exacerbate the problem. TAG concentrations 4–8 h after a meal may be the most telling (1, 22), and changes of the magnitude noted in the present study are predictive of increased CVD risk (13). The first-phase response has not been evaluated in clinical trials to date since blood samples have not been drawn during this time frame. Thus the clinical significance of the first-phase response is unknown. However, in this and earlier work (20), a significant correlation has been documented between the magnitude of the first- and second-phase responses. Whether the first-phase response holds predictive, and possibly, a causal relation to CVD risk through modulation of the second-phase TAG concentration warrants further study.
Early work with rats revealed that oral exposure to corn oil with gastric lipid loading leads to a significantly prolonged serum TAG elevation (27). Subsequent human trials replicated this finding, identified the biphasic nature of the response, and elucidated aspects of the mechanism (18–21, 25, 28, 30). However, information about the effective stimulus attributes, required exposure times, and response reliability was lacking and precluded assessment of the practical implications of oral fat exposures.
The present study documented that oral exposure to dietary fat prompted a first-phase TAG response in 88% of the participants, and about 80% responded to an exposure of ≤ 20 min. This was significantly greater than chance, and the mean and peak responses were significantly greater than values after no oral stimulation. Thus the incidence of a first-phase TAG response is high with minimal oral exposure to fat. However, about half of the first-phase TAG rise may be related to tactile or other stimulatory aspects of the procedure. The incidence of responses after ≤ 20 min of oral exposure to the nonfat cream cheese was about 70% and 50% of participants exhibited a response after only swallowing the capsules. The 10-mg/dl criterion for a response was adopted since it represents the upper limit of the 95% confidence interval for resting serum TAG concentration, but, in retrospect, with a high incidence of TAG elevation associated with nonspecific oral stimulation, a higher criterion may be useful in future studies.
Exposure with both full-fat and nonfat stimuli reduced the lag time preceding a TAG rise compared with no oral exposure. This is suggestive of a signaling role. Findings from other human studies are consistent with this observation. Compared with fasting, modified sham feeding (MSF) of cake containing fat led to a significantly greater pancreatic polypeptide concentration, an index of vagal activation, whereas sampling cake without fat led to an intermediate response over 20 min (4). Furthermore, MSF of a high-fat meal an hour before actual ingestion of a meal led to an earlier and greater TAG response than when there was no MSF (28). The TAG response was intermediate when the MSF followed the meal by 1 h. Thus brief (nutritionally relevant), general oral stimulation appears to prompt a small TAG response, but the magnitude is greater when the oral stimulus contains fat. The observation of a trend toward lower magnitude of the first-phase response with increased exposure times specifically to the nonfat cream cheese was unexpected. However, because of the limited number of participants tested, this trend cannot presently be deemed reliable. However, a lower first-phase response to nonfat vs. full-fat stimuli has been reported in other trials where the exposure time was comparable to our longer trials (25).
Differences in the reliability of the first-phase responses to the full-fat and nonfat stimuli further support the validity of the distinction. With retesting of those responding to the first exposures, 75% of individuals responded again when exposed to the full-fat stimulus, whereas the incidence was only 45% with the nonfat stimulus. In addition, test-retest correlations for both mean and peak serum TAG concentrations were significant after oral fat exposure but not with nonfat exposure. Thus the first-phase TAG response to a single 10-s oral exposure to dietary fat is also moderately reliable (correlation coefficient of ∼0.6). We hypothesize that oral sensory systems are present to detect relevant stimuli and generate signals that allow an organism to initiate and gauge the magnitude of suitable adaptive responses (e.g., salivate, swallow, secrete insulin). The first-phase response may reflect this signaling role, so a better understanding of its characteristics may promote understanding of its function and the implications of its manipulation.
Among the total sample, the mean second-phase TAG response was significantly greater after 10 s of oral stimulation with either full-fat or nonfat cream cheese compared with no oral stimulation. However, the response to the two stimuli did not differ. In contrast, a significantly greater TAG response was observed with 20 min of oral exposure to the full-fat stimulus compared with the nonfat version. Although the sample size of the later tests was limited (N = 14), the stronger effect of oral fat exposure on the postprandial TAG concentration may require longer exposure, albeit still within the time frame of a meal. This finding replicates multiple observations of an augmented response to full-fat cream cheese relative to a nonfat version with longer exposure times (18–20, 25). The stronger response to full-fat vs. nonfat exposure with longer stimulation times (i.e., 20 min and 2 h) is also noted in analyses of only those showing a response. Whether a differential response would occur with exposure between 10 s and 20 min cannot be determined because of the limited number of participants (N = 7) tested for 5 min.
Work with rats indicated that a TAG response was only obtained in animals familiar with the testing protocol (27). Therefore, to determine whether an individual in the present study may be a nonresponder, those who failed to respond to the initial 10-s exposure were retested at least two additional times, once for 20 min and once for 2 h. A response to some level of exposure was noted in 88% of participants, so, at a minimum, nonresponders would seem to be a small subgroup. However, this study used a convenience sample of normolipemic adults, so it is possible the proportion could be high in some subgroup of the population. Given the nature of the response, it would be most important to assess the prevalence of responders in those with a predisposition toward dyslipidemias. It could also help to elucidate the significance of polymorphisms for putative fat taste transduction mechanisms [e.g., CD36 (15, 31)].
On the basis of detection thresholds for linoleic acid, others have proposed that the prevalence of fat tasters may be 46–58% (10) and they do not differ from nontasters in age, BMI, dietary restraint, disinhibition, hunger, or PROP taster status. Another trial noted 80% of PROP tasters could discriminate conjugated linoleic acid in ice cream, whereas only 17% of nontasters could do so (23). If it is assumed that ∼70% of the population tested was comprised of PROP tasters, this would correspond to about 60% of the population as fat tasters. Both of these studies assessed sensitivity by detection of a single test concentration. We have measured detection thresholds with the use of an ascending, three-alternative, forced-choice, sip-and-spit procedure and measured thresholds in nearly all individuals tested (2, 3, 17). However, the range of values covers three to four orders of magnitude (17), indicating there is large individual variability in sensitivity. How threshold sensitivity relates to TAG responsiveness warrants further study since it will help to determine the prevalence and implications of fat-taster status.
The present study has a number of important limitations. First, the exposure times were not randomly administered. Given evidence that there may be learning effects (27) and, potentially, extinction effects with cephalic-phase testing, the true proportion of responders at any given level of exposure cannot be determined from the present data. The response-driven exposure protocol may also have introduced a bias toward minimizing exposure effects with the longer exposures. Only individuals failing to respond to shorter exposures received longer exposure, so these groups were comprised of those that may be less responsive. It should also be noted that these data are based on responses to a single food. Items with different fat composition and concentration may yield different results.
In summary, this work further confirms that oral fat exposure independently increases serum TAG concentrations. It demonstrates that stimulation durations likely to be encountered in normal eating (10 s to 20 min) are sufficient to elicit the response and that it is reliable. Although progress is being made on the mechanisms responsible for the effect (e.g., Refs. 25 and 29), its physiological function and contribution to pathophysiology (e.g., CVD risk) remain unknown.
GRANTS
This work was supported by a grant from the National Institutes of health (PHS R01 DK45294).
Acknowledgments
Invaluable technical assistance was provided Judy George, Robin Rhine, and Christen Wood.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 298: 309–316, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Chale-Rush A, Burgess JR, Mattes RD. Evidence for human orosensory (taste?) sensitivity to free fatty acids. Chem Senses 32: 423–431, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Chale-Rush A, Burgess JR, Mattes RD. Multiple routes of chemosensitivity to free fatty acids in humans. Am J Physiol Gastrointest Liver Physiol 292: G1206–G1212, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Crystal SR, Teff KL. Tasting fat: cephalic phase hormonal responses and food intake in restrained and unrestrained eaters. Physiol Behav 89: 213–220, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Fielding BA, Callow J, Owen RM, Samra JS, Matthews DR, Frayn KN. Postprandial lipemia: the origin of an early peak studied by specific dietary fatty acid intake during sequential meals. Am J Clin Nutr 63: 36–41, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, Nordestgaard BG. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA 300: 2142–2152, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Gaillard D, Laugerette F, Darcel N, El-Yassimi A, Passilly-Degrace P, Hichami A, Khan NA, Montmayeur JP, Besnard P. The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J 22: 1458–1468, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Heath RB, Jones R, Frayn KN, Robertson MD. Vagal stimulation exaggerates the inhibitory ghrelin response to oral fat in humans. J Endocrinol 180: 273–281, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Hiraoka T, Fukuwatari T, Imaizumi M, Fushiki T. Effects of oral stimulation with fats on the cephalic phase of pancreatic enzyme secretion in esophagostomized rats. Physiol Behav 79: 713–717, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Kamphuis MM, Saris WH, Westerterp-Plantenga MS. The effect of addition of linoleic acid on food intake regulation in linoleic acid tasters and linoleic acid non-tasters. Br J Nutr 90: 199–206, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Kamphuis MM, Westerterp-Plantenga MS. PROP sensitivity affects macronutrient selection. Physiol Behav 79: 167–172, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan JM, Siemers W, Grill HJ. Effect of oral versus gastric delivery on gastric emptying of corn oil emulsions. Am J Physiol Regul Integr Comp Physiol 273: R1263–R1270, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Langsted A, Freiberg JJ, Nordestgaard BG. Fasting and nonfasting lipid levels. Circulation 118: 2047–2056, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest 115: 3177–3184, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee K, Godeau B, Fromont P, Plonquet A, Debili N, Bachir D, Reviron D, Gourin J, Fernandez E, Galacteros F, Bierling P. CD36 deficiency is frequent and can cause platelet immunization in Africans. Transfusion 39: 873–879, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Mattes RD Fat preference and adherence to a reduced-fat diet. Am J Clin Nutr 57: 373–381, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Mattes RD Human detection of free fatty acids (Abstract). International Symposium on Olfaction and Taste, XV: San Francisco, CA, July 2008.
- 18.Mattes RD Oral exposure to butter, but not fat replacers elevates postprandial triacylglycerol concentration in humans. J Nutr 131: 1491–1496, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Mattes RD Oral fat exposure alters postprandial lipid metabolism in humans. Am J Clin Nutr 63: 911–917, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Mattes RD Oral fat exposure increases the first phase triacylglycerol concentration due to release of stored lipid in humans. J Nutr 132: 3656–3662, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Mattes RD The taste of fat elevates postprandial triacylglycerol. Physiol Behav 74: 343–348, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Mora S, Rifai N, Buring JE, Ridker PM. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation 118: 993–1001, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nasser JA, Kissileff HR, Boozer CN, Chou CJ, Pi-Sunyer FX. PROP taster status and oral fatty acid perception. Eat Behav 2: 237–245, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 298: 299–308, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Parks EJ Oral fat sensory exposure elevates chylomicron-TG and delays subsequent glucose metabolism (Abstract). Am Diabetes Assoc. 68th Annual Meeting: San Francisco, CA, June 2008.
- 26.Prescott J, Tepper BJ, eds. Genetic Variation in Taste Sensitivity. New York: Marcel Dekker, 2004, pp. 255.
- 27.Ramirez I Oral stimulation alters digestion of intragastric oil meals in rats. Am J Physiol Regul Integr Comp Physiol 248: R459–R463, 1985. [DOI] [PubMed] [Google Scholar]
- 28.Robertson MD, Mason AO, Frayn KN. Timing of vagal stimulation affects postprandial lipid metabolism in humans. Am J Clin Nutr 76: 71–77, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Robertson MD, Parkes M, Warren BF, Ferguson DJ, Jackson KG, Jewell DP, Frayn KN. Mobilisation of enterocyte fat stores by oral glucose in humans. Gut 52: 834–839, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smeets AJ, Westerterp-Plantenga MS. Satiety and substrate mobilization after oral fat stimulation. Br J Nutr 95: 795–801, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka T, Sohmiya K, Kawamura K. Is CD36 deficiency an etiology of hereditary hypertrophic cardiomyopathy? J Mol Cell Cardiol 29: 121–127, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Teff KL, Mattes RD, Engelman K. Cephalic phase insulin release in normal weight males: verification and reliability. Am J Physiol Endocrinol Metab 261: E430–E436, 1991. [DOI] [PubMed] [Google Scholar]
- 33.Tirosh A, Rudich A, Shochat T, Tekes-Manova D, Israeli E, Henkin Y, Kochba I, Shai I. Changes in triglyceride levels and risk for coronary heart disease in young men. Ann Intern Med 147: 377–385, 2007. [DOI] [PubMed] [Google Scholar]