Abstract
ERM (ezrin, radixin, and moesin) proteins play critical roles in epithelial and endothelial cell polarity, among other functions. In gastric glands, ezrin is mainly expressed in acid-secreting parietal cells, but not in mucous neck cells or zymogenic chief cells. In looking for other ERM proteins, moesin was found lining the lumen of much of the gastric gland, but it was not expressed in parietal cells. No significant radixin expression was detected in the gastric glands. Moesin showed an increased gradient of expression from the neck to the base of the glands. In addition, the staining pattern of moesin revealed a branched morphology for the gastric lumen. This pattern of short branches extending from the glandular lumen was confirmed by using antibody against zonula occludens-1 (ZO-1) to stain tight junctions. With a mucous neck cell probe (lectin GSII, from Griffonia simplicifolia) and a chief cell marker (pepsinogen C), immunohistochemistry revealed that the mucous neck cells at the top of the glands do not express moesin, but, progressing toward the base, mucous cells showing decreased GSII staining had low or moderate level of moesin expression. The level of moesin expression continued to increase toward the base of the glands and reached a plateau in the base where chief cells and parietal cells abound. The level of pepsinogen expression also increased toward the base. Pepsinogen C was located on cytoplasmic granules and/or more generally distributed in chief cells, whereas moesin was exclusively expressed on the apical membrane. This is a clear demonstration of distinctive cellular expression of two ERM family members in the same tissue. The results provide the first evidence that moesin is involved in the cell biology of chief cells. Novel insights on gastric gland morphology revealed by the moesin and ZO-1 staining provide the basis for a model of cell maturation and migration within the gland.
Keywords: gastric gland structure, tight junction, development, ezrin, radixin
in the last decade the ERM (ezrin, radixin, and moesin) membrane cytoskeleton linker protein family (33) has been a subject of intensive study because of their versatile functions in various tissue models (5, 11, 19). It was postulated that the ERM proteins arose by gene duplication because of the high degree of structural conservation among them (4). Each of the ERM proteins has an NH2-terminal membrane binding domain [termed FERM domain (8)] and a COOH-terminal F-actin binding domain (1, 35), connected by an α-helical domain actively participating in the regulation of ERM activities (27). Extensive homologies exist in the amino acid sequences among ERM proteins: moesin has a 72% sequence identity to ezrin (26), whereas radixin and ezrin sequences are 75% common (13). The ability of the NH2-terminal FERM domain to bind to the COOH-terminus allowed an elegant mechanism for the regulation of ERM activities. In the dormant state, ERM proteins take an NH2 terminal-to-COOH terminal binding conformation, which can be opened up by phosphorylation at a conserved COOH-terminal threonine site (18, 28, 38). The opened conformation of ERM proteins allows stronger interaction of ezrin with F-actin and the plasma membrane.
Structural similarities among ERM proteins seem to lead to similar functions, as suggested in the kidney proximal tubule cells, where ezrin and moesin are both expressed at high levels in the brush border microvilli (3). However, in most other tissues, ezrin, radixin, and moesin are differentially expressed (3), indicating a specialized function for each of these proteins. For instance, the only ERM protein in the brush border membrane of the small intestine is ezrin (3, 16). The liver is an interesting example of cell-specific expression where the dominant ERM protein in the hepatocytes is radixin, liver cholangiocytes express only ezrin (12), liver stellate cells express only moesin (30), and the only abundant ERM protein in endothelial cells is moesin (3). In addition, gene knockout experiments of these proteins caused very different phenotypes, further supporting different functions of ERM proteins. Ezrin knockout mice did not grow after birth and died within 10 days of parturition, showing abnormal epithelial organization and villus morphogenesis in the developing intestine (32). A conditional knockout model (“knockdown”) of ezrin was made to study ezrin function in adult mice where the low level of ezrin expression allowed animals to achieve some level of postnatal maturity (34). In these animals, defects in the stimulation-dependent expansion of apical canalicular membranes in gastric parietal cells was observed, which resulted in the loss of gastric acid secretion. Knocking out radixin in mice is not as lethal as knocking out ezrin. Mice without radixin grew and developed normally as wild-type ones, although mild liver injury, due to impaired secretion of conjugated bilirubin, was observed (25). No abnormal phenotype was observed in moesin knockout mice (9). So far, the only effect of knocking out moesin was impaired stellate cell migration and decreased fibrosis in response to liver injury (30).
In parietal cells ezrin is predominantly expressed on the apical canalicular membrane (17, 36). A lower level of ezrin expression was also detected on surface mucous cell membranes, but ezrin was not detected in mucous neck cells or chief cells (17). As ERM proteins are broadly implicated in the establishment and maintenance of the polarity of epithelial cells (15, 32, 37) and the membrane activities of secreting cells (e.g., Ref. 12), we were motivated to look for ERM proteins (namely radixin and moesin) in the gastric glands. We report here the expression pattern of moesin in gastric glands, including the first evidence that moesin participates in the biology of the pepsinogen-secreting chief cells, as well as novel insights on the morphology of the gastric glands revealed in the course of this study.
Because the experimental objectives of this work are so dependent on the cellular organization of gastric glands, we will briefly review the structure of gastric glands located in the main body of the stomach, which are provided in much greater detail in the comprehensive works of Karam and LeBlond (20–24). The tubular epithelial glands are anatomically and functionally divided into four different areas: 1) the pit region, consisting mainly of columnar surface mucous cells that constitute the upper ∼10–15% of the gland length in the rabbit; 2) a relatively small but important region called the isthmus where the multipotent progeny cells reside; 3) the neck region abundant with mucous neck cells, which are distinct from surface mucous cells and are the transitioning cells destined to become chief cells as they divide and migrate down the length of the gland; and 4) the base region abundant with zymogenic chief cells. Acid-secreting parietal cells are distributed along much of the length of the gastric gland; although they are rare in the pit area they abound in both neck and base regions.
MATERIALS AND METHODS
Reagents.
The monoclonal anti-moesin (O.N.454) antibody and the polyclonal goat anti-pepsin C (I-19) antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). This anti-pepsin C antibody recognizes both pepsin C and pepsinogen C in Western blot and immunostaining. The monoclonal anti-ezrin (4A5) antibody was from Covance (Berkeley, CA). Horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Jackson ImmunoResearch Laboratories, West Grove, PA. Rabbit anti-radixin (R3653) and FITC-labeled phalloidin were from Sigma (P5282). Alexa Fluor 555 and Alexa Fluor 488-conjugated secondary antibodies were products of Invitrogen (Eugene, OR). Alexa Fluor 488-conjugated lectin GSII (from Griffonia simplicifolia) was a product from Invitrogen.
Isolation of gastric glands and parietal cells from rabbits.
All procedures and treatments for handling animals were reviewed and approved by the Berkeley Animal Care and Use Committee. Gastric glands were isolated from New Zealand White rabbits (Oryctolagus cuniculus) by collagenase digestion as previously described (2). Although the majority of the isolated glands are not in full length (both the surface/pit cells and the cells at the bottom of the glands tend to dissociate), there is sufficient amount (∼5% of total) of full-length glands for immunofluorescence studies. Collagenase digestion also yields isolated cells from gastric glands. These cells were cultured for designated time periods on Matrigel-coated coverslips according to the method of Chew et al. (6), except that Nycodenz gradient separation was not performed to enrich parietal cells since all cell types were needed in this study. In some experiments, the freshly isolated cells were immediately attached to poly-l-lysine (P1399, Sigma)-coated coverslips and directly processed for immunofluorescence staining.
Immunoblotting.
Gastric mucosa, kidney cortex, and liver tissue were lysed with SDS-PAGE loading buffer, boiled, and separated on SDS-PAGE. Proteins on the gels were then transferred onto nitrocellulose membranes. Blots were blocked with 5% fat-free milk and probed with anti-ezrin, anti-moesin, or anti-radixin antibodies (1:5,000 dilution for all antibodies). HRP-conjugated secondary antibodies (1:10,000 dilution for all secondary antibodies) were used to reveal antibody specific signals with Western Lightning Chemiluminescence substrate (PerkinElmer Life Sciences, Boston, MA).
Immunofluorescence.
Isolated gastric glands or cells were attached to the poly-l-lysine-coated glass slides, fixed by 3.7% formaldehyde, and permeabilized with 0.5% Triton X-100. Samples were then probed with antibodies [anti-moesin, anti-ezrin, anti-pepsinogen C, and anti-zonula occludens-1 (ZO-1) used at 1:50 dilution]. Afterward, the cells were incubated with fluorescein-conjugated secondary antibodies (used at 1:200 dilution), FITC-phalloidin (1:200), or Alexa Fluor 488-GSII (1:50). Images of Alexa Fluor 555 (excitation with 543-nm laser, emission from 590 to 655 nm) and FITC/Alexa Fluor 488 (excitation with 488-nm laser, emission from 505 to 580 nm) were collected at one Airy unit pinhole with Plan-Neofluar ×40/1.3 oil differential interference contrast objective on a Zeiss LSM 510 Meta confocal microscope. Cells grown on Matrigel-coated coverslips were fixed by 3.7% formaldehyde, permeabilized with 0.1% Triton X-100, and then followed the same procedure as described above.
For tissue sections, rabbit stomach was excised immediately after euthanasia. After removing food, the oxyntic mucosa was cut into small pieces smaller than 5 mm in any dimension for efficient fixation with 3.7% paraformaldehyde. After fixation, tissue was immersed in 30% sucrose and then frozen in Optimal Cutting Temperature media (Sakura Finetek USA, Torrance, CA); 15-mm sections were prepared from these frozen blocks with a cryostat (Leitz 1720 digital Kryostat, Leitz, Germany). These sections were then rehydrated in PBS, permeabilized with 0.5% Triton X-100 in PBS with 1% BSA, and stained with antibodies followed by fluorescent secondary antibodies and other fluorescent reagents as specified in figure legends. Images were collected with a Zeiss LSM 510 Meta confocal microscope with the settings specified above.
RESULTS
Moesin, but not radixin, is expressed in gastric glands.
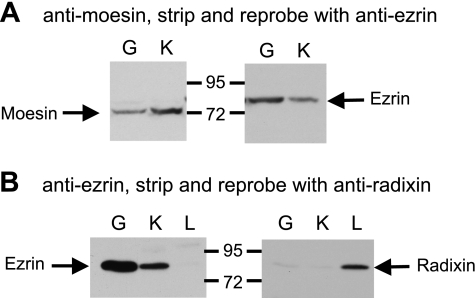
In gastric glands, ezrin is expressed in acid-secreting parietal cells, but not in pepsinogen-secreting chief cells or mucous neck cells (17). Therefore, it was of interest to discover whether the parietal cells also express other ERM proteins and whether chief cells and mucous neck cells express any of the ERM proteins. To approach these questions, lysates made from rabbit gastric glands were analyzed by Western blots with a monoclonal anti-moesin antibody (Fig. 1A). The lysate made from rabbit kidney proximal tubules was used as a positive control since the brush border membranes of the proximal tubules are known to be a rich source of moesin. A band that migrated slightly above the 72-kDa standard was detected in the gastric sample similar to moesin from proximal tubules. Stripping and reprobing with a monoclonal anti-ezrin antibody indicated that the anti-moesin antibody specific band differed from the 80-kDa ezrin band (Fig. 1A).
Fig. 1.
Detection of ezrin and moesin, but not radixin, in rabbit gastric glands. A: gastric glands (G) and kidney cortex (K) were lysed with SDS-PAGE loading buffer, separated on a 10% SDS-PAGE gel, and analyzed by Western blot with anti-moesin antibody. The blot was then stripped and reprobed with anti-ezrin antibody. B: gastric glands, kidney cortex, and liver samples (L) were analyzed by Western blot with anti-ezrin antibody, stripped, and reprobed with anti-radixin antibody.
To look for radixin expression, lysates made from rabbit gastric glands, kidney proximal tubule, and liver were first analyzed by Western blot with anti-ezrin antibody, then stripped and reprobed with a rabbit anti-radixin antibody (Fig. 1B). With the same amount of total protein loaded for each sample, strong ezrin signals were detected in gastric and renal samples, but not in liver samples. Liver is known to be a rich source of radixin. In liver sample, Western blot analysis with anti-radixin antibody revealed a band with the same apparent mass of ezrin (80 kDa). Small negligible signals were also detected with gastric glands and kidney samples.
Cellular localization of moesin is different from ezrin in gastric glands.
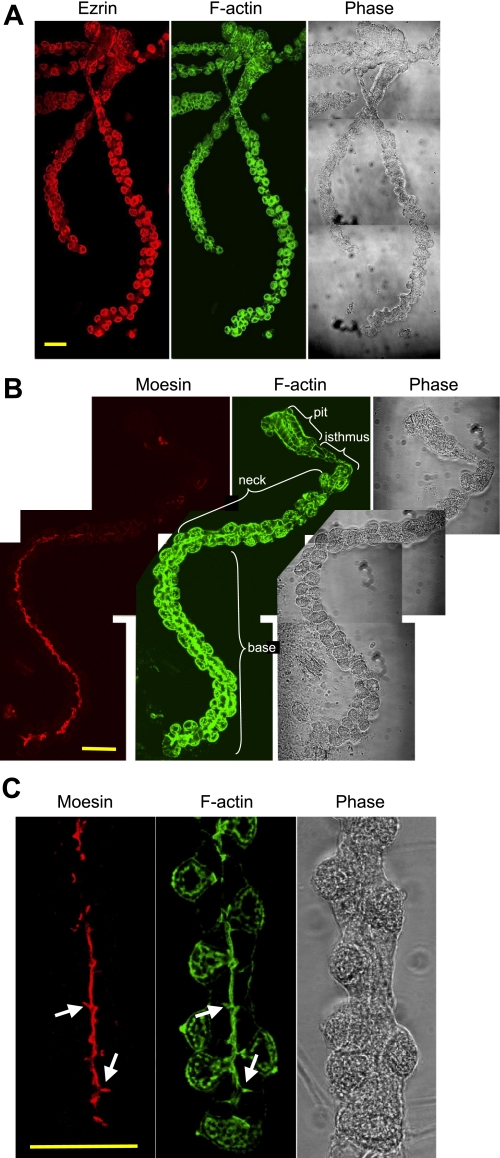
To make sure that the moesin signal was not from a small contamination of endothelial cells and to look for the cellular distribution of moesin in gastric glands, immunofluorescence stainings were performed with the anti-moesin and the anti-ezrin antibodies. Costaining F-actin with fluorescent phalloidin in both cases provided global views of whole gastric glands. The ezrin signal was mainly found in parietal cells, which were bulging cells found mainly at the periphery of the glands (Fig. 2A). Parietal cells from the neck area to the base of the glands showed similar intensity of the ezrin signal. The contour of the surface mucous cells was also stained by the ezrin antibody, with a lower intensity than that of parietal cells. Ezrin signal was not detected in chief cells or mucous neck cells. F-actin was found in all cells, particularly rich at the apical membrane borders of all cells, but also seen to some extent at basolateral membrane surfaces.
Fig. 2.
Distribution of moesin in gastric glands is different from that of ezrin. Gastric glands isolated from rabbit were fixed, permeabilized, and stained with an anti-ezrin antibody (A) or an anti-moesin antibody (B and C). Ezrin and moesin staining was revealed with an Alexa Fluor 555-conjugated secondary antibody. All the glands were also stained with FITC-conjugated phalloidin for F-actin. Images were collected with a confocal microscope. To obtain the complete images of a full-length gland, pictures of different area were taken separately and pieced together with Photoshop software (A and B). In B a single gland is shown with the 4 major anatomical regions noted by brackets. C: a higher magnification is shown for the moesin staining in the base of the gland. Arrows point to the branched lumen of the gland, with moesin staining not connected to any parietal cells. Bar = 50 μm.
Moesin staining of the gastric glands was remarkable for several reasons (Fig. 2B). For example, moesin staining sharply differed from ezrin staining in that most of the moesin signal was found at the apical membrane of cells lining the lumen of the gastric glands, principally expressed in cells lining the base of the glands. Very little moesin staining was observed in the pit or isthmus regions, but frequently some moesin staining was seen at the luminal surface of cells in the lower neck region of the glands. This latter signal was not as intense as in the base but seemed to grow as the cells transitioned from neck to base. Although we could not initially rule out the possibility of moesin expression in the outmost apical membrane of the parietal cells, the majority of the canalicular apical membrane of parietal cells was not stained. An additional feature of moesin staining was a pattern of branching out from the luminal canal, with branches often pointing toward the parietal cells. These “branches” were also stained for F-actin, but they were not likely part of parietal cells as they were also detected at places where parietal cells were absent (Fig. 2C). Thus the moesin staining revealed a novel structural feature of gastric glands, which may be a way of further expanding the surface area of the glandular cell apical membrane.
Localization of moesin on the apical membrane of chief cells, but not parietal cells.
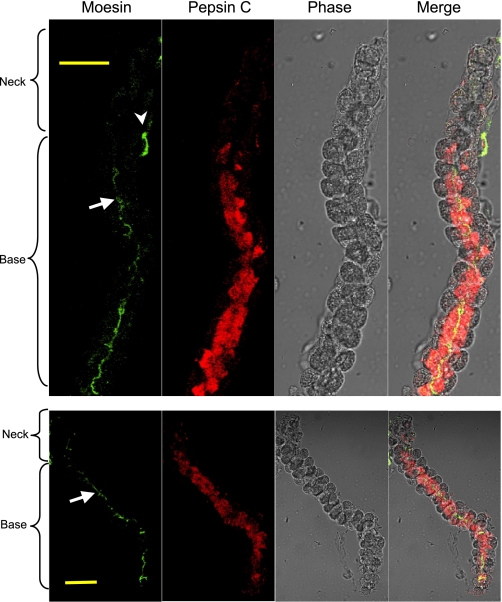
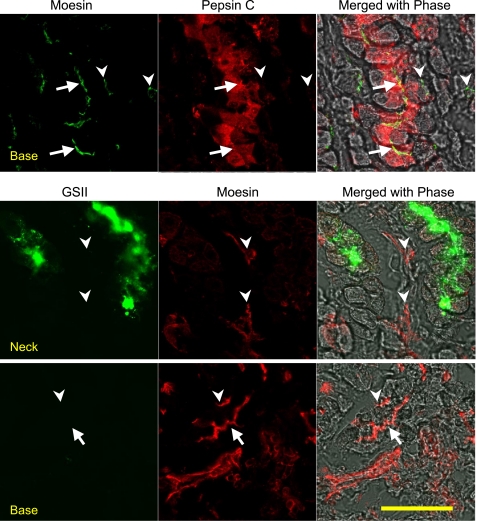
Studies on the development of gastric glands suggested that the stem cells in the isthmus region differentiate into preparietal cells and mucous neck cells, the latter in turn differentiate and mature into chief cells (23). During the course of this maturation, chief cells migrate to the base of the glands. The moesin staining pattern in the gastric glands strongly suggests an association of moesin expression with chief cell function. For direct evidence that chief cells express moesin, gastric glands were costained with anti-moesin and anti-pepsin C antibodies (Fig. 3). Again, moesin was observed on the apical membranes facing the gland lumen and most of the moesin staining was localized to the base of the glands. Pepsin C staining seemed to take a similar pattern. A few cells in the neck area showed low or moderate expression of pepsinogen C. Toward the base of the glands, the expression level of pepsinogen C seemed to be constant. No significant staining was detected in the parietal cells at the periphery of the glands. Although moesin was located on the apical membrane of the chief cells, pepsinogen C was located throughout the cells, consistent with its expected subcellular localizations in rough endoplasmic reticulum (rER), Golgi apparatus, and zymogen granules.
Fig. 3.
Similar cellular distribution of moesin and pepsinogen in gastric glands. Glands were costained with an anti-moesin antibody and an anti-pepsin C antibody to compare the cellular distribution of moesin and pepsinogen C in the glands. An Alexa Fluor 488-conjugated secondary antibody was used for moesin staining, and an Alexa Fluor 555-conjugated secondary antibody was used to stain pepsinogen C. Images were collected with a confocal microscope. Arrows point to the lumen of the gastric gland (note the moesin staining of chief cell apical membrane); arrowhead points to moesin staining in the endothelial cells. Bar = 50 μm.
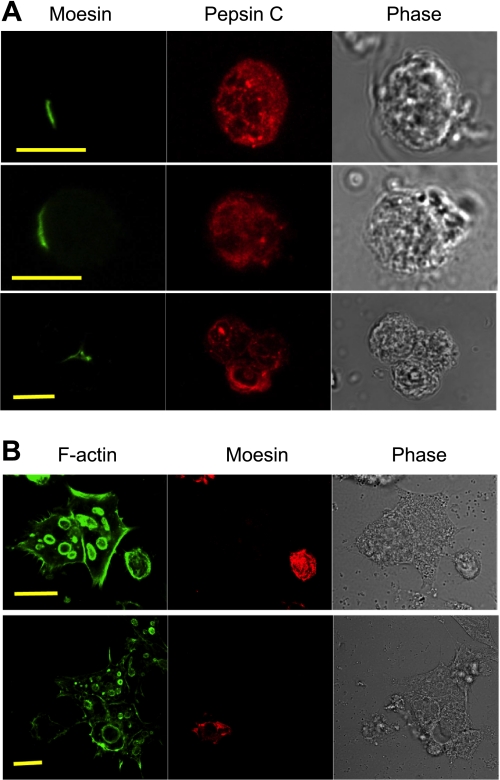
To be certain of this relative position of moesin and pepsinogen C in the chief cells, isolated glandular cells were fixed and immunostained immediately after isolation (Fig. 4A). Punctuate pepsinogen staining suggesting zymogen granules was observed in the chief cells, which also showed weak cloudy staining likely representing pepsinogen within the rER. Moesin staining was nicely enriched at limited areas of the plasma membrane, which we project to correspond to apical plasma membrane patches. In Fig. 4A, bottom, where three chief cells were joining each other surrounding the gland lumen, moesin was clearly located only at the apical membrane junction of these cells.
Fig. 4.
Comparison of freshly isolated glandular cells and cultured glandular cells. A: cells isolated from gastric glands were immediately attached onto poly-l-lysine-coated coverslips before fixation, permeabilization, and immunostaining with the anti-moesin antibody (followed by an Alexa Fluor 488-conjugated secondary antibody) and an anti-pepsin C antibody (followed by an Alexa Fluor 555-conjugated secondary antibody). Bar = 10 μm. B: cells isolated from gastric glands were settled on Matrigel-coated coverslips and cultured for 24 h before fixation, permeabilization, and immunostaining. Cells were stained with the anti-moesin antibody (followed by an Alexa Fluor 555-conjugated secondary) and the FITC-conjugated phalloidin. Bar = 20 μm.
To ascertain whether the moesin staining at the branches of the gastric lumen had some contribution from parietal cells, isolated glandular cells were cultured for 24 h prior to staining for moesin and F-actin. Cultured parietal cells form intracellular F-actin-rich vacuoles, which represent the invaginated apical membrane of these cells. These so-called apical membrane vacuoles and the large size of parietal cells provided convenient ways to locate parietal cells in the mixture of isolated glandular cells. No moesin signal was detected from the isolated parietal cells by immunofluorescence staining with the anti-moesin antibody (Fig. 4B). Instead, moesin staining was often observed in smaller cells not carrying any apical membrane vacuoles (Fig. 4B). These isolated cells underwent significant change in morphology and membrane polarity so that the moesin signal was more broadly distributed compared with cells fixed immediately after isolation (cf. Fig. 4A).
Expression of moesin in mucous neck cells.
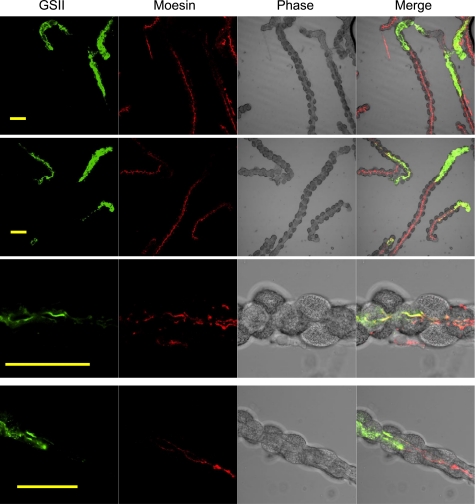
The above results could not exclude the possibility that moesin is also expressed in the mucous neck cells. To look into this possibility, gastric glands were costained with moesin antibody and Alexa Fluor 488-conjugated lectin GSII, which is a well-established marker for mucous neck cells (7, 10). As expected, GSII heavily stained the neck area of gastric glands (Fig. 5, top). Surface cells and parietal cells were not stained. A little GSII staining was found below the neck area, indicating decreased GSII staining in the mucous-to-chief transitional cells as well as chief cells. Chief cells at the more basal of the glands were not stained by GSII lectin.
Fig. 5.
Limited expression of moesin in mucous neck cells. Gastric glands were costained with the anti-moesin antibody and the Alexa Fluor 488-conjugated GSII lectin (from Griffonia simplicifolia) to study the possible distribution of moesin in mucous neck cells in the gastric glands. An Alexa Fluor 555-conjugated secondary antibody was used for moesin staining. Images were collected with a confocal microscope. Top 2 rows show the distinct distributions of GSII signals (mainly staining the neck) and moesin signals (mainly staining the body). Bottom 2 rows show the transitioning area of the gastric glands with overlapping distribution of both GSII signals and moesin signals. Bar = 50 μm.
Similar to previous observation, moesin antibody stained the opposite end of the gastric glands. Although the staining patterns were different between moesin- and GSII-specific probes, these two stainings were obviously overlapping (Fig. 5, bottom). In the lower neck regions, where GSII staining was much lower in intensity than the GSII staining in the upper neck regions, weak moesin staining was detected. Colocalization of these two stainings in the same cells was made obvious by merging all the channels.
Mucous-to-zymogenic transitioning cells.
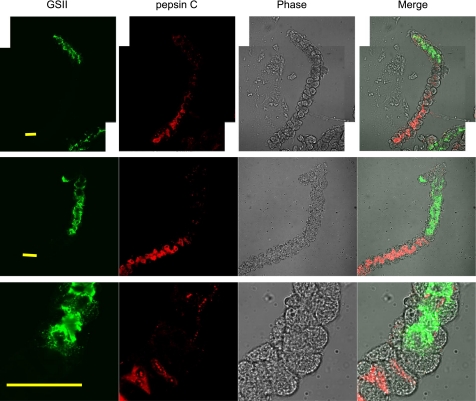
We hypothesize that cells that show both GSII and moesin staining are mucous-to-zymogenic transitioning cells. To seek more direct support for this hypothesis, gastric glands were stained with GSII and anti-pepsin C antibody (Fig. 6). The overall impression is that the GSII lectin stained the neck ends of the glands whereas anti-pepsin C antibody stained the opposite basal ends (Fig. 6, top and middle). Toward the base of the gastric glands, the GSII staining always exhibited a sudden drop whereas the pepsinogen C staining did not increase immediately. Often, the pepsinogen C expression increased slowly toward the base of the glands (Fig. 6, top). Cells exhibiting little GSII staining while the pepsinogen C expression was not yet fully powered are considered to be mucous-to-zymogenic transitioning cells. Occasionally, the transitioning cells seem to be few, as shown in Fig. 6, middle. As shown from the higher magnification of this gland (Fig. 6, bottom), only one cell showed weak staining for both GSII and pepsin C antibody. Interestingly, a small amount of pepsinogen C was found in many of the GSII-positive neck cells, supporting the idea that zymogenic chief cells are derived from mucous neck cells.
Fig. 6.
Cellular distribution of pepsinogen C is similar to that of moesin. Gastric glands were costained with the anti-pepsin C antibody and the Alexa Fluor 488-conjugated GSII lectin to study the cellular distribution of pepsinogen C in the gastric glands. An Alexa Fluor 555-conjugated secondary antibody was used for pepsinogen C staining. Images were collected with a confocal microscope. Top and middle rows show the distinct distributions of GSII signals (mainly staining the neck) and moesin signals (mainly staining the body). In the top row, 2 image fields are superimposed to obtain the full length of a gland. Bottom shows the transitioning area of the gastric glands with overlapping distribution of both GSII signals and pepsinogen C signals. Bar = 50 μm.
Fine organization of gastric glands.
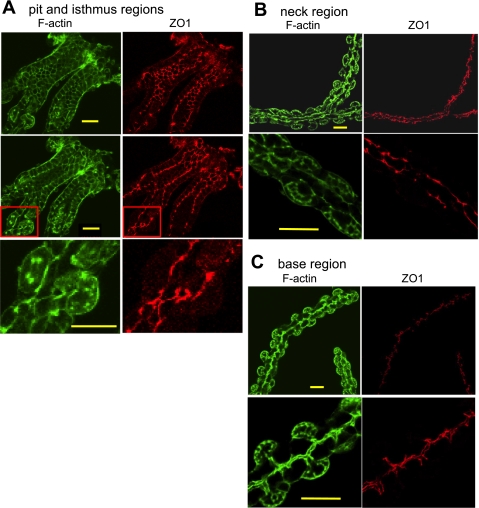
Moesin staining revealed the novel morphology of the gastric gland lumen with many small branches or “spikes.” Because of the absence of moesin at the upper gastric glands, we were prompted to investigate the gastric gland morphology with another marker: antibodies against the tight junction protein ZO-1 (Fig. 7). The pattern of ZO-1 staining of the pit area depended on the level of optical sectioning through the gland. When the optical section was relatively en face to a layer of cells near the apical border, tight junctional staining exhibited a typical chicken-wire pattern surrounding cells lining the gland lumen. When the plane of the image included a cross section running along the pit cells, the staining pattern was typical of tightly aligned columnar epithelial cells. Note that there were a few parietal cells in the pit or isthmus regions, as revealed by their intracellular canalicular apical membranes, but where they occurred these parietal cells formed a typical epithelial cell morphology in that they stand side by side with pit cells (Fig. 7, top). The ZO-1 staining of the upper neck area is similar to that of the pit and isthmus regions: parietal cells and mucous neck cells tend to be lined up along the gland lumen. At the lower neck region and the base of the glands, the ZO-1 staining pattern took on a different form and clearly affirmed the gland lumen structure with short branches projecting from a relatively smooth lumen, becoming even more prominent toward the base of the gland (Fig. 7, bottom). The morphology and location of the short projections is consistent with their constitution by the apical plasma membranes of neighboring chief or mucous neck cell (also consistent with moesin staining). Overall, the ZO-1 staining of the upper part of the gastric glands was different from the bottom part of the glands in two aspects: 1) the gland lumen was wider in the pit region than that in the more basal regions; and 2) the parietal cells (or preparietal cells) in the upper glands were closer to the gland lumen compared with more bloated parietal cells in the lower gland regions, and thus branches were minimal in the upper glands.
Fig. 7.
Zonula occludens-1 (ZO-1) staining reveals novel details on the organization of gastric glands. Gastric glands were stained with an anti-ZO-1 antibody followed by the Alexa Fluor 555-conjugated secondary antibody. The glands were also stained with FITC-conjugated phalloidin for F-actin. Images were collected with a confocal microscope. Different patterns of ZO-1 staining were observed in different gland regions. A: top and middle show pit and isthmus regions at 2 different optical sections separated by 3 μm; bottom shows a region near the isthmus at higher magnification from the area circumscribed by the red square in the middle. B: staining of the neck region shown at low power and higher magnification. C: staining of the base region shown at low power and higher magnification. Bar = 20 μm in all cases.
Immunofluorescence staining of gastric mucosal sections.
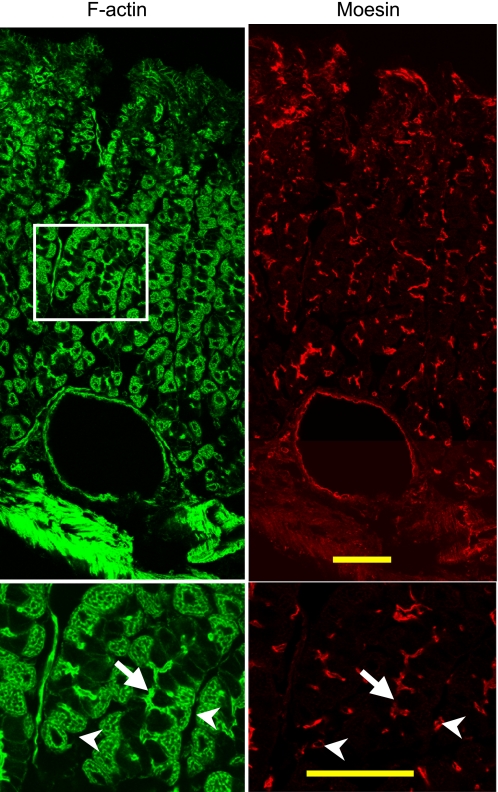
To examine whether the observations described on isolated glands and cells were representative of intact tissue, cryosections of fresh stomach tissue were immunostained with anti-moesin antibody and phalloidin (Fig. 8). Moesin antibody clearly stained the gastric lumen in the base area (Fig. 8, bottom), indicating chief cell apical membrane. In contrast to the staining pattern of isolated glands, the mucosal sections also had strong staining in the surface and neck areas. Careful examination indicates that the majority of the moesin staining in surface/neck area is from capillary endothelial cells (Fig. 8, top). Figure 8, top also showed moesin staining in the endothelial cells of a large venous blood vessel near the serosal surface (bottom of Fig. 8, top).
Fig. 8.
Moesin is detected on both endothelial cells and the luminal surface of cells in the base of gastric glands. Cryosections 15 μm thick were prepared from fresh stomach tissue for immunofluorescence staining with mouse anti-moesin antibody followed by the Alexa Fluor 555-conjugated donkey anti-mouse antibody and FITC-conjugated phalloidin. Bottom: higher magnification of the boxed area in the top. Note the moesin staining of a large blood vessel (close to the muscularis externa) in the top. Arrows point to the lumen of the gastric gland (note the moesin staining of the gland lumen) whereas arrowheads point to moesin staining in the endothelial cells. Bar = 50 μm in all cases.
For a more accurate localization of moesin, stomach sections were costained with the anti-moesin antibody and the anti-pepsin C antibody (Fig. 9, top). Pepsin C-positive cells are mainly found in the base area of the gastric glands (data not shown), as expected. Similar to Fig. 8, moesin was found in both cells lining the lumen of the gastric gland and the endothelial cells. Most, if not all, pepsin C-positive chief cells showed moesin staining. Similar to immunostaining of the isolated glands, the intensity of pepsin C staining varies among chief cells with some cells showing punctate granule staining whereas others showed a more distributed cytosolic staining, suggesting endoplasmic reticulum localization. Moesin was clearly expressed on the apical membranes of chief cells. The apparent impression that some chief cells are negative for moesin staining is because the apical membranes of many chief cells are out of the focal plane since a different focal plane would show moesin staining in different subgroup of chief cells.
Fig. 9.
Stomach sections confirmed the polarized distribution of moesin along gastric glands. Top: cryosections were stained with mouse anti-moesin and goat anti-pepsin C antibodies followed by the Alexa Fluor 488-conjugated donkey anti-mouse and Alexa Fluor 555 donkey anti-goat antibodies. A typical image of base region is shown. Arrows point to the lumen of the gastric gland (note the moesin staining of chief cell apical membrane), and arrowheads point to moesin staining in the endothelial cells. Middle (neck area) and bottom (base area): cryosections were stained with mouse anti-moesin followed by the Alexa Fluor 555-conjugated goat anti-mouse antibody and Alexa Fluor 488-conjugated lectin GSII. In the neck region (middle) arrows point to the lumen of gastric glands (note the moesin staining of chief cell apical membrane), and arrowheads point to the endothelial cells (also note the moesin staining). Note that the moesin staining in the neck area is always from endothelial cells but not from GSII-positive mucous neck cells. In the bottom from the base region there was no GSII staining, but moesin staining is obvious in the gland lumen (very likely chief cells) as well as endothelial cells. The same magnification was used for all the micrographs: bar = 50 μm.
Stomach sections were also costained with anti-moesin antibody and Alexa Fluor 488-conjugated lectin GSII (mucous neck cell marker). GSII staining was bright and specific in the neck area of the gastric glands (data not shown). Although abundant moesin staining was also detected in the upper part of gastric mucosa, careful examination of the staining indicated that those moesin signal was mainly from endothelial cells in the connective tissue between gastric glands (Fig. 9, middle). Weak moesin staining in the transitioning mucous neck cells could be found (data not shown), but not as apparent with the isolated glands. In the lower part of gastric mucosa, where GSII staining was absent, moesin staining was found rich in both chief cells and endothelial cells (Fig. 9, bottom).
DISCUSSION
ERM proteins in the gastric glands.
The results reported here clearly demonstrate the expression of both moesin and ezrin, but not radixin, in gastric glands. Ezrin was mainly expressed in parietal cells, but not in chief cells nor in precursor mucous neck cells. On the other hand, moesin was expressed in mucous neck cells and more heavily in chief cells. Immunostaining of stomach sections confirmed these observations. Considering the common origin of parietal cells and chief cells (7, 23), and the similarities in the structure and function of ERM proteins, this differential expression of ezrin and moesin was a very fascinating observation worthy of further investigation. One possible direction for further study could be the differential role of ERM proteins in the development of the gastric glands because the differential expression of ezrin and moesin occur early in the development of gastric glands: moesin was not detected in the isthmus area or in young parietal cells, nor was ezrin detected in the mucous neck (prechief) cells in the neck area.
Expression of moesin on the apical membrane of gastric chief cells.
This study suggests that moesin is associated with chief cell functions: 1) Moesin was colocalized with pepsinogen C at the base area of the gastric glands. Often, an increasing gradient of moesin expression was observed from the neck area to the bottom of the glands. 2) Moesin is localized exclusively on the apical membrane of the chief cells. 3) Whereas ezrin showed a parietal cell-positive, chief cell-negative staining pattern, moesin showed the opposite staining pattern: parietal cell negative and chief cell positive. Because of its apical membrane location and the well-known function of chief cells to secrete pepsinogen granules, we were initially drawn to an interpretation of moesin acting as a membrane-cytoskeleton support element for pepsinogen secretion. However, an earlier study reported that there was no moesin expression in gastric glands, including chief cells, of the mouse (3). This discrepancy may reflect a species difference in ERM protein expression since these authors were studying ERM proteins in a mouse model whereas the results reported here were conducted with rabbits. Since moesin is absent from the mouse chief cells, and moesin knockout mice did not show any apparent phenotype (9), the function of moesin becomes highly intriguing. Such differences in ERM proteins among different species is an interesting subject for further study.
Novel insights on gastric gland morphology: the lumen of the gastric glands is not a smooth interior.
Gastric glands are epithelial indentations and extensions of the stomach lumen to expand the functional surface of the stomach. Careful analysis of the ZO-1 staining pattern clearly reaffirms the classical view that the glands comprise a single layer of mixed epithelial cells joined by tight junctions. However, the staining of moesin and ZO-1, with those spikes between chief cells and mucous neck cells, indicate that there are secondary mini-invaginations in the organization of gastric glands. The majority of parietal cells have their apical canalicular openings at termini of these secondary indentations, which themselves are formed by the apical membranes of chief cells or mucous neck cells (Fig. 8, bottom). There may be some advantages for this arrangement. For example, the secretory membrane surfaces of chief cells and mucous neck cells are extended without increasing the volume of the gastric glands. Also, one could view the indentations as part of an apical extracellular volume network of the individual parietal cell that most directly includes the complexity and volume of its secretory canaliculi. It is within this unique extracellular compartment that ion transport and ATP-driven exchange can produce the HCl secretory product relatively independent of the streaming bulk flow within the gastric lumen per se (29).
Development of gastric glands.
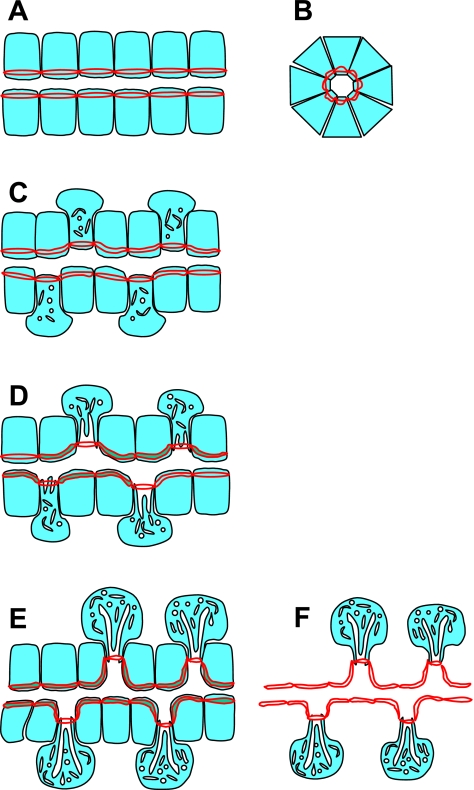
In studying the morphology of the gastric glands using moesin and ZO-1 as markers, we observed different organizations for the upper glands and the lower glands. In the neck area of gastric glands, the lumens of the glands are larger than those of the lower part of the glands, which is expected, since there is a higher trafficking of the gastric secretion close to the opening of the glands. A more interesting difference is that the gland lumens in the pit and the upper neck regions do not have branches or spikes, which are common features of the lower part of the gastric glands. Since all the gastric gland cells are believed to be derived from a common ancestor cell (7, 23), and the gastric cells are migrating from the isthmus area to the other parts of the glands during development (14, 23, 31), we propose a model for the development of gastric glands explaining the morphological differences between the neck region and the base of the glands (Fig. 10). The cells in the isthmus and proximal neck region are in the early stage of development. They are more homogeneous in morphology and cellular activity and take on a uniform organization of typical epithelial cells. As the cells differentiate, those destined for the parietal cell lineage undergo drastic changes in intracellular morphology, volume, and cellular activity. Mitochondria become larger and more numerous, cells become engorged with newly synthesized H-K-ATPase-rich membranes of the tubulovesicular compartment, and secretory canaliculi develop. The increased volume of the parietal cells cannot find space directly along the gastric gland lumen; however, since the cells maintain tight junctional contact with neighbors they find space to expand laterally along the walls of the gland, from whence they were originally named parietal. On the other hand, the differentiation and development of mucous neck cells and chief cells do not involve a large change in cell volume, but these cells continue to multiply and take more of the surface area facing the gland lumen, which may represent a force pushing parietal cells further to the gland periphery. Earlier studies indicated that parietal cells and preparietal cells are nondividing cells, since they do not take in any [3H]thymidine label, whereas mucous neck cells and chief cells are both dividing and take in [3H]thymidine (21). The structural differences between the neck and base of the gastric glands are consistent with the existing model for the development of the glands and provide supportive evidence for the model.
Fig. 10.
Cartoon depicting an hypothesis for morphogenesis of gastric glands. All the cells in gastric glands are derived from stem cells that reside in the isthmus of the gland. The differential patterns for the staining of ZO-1 and moesin in the neck and the body of the glands implicate an interesting course for the continued development of glandular morphology in both space (isthmus to base) and time (shown here as the progressive change from A to D). A and B show respective longitudinal and cross-sectional regions from the isthmus where all cells are undifferentiated. Cytoplasmic matrix of cells is shown in light blue; red lines represent tight junctions with the double red line taking artistic license to indicate staining around the entire periphery of each cell. In early development (C), preparietal cells differentiate from stem cells initiating organellar volume expansion largely projected toward the glandular periphery. With further differentiation (D), parietal cells migrate toward the base of the gland, continuing to grow and expand in the peripheral direction and forming intracellular canaliculi to expand their apical membrane surfaces. This retreat of parietal cells from the lumen of the glands is likely to exert a pulling effect on the gland lumen, making secondary indentations to further expand total glandular luminal secretory surface area (E). The replication, basal migration, and the growth of mucous neck cells and then differentiation into chief cells, in concert with parietal cell development, facilitate the formation of the mature glandular morphology. In F all cells other than parietal cells have been deleted to emphasize the gland luminal structure including the surface indentations projecting toward parietal cells.
In summary, distinct cellular distributions of moesin and ezrin were observed in gastric glands, with moesin specifically located on the apical membrane of pepsinogen secreting chief cells. The increased expression of moesin accompanying the differentiation and development from mucous neck cells to chief cells, and its apical localization, suggested a role for moesin in chief cell function. The moesin staining and the subsequent ZO-1 staining revealed a small secondary invagination in the organization of the gastric epithelium (in addition to the primary invagination representing the glands themselves; and tertiary invagination formed by intracellular canalicular membrane of parietal cells). This structural feature of the gastric glands is also consistent with the pattern of continuous differentiation and development of the various cell types in the glands.
GRANTS
This work was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases, 5RO1DK10141-42.
Supplementary Material
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Algrain M, Turunen O, Vaheri A, Louvard D, Arpin M. Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membrane-cytoskeletal linker. J Cell Biol 120: 129–139, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berglindh T Gastric glands and cells: preparation and in vitro methods. Methods Enzymol 192: 93–107, 1990. [DOI] [PubMed] [Google Scholar]
- 3.Berryman M, Franck Z, Bretscher A. Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J Cell Sci 105: 1025–1043, 1993. [DOI] [PubMed] [Google Scholar]
- 4.Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol 3: 586–599, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Bretscher A, Reczek D, Berryman M. Ezrin: a protein requiring conformational activation to link microfilaments to the plasma membrane in the assembly of cell surface structures. J Cell Sci 110: 3011–3018, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Chew CS, Ljungstrom M, Smolka A, Brown MR. Primary culture of secretagogue-responsive parietal cells from rabbit gastric mucosa. Am J Physiol Gastrointest Liver Physiol 256: G254–G263, 1989. [DOI] [PubMed] [Google Scholar]
- 7.Chew CS, Okamoto CT, Chen X, Qin HY. IQGAPs are differentially expressed and regulated in polarized gastric epithelial cells. Am J Physiol Gastrointest Liver Physiol 288: G376–G387, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Chishti AH, Kim AC, Marfatia SM, Lutchman M, Hanspal M, Jindal H, Liu SC, Low PS, Rouleau GA, Mohandas N, Chasis JA, Conboy JG, Gascard P, Takakuwa Y, Huang SC, Benz EJ Jr, Bretscher A, Fehon RG, Gusella JF, Ramesh V, Solomon F, Marchesi VT, Tsukita S, Tsukita S, Arpin M, Louvard D, Tonks NK, Anderson JM, Fanning AS, Bryant PJ, Woods DF, Hoover KB. The FERM domain: a unique module involved in the linkage of cytoplasmic proteins to the membrane. Trends Biochem Sci 23: 281–282, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Doi Y, Itoh M, Yonemura S, Ishihara S, Takano H, Noda T, Tsukita S. Normal development of mice and unimpaired cell adhesion/cell motility/actin-based cytoskeleton without compensatory up-regulation of ezrin or radixin in moesin gene knockout. J Biol Chem 274: 2315–2321, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Falk P, Roth KA, Gordon JI. Lectins are sensitive tools for defining the differentiation programs of mouse gut epithelial cell lineages. Am J Physiol Gastrointest Liver Physiol 266: G987–G1003, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Fievet B, Louvard D, Arpin M. ERM proteins in epithelial cell organization and functions. Biochim Biophys Acta 1773: 653–660, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Fouassier L, Duan CY, Feranchak AP, Yun CH, Sutherland E, Simon F, Fitz JG, Doctor RB. Ezrin-radixin-moesin-binding phosphoprotein 50 is expressed at the apical membrane of rat liver epithelia. Hepatology 33: 166–176, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Funayama N, Nagafuchi A, Sato N, Tsukita S. Radixin is a novel member of the band 4.1 family. J Cell Biol 115: 1039–1048, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ge YB, Ohmori J, Tsuyama S, Yang DH, Kato K, Miyauchi M, Murata F. Immunocytochemistry and in situ hybridization studies of pepsinogen C-producing cells in developing rat fundic glands. Cell Tissue Res 293: 121–131, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Gobel V, Barrett PL, Hall DH, Fleming JT. Lumen morphogenesis in C. elegans requires the membrane-cytoskeleton linker erm-1. Dev Cell 6: 865–873, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Gorham DA, Bretscher A, Carey HV. Hibernation induces expression of moesin in intestinal epithelial cells. Cryobiology 37: 146–154, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Hanzel D, Reggio H, Bretscher A, Forte JG, Mangeat P. The secretion-stimulated 80K phosphoprotein of parietal cells is ezrin, and has properties of a membrane cytoskeletal linker in the induced apical microvilli. EMBO J 10: 2363–2373, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi K, Yonemura S, Matsui T, Tsukita S. Immunofluorescence detection of ezrin/radixin/moesin (ERM) proteins with their carboxyl-terminal threonine phosphorylated in cultured cells and tissues. J Cell Sci 112: 1149–1158, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Hughes SC, Fehon RG. Understanding ERM proteins—the awesome power of genetics finally brought to bear. Curr Opin Cell Biol 19: 51–56, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Karam SM Dynamics of epithelial cells in the corpus of the mouse stomach. IV. Bidirectional migration of parietal cells ending in their gradual degeneration and loss. Anat Rec 236: 314–332, 1993. [DOI] [PubMed] [Google Scholar]
- 21.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec 236: 259–279, 1993. [DOI] [PubMed] [Google Scholar]
- 22.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. II. Outward migration of pit cells. Anat Rec 236: 280–296, 1993. [DOI] [PubMed] [Google Scholar]
- 23.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec 236: 297–313, 1993. [DOI] [PubMed] [Google Scholar]
- 24.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. V. Behavior of entero-endocrine and caveolated cells: general conclusions on cell kinetics in the oxyntic epithelium. Anat Rec 236: 333–340, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Kikuchi S, Hata M, Fukumoto K, Yamane Y, Matsui T, Tamura A, Yonemura S, Yamagishi H, Keppler D, Tsukita S. Radixin deficiency causes conjugated hyperbilirubinemia with loss of Mrp2 from bile canalicular membranes. Nat Genet 31: 320–325, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Lankes WT, Furthmayr H. Moesin: a member of the protein 4.1-talin-ezrin family of proteins. Proc Natl Acad Sci USA 88: 8297–8301, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, Nance MR, Kulikauskas R, Nyberg K, Fehon R, Karplus PA, Bretscher A, Tesmer JJ. Self-masking in an intact ERM-merlin protein: an active role for the central alpha-helical domain. J Mol Biol 365: 1446–1459, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol 140: 647–657, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okamoto CT, Karvar S, Forte JG. The cell biology of gastric acid secretion. In: Physiology of the Gastrointestinal Tract (4th ed.), edited by Johnson LR. Burlington, MA: Elsevier Academic, 2006, p. 1189–1221.
- 30.Okayama T, Kikuchi S, Ochiai T, Ikoma H, Kubota T, Ichikawa D, Fujiwara H, Okamoto K, Sakakura C, Sonoyama T, Kokuba Y, Doi Y, Tsukita S, Bissell DM, Otsuji E. Attenuated response to liver injury in moesin-deficient mice: Impaired stellate cell migration and decreased fibrosis. Biochim Biophys Acta 1782: 542–548, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Ramsey VG, Doherty JM, Chen CC, Stappenbeck TS, Konieczny SF, Mills JC. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development 134: 211–222, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Saotome I, Curto M, McClatchey AI. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev Cell 6: 855–864, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Sato N, Funayama N, Nagafuchi A, Yonemura S, Tsukita S. A gene family consisting of ezrin, radixin and moesin. Its specific localization at actin filament/plasma membrane association sites. J Cell Sci 103: 131–143, 1992. [DOI] [PubMed] [Google Scholar]
- 34.Tamura A, Kikuchi S, Hata M, Katsuno T, Matsui T, Hayashi H, Suzuki Y, Noda T, Tsukita S. Achlorhydria by ezrin knockdown: defects in the formation/expansion of apical canaliculi in gastric parietal cells. J Cell Biol 169: 21–28, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turunen O, Wahlstrom T, Vaheri A. Ezrin has a COOH-terminal actin-binding site that is conserved in the ezrin protein family. J Cell Biol 126: 1445–1453, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urushidani T, Hanzel DK, Forte JG. Characterization of an 80-kDa phosphoprotein involved in parietal cell stimulation. Am J Physiol Gastrointest Liver Physiol 256: G1070–G1081, 1989. [DOI] [PubMed] [Google Scholar]
- 37.Van Furden D, Johnson K, Segbert C, Bossinger O. The C. elegans ezrin-radixin-moesin protein ERM-1 is necessary for apical junction remodelling and tubulogenesis in the intestine. Dev Biol 272: 262–276, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Zhu L, Zhou R, Mettler S, Wu T, Abbas A, Delaney J, Forte JG. High turnover of ezrin T567 phosphorylation: conformation, activity, and cellular function. Am J Physiol Cell Physiol 293: C874–C884, 2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.