Abstract
The third meeting of the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) was focused on selecting promising measures for each of the cognitive constructs selected in the first CNTRICS meeting. In the domain of executive control, the 2 constructs of interest were “rule generation and selection” and “dynamic adjustments in control.” CNTRICS received 4 task nominations for each of these constructs, and the breakout group for executive control evaluated the degree to which each of these tasks met prespecified criteria. For rule generation and selection, the breakout group for executive control recommended the intradimensional/extradimensional shift task and the switching Stroop for translation for use in clinical trial contexts in schizophrenia research. For dynamic adjustments in control, the breakout group recommended conflict and error adaptation in the Stroop and the stop signal task for translation for use in clinical trials. This article describes the ways in which each of these tasks met the criteria used by the breakout group to recommend tasks for further development.
Keywords: cognition, clinical trials, schizophrenia
Deficits in executive control have long been thought to be one of the hallmark cognitive characteristics of schizophrenia. Executive control processes are those mechanisms that allow individuals to flexibly and dynamically adjust their performance in response to changing environmental demands and changing internal goal states. Numerous empirical studies have shown that individuals with schizophrenia show deficits on tasks designed to measure such executive control processes.1 Deficits on such executive control tasks are associated with both negative and disorganized symptoms in schizophrenia and those at risk for schizoprenia,2–4 and deficits in executive control are associated with poor functional outcome.5
Given the prominence of executive control deficits in schizophrenia, it is not surprising that this was one of the cognitive domains upon which both the MATRICS and Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) initiatives have focused. As described by Kerns et al,6 a great deal of basic research in cognitive neuroscience has focused on elucidating the cognitive and neurobiological mechanisms that provide humans with the ability to flexibly shift and adapt their behavior over time and over different internal and external contingencies. As part of the first CNTRICS meeting, it was suggested that 2 components of executive control were ready for immediate translation for development and use in clinical trials in schizophrenia: (1) “rule generation and selection” and (2) “dynamic adjustments in control.” Rule generation and selection was defined as “the processes involved in activating task-related goals or rules based on endogenous or exogenous cues, actively representing them in a highly accessible form, and maintaining this information over an interval during which that information is needed to bias and constrain attention and response selection.7”
Dynamic adjustments in control were defined as “the processes involved in detecting the occurrence of conflict or errors in ongoing processing, identifying the type of control adjustments needed, and recruiting additional control processes.7”
The goal of the third CNTRICS meeting was to solicit and evaluate nominations for promising tasks that would measure each of the constructs selected in the first CNTRICS meeting (see Carter and Barch8 for a full list of all the constructs), including the 2 constructs selected under executive control. Nominations were solicited from both basic and clinical scientists, from both academia and industry. The nominators were asked to provide a description of the task and to provide information on 6 domains relevant to selecting the most promising tasks: (1) cognitive construct validity; (2) neural construct validity; (3) pharmacological sensitivity; (4) reliability; (5) other psychometric characteristics; and (6) the availability of animal models. The overview article at the beginning of this special section outlines why and how these specific criteria were selected and discusses the ways in which these criteria were used to select among tasks. In addition, the overview article describes additional criteria that could be used to adjudicate between tasks that appeared similar on the properties described above, such as evidence for impairment in schizophrenia. However, importantly, evidence for impairment in schizophrenia was not considered a requisite feature so as to facilitate the inclusion of potentially promising paradigms that have yet to be studied in schizophrenia. The goal of the current article is to provide a brief summary of the tasks that were nominated, the reasons as to why some tasks were not selected as “recommended for immediate translation” and to provide an overview of the data used to select the tasks. This overview is not meant to be an exhaustive review of the literature on each task, but rather to give the reader a starting point for understanding what each task is, how it is administered, why it was chosen, what the evidence is for construct and neural validity, what challenges the task faces in the translation process, and pointers to the primary literature for further consideration.
Rule Generation and Selection
CNTRICS received 4 initial nominations for the rule generation and selection construct: (1) the 1-2 AX-CPT; (2) the Groton Maze learning test; (3) CANTAB intradimensional/extradimensional (ID/ED) task; and (4) the switching Stroop. The executive control breakout group was tasked with the need to evaluate these nominations. Following animated discussion, the group decided that 2 of these tasks (CANTAB ID/ED and the switching Stroop) should be nominated for further development. Table 1 provides a brief overview of these 4 tasks and a very brief summary of their pros and cons in regards to the selection criteria.
Table 1.
Description of Tasks Nominated for Rule Generation and Selection Construct Definition: The Processes Involved in Activating Task-Related Goals or Rules Based on Endogenous or Exogenous Cues, Actively Representing Them in a Highly Accessible Form, and Maintaining This Information Over an Interval During Which That Information Is Needed to Bias and Constrain Attention and Response Selection7
| Tasks Recommended For Translation |
| CANTAB ID/ED task |
| Construct validity |
| Supported by both human and animal research |
| Designed to decompose the WCST |
| Neural systems |
| Evidence for role of ventro- and dorslolateral prefrontal cortex in extradimensional shifting (rule selection) from human and animal research |
| Evidence for role of dopamine and norepinephrine in extradimensional shifting (rule selection) from human and animal research |
| Pharmacological sensitivity |
| Numerous studies in humans and animals showing that ID/ED performance is sensitive to a variety of pharmacological manipulations |
| Evidence that modafinal can improve ID/ED performance in schizophrenia |
| Animal models |
| Animal models available for rhesus monkey, marmoset, and rat |
| Performance in schizophrenia |
| Consistent evidence for impairments in extradimensional shifting |
| Mixed evidence for impairments in intradimensional shifting |
| Psychometric data |
| Low test-retest reliability |
| Practice effects influenced by insight and learning on task over time |
| Potential confounds of generalized deficits with differences in discriminating power across conditions |
| Future directions |
| Modifications needed to improve test-retest reliability and to reduce practice effects |
| Evaluation of discriminating power across conditions is needed. If not equal, modifications will be needed to improve ability to detect differential deficits |
| Switching stroop |
| Construct validity |
| Supported by computational modeling and individual differences research |
| Is a good measure of rule selection, but not of rule generation |
| Neural systems |
| Evidence from functional neuroimaging on the importance of DLPFC and ACC |
| Evidence from lesions studies for importance of DLPFC |
| Pharmacological sensitivity |
| Evidence that dopamine augmentation can improve Stroop performance in schizophrenia, but may not be specific to rule selection |
| Animal models |
| There are rat and primate models of paradigms described as measuring similar constructs |
| The degree to which the animal models (especially the rat models) are homologous to the human paradigm is an open question |
| Performance in schizophrenia |
| Evidence for deficits in schizophrenia in the ability to select and apply the appropriate cue on the switching Stroop |
| Evidence for reduced DLPFC and ACC activity in schizophrenia during the performance of incongruent Stroop trials |
| Psychometric data |
| Little psychometric data available on test-rest reliability, practice effects, or floor/ceiling effects |
| Future directions |
| Research needed on assessing and improving the psychometric characteristics of the switching Stroop |
| Research needed on ways to make the use of the Switch Stroop feasible in clinical trials contexts (eg, task length, standardized administration, enhanced sensitivity to detect deficits, etc.) |
| Evaluation of discriminating power across conditions is needed. If not equal, modifications will be needed to improve ability to detect differential deficits |
| Evaluation of the homology of putative animal models and development of better animal models if needed |
| Other nominated tasks |
| 1-2 AX-CPT |
| Construct validity |
| Task modeled in a computational model of prefrontal-basal ganglia contributions to working memory |
| Neural systems |
| No published data |
| Pharmacological sensitivity |
| No published data |
| Animal models |
| No published data |
| Performance in schizophrenia |
| No published data |
| Psychometric data |
| No published data |
| Groton maze learning task |
| Construct validity |
| Not clear that the task selectively measures rule selection or generation as performance may be strongly influenced by the ability to learn from feedback and visual spatial processing abilities |
| Neural systems |
| Evidence that performance can elicit feedback-related error negatives in ERP studies9 |
| Pharmacological sensitivity |
| Evidence that speed and efficiency of performance can be improved10 |
| Animal models |
| Cincinnati Water Maze test in rodents may be an animal homologue11 |
| Performance in schizophrenia |
| Evidence for excessive rule-breaking errors in schizophrenia12 |
| Psychometric data |
| Preliminary evidence of good psychometric characteristics13 |
Tasks not Selected as Recommended for Development
In the 1-2 AX-CPT,14 stimuli are presented one at a time in a sequence and subjects must press one button for a target and another for a nontarget. The stimuli are letters, and subjects are told to look for specific target sequences (eg, A followed by an X). Interleaved with the letters are the numbers “1” or “2.” These numbers instruct the subject to focus on one or another target sequence. For example, if the most recent number was a 1, the target sequence would be an A followed by an X, but if it had been a 2, the target sequence would be a B followed by a Y. This task is designed to test the subject's ability to generate or select the appropriate rule for responding based on the previously presented number. Performance on this task has been modeled in a neural network model of frontal-basal ganglia interactions and their contribution to working memory.14 The breakout group felt that the 1-2 AX-CPT was an interesting and promising task but that it needed more research at both the basic and clinical levels before it was ready for translation. Although there is encouraging pilot imaging work with this task, and pilot work in patients with schizophrenia, neither of these have been published yet.
The Groton Maze learning test15–17 is one in which subjects must learn a series of hidden mazes based on feedback. Although the breakout group also felt that this was an interesting task, the group felt that it was not clear that it was a specific measure of the construct of interest (rule generation and selection) as poor performance on the Groton Maze learning test could reflect a number of different factors in addition to rule generation and selection, including problems learning from feedback or spatial processing difficulties.
Tasks Recommended for Immediate Translation for Rule Generation and Selection CANTAB ID/ED Task
Description
The CANTAB intra/extradimensional set shift is implemented on a computer-controlled touch screen and takes approximately 7 min to administer, depending on the level of impairment of the individual performing the task (see also www.camcog.com for description and screen shot figure). Two artificial visual perceptual dimensions are used in the test: (1) color-filled shapes and (2) white lines. The simple stimuli in the task are made up of just one of these perceptual dimensions, whereas compound stimuli are made up of both, namely white lines overlying color-filled shapes (see stimuli on www.camcog.com Web site). The subject starts by seeing 2 simple color-filled shapes (presented in 2 of 4 locations on the screen, randomly on each trial) and must learn which one is correct by touching it (simple discrimination stage: SD). Subjects progress through the test by satisfying a set criterion of learning at each stage (6 consecutive correct responses). If at any stage the subject fails to reach this criterion after 50 trials, the test terminates. Computer-provided feedback teaches the subject which stimulus is correct, and after 6 correct responses for the initial simple discrimination stage, there is a reversal (simple discrimination reversal stage: SDR). Following acquisition of this reversal stage (which teaches the subject that different stimuli within a perceptual dimension are relevant to the rule), the subject is asked to complete compound discriminations in which each stimulus now has 2 potentially relevant perceptual features (lines and color-filled shapes), initially with spatially separated (C-D stage) and then superimposed (CD stage) stimuli. The subject then must perform a reversal on the compound discrimination stimuli (compound discrimination reversal: CDR stage) before the stimulus exemplars change for an intradimensional shift. In the intradimensional shift stage, the same dimension of the stimulus remains relevant (eg, lines or shapes), but the specific feature within the dimension that is the target changes (eg, which colored shape). This stage is designed to reinforce perceptual dimension learning and to provide a control for the crucial extradimensional shift stage. For the initial intradimensional shift (IDS stage), color-filled shapes remain the only relevant dimension, then later after intradimensional shift reversal (IDR stage), again there are new stimuli/exemplars for the extradimensional shift (EDS state) in which the white lines become the only relevant dimension. This is followed by the extradimensional reversal stage (EDR), in which subjects must shift between dimensions. There are thus 9 stages in all, designated SD, SDR, C-D, CD, CDR, IDS, IDR, EDS, and EDR. The task has 2 modes: a clinical mode for testing only once and 7 parallel modes for repeated testing. The task has 18 outcome measures, assessing errors, and numbers of trials and stages completed.
From extensive experience with the task, the developers have a number of recommendations for optimal task use. First, although it is feasible to begin the task with lines and shift to shapes, it is recommended that the initial learning be fixed at shape. Second, they recommend, time permitting, that the subjects are screened with a short instructional test (Big circle/little circle discrimination) which tests the subjects’ ability to understand what a rule is and to be able to reverse it on command. Third, there is a fixed rubric for the verbal instructions for this test which are minimal but which should be followed closely.
Construct Validity
The theoretical validity of the test was established over 30 years ago and stems from early animal and human learning theory on discrimination learning, reversal, and dimensional shifting. More recent computational approaches have confirmed for example that reversal learning and extradimensional shifting necessarily engage different processes and have also considered the paradigm in the context of a theory of frontal lobe function based on working memory.18 The CANTAB test was designed partly to decompose the well-known Wisconsin Card Sort test (WCST) into its constituent parts including a critical extradimensional shift stage that is probably equivalent to a category shift on the WCST. The initial validation study demonstrating cross-species advantage for IDS over EDS performance in humans and marmosets was by Roberts et al.19 There is an extensive and growing bibliography of the ID/ED task, and the reader is referred to www.camcog.com for references and abstracts. The following constitutes a brief overview.
Neural Systems
The neural substrates of the test (or similar variants of it, with changes in the stimuli but not the basic principles of test design) have been defined on the basis of human neuropsychological data in brain damaged patients, functional imaging data (both functional magnetic resonance imaging [fMRI] and positron emission tomography),20,21 and animal (monkey, rat, mouse) lesion studies. There is converging evidence that reversal learning implicates orbitofrontal cortex function in humans, monkeys, and rats.20,22–27 There is also additional evidence for ventromedial striatal and posterior parietal involvement.20,21,28 There is considerable evidence for a role for the primate ventrolateral prefrontal cortex in extradimensional shift learning20,22 as well as other posterior cortical (posterior parietal, temporal) areas. The neuroanatomical dissociation between EDS and reversal learning in marmoset also holds for the rat.29 The precise substrates of discrimination learning and intradimensional shift learning are less clear, but see Rogers et al21 for some initial observations.
Pharmacological and Behavioral Manipulation of Task Performance
In humans, there is evidence for double dissociation in effects of indoleamine (serotonin, 5-HT) and catecholamine (dopamine, DA), noradrenaline (NA) manipulations on reversal learning, and extradimensional shifting, respectively, in the ID/ED task. Thus, tryptophan depletion (implicating 5-HT) selectively impairs reversal learning in humans, whereas the catecholamine agent methylphenidate improves extradimensional shifting.30,31 Noradrenergic manipulations appear particularly sensitive to human extradimensional shifting performance.32 There is only weak evidence of effects on extradimensional shifting of DA receptor antagonism33 or L-Dopa medication (in Parkinson's disease).34
In animals, as in humans, there is considerable evidence for an involvement of 5-HT in reversal learning. In marmosets, selective depletion of 5-HT (and not DA) in the orbitofrontal cortex impairs reversal learning.24–26 Orbitofrontal 5-HT is also implicated in reversal learning in the rat.35 In monkeys, DA depletion impairs intradimensional shifting but not reversal or extradimensional shifting (which may even be artifactually improved in certain instances).36,37 In rats, there is evidence that the noradrenergic system is implicated specifically in the extradimensional shifting.38,39 However, there is some evidence in the rat and mouse of involvement of prefrontal cortex (PFC) DA in extradimensional shifting (based on genetic studies with COMT polymorphisms40).
In addition, there are, approximately 30 studies of pharmacological effects of drugs, including many candidates for the medication of cognitive deficits in schizophrenia on intradimensional and extradimensional shifting in the rat model to be described below. These drugs include the following: 5-HT6 receptor antagonist, NA agonist, alpha-7 subunit nicotinic agonist, D1 receptor agonist, PDE inhibitors, and modafinil. Many of these compounds selectively improve extradimensional shifting performance, which may be most closely tied to rule generation and selection. There are also selective demonstrations of effects of stress on extradimensional shifting performance and neuronal morphology in the medial PFC in rats.41
Animal Models
There are number of different animal models of ID/ED task performance that have been developed. For example, there is a paradigm for rhesus monkeys,42 and there have been a number of studies of ID/ED performance in marmosets.19,22 In addition, Birrell and Brown established a rat model based on textures and odors rather than visual stimuli.29 This has been used to establish the neuroanatomical substrates of the task in rats (see above) and for pharmacological studies. It has also been used for models of schizophrenia (eg, chronic PCP treatment, amphetamine sensitization, isolation rearing, MAM pretreatment). A typical study has shown benefit of a PDE inhibitor or modafinil against deficits produced by PCP at the extradimensional shift stage that are not remediated by conventional antipsychotic DA receptor antagonists.43
Performance in Schizophrenia
There have been over 20 studies so far listed in the CANTAB bibliography on the ID/ED test in schizophrenia, beginning with Elliott et al.44,45 These studies have been not only in chronic schizophrenia46,47 but also in first-episode psychosis.48,49 Comparisons with other disorders have included comparisons with bipolar disorder and depression, Alzheimer's, Parkinson's, and Huntington's diseases, OCD and ADHD, neurosurgical patients with damage to the frontal and temporal lobes, as well as amygdalo-hippocampectomy (see CANTAB Web site). An explicit comparison of chronic schizophrenia with frontal and temporal lobe lesions was published by Pantelis et al.46 That study showed that people with chronic schizophrenia were severely impaired at both the intradimensional and extradimensional shifting stages, compared with both patients with lateral PFC damage (who were mainly impaired at the extradimensional shifting stage) and temporal lobe lesioned patients (who did not differ from age- and IQ-matched controls). Other studies have shown that people with chronic schizophrenia can also fail at the simple reversal stage or compound discrimination stages.44,50 Jazbec et al provided one of the first direct comparisons of ID/ED performance and the WCST in schizophrenia, with significant correlations at the extradimensional shift stage.50
In terms of pharmacological treatment, Turner et al47 showed, in a double-blind placebo-controlled proof of concept study, that acutely administered modafinil added to conventional antipsychotic medication in schizophrenia improved several aspects of performance including the ID/ED test. This “proof-of-concept” study demonstrated that it was indeed plausible to improve aspects of cognitive function in schizophrenia and was subsequently ‘back translated’ to show improvement in the rat plus PCP model.43
Psychometric Data
Standardized data on 341 elderly subjects were published in Robbins et al.51 Performance on the ID/ED test declined quiet sharply in the eldest cohort (>70) especially at the extradimensional shift stage.51 There are limited data on test-retest reliability; Lowe and Rabbitt found quite low (r = 0.4) test-retest correlations for some variables of the ID/ED test in normal volunteers.52 This is not surprising in view of the fact that the WCST has very low test-retest reliability. Thus, significant developmental work is clearly needed to determine how reliability on this test might be improved and what reliability is like in patient groups. In general, this class of tests does depend on insight and/or procedural learning or other forms of practice effects derived from previous experience of the tests, and between-group comparisons are recommended for pharmacological studies to alleviate this problem (as compared with within subject designs that require multiple testing sessions per person). The use of parallel versions of the task may help with this concern but may not entirely reduce the potentially long-acting influence of insight into the basic nature and structure of the task. In addition, the various conditions on the task are not matched for discriminating power, with the conditions that are often most impaired in schizophrenia likely those with the greatest discriminating power (eg, extradimensional shift stages). Thus, work is needed to determine whether greater impairments at the extradimensional shift stage in schizophrenia reflect a specific impairment in generating and/or selecting rules or a generalized deficit that interacts with the psychometric characteristics of this stage of the task.
Future Directions
More analysis of the neural and neurochemical substrates of performance is needed in both humans and animal subjects. Several specific issues require resolution or consolidation, as indicated above. These can be pursued using functional neuroimaging approaches in humans and lesions or neuropharmacological manipulations in experimental animals. Longitudinal studies are also required for schizophrenia, and other studies of pharmacological effects, though proof of concept that ID/ED performance can be enhanced has already been demonstrated. In psychometric terms, more data are required. The test may have to be modified to provide good test-retest reliability. For example, the fixed sequence of testing of the component processes ID shifting and ED shifting may need to be replaced by a version of the test that interleaves the 2 forms of shifting. In addition, research is needed to determine whether deficits on specific stages of the task reflect differential deficits or generalized deficits that reflect the greater discriminating power of the ED compared with the ID, CD, or SD conditions. If ED conditions indeed have greater discriminating power, the test will need to be modified to either match the ID and ED measures on discriminating power or introduce another control (eg, one in which set shifting deficits convey a performance advantage).
The Switching Stroop
Description
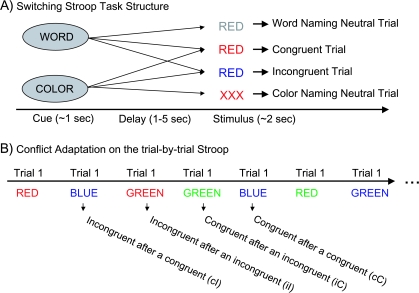
The “switching Stroop” is a variation on the classic Stroop task. It was nominated as a measure of the rule generation and selection construct as an example of an asymmetric task-switching paradigm. An asymmetric paradigm is one in which one of the task involves a more prepotent dimensions than another. In such task-switching paradigms, subjects must use the cue to generate and/or select the appropriate rule to govern response selection for the upcoming stimulus. Thus, although this description will focus on the Stroop specifically, it is possible that other asymmetric task-switching paradigms may be just as promising. In the classic Stroop,53 participants are presented with stimuli that vary on 2 dimensions (eg, color words written in different colors). Participants are told to respond to one of the dimensions and to ignore the other. Typically, one of the dimensions is more “prepotent” (ie, word reading), making it more difficult for participants to ignore that dimension and leading to more interference from the prepotent dimension when trying to attend to and respond based on the less dominant dimension (eg, color naming). This typically leads to slower responding and more errors when participants are told to respond on the basis of the less dominant dimension.54 To perform the task correctly, especially when responding to the less prepotent dimension, participants need to use task instructions to select the appropriate rule for which stimulus dimension to use for responding. However, standard versions of this task do not sensitively probe the selection or maintenance of rule information. This is because the task is typically kept constant across blocks of trials, so that each trial reinforces the correct response dimension and thus the correct rule. To make the task more sensitive to the ability to select and maintain the appropriate rule, one can vary the task (ie, the dimension to be responded to—word or color) on a trial-by-trial basis55 (see figure 1). In such “switching” versions of the Stroop, the subject is cued to the relevant dimension (rule) prior to each trial. This can be done either via an auditory cue or a visual cue. In addition, one can vary the delay (1 vs 5 s) between the task cue and the stimulus. This allows one to assess whether rule maintenance is problematic by comparing performance over a longer rule maintenance delay vs a shorter rule maintenance delay.
Fig. 1.
(A) Illustration of the Structure of the switching Stroop Task. The cue is either to “color” or “word” read and then the stimulus is congruent, neutral, or incongruent. (B) An illustration of trial structure for computing conflict adaptation effects in the trial-by-trial Stroop.
In most versions of the Stroop, including the switching Stroop, experimenters typically include 3 types of conditions (see figure 1): (1) congruent, in which the 2 dimensions point to the same response (eg, the word “red” written in red); (2) neutral, in which response relevant information is present in only one dimension (eg, a row of Xs printed in red—see Barch and Carter for discussion of factors influencing choice of neutral condition56); and (3) incongruent, in which the 2 dimensions point to different responses (eg, the word red written in blue). In addition, performance on most versions of the Stroop can be measured in terms of either reaction times or errors. Experimenters typically look at one of 3 variables: (1) total Stroop effect (performance in the incongruent condition—performance in the congruent condition); (2) interference (performance in the incongruent condition—performance in the neutral condition); or (3) facilitation (performance in the congruent condition—performance in the neutral condition).
Construct Validity
There is a long history of research on standard versions of the Stroop task that provides evidence for the construct validity of this task as a measure of the ability of an individual to select and apply the appropriate rule for response selection. For example, there is a good deal of computational modeling work demonstrating that performance on the Stroop task across many experimental manipulations, including switching manipulations, can be accounted for by mechanisms that provide for the active selection and maintenance of the appropriate task rule.57,58 Such mechanisms have been implemented in connectionist interactive activation models and have been used to make predictions about specific patterns of fMRI data.58,59 These models have typically assumed that such task rule representations are stored in prefrontal cortex, maintained through recurrent connections that provide “top-down” support for lateral inhibition mechanisms in relevant processing pathways that allow the less dominant pathway (eg, color naming) to compete successfully with the more dominant pathway (eg, word naming). In addition, there are data suggesting that individual differences in the ability to actively maintain representations in working memory predicts individual differences in the ability to select and effect use the appropriate task rule during Stroop task performance.60 As with the computational modeling work, these data have been interpreted as reflecting an important role for prefrontally maintained representations in successful Stroop performance. One thing to note with the switching Stroop is that while it is a good measure of the ability to “select” task rules, it is not a good measure of the ability to “generate” rules as the experimenter provides the rule. In addition, there may be some confounding of task-switching demands with rule selection demands in the switching Stroop, given that RTs could also reflect the time it takes to switch from one rule to another (eg, color vs word), as well as the ability to maintain and apply the appropriate rule. Thus, care would need to be taken to dissociate these effects in future work with the switching Stroop.
Neural Systems
There have been only a handful of studies using functional imaging to examine the neural substrates of performance on the switching Stroop. However, these studies have been particularly informative as to the specific roles played by dorsolateral prefrontal cortex (DLPFC) vs anterior cingulate cortex (ACC) in task performance. Specifically, these studies have demonstrated that cue-related activity in DLPFC was enhanced on trials in which the participants were cued to color name as compared with word read, consistent with the hypothesis that greater top-down control to support rule representation is needed on color-naming trials.61,62 In contrast, cue-related anterior cingulate (ACC) activity did not vary as a function of task, but ACC activity was greater on incongruent relative to congruent trials, consistent with a role of for ACC in monitoring for competition during responding.61
Research has also examined the degree to which lesions to the frontal cortex impair performance on standard versions of the Stroop task, though to our knowledge no such studies have been conducted with switching Stroop tasks. Although the evidence in this regard is mixed, some meta-analytic work suggests that frontal lesions are reliably associated with impaired performance on the Stroop task,63 though it is not clear that such deficits are isolated to conditions in which rule selection and maintenance are most critical (eg, incongruent conditions or color naming specifically).63
Pharmacological and Behavioral Manipulation of Task Performance
A number of different variations on the Stroop task have been used in a range of pharmacological and behavioral manipulation studies. Of most relevance to the current topic are those that involved individuals with schizophrenia. For example, work by Barch and Carter demonstrated that low doses of amphetamine in patients taking stable doses of typical antipsychotics improve reaction times on a standard Stroop task,64 though this improvement was found in all conditions, not just the incongruent condition. In addition, research has shown that nicotine administration can improve Stroop interference, with evidence of greater improvements in individuals with schizophrenia than in controls.65 Thus, prior research indicates that Stroop performance is amenable to change with pharmacological interventions in individuals with schizophrenia. However, to our knowledge, research has not yet examined the amenability of switching Stroop performance specifically to change via either pharmacological or behavioral manipulations.
Animal Models
A number of paradigms for use in animals have been developed that are designed to capture the type of response competition that requires the type of appropriate rule selection required by the switching Stroop in humans. For example, work in rats has focused on biconditional discrimination tasks that require animals to use contextual information to modify responses to specific stimuli.66–68 In addition, paradigms have been developed for monkeys that are designed to mimic critical features of the Stroop task, including the need to selectively attend to one dimension of stimulus and the inclusion of different feature dimensions that provide conflicting response information.65,69,70 The degree of homology between the cognitive and neural mechanisms engaged by these paradigms in animals vs humans needs further investigation. However, these studies provide encouraging evidence of the ability to model “Stroop-like” task performance in animals.
Performance in Schizophrenia
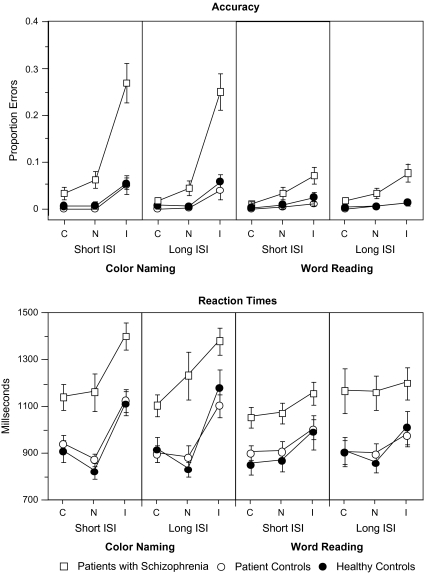
At least one study has specifically examined switching Stroop performance in patients with schiophrenia.55 This work used a version of the switching Stroop in which: (1) an auditory cue signaled whether participants should word read or color name, (2) there were 2 different delays between the cue and the onset of the stimulus (1 vs 5 s); and (3) the neutral stimuli for color naming were rows of Xs. The schizophrenia patients showed disproportionate interference in errors compared with controls for color naming as well as a disproportionate total Stroop effect in reaction times compared with controls for color naming (see figure 2). These effects did not interact with delays, suggesting that the magnitude of the impairments was similar for both short and long delays (see figure 2). Functional imaging work with the Stroop has consistently revealed deficits in the activation of DLPFC and ACC in schizophrenia. For example, Kerns et al71 found reduced anterior cingulate activity during incongruent Stroop trials in schizophrenia, Weiss et al72 found underactivation of DLPFC and anterior cingulate in schizophrenia during performance of a Stroop task, and Yucel et al73 found underactivation of paracingulate cortex in schizophrenia. These studies did not use switching Stroop tasks specifically and instead used standard Stroop versions. However, standard Stroop versions do still assess the ability to select and apply the appropriate rule, though they may just not be as sensitive to this process as the switching Stroop. Deficits on the Stroop task are also present in the relatives of individuals with schizophrenia, suggesting that they are associated with vulnerability to the disorder.74,75 In addition, recent work has shown reduced DLPFC activity during performance of the Stroop in unaffected first-degree relatives of patients, though intact ACC activity.76 However, to our knowledge, the switching Stroop has not been used with relatives of individuals with schizophrenia.
Fig. 2.
Stroop Task Accuracy and Reaction Times from Cohen et al.55 Reprinted with permission from the American Psychological Association. C = congruent, N = neutral, I = incongruent.
Psychometric Data
There is very little information about any psychometric characteristics of the switching Stroop. Cohen et al did report an internal consistently of 0.9 for incongruent color-naming errors at a 5-s cue-probe delay and an internal consistency of 0.53 for color-naming facilitation at a 5-s delay.
Future Directions
There are a number of different important future directions for research on the switching Stroop for use in clinical trial contexts in schizophrenia. As noted above, little is known about the psychometrics of this task, and thus, studies examining test-retest reliability, practice effects, floor, and ceiling effect, etc are needed. In addition, research is needed to establish the most sensitive neutral condition to use in studies of schizophrenia. In addition, the ways in which the task has been presented in prior studies involve many trials and a lengthy presentation. This may not be practically feasible in clinical trial contexts. As such, work on ways in which to shorten and enhance the sensitivity of the task is needed. Although standard versions of the Stroop task have been shown to be amenable to pharmacological manipulation, work is needed to demonstrate that performance on the switching Stroop can be enhanced either pharmacologically or behaviorally. In addition, work is needed to establish that the greater deficit on color-naming trial references a differential deficit rather than a generalized deficit given that the color-naming condition may have greater discriminating power.
Dynamic Adjustments in Control
CNTRICS received 4 initial nominations for the dynamic adjustments in control construct: (1) the attention networks task (ANT); (2) the Simon task; (3) the trial-by-trial Stroop task; and (4) the stop signal task. In the stop signal task, as described below, dynamic adjustments in control are examined by determining how individuals modulate their behavior when given a cue to interpret an ongoing response. In the ANT, the Simon task, and the trial-by-trial Stroop, dynamic adjustments of control are examined by investigating 2 effects: (1) conflict adaptation77 and (2) posterror adjustments.78,79 Conflict adaptation is thought to reflect behavioral adjustments following trials with high conflict (eg, incongruent trials) vs those with low conflict (eg, neutral or congruent trials). In other words, participants are faster to response to an incongruent trial that is preceded by an incongruent trial compared with an incongruent trial preceded by a congruent trial.77,80 The interpretation of this result has been that the conflict engendered by the first incongruent trial leads to an upregulation in control that reduces the conflict induced by the next incongruent trial.80 Posterror adjustments are thought to reflect a similar process. Participants are typically slower, but more accurate, on trials following errors vs trials following correct responses.78,79 This is again thought to reflect an upregulation of control engendered by the awareness (not necessarily “conscious”) of having made an error.
The executive control breakout group felt that all 4 tasks were reasonable measures of the construct of interest. However, the breakout group felt that the ANT, the Simon, and the Stroop were all variations on a theme, with a similar deep structure in terms of assessing dynamic adjustments in control. Of the 3, the breakout group felt that the Stroop version has the largest extant literature in terms of the cognitive and neural bases of dynamic adjustments in control and the largest existing literature in schizophrenia. Thus, the breakout group recommended that the Stroop version be put forth for immediate translation for use in clinical trials in schizophrenia. In addition, the breakout group made a similar recommendation for the stop signal task, given that it measured a somewhat different aspect of dynamic adjustments in control and thus might provide complimentary information. Table 2 provides a brief overview of these 4 tasks and a very brief summary of their pros and cons in regards to the selection criteria.
Table 2.
Description of Tasks Nominated for Dynamic Adjustments in Control Construct Definition: The Processes Involved in Detecting the Occurrence of Conflict or Errors in Ongoing Processing, Identifying the Type of Control Adjustments Needed, and Recruiting Additional Control Processes7
| Tasks Recommended For Translation |
| Conflict and Error Adaptation in the Stroop Task |
| Construct validity |
| Supported by computational modeling work and individual differences research |
| Neural systems |
| Evidence for the role of ACC in conflict detection and DLPFC in control adjustments from human neuroimaging studies |
| Mixed evidence for the role of DLPFC in control adjustments from lesion research |
| Mixed for the role of ACC in conflict detection from lesion research |
| Pharmacological sensitivity |
| Consistent evidence that basic ERN effects to errors can be modulated via pharmacological challenges |
| Mixed evidence on the degree to which behavioral performance adjustments can be manipulated pharmacologically |
| Evidence for dissociations in the effects of pharmacological manipulations on ERN indices vs behavioral indices |
| Animal models |
| Animal models available for monkey and rat, though the degree of homology needs further research |
| Performance in schizophrenia |
| Evidence for reduced conflict adaptation and reduced posterror slowing (mixed) in schizophrenia |
| Evidence for reduced ACC responses to errors and conflict |
| Psychometric data |
| Little to none currently available |
| Future directions |
| Research needed on assessing and improving the psychometric characteristics of the error and conflict adaptation effects |
| Research needed on ways to make the use of conflict and error adaptation effects feasible in clinical trials contexts (task length, standardized administration, enhanced sensitivity to detect deficits, etc.) |
| Evaluation of discriminating power across conditions is needed. If not equal, modifications will be needed to improve ability to detect differential deficits |
| Evaluation of antipsychotic effects on error and conflict adaptation effects |
| Stop signal task |
| Construct validity |
| Supported by evidence of stopping deficits in populations thought to have impulse control problems, such as ADHD |
| Neural systems |
| Evidence from human imaging and lesion studies on the role of inferior frontal gyrus in inhibition |
| Evidence from human imaging and neurological disorder studies on the role of the subthalamic nucleus in inhibition |
| Pharmacological sensitivity |
| Strong evidence that inhibition on the stop signal task is affected by manipulations of catecholamines, which may involve both dopamine and norepinephrine |
| Animal models |
| There is a primate model that involves countermanding of eye movements |
| There is a rodent model that involves an auditory stop signal and button press |
| Performance in schizophrenia |
| Evidence for reduced stopping accuracy and elongated stop signal reaction times |
| Psychometric data |
| The use of an adaptive stop signal reaction time estimate procedure reduces floor and ceiling effects |
| Preliminary positive evidence for good test-retest reliability and small practice effects |
| Future directions |
| Need to develop better adaptive methods for stop signal reaction time estimation that reduce or eliminate strategy changes on the part of the participant |
| Need to directly compare different versions of the stop signal task in terms of sensitivity and psychometric characteristics |
| Need an evaluation of the degree of homology between the human and animal versions |
| Other nominated tasks |
| Attention networks task |
| Construct validity |
| Evidence from individual differences research and computational modeling |
| Neural systems |
| Evidence for activation of the ACC during conflict81,82 |
| Polymorphisms in dopamine receptor 4 and monoamine oxidase A genes were found to be related to differential behavioral conflict response83 as well as differential ACC conflict response in an fMRI study84 |
| Pharmacological sensitivity |
| No published data |
| Animal models |
| Animal models available for monkey and rat, though the degree of homology needs further research |
| Performance in schizophrenia |
| A selective impairment of ANT conflict processing in schizophrenia has been reported85 |
| Psychometric data |
| Preliminary evidence of good test-retest reliablity86 |
| Simon task |
| Construct validity |
| Behavioral studies have shown robust postconflict and posterror adaptation with this task87 |
| Neural systems |
| Human fMRI studies have linked the ACC to conflict-related activity and subsequent adjustments in control and the DLPFC to supporting those adjustments as for the Stroop88,89 |
| Individuals with lesions to rostral ACC show impaired conflict adaptation on the Simon task 96 |
| Pharmacological sensitivity |
| Unknown |
| Animal models |
| Animal models available for monkey and rat, though the degree of homology needs further research |
| Performance in schizophrenia |
| Unknown |
| Psychometric data |
| Unknown |
Tasks Recommended for Immediate Translation for Dynamic Adjustments of Control Conflict and Error Adaptation in the Stroop Task
Description
The basic design of a Stroop task was described above under the switching Stroop description. In addition, the basic approach to assessing dynamic adjustments in control in the context of conflict tasks was described above. To clarify, in the Stroop task, a high conflict trial for color naming is an incongruent trial in which the word and the color are different (eg, RED written in blue). A low conflict trial is a congruent trial in which the word and the color are the same (RED written in red). In previous studies, dynamic adjustments in control via conflict adaptation have been studied in the Stroop task by comparing 2 sets of trials (see figure 1B): (1) incongruent following an incongruent (iI) vs incongruent following a congruent (cI) and (2) congruent following a congruent (cC) vs congruent following an incongruent (iC).90–93 Dynamic adjustments in control via posterror adjustments are examined as described above. There are several design aspects that one needs to attend in designing a Stroop study for use in examining dynamic adjustments in control. Most importantly is the need to ensure that the sequences of trials are set up in a way to allow sufficient numbers of the relevant “pairings” of trials, as described above. This may require a pseudorandom trial sequence generation that ensures a lack of predictability on the part of the participants coupled with a sufficient number of trials in each cell.
Construct Validity
The construct validity of conflict and error adaptations as measures of dynamic adjustments in control has been supported from a number of different types of studies. For example, several different computational models have been developed that account for both error and conflict adaptation effects in terms of the detection of conflict and the use of such conflict detection to adjust and regulate the amount of control. These models are framed in terms of control being implemented via active representations of task rules or S-R mappings (potentially housed in prefrontal cortex) that provide feedback over posterior cortical–processing areas,94–97 with conflict detection occurring via anterior cingulate (ACC) mechanisms that monitor for the degree of conflict between competing response options. One potential threat to the construct validity of conflict adaptation effects is the degree to which the improvement in performance on iI trials compared with cI trials or on cC vs iC trials can be accounted for by repetition priming instead of increases in cognitive control.98 Although it is clear that such repetition priming can facilitate performance on subsequent trials, research has also shown that conflict adaptation effects are present even when no exact repetitions are used in the calculation of such effects.90,99,100
Neural Systems
There is a burgeoning literature on the neural substrates of such conflict adaptation and error adaptation effects as measures of control adjustments, stemming from both ERP and fMRI research in humans. In the evoked response potential (ERP) literature, components such as the error-related negativity (ERN) and the N2 (or N450 during the Stroop task) have been localized to the ACC and have been frequently investigated in the context of conflict and error monitoring and control adjustments. In addition, the conflict-related negativity (CRN) has also been investigated in relationship to conflict monitoring and control adjustments. Further, there is relevant lesion work as well as recent nonhuman primate work. Much of this literature suggests that the dorsal anterior cingulate (ACC), via inputs from the midbrain dopamine system, monitors for and detects conflict.80,94,95,97,101 In the discussion below, the term ACC will be used to refer both to activation in fMRI studies and to ERP studies examining the ERN, the N2, and the CRN, given the large literature linking these components to an ACC generator. Although there is some debate as to the precise interpretation of the role of ACC in cognition,102 it is clear empirically that the ACC is more active on high vs low conflict trials in the Stroop task and that it is more active on cI than iI trials in the Stroop task.90,92 Further, the magnitude of ACC activity on trial N predicts the degree of behavioral adjustment on trial N + 1, with greater behavioral adjustments associated with greater ACC activity on the previous trial in the Stroop.90 In addition, the ACC is strongly active on error trials in the Stroop, and the degree of ACC activity errors predicts the magnitude of posterror slowing (more ACC → more slowing).90,103,104
The actual implementation of control adjustments following conflict detection has been linked to the function of DLPFC. For example, DLPFC activity is greater on iI than on cI trials, putatively reflecting the increased control necessary to execute conflict adaptation,92,105 and the degree of DLPFC activity predicts the degree of conflict adaptation.88,90,92,106 Further, the degree of ACC activity on previous high conflict trials predicts the degree of DLPFC activity on the subsequent trial.88,90
The lesion data on the role of ACC and DLPFC in control adjustments is mixed. For example, Fellows and Farah107 did not find evidence that ACC lesions influenced Stroop performance of posterror slowing. However, recent work by di Pellegrino showed reductions in both conflict adaptation and posterror slowing in ACC lesion patients.108 Damage to PFC does seem to reduce the likelihood that individuals will correct their errors, but does not appear to reduce post error slowing, which is not consistent with the hypothesis that DLPFC is necessary for conflict-related adjustments in control.109
The above discussion perhaps implies more consistency in the literature on the neural substrates of dynamics adjustments of control than would be apparent based on a thorough review of the literature. Although there is a large amount of data to support important roles for DLPFC and ACC in dynamic adjustments of control, there is also conflicting data and data that suggest other roles for ACC in action monitoring102,110,111 (for a review, see Carter and van Veen112). As noted above, some of the open questions involve the degree to which ACC and/or DLPFC function is necessary for dynamic adjustments of control and the degree to which posterror and conflict adaptation effects reflect the same mechanisms. This is still a rich and active area of investigation that is generating much interesting data about the neutral substrates of cognitive control.
Pharmacological and Behavioral Manipulation
A number of studies have examined pharmacological influences on the ERN and both conflict adaptation and posterror slowing, with mixed results. For example, benzodiazepines reduce the magnitude of the ERN113,114 and show some evidence of reducing the N2 congruency effect on the Stroop.114 Benzodiazepines do not appear to reduce posterror slowing but do reduce the influence of errors on conflict adaptation114 and reduce the number of errors that are corrected.113 Yohimbine (a selective alpha-2 adrenergic receptor antagonist) increases the ERN but does not change posterror slowing. However, Yohimbine does lead to fewer errors following errors, another reflection of control adaptation.115 Caffeine also increases the ERN but does not influence posterror slowing.116 In terms of dopaminergic modulation, haldol (a D2 antagonist) consistently reduces the magnitude of the ERN,117,118 while amphetamine (indirect dopamine agonist) increases it.118 However, neither appears to influence N2 congruency effects or posterror slowing or conflict adaptation effects.114,117,118 Olanzapine reduced the ERN and reduced the degree of posterror slowing.118 Thus, these studies provide strong evidence that basic ERN effects to errors can be modulated via pharmacological challenges, both in terms of enhancement and reduction. However, there is mixed evidence as to the degree to which behavioral performance adjustments can be manipulated pharmacologically and evidence for dissociations in the effects of pharmacological manipulations on ERN indices vs behavioral indices.
Animal Models
A growing number of studies have examined conflict- and error-related processing in nonhuman primate models. These studies indicate that neurons in a number of medial prefrontal regions are modulated in response to errors, including anterior cingulate, supplementary motor regions including the supplementary eye fields, and the superior frontal gyrus.119–121 However, it is less clear in monkeys that these regions also signal nonerror-related conflict, with some studies suggesting conflict-related activity122 and other not,121,123 and the temporal dynamics of the error-related signal may differ from that seen in humans. Some work has also examined the role of DLPFC in conflict adaptation in nonhuman primates. This work demonstrated that lesions to DLPFC reduced postconflict behavioral adjustments, but that lesions to the ACC did not.124 There is also recent work in a rat model of posterror slowing. In this study, rats were trained a simple reaction time task, in which they had to hold a lever for a specific period of time. The rats showed longer RTs on trials following an error vs those trials following a correct response. Further, inactivation of dorsal medial PFC reduced the amount of posterror slowing shown by the rats.125
Performance in Schizophrenia
A growing number of studies have examined fMRI and ERP measures of error and conflict processing in schizophrenia as well as indices of dynamic adjustments in control. Several studies suggest that individuals with schizophrenia show reduced error-related ACC responses71,126–132 as well as reduced posterror slowing71,126 on the Stroop task as well as other tasks. However, there is also evidence that patients with schizophrenia can show normal error correction performance even in the context of reduced ACC responses to errors127,133 and that the relationship between the magnitude of the ERN and error-related behaviors is intact in schizophrenia.130 Individuals with schizophrenia also show reduced conflict-related ACC activation on the Stroop task71,127 as well as reductions in conflict adaptation effects.71
Psychometric Characteristics
There is little published psychometric data on either ERN or ACC responses to error- or conflict-related processes or behavioral indices of conflict adaptation or posterror slowing.
Future Directions
As noted above, there are still open questions about the specific neural circuits that underlie dynamic adjustments in control and open questions about the degree to which conflict adaptation and posterror changes in behavior reflect the same or dissociable mechanisms. There is also a large need for psychometric research on the conflict adaptation and posterror change measures, as well as for ERN and ACC responses. This includes research on test-retest reliability, practice effects, and floor and ceiling effects. In addition, work is needed to determine the optimal number of trials needed to obtain robust estimate of conflict- and error-related behavioral adjustments. In addition, more information is needed on the degree to which reductions in ACC responses (measured either by fMRI or ERPs) or behavioral indices of dynamic adjustment of control are negatively influenced by either typical or atypical antipsychotics in individuals with schizophrenia.
The Stop Signal Task
Description
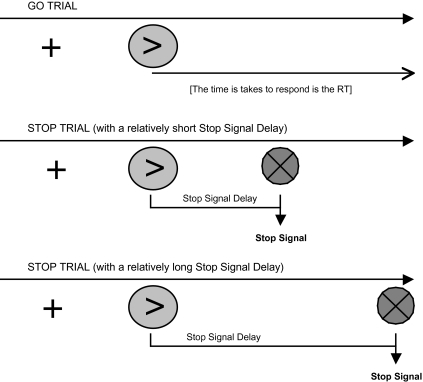
The stop signal paradigm measures an individual's ability to interrupt a motor response after it has been initiated. The subject is presented with a primary task that generally involves a 2-choice response, such as deciding which of 2 letters is presented and pressing the appropriate button. On a proportion of trials, the subject is presented with a “stop signal,” in which case the subject is instructed to withhold their response if possible (see figure 3). The likelihood of stopping is determined by the delay at which the stop signal is presented; if the signal occurs soon after the stimulus, then the subject is very likely to stop, whereas successful stopping becomes much less likely if the signal occurs later in the trial.
Fig. 3.
An Illustration of the Structure of the Stop Signal Task. On each trial, subjects are presented with a stimulus to which they must respond. On some trials they receive a subsequent cue that indicates that they have to stop their response (the stop signal). If the stop signal comes quickly after the initial stimulus, it is a short stop signal delay and it is easier for participants to halt their response. If the stop signal is presented at a longer time following the initial stimulus, it is harder for participants to halt their response.
The stop signal paradigm allows one to estimate the time needed to stop a behavior. Although this is not an observable quantity (ie, it is a parameter regarding trials where no behavior was made), it can be estimated from observed behavior if certain assumptions are made. These assumptions comprise the so-called “race model” of the stop signal reaction time.134 This model assumes that response production and response inhibition processes race against one another and that the first one to finish determines whether a response is executed or inhibited. Although the strong assumption of independence is unlikely to be correct,135 it nonetheless does a good job of characterizing behavior in the stop signal task.136
The goal of the race model is to allow estimation of the stop signal reaction time (SSRT), which is the amount of time needed by the stop process to successfully inhibit a response. There are a number of ways to estimate SSRT;134 a commonly used method does this by determining the stop signal delay at which subjects fail to stop on 50% of trials. This value can be estimated either by using an adaptive staircase method to estimate it directly or by evaluating performance at a range of stop signal delay (SSD) values and interpolating to estimate it. Once is known, then the SSRT can be estimated as, since failing on 50% of trials means that half of the responses are faster than SSRT + and half are slower, which is equivalent to the median RT.
A comprehensive “user's guide” to the stop signal task was published by Logan and Cowan,134 Once the range of SSD values are known, then the SSRT can be estimated, since failing on 50% of trials means that half of the responses are faster than SSRT and half are slower, which is equivalent to the median RT. which should be read closely by any prospective user of the task. It is important to highlight that this task is highly sensitive to the instructions and feedback that are given to the subject. In particular, many subjects will have the tendency to delay their responses in order to wait for the stop signal. If an adaptive staircase method is used, then this can result in SSD values that do not converge but rather continue increasing across the entire task. When this occurs, it is impossible to obtain an accurate estimate of SSRT because there is no SSD for which the proportion of successful stops is known accurately. These strategies can be reduced by carefully instructing the subjects, and also by providing feedback on go task response time after every block, to ensure that subjects do not slow down on go trials. It is also critical to examine the resulting staircases when adaptive methods are used, to ensure that they have converged to a stable estimate of. It is particularly useful to use multiple staircases, so that reliability can be assessed across them and so that subjects cannot easily predict changes in SSDs.
One particular issue that has arisen in the literature is whether SSRT should be estimated using adaptive methods or using a set of fixed SSDs. Although more complicated to implement, the adaptive staircase method has a number of benefits. Psychologically, it ensures that subjects with very different levels of inhibitory function exhibit the same proportion of failed inhibitory trials, which eliminates confounds regarding frustration or error likelihood prediction. Psychometrically, it ensures roughly equal numbers of successful and unsuccessful stop trials, which provides increased power to contrast these conditions; contrast this with the go/no-go paradigm, in which there is generally a very low rate (∼10%) of commission errors. Simulation studies136 also show that SSRT is more reliably estimated using an adaptive tracking method than using a set of fixed SSDs. The one caution, as noted above, is that adaptive methods may be more likely to lead subjects to adopt waiting strategies; careful instruction and continuous feedback throughout testing can help avoid this problem.
Construct Validity
The stop signal task measures the ability to reactively inhibit a response once it has been initiated. Its construct validity as a measure of control has been demonstrated primarily in the context of impulsivity and behavioral control. In the normal population, SSRT is correlated with real-world impulsivity as measured using paper-and-pencil impulsivity scales.137 A large body of research has linked performance on the stop signal task to disorders of impulse control, including attention deficit hyperactivity disorder (ADHD) (reviewed by Lijffijt et al138), obsessive-compulsive disorder and trichotillomania,139 methamphetamine abuse,140 and cocaine use.141,142
Neural Systems
Evidence regarding the neural systems supporting stop signal performance in humans comes from both lesion and imaging studies. A study of frontal lesion patients143 provided evidence that the right inferior frontal gyrus is critical for stopping in this task; patients with lesions to this area were showed lengthened SSRT values, and SSRT was correlated with the amount of lesion in the region. However, another study found that the medial superior prefrontal cortex (supplementary and pre-supplementary motor areas) is also important for response inhibition.144,145 Neuroimaging studies have also implicated both of these prefrontal regions, which show activity on stop trials in comparison to go trials that are correlated across subjects with SSRT.146–149 These studies have not generally found activity in right inferior prefrontal or medial superior prefrontal cortex for the comparison of successful vs unsuccessful inhibition; this is predicted by the race model because that model proposes that successful inhibition is primarily driven by the speed of the go process rather than differences in the effectiveness of the stop process.
There is also evidence that basal ganglia regions may be involved in stop signal inhibition, specifically the subthalamic nucleus (STN). Stimulation of the STN in individuals with Parkinson's disease resulted in improved stop signal reaction time.150 Neuroimaging studies have also found that STN is activated for successful stop vs go trials and that its activation is also correlated with SSRT across subjects.146,147 The role of the STN in stopping is consistent with its excitation of pallidal neurons, which results in thalamic inhibition and cortical deactivation.151 There is also evidence that STN receives direct projections from both of the prefrontal regions implicated in stopping,147 as part of the “hyperdirect” pathway into the basal ganglia.152
Pharmacological or Behavioral Manipulation
There is strong evidence that inhibition on the stop signal task is affected by manipulations of catecholamines. SSRT is improved by methylphenidate in both children153 and adults154 with ADHD. It is likely that this reflects both dopaminergic and noradrenergic effects: SSRT is improved by low doses of cocaine155 and by specific inhibition of noradrenergic reuptake using atomoxetine156 and desipramine.157 There is also evidence for association between stop signal reaction time and genetic polymorphisms in the D4 dopamine receptor and dopamine transporter genes.158
Animal Models
One of the strong aspects of the stop signal task for translational research is the existence of animal models in both nonhuman primates and rodents. The primate model involves countermanding of eye movements; after fixation on a central point, a lateral stimulus is presented and the animal makes an eye movement to that stimulus. On a small proportion of stop trials, the central fixation point reappears, and the animal is trained to countermand its eye movement on those trials. Extensive work has characterized the neural systems involved in this process (reviewed by Schall et al120). This model has provided a great deal of knowledge about how inhibition occurs in eye movements, but there are reasons to believe that the countermanding model may not be applicable to other forms of the stop signal task.
A rodent model of the stop signal paradigm has been developed by Eagle et al.159,160 In this paradigm, the rat first presses a button to start the trial and then has to press a second button as the “go” response; on trials when an auditory stop signal is presented, the rat must withhold response in order to obtain reward. Studies using this paradigm have shown that stopping is affected by lesions to the orbitofrontal cortex and STN161 and that it is modulated by d-amphetamine,159 modafinil, and methylphenidate.162 These results are largely consistent with the human literature and provide strong support for this paradigm as a model system for translational research on response inhibition.
Performance in Schizophrenia
There are relatively limited data on stop signal performance in schizophrenia. Badcock et al163 found that patients with schizophrenia, as well as subjects with other forms of psychosis, exhibited impaired response inhibition (as measured by the slope of inhibition functions because a fixed SSD method was used). Vink et al164 examined inhibitory control using an adaptation of the stop signal task with a visual stop signal, in patients with schizophrenia and unaffected siblings. SSRT was not estimated, but instead the accuracy of stopping was reported. They found that patients were impaired at stopping, particularly when stop signals became more likely (ie, after a larger number of go trials). Siblings showed a marginally significant effect in the same direction. Bellgrove et al165 examined stop signal performance in a group of early-onset schizophrenic patients diagnosed as either paranoid or undifferentiated. SSRT was estimated using an adaptive staircase method, with separate SSRT estimates for each hand. The undifferentiated patients exhibited a substantial deficit in SSRT for the left hand; no deficits were observed for the paranoid group. Finally, Enticott et al166 compared SSRT in schizophrenic inpatients and matched controls and found that schizophrenics showed increased SSRT. Thus, although not conclusive, there is evidence from several studies for deficits in response inhibition in schizophrenic patients and, perhaps more important, in unaffected siblings as well.
Psychometric Characteristics
As noted above, the use of adaptive SSRT estimation methods imbues the stop signal task with desirable psychometric characteristics. By adapting the SSD to each subject to ensure 50% successful inhibition, it prevents ceiling and floor effects (which are problematic in the go/no-go task). In unpublished work (J. R. Cohen & R. A. Poldrack, unpublished), there was no evidence of practice effects in SSRT estimates obtained using adaptive methods. Previous work has also shown that the mean proportion of inhibition, when measured using a fixed set of SSDs, exhibits high test-retest reliability.
Future Directions
Future work on the stop signal task should focus on at least 4 areas. First, a better characterization of response inhibition function across a range of neuropsychiatric disorders is needed, in order to characterize whether there is any specificity to the inhibitory deficits suggested by the existing literature. These studies should also include unaffected relatives, particularly given the potential for antipsychotic drugs to interfere with inhibitory functions. Second, research is needed on ways to develop better adaptive methods for estimation of SSRT and better procedures to prevent strategic adaptations that result in slowing of go response times. Third, work is needed on ways to better delineate different aspects of the stop signal paradigm and their potential effects on reliability and neural systems validity. Relatively little work to date has directly compared different forms of the stop signal task (eg, using visual vs auditory stop signals or using standard vs selective stopping tasks). Fourth, the field needs a better characterization of the genetic architecture of response inhibition. There is evidence from candidate gene studies for association with specific genes, but these await validation by genome-wide association studies.
General Conclusion
The discussion above aimed to present a summary of the data upon which decisions were made at the third CNTRICS meeting in regards to which tasks to nominate for translation into clinical trials. In this selection process and in the descriptions provided above, much emphasis was placed on the evidence regarding construct validity, the neural substrates of the tasks, and the availability of animal models. This emphasis was intentional because the guiding theme of the CNTRICS initiative has been that we need to leverage the advances in cognitive neuroscience to develop better and more effective treatments for cognitive impairment in schizophrenia. One thing that should be clear from the above descriptions though is that much work is needed to make these paradigms—derived from the basic science literature—practical and feasible for use in clinical trials. For some, this process might seem daunting and might lead one to fall back on traditional tasks for which such psychometric and practicality information is already available. However, we would argue strongly that the hard work that lies ahead is well worth it and critically necessary so as to move the field forward. The use of paradigms with a tight grounding in modern theories of human and animal cognitive neuroscience may allow us to pursue a more rationally guided pathway to drug development and testing that has the potential to point the way toward more effective treatment pathways. Yes, it is true that development work for some of these tasks will not end up being successful and some may not “survive” the translation process in a way that allows them to be put to good use in clinical trials. However, even if only some tasks are successfully translated for use in clinical trials, and these paradigms end up improving our ability to design and evaluate effective cognitive treatments, then the whole process will have been worth the time and effort of the many people involved.
Funding
NIH, the McDonnell Center for Higher Brain Function, and NARSAD to D.M.B; NIMH, the Robert Wood Johnson Foundation and NARSAD to C.S.C; C.S.C has served as a consultant for Pfizer, Eli Lilly, and Roche. NIMH, the McDonnell Foundation, and NARSAD to D.M.B; NIH to T.S.B; NIH; James S. McDonnell Foundation for Dr. Poldrack to R.A.P; MRC, Wellcome Trust, McDonnell Foundation, and E.U. (FP6) to T.W.R. He consults for Cambridge Cognition, E. Lilly, GlaxoSmithKline and Allon Therapeutics.
Acknowledgments
We would like to thank Jody Conrad, Carol Cox, and Deb Tussing, whose efforts have been invaluable in the CNTRICS process. In addition, we would like to thank the other members of the executive control breakout group, including Andrew Blackwell, Nelson Cowan, Randy Engle, Judith Jaeger, Rich Keefe, John Kerns, Ann Kring, Gina Kuperberg, Steve Luck, Henry Mahncke, Paul Maruff, Michael Minzenberg, Holly Moore, Venkatesha Murthy, Keith Nuechterlein, Patricio O'Donnell, Brad Postle, Nathan Pradeep, Charan Ranganath, Martin Sarter, Larry Seidman, Magnus Sjogren, Ed Smith, Mark Tricklebank, Sophia Vinogradov, and Andy Yonelinas.
References
- 1.Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: integration of performance-based and brain imaging findings. Psychol Bull. 2007;133:833–858. doi: 10.1037/0033-2909.133.5.833. [DOI] [PubMed] [Google Scholar]
- 2.Kerns JB, Berenbaum H. Cognitive impairments associated with formal thought disorder in people with schizophrenia. J Abnorm Psychol. 2002;111:211–224. [PubMed] [Google Scholar]
- 3.Delawalla Z, Barch DM, Fisher Eastep JL, et al. Factors mediating cognitive deficits and psychopathology among siblings of individuals with schizophrenia. Schizophr Bull. 2006;32:525–537. doi: 10.1093/schbul/sbj082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barch DM, Mitropoulou V, Harvey PD, New AS, Silverman JM, Siever LJ. Context-processing deficits in schizotypal personality disorder. J Abnorm Psychol. 2004;113:556–568. doi: 10.1037/0021-843X.113.4.556. [DOI] [PubMed] [Google Scholar]
- 5.McClure MM, Bowie CR, Patterson TL, et al. Correlations of functional capacity and neuropsychological performance in older patients with schizophrenia: evidence for specificity of relationships? Schizophr Res. 2007;89:330–338. doi: 10.1016/j.schres.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 6.Kerns JG, Nuechterlein KH, Braver TS, Barch DM. Executive functioning component mechanisms and schizophrenia. Biol Psychiatry. 2008;64:26–33. doi: 10.1016/j.biopsych.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 7.Carter CS, Barch DM, Buchanan RW, et al. Identifying cognitive mechanisms targeted for treatment development in schizophrenia: an overview of the first meeting of the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia Initiative. Biol Psychiatry. 2008;64:4–10. doi: 10.1016/j.biopsych.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33:1131–1137. doi: 10.1093/schbul/sbm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathewson K, Dywan J, Snyder PJ, Tays W, Segalowitz S. Electrophysiological correlates of older and younger adults’ response to error feedback using a maze learning task. Psychophysiology. doi: 10.1111/j.1469-8986.2008.00699.x. in press. [DOI] [PubMed] [Google Scholar]
- 10.Snyder AM, Maruff P, Pietrzak RH, Cromer JR, Snyder PJ. Effect of treatment with stimulant medication on nonverbal executive function and visuomotor speed in children with attention deficit/hyperactivity disorder (ADHD) Child Neuropsychol. 2008;14:211–226. doi: 10.1080/09297040701220005. [DOI] [PubMed] [Google Scholar]
- 11.Williams MT, Morford LL, McCrea AE, Wood SL, Vorhees CV. Administration of D,L-fenfluramine to rats produces learning deficits in the Cincinnati water maze but not the Morris water maze: relationship to adrenal cortical output. Neurotoxicol Teratol. 2002;24:783–796. doi: 10.1016/s0892-0362(02)00318-5. [DOI] [PubMed] [Google Scholar]
- 12.Snyder PJ, Jackson CE, Piskulic D, Olver J, Norman TR, Maruff P. Spatial working memory and problem solving in schizophrenia: the effect of symptom stabilization with atypical antipsychotic medication. Psychiatry Res. doi: 10.1016/j.psychres.2007.07.011. in press. [DOI] [PubMed] [Google Scholar]
- 13.Pietrzak RH, Maruff P, Mayes LC, Roman SA, Sosa JA, Snyder PJ. An examination of the construct validity and factor structure of the Groton Maze Learning Test, a new measure of spatial working memory, learning efficiency, and error monitoring. Arch Clin Neuropsychol. 2008;23:433–445. doi: 10.1016/j.acn.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Frank MJ, Loughry B, O'Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn Affect Behav Neurosci. 2001;1:137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- 15.Pietrzak RH, Cohen H, Snyder PJ. Spatial learning efficiency and error monitoring in normal aging: an investigation using a novel hidden maze learning test. Arch Clin Neuropsychol. 2007;22:235–245. doi: 10.1016/j.acn.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Pietrzak RH, Maruff P, Mayes LC, Roman S, Sosa J, Snyder PJ. Construct validity and factor structure of the groton maze learning test: a new measure of spatial working memory, learning efficiency, and error monitoring. under review. doi: 10.1016/j.acn.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Snyder AM, Maruff P, Pietrzak RH, Cromer JR, Snyder PJ. Effect of treatment with Stimulant Medication on Nonverbal Executive Function and Visuomotor Speed in Children with Attention Deficit/Hyperactivity Disorder (ADHD) Child Neuropsychol. 2008;14(3):211–226. doi: 10.1080/09297040701220005. [DOI] [PubMed] [Google Scholar]
- 18.O'Reilly RC, Noelle DC, Braver TS, Cohen JD. Prefrontal cortex and dynamic categorization tasks: representational organization and neuromodulatory control. Cereb Cortex. 2002;12:246–257. doi: 10.1093/cercor/12.3.246. [DOI] [PubMed] [Google Scholar]
- 19.Roberts AC, Robbins TW, Everitt BJ. The effects of intradimensional and extradimensional shifts on visual discrimination learning in humans and non-human primates. Q J Exp Psychol B. 1988;40:321–341. [PubMed] [Google Scholar]
- 20.Hampshire A, Owen AM. Fractionating attentional control using event-related fMRI. Cereb Cortex. 2006;16:1679–1689. doi: 10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- 21.Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW. Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. J Cogn Neurosci. 2000;12:142–162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- 22.Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 23.Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- 25.Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, Roberts AC. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J Neurosci. 2005;25:532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- 27.Chamberlain SR, Menzies L, Hampshire A, et al. Orbitofrontal dysfunction in patients with obsessive-compulsive disorder and their unaffected relatives. Science. 2008;321:421–422. doi: 10.1126/science.1154433. [DOI] [PubMed] [Google Scholar]
- 28.Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci. 2002;22:4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park SB, Coull JT, McShane RH, et al. Tryptophan depletion in normal volunteers produces selective impairments in learning and memory. Neuropharmacology. 1994;33:575–588. doi: 10.1016/0028-3908(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 31.Rogers RD, Blackshaw AJ, Middleton HC, et al. Tryptophan depletion impairs stimulus-reward learning while methylphenidate disrupts attentional control in healthy young adults: implications for the monoaminergic basis of impulsive behaviour. Psychopharmacology (Berl) 1999;146:482–491. doi: 10.1007/pl00005494. [DOI] [PubMed] [Google Scholar]
- 32.Middleton HC, Sharma A, Agouzoul D, Sahakian BJ, Robbins TW. Idazoxan potentiates rather than antagonizes some of the cognitive effects of clonidine. Psychopharmacology (Berl) 1999;145:401–411. doi: 10.1007/s002130051074. [DOI] [PubMed] [Google Scholar]
- 33.Mehta MA, Manes FF, Magnolfi G, Sahakian BJ, Robbins TW. Impaired set-shifting and dissociable effects on tests of spatial working memory following the dopamine D2 receptor antagonist sulpiride in human volunteers. Psychopharmacology (Berl) 2004;176:331–342. doi: 10.1007/s00213-004-1899-2. [DOI] [PubMed] [Google Scholar]
- 34.Lange KW, Paul GM, Robbins TW, Marsden CD. L-Dopa and frontal cognitive function in Parkinson’s disease. Adv Neurol. 1993;60:475–478. [PubMed] [Google Scholar]
- 35.Boulougouris V, Glennon JC, Robbins TW. Dissociable effects of selective 5-HT(2A) and 5-HT(2C) receptor antagonists on serial spatial reversal learning in rats. Neuropsychopharmacology. 2007;33:2007–2019. doi: 10.1038/sj.npp.1301584. [DOI] [PubMed] [Google Scholar]
- 36.Roberts AC, De Salvia MA, Wilkinson LS, et al. 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort Test: possible interactions with subcortical dopamine. J Neurosci. 1994;14:2531–2544. doi: 10.1523/JNEUROSCI.14-05-02531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crofts HS, Dalley JW, Collins P, et al. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb Cortex. 2001;11:1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- 38.Lapiz MD, Bondi CO, Morilak DA. Chronic treatment with desipramine improves cognitive performance of rats in an attentional set-shifting test. Neuropsychopharmacology. 2007;32:1000–1010. doi: 10.1038/sj.npp.1301235. [DOI] [PubMed] [Google Scholar]
- 39.Tait DS, Brown VJ, Farovik A, Theobald DE, Dalley JW, Robbins TW. Lesions of the dorsal noradrenergic bundle impair attentional set-shifting in the rat. Eur J Neurosci. 2007;25:3719–3724. doi: 10.1111/j.1460-9568.2007.05612.x. [DOI] [PubMed] [Google Scholar]
- 40.Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci. 2004;24:5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liston C, Miller MM, Goldwater DS, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weed MR, Taffe MA, Polis I, et al. Performance norms for a rhesus monkey neuropsychological testing battery: acquisition and long-term performance. Cogn Brain Res. 1999;8:185–201. doi: 10.1016/s0926-6410(99)00020-8. [DOI] [PubMed] [Google Scholar]
- 43.Broberg BV, Dias R, Glenthoj BY, Olsen CK. Evaluation of a neurodevelopmental model of schizophrenia—early postnatal PCP treatment in attentional set-shifting. Behav Brain Res. 2008;190:160–163. doi: 10.1016/j.bbr.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 44.Elliott R, McKenna PJ, Robbins TW, Sahakian BJ. Neuropsychological evidence for frontostriatal dysfunction in schizophrenia. Psychol Med. 1995;25:619–630. doi: 10.1017/s0033291700033523. [DOI] [PubMed] [Google Scholar]
- 45.Elliott R, Sahakian BJ, McKay AP, Herrod JJ, Robbins TW, Paykel ES. Neuropsychological impairments in unipolar depression: the influence of perceived failure on subsequent performance. Psychol Med. 1996;26:975–989. doi: 10.1017/s0033291700035303. [DOI] [PubMed] [Google Scholar]
- 46.Pantelis C, Barber FZ, Barnes TR, Nelson HE, Owen AM, Robbins TW. Comparison of set-shifting ability in patients with chronic schizophrenia and frontal lobe damage. Schizophr Res. 1999;37:251–270. doi: 10.1016/s0920-9964(98)00156-x. [DOI] [PubMed] [Google Scholar]
- 47.Turner DC, Clark L, Pomarol-Clotet E, McKenna PJ, Robbins S, Sahakian B. Modafinil improves cognition and attentional set shifting in patients with chronic schizophrenia. 2004;29(7):1363–1373. doi: 10.1038/sj.npp.1300457. [DOI] [PubMed] [Google Scholar]
- 48.Hutton SB, Puri BK, Duncan LJ, Robbins TW, Barnes TR, Joyce EM. Executive function in first-epsiode schizophrenia. Psychol Med. 1998;28:463–473. doi: 10.1017/s0033291797006041. [DOI] [PubMed] [Google Scholar]
- 49.Joyce E, Hutton S, Mutsatsa S, et al. Executive dysfunction in first-episode schizophrenia and relationship to duration of untreated psychosis: the West London Study. Br J Psychiatry Suppl. 2002;43:s38–s44. doi: 10.1192/bjp.181.43.s38. [DOI] [PubMed] [Google Scholar]
- 50.Jazbec S, Pantelis C, Robbins T, Weickert T, Weinberger DR, Goldberg TE. Intra-dimensional/extra-dimensional set-shifting performance in schizophrenia: impact of distractors. Schizophr Res. 2007;89:339–349. doi: 10.1016/j.schres.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 51.Robbins TW, James M, Owen AM, et al. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: implications for theories of executive functioning and cognitive aging. J Int Neuropsycho Soc. 1998;4:474–490. doi: 10.1017/s1355617798455073. [DOI] [PubMed] [Google Scholar]
- 52.Lowe C, Rabbitt P. Test-re-test reliability of the CANTAB and ISPOCD neuropsychological batteries: theoretical and practical issues. Neuropsychologia. 1998;36:915–923. doi: 10.1016/s0028-3932(98)00036-0. [DOI] [PubMed] [Google Scholar]
- 53.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 54.MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- 55.Cohen JD, Barch DM, Carter C, Servan-Schreiber D. Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. J Abnorm Psychol. 1999;108:120–133. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- 56.Barch DM, Carter CS, Cohen JD. Factors influencing Stroop performance in schizophrenia. Neuropsychology. 2004;18:477–484. doi: 10.1037/0894-4105.18.3.477. [DOI] [PubMed] [Google Scholar]
- 57.Cohen JD, Dunbar K, McClelland JL. On the control of automatic processes: a parallel distributed processing account of the Stroop effect. Psychol Rev. 1990;97:332–361. doi: 10.1037/0033-295x.97.3.332. [DOI] [PubMed] [Google Scholar]
- 58.Herd SA, Banich MT, O'Reilly RC. Neural mechanisms of cognitive control: an integrative model of stroop task performance and fMRI data. J Cogn Neurosci. 2006;18:22–32. doi: 10.1162/089892906775250012. [DOI] [PubMed] [Google Scholar]
- 59.Dehaene S, Kerszberg M, Changeux JP. A neuronal model of a global workspace in effort cognitive tasks. Proc Natl Acad Sci U S A. 1998;95:14529–14534. doi: 10.1073/pnas.95.24.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kane MJ, Engle RW. Working-memory capacity and the control of attention: the contributions of goal neglect, response competition, and task set to Stroop interference. J Exp Psychol Gen. 2003;132:47–70. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- 61.MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 62.Donohue SE, Wendelken C, Bunge SA. Neural correlates of preparation for action select as a function of specific task demands. J Cogn Neurosci. 2008;20:694–706. doi: 10.1162/jocn.2008.20042. [DOI] [PubMed] [Google Scholar]
- 63.Demakis GJ. Frontal lobe damage and tests of executive processing: a meta-analysis of the category test, Stroop test and trail-making test. J Clin Exp Neuropsychol. 2004;26:441–450. doi: 10.1080/13803390490510149. [DOI] [PubMed] [Google Scholar]
- 64.Barch DM, Carter CS. Amphetamine improves cognitive function in medicated individuals with schizophrenia and in healthy volunteers. Schizophr Res. 2005;77:43–58. doi: 10.1016/j.schres.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 65.Henik A, Salo R. Schizophrenia and the stroop effect. Behav Cogn Neurosci Rev. 2004;3:42–59. doi: 10.1177/1534582304263252. [DOI] [PubMed] [Google Scholar]
- 66.Haddon JE, George DN, Killcross AS. Contextual control of biconditional task performance: evidence for cue and response competition in rats. Q J Exp Psychol (Colchester) 2007;61 doi: 10.1080/17470210701515819. [DOI] [PubMed] [Google Scholar]
- 67.Haddon JE, Killcross S. Contextual control of choice performance: behavioral, neurobiological, and neurochemical influences. Ann N Y Acad Sci. 2007;1104:250–269. doi: 10.1196/annals.1390.000. [DOI] [PubMed] [Google Scholar]
- 68.Marquis JP, Killcross S, Haddon JE. Inactivation of the prelimbic, but not infralimbic, prefrontal cortex impairs the contextual control of response conflict in rats. Eur J Neurosci. 2007;25:559–566. doi: 10.1111/j.1460-9568.2006.05295.x. [DOI] [PubMed] [Google Scholar]
- 69.Stoet G, Snyder LH. Correlates of stimulus-response congruence in the posterior parietal cortex. J Cogn Neurosci. 2007;19:194–203. doi: 10.1162/jocn.2007.19.2.194. [DOI] [PubMed] [Google Scholar]
- 70.Stoet G, Snyder LH. Effects of the NMDA antagonist ketamine on task-switching performance: evidence for specific impairments of executive control. Neuropsychopharmacology. 2006;31:1675–1681. doi: 10.1038/sj.npp.1300930. [DOI] [PubMed] [Google Scholar]
- 71.Kerns JG, Cohen JD, MacDonald AW, III, et al. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. Am J Psychiatry. 2005;162:1833–1839. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- 72.Weiss EM, Siedentopf C, Golaszewski S, et al. Brain activation patterns during a selective attention test—a functional MRI study in healthy volunteers and unmedicated patients during an acute episode of schizophrenia. Psychiatry Res. 2007;154:31–40. doi: 10.1016/j.pscychresns.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 73.Yucel M, Pantelis C, Stuart GW, et al. Anterior cingulate activation during Stroop task performance: a PET to MRI coregistration study of individual patients with schizophrenia. Am J Psychiatry. 2002;159:251–254. doi: 10.1176/appi.ajp.159.2.251. [DOI] [PubMed] [Google Scholar]
- 74.Fields RB, Van Kammen DP, Peters JL, et al. Clonidine improves memory function in schizophrenia independently from change in psychosis. Schizoph Res. 1988;1:417–423. doi: 10.1016/0920-9964(88)90024-2. [DOI] [PubMed] [Google Scholar]
- 75.Snitz BE, Macdonald AW, III, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32:179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Becker TM, Kerns JG, Macdonald AW, III, Carter CS. Prefrontal dysfunction in first-degree relatives of schizophrenia patients during a Stroop task. Neuropsychopharmacology. 2008 doi: 10.1038/sj.npp.1301673. [DOI] [PubMed] [Google Scholar]
- 77.Gratton G, Coles MG, Donchin E. Optimizing the use of information: strategic control of activation of responses. J Exp Psycho Gen. 1992;121:480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- 78.Rabbitt PMA. Errors and error correction in choice-response tasks. J Exp Psychol. 2008;33(11):2619–2625. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- 79.Laming D. Choice reaction performance following an error. Acta Psychol. 1979;43:199–224. doi: 10.1016/0001-6918(79)90032-5. [DOI] [PubMed] [Google Scholar]
- 80.Botvinick MM, Braver TS, Carter CS, Barch DM, Cohen JC. Report nr Technical Report 98.1. Pittsburgh, PA: Center for the Neural Basis of Cognition; 1998. Evaluating the demand for control: Anterior cingulate cortex and conflict monitoring. [Google Scholar]
- 81.Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 82.Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. Neuroimage. 2003;18:42–57. doi: 10.1006/nimg.2002.1319. [DOI] [PubMed] [Google Scholar]
- 83.Fossella J, Sommer T, Fan J, et al. Assessing the molecular genetics of attention networks. BMC Neurosci. 2002;3:14. doi: 10.1186/1471-2202-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fan J, Fossella J, Sommer T, Wu Y, Posner MI. Mapping the genetic variation of executive attention onto brain activity. Proc Natl Acad Sci U S A. 2003;100:7406–7411. doi: 10.1073/pnas.0732088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang K, Fan J, Dong Y, Wang CQ, Lee TM, Posner MI. Selective impairment of attentional networks of orienting and executive control in schizophrenia. Schizophr Res. 2005;78:235–241. doi: 10.1016/j.schres.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 86.Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- 87.Ridderinkhof KR. Micro- and macro-adjustments of task set: activation and suppression in conflict tasks. Psychol Res. 2002;66:312–323. doi: 10.1007/s00426-002-0104-7. [DOI] [PubMed] [Google Scholar]
- 88.Kerns JG. Anterior cingulate and prefrontal cortex activity in an FMRI study of trial-to-trial adjustments on the Simon task. Neuroimage. 2006;33:399–405. doi: 10.1016/j.neuroimage.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 89.Sturmer B, Leuthold H, Soetens E, Schroter H, Sommer W. Control over location-based response activation in the Simon task: behavioral and electrophysiological evidence. J Exp Psychol Hum Percept Perform. 2002;28:1345–1363. doi: 10.1037//0096-1523.28.6.1345. [DOI] [PubMed] [Google Scholar]
- 90.Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 91.Carter CS, Macdonald AM, Botvinick M, et al. Parsing executive processes: strategic versus evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Egner T, Hirsch J. The neural correlates and functional integration of cognitive control in a Stroop task. Neuroimage. 2005;24:539–547. doi: 10.1016/j.neuroimage.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 93.Egner T, Delano M, Hirsch J. Separate conflict-specific cognitive control mechanisms in the human brain. Neuroimage. 2007;35:940–948. doi: 10.1016/j.neuroimage.2006.11.061. [DOI] [PubMed] [Google Scholar]
- 94.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JC. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 95.Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- 96.Brown JW, Reynolds JR, Braver TS. A computational model of fractionated conflict-control mechanisms in task-switching. Cognit Psychol. 2007;55:37–85. doi: 10.1016/j.cogpsych.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 97.Brown JW, Braver TS. A computational model of risk, conflict, and individual difference effects in the anterior cingulate cortex. Brain Res. 2008;1202:99–108. doi: 10.1016/j.brainres.2007.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mayr U, Awh E, Laurey P. Conflict adaptation effects in the absence of executive control. Nat Neurosci. 2003;6:450–452. doi: 10.1038/nn1051. [DOI] [PubMed] [Google Scholar]
- 99.Ullsperger M, Bylsma LM, Botvinick MM. The conflict adaptation effect: it’s not just priming. Cogn Affect Behav Neurosci. 2005;5:467–472. doi: 10.3758/cabn.5.4.467. [DOI] [PubMed] [Google Scholar]
- 100.Notebaert W, Gevers W, Verbruggen F, Liefooghe B. Top-down and bottom-up sequential modulations of congruency effects. Psychon Bull Rev. 2006;13:112–117. doi: 10.3758/bf03193821. [DOI] [PubMed] [Google Scholar]
- 101.Botvinick MM, Nystrom L, Fissel K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- 102.Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- 103.Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- 104.Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci. 2005;25:11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat Neurosci. 2005;8:1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- 106.Marco-Pallares J, Camara E, Munte TF, Rodriguez-Fornells A. Neural mechanisms underlying adaptive actions after slips. J Cogn Neurosci. 2008;20:1595–1610. doi: 10.1162/jocn.2008.20117. [DOI] [PubMed] [Google Scholar]
- 107.Fellows LK, Farah MJ. Is anterior cingulate cortex necessary for cognitive control? Brain. 2005;128:788–796. doi: 10.1093/brain/awh405. [DOI] [PubMed] [Google Scholar]
- 108.di Pellegrino G, Ciaramelli E, Ladavas E. The regulation of cognitive control following rostral anterior cingulate cortex lesion in humans. J Cogn Neurosci. 2007;19:275–286. doi: 10.1162/jocn.2007.19.2.275. [DOI] [PubMed] [Google Scholar]
- 109.Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nat Neurosci. 2000;3:516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- 110.Burle B, Roger C, Allain S, Vidal F, Hasbroucq T. Error negativity does not reflect conflict: a reappraisal of conflict monitoring and anterior cingulate cortex activity. J Cogn Neurosci. 2008;20:1637–1655. doi: 10.1162/jocn.2008.20110. [DOI] [PubMed] [Google Scholar]
- 111.Botvinick MM. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci. 2007;7:356–366. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- 112.Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- 113.Riba J, Rodriguez-Fornells A, Munte TF, Barbanoj MJ. A neurophysiological study of the detrimental effects of alprazolam on human action monitoring. Brain Res Cogn Brain Res. 2005;25:554–565. doi: 10.1016/j.cogbrainres.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 114.de Bruijn ER, Hulstijn W, Verkes RJ, Ruigt GS, Sabbe BG. Drug-induced stimulation and suppression of action monitoring in healthy volunteers. Psychopharmacology (Berl) 2004;177:151–160. doi: 10.1007/s00213-004-1915-6. [DOI] [PubMed] [Google Scholar]
- 115.Riba J, Rodriguez-Fornells A, Morte A, Munte TF, Barbanoj MJ. Noradrenergic stimulation enhances human action monitoring. J Neurosci. 2005;25:4370–4374. doi: 10.1523/JNEUROSCI.4437-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tieges Z, Richard Ridderinkhof K, Snel J, Kok A. Caffeine strengthens action monitoring: evidence from the error-related negativity. Brain Res Cogn Brain Res. 2004;21:87–93. doi: 10.1016/j.cogbrainres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 117.Zirnheld PJ, Carroll CA, Kieffaber PD, O'Donnell BF, Shekhar A, Hetrick WP. Haloperidol impairs learning and error-related negativity in humans. J Cogn Neurosci. 2004;16:1098–1112. doi: 10.1162/0898929041502779. [DOI] [PubMed] [Google Scholar]
- 118.de Bruijn ER, Sabbe BG, Hulstijn W, Ruigt GS, Verkes RJ. Effects of antipsychotic and antidepressant drugs on action monitoring in healthy volunteers. Brain Res. 2006;1105:122–129. doi: 10.1016/j.brainres.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 119.Amiez C, Joseph JP, Procyk E. Anterior cingulate error-related activity is modulated by predicted reward. Eur J Neurosci. 2005;21:3447–3452. doi: 10.1111/j.1460-9568.2005.04170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schall JD, Stuphorn V, Brown JW. Monitoring and control of action by the frontal lobes. Neuron. 2002;36:309–322. doi: 10.1016/s0896-6273(02)00964-9. [DOI] [PubMed] [Google Scholar]
- 121.Emeric EE, Brown JW, Leslie M, Pouget P, Stuphorn V, Schall JD. Performance monitoring local field potentials in the medial frontal cortex of primates: anterior cingulate cortex. J Neurophysiol. 2008;99:759–772. doi: 10.1152/jn.00896.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Olson CR, Gettner SN. Neuronal activity related to rule and conflict in macaque supplementary eye field. Physiol Behav. 2002;77:663–670. doi: 10.1016/s0031-9384(02)00945-9. [DOI] [PubMed] [Google Scholar]
- 123.Nakamura K, Roesch MR, Olson CR. Neuronal activity in macaque SEF and ACC during performance of tasks involving conflict. J Neurophysiol. 2005;93:884–908. doi: 10.1152/jn.00305.2004. [DOI] [PubMed] [Google Scholar]
- 124.Mansouri FA, Buckley MJ, Tanaka K. Mnemonic function of the dorsolateral prefrontal cortex in conflict-induced behavioral adjustment. Science. 2007;318:987–990. doi: 10.1126/science.1146384. [DOI] [PubMed] [Google Scholar]
- 125.Narayanan NS, Laubach M. Neuronal correlates of post-error slowing in the rat dorsomedial prefrontal cortex. J Neurophysiol. 2008;100:520–525. doi: 10.1152/jn.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Alain C, McNeely HE, Yu H, Christensen BK, West R. Neurophysiological evidence of error monitoring deficits in patients with schizophrenia. Cereb Cortex. 2002;12:840–846. doi: 10.1093/cercor/12.8.840. [DOI] [PubMed] [Google Scholar]
- 127.Kopp B, Rist F. An event-related brain potential substrate of disturbed response monitoring in paranoid schizophrenic patients. J Abnorm Psychol. 1999;108:337–346. doi: 10.1037//0021-843x.108.2.337. [DOI] [PubMed] [Google Scholar]
- 128.Mathalon DH, Dedor M, Faustman WO, Gray M, Askari N, Ford JM. Response-monitoring dysfunction in schizophrenia: an event-related brain potential study. J Abnorm Psychol. 2002;111:22–41. [PubMed] [Google Scholar]
- 129.Morris SE, Heerey EA, Gold JM, Holroyd CB. Learning-related changes in brain activity following errors and performance feedback in schizophrenia. Schizophr Res. 2008;99:274–285. doi: 10.1016/j.schres.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Morris SE, Yee CM, Nuechterlein KH. Electrophysiological analysis of error monitoring in schizophrenia. J Abnorm Psychol. 2006;115:239–250. doi: 10.1037/0021-843X.115.2.239. [DOI] [PubMed] [Google Scholar]
- 131.Laurens KR, Ngan ETC, Bates AT, Kiehl KA, Liddle PF. Rostral anterior cingulate cortex dysfunction during error processing in schizophrenia. Brain. 2003;126:610–622. doi: 10.1093/brain/awg056. [DOI] [PubMed] [Google Scholar]
- 132.Polli FE, Barton JJ, Thakkar KN, et al. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain. 2008;131:971–986. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- 133.Polli FE, Barton JJ, Vangel M, Goff DC, Iguchi L, Manoach DS. Schizophrenia patients show intact immediate error-related performance adjustments on an antisaccade task. Schizophr Res. 2006;82:191–201. doi: 10.1016/j.schres.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 134.Logan G, Cowan N. On the ability to inhibit thought and action: a theory of an act of control. Psychol Rev. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- 135.Boucher L, Palmeri TJ, Logan GD, Schall JD. Inhibitory control in mind and brain: an interactive race model of countermanding saccades. Psychol Rev. 2007;114:376–397. doi: 10.1037/0033-295X.114.2.376. [DOI] [PubMed] [Google Scholar]
- 136.Band GP, van der Molen MW, Logan GD. Horse-race model simulations of the stop-signal procedure. Acta Psychol (Amst) 2003;112:105–142. doi: 10.1016/s0001-6918(02)00079-3. [DOI] [PubMed] [Google Scholar]
- 137.Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychol Sci. 1997;8:60–64. [Google Scholar]
- 138.Lijffijt M, Kenemans JL, Verbaten MN, van Engeland H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: deficient inhibitory motor control? J Abnorm Psychol. 2005;114:216–222. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- 139.Chamberlain SR, Fineberg NA, Blackwell AD, Robbins TW, Sahakian BJ. Motor inhibition and cognitive flexibility in obsessive-compulsive disorder and trichotillomania. Am J Psychiatry. 2006;163:1282–1284. doi: 10.1176/ajp.2006.163.7.1282. [DOI] [PubMed] [Google Scholar]
- 140.Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79:273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 141.Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 2002;66:265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- 142.Colzato LS, van den Wildenberg WP, Hommel B. Impaired inhibitory control in recreational cocaine users. PLoS ONE. 2007;2:e1143. doi: 10.1371/journal.pone.0001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- 144.Floden D, Stuss DT. Inhibitory control is slowed in patients with right superior medial frontal damage. J Cogn Neurosci. 2006;18:1843–1849. doi: 10.1162/jocn.2006.18.11.1843. [DOI] [PubMed] [Google Scholar]
- 145.Nachev P, Wydell H, O'Neill K, Husain M, Kennard C. The role of the pre-supplementary motor area in the control of action. Neuroimage. 2007;36(suppl. 2):T155–T163. doi: 10.1016/j.neuroimage.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chevrier AD, Noseworthy MD, Schachar R. Dissociation of response inhibition and performance monitoring in the stop signal task using event-related fMRI. Hum Brain Mapp. 2007;28:1347–1358. doi: 10.1002/hbm.20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- 150.van den Wildenberg WP, van Boxtel GJ, van der Molen MW, Bosch DA, Speelman JD, Brunia CH. Stimulation of the subthalamic region facilitates the selection and inhibition of motor responses in Parkinson’s disease. J Cogn Neurosci. 2006;18:626–636. doi: 10.1162/jocn.2006.18.4.626. [DOI] [PubMed] [Google Scholar]
- 151.Hershey T, Revilla FJ, Wernle AR, et al. Cortical and subcortical blood flow effects of subthalamic nucleus stimulation in PD. Neurology. 2003;61:816–821. doi: 10.1212/01.wnl.0000083991.81859.73. [DOI] [PubMed] [Google Scholar]
- 152.Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci Res. 2002;43:111–117. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 153.Tannock R, Schachar RJ, Carr RP, Chajczyk D, Logan GD. Effects of methylphenidate on inhibitory control in hyperactive children. J Abnorm Child Psychol. 1989;17:473–491. doi: 10.1007/BF00916508. [DOI] [PubMed] [Google Scholar]
- 154.Aron AR, Dowson JH, Sahakian BJ, Robbins TW. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2003;54:1465–1468. doi: 10.1016/s0006-3223(03)00609-7. [DOI] [PubMed] [Google Scholar]
- 155.Fillmore MT, Rush CR, Hays L. Acute effects of cocaine in two models of inhibitory control: implications of non-linear dose effects. Addiction. 2006;101:1323–1332. doi: 10.1111/j.1360-0443.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- 156.Chamberlain SR, Muller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science. 2006;311:861–863. doi: 10.1126/science.1121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Overtoom CC, Verbaten MN, Kemner C, et al. Effects of methylphenidate, desipramine, and L-dopa on attention and inhibition in children with Attention Deficit Hyperactivity Disorder. Behav Brain Res. 2003;145:7–15. doi: 10.1016/s0166-4328(03)00097-4. [DOI] [PubMed] [Google Scholar]
- 158.Congdon E, Lesch KP, Canli T. Analysis of DRD4 and DAT polymorphisms and behavioral inhibition in healthy adults: implications for impulsivity. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:27–32. doi: 10.1002/ajmg.b.30557. [DOI] [PubMed] [Google Scholar]
- 159.Eagle DM, Robbins TW. Inhibitory control in rats performing a stop-signal reaction-time task: effects of lesions of the medial striatum and d-amphetamine. Behav Neurosci. 2003;117:1302–1317. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- 160.Eagle DM, Robbins TW. Lesions of the medial prefrontal cortex or nucleus accumbens core do not impair inhibitory control in rats performing a stop-signal reaction time task. Behav Brain Res. 2003;146:131–144. doi: 10.1016/j.bbr.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 161.Eagle DM, Baunez C, Hutcheson DM, Lehmann O, Shah AP, Robbins TW. Stop-signal reaction-time task performance: role of prefrontal cortex and subthalamic nucleus. Cereb Cortex. 2008;18:178–188. doi: 10.1093/cercor/bhm044. [DOI] [PubMed] [Google Scholar]
- 162.Eagle DM, Tufft MR, Goodchild HL, Robbins TW. Differential effects of modafinil and methylphenidate on stop-signal reaction time task performance in the rat, and interactions with the dopamine receptor antagonist cis-flupenthixol. Psychopharmacology (Berl) 2007;192:193–206. doi: 10.1007/s00213-007-0701-7. [DOI] [PubMed] [Google Scholar]
- 163.Badcock JC, Michie PT, Johnson L, Combrinck J. Acts of control in schizophrenia: dissociating the components of inhibition. Psychol Med. 2002;32:287–297. doi: 10.1017/s0033291701005128. [DOI] [PubMed] [Google Scholar]
- 164.Vink M, Ramsey NF, Raemaekers M, Kahn RS. Striatal dysfunction in schizophrenia and unaffected relatives. Biol Psychiatry. 2006;60:32–39. doi: 10.1016/j.biopsych.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 165.Bellgrove MA, Chambers CD, Vance A, Hall N, Karamitsios M, Bradshaw JL. Lateralized deficit of response inhibition in early-onset schizophrenia. Psychol Med. 2006;36:495–505. doi: 10.1017/S0033291705006409. [DOI] [PubMed] [Google Scholar]
- 166.Enticott PG, Ogloff JR, Bradshaw JL. Response inhibition and impulsivity in schizophrenia. Psychiatry Res. 2008; 15;157(1–3):251–4. doi: 10.1016/j.psychres.2007.04.007. [DOI] [PubMed] [Google Scholar]