Abstract
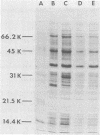
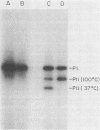
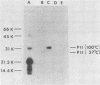
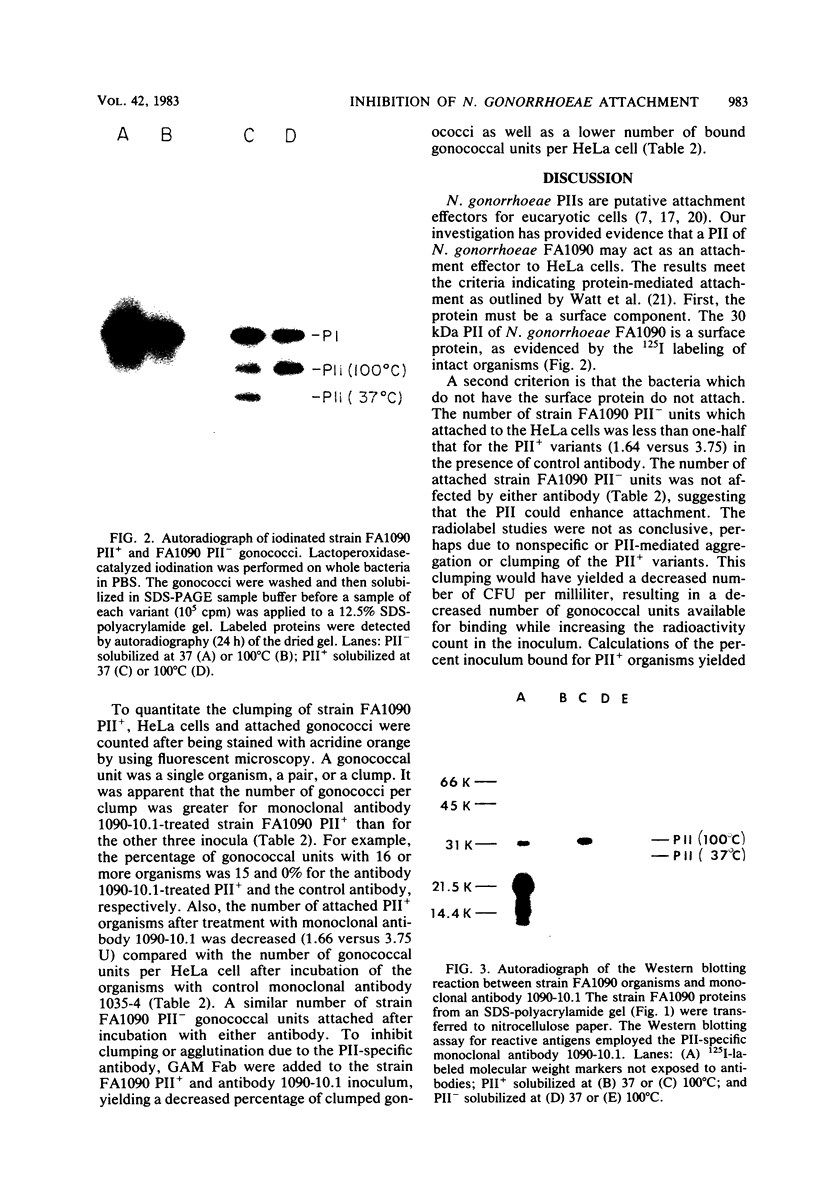
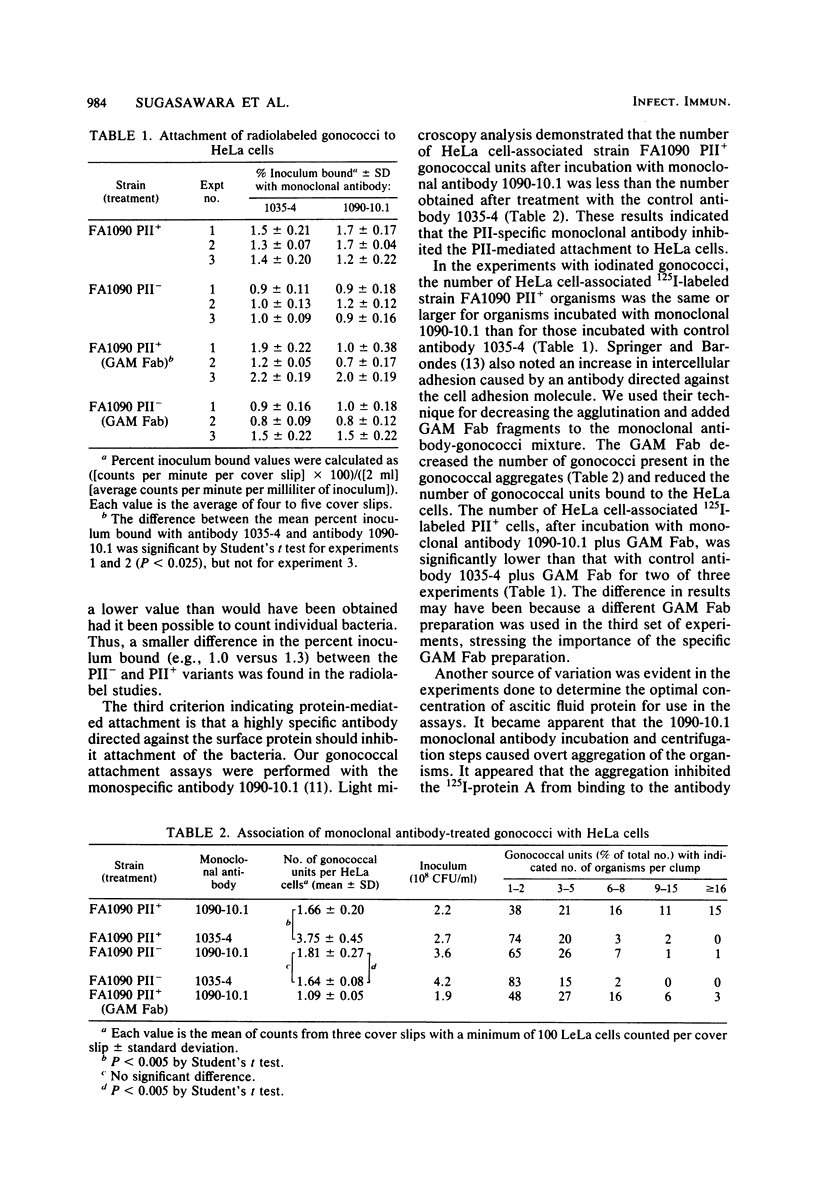
This study showed that a protein II (PII) of Neisseria gonorrhoeae FA1090 appeared to act as a mediator of attachment to HeLa cells. Two colony variants of FA1090 were selected. Both gonococcal variants were nonpiliated, but one contained a PII and the other did not. A monoclonal antibody (1090-10.1), which was directed against the PII, inhibited the apparent PII-mediated attachment to HeLa cells. Antibodies produced from clone 1035-4, which had no PII specificity, did not inhibit the attachment and were used as controls. Inhibition of gonococcal attachment by the 1090-10.1 monoclonal antibodies was demonstrated by fluorescent microscopy analysis. Monoclonal antibody 1090-10.1 appeared to cause agglutination of the PII-containing organism. To block the clumping caused by the PII-specific monoclonal antibodies, Fab fragments of goat anti-mouse IgG were incubated with gonococci and the 1090-10.1 monoclonal antibodies. The results showed that the goat anti-mouse IgG Fab fragments partially blocked the agglutination caused by the PII-specific monoclonal antibody. The effect of the 1090-10.1 antibodies on attachment was also determined by monitoring the HeLa cells with attached iodinated gonococci. The monoclonal antibody appeared to inhibit the PII-mediated attachment.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. P., Klitzman J. M., Hellström K. E. A microassay for antibody binding to tumor cell surface antigens using 125I-labelled protein a from Staphylococcus aureus. J Immunol Methods. 1977;15(1):57–66. doi: 10.1016/0022-1759(77)90017-5. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- James J. F., Zurlinden E., Lammel C. J., Brooks G. F. Relation of protein I and colony opacity to serum killing of Neisseria gonorrhoeae. J Infect Dis. 1982 Jan;145(1):37–44. doi: 10.1093/infdis/145.1.37. [DOI] [PubMed] [Google Scholar]
- King G. J., Swanson J. Studies on gonococcus infection. XV. Identification of surface proteins of Neisseria gonorrhoeae correlated with leukocyte association. Infect Immun. 1978 Aug;21(2):575–584. doi: 10.1128/iai.21.2.575-584.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G., James J. F., Swanson J. Studies on gonococcus infection. XI. Comparison of in vivo and vitro association of Neisseria gonorrhoeae with human neutrophils. J Infect Dis. 1978 Jan;137(1):38–43. doi: 10.1093/infdis/137.1.38. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambden P. R., Heckels J. E., James L. T., Watt P. J. Variations in surface protein composition associated with virulence properties in opacity types of Neisseria gonorrhoeae. J Gen Microbiol. 1979 Oct;114(2):305–312. doi: 10.1099/00221287-114-2-305. [DOI] [PubMed] [Google Scholar]
- NISONOFF A., WISSLER F. C., LIPMAN L. N., WOERNLEY D. L. Separation of univalent fragments from the bivalent rabbit antibody molecule by reduction of disulfide bonds. Arch Biochem Biophys. 1960 Aug;89:230–244. doi: 10.1016/0003-9861(60)90049-7. [DOI] [PubMed] [Google Scholar]
- Nachamkin I., Cannon J. G., Mittler R. S. Monoclonal antibodies against Neisseria gonorrhoeae: production of antibodies directed against a strain-specific cell surface antigen. Infect Immun. 1981 May;32(2):641–648. doi: 10.1128/iai.32.2.641-648.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer W. R., Barondes S. H. Cell adhesion molecules: detection with univalent second antibody. J Cell Biol. 1980 Dec;87(3 Pt 1):703–707. doi: 10.1083/jcb.87.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Colony opacity and protein II compositions of gonococci. Infect Immun. 1982 Jul;37(1):359–368. doi: 10.1128/iai.37.1.359-368.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., King G., Zeligs B. Studies on gonococcus infection. VIII. 125Iodine labeling of gonococci and studies on their in vitro interactions with eukaryotic cells. Infect Immun. 1975 Mar;11(3):453–459. doi: 10.1128/iai.11.3.453-459.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Kraus S. J., Gotschlich E. C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J Exp Med. 1971 Oct 1;134(4):886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Sparks E., Young D., King G. Studies on Gonococcus infection. X. Pili and leukocyte association factor as mediators of interactions between gonococci and eukaryotic cells in vitro. Infect Immun. 1975 Jun;11(6):1352–1361. doi: 10.1128/iai.11.6.1352-1361.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramont E. C. Specificity of inhibition of epithelial cell adhesion of Neisseria gonorrhoeae. Infect Immun. 1976 Aug;14(2):593–595. doi: 10.1128/iai.14.2.593-595.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Mazauskas C. Immunological properties of murine thymus-dependent lymphocyte surface glycoproteins. Eur J Immunol. 1976 Aug;6(8):557–562. doi: 10.1002/eji.1830060806. [DOI] [PubMed] [Google Scholar]