Abstract
Deficient sensorimotor gating as indexed by prepulse inhibition (PPI) of the startle response has been reported repeatedly in patients suffering from schizophrenia. According to the widely accepted “protective hypothesis,” PPI reflects the protection of ongoing information processing against interference by other stimuli. Alternatively, it has been proposed that PPI might be regulated by startle reflex circuit excitability. In the present study, we evaluated these 2 conceptually divergent approaches underlying the regulation of PPI. To this end, we assessed sensorimotor gating as indexed by PPI, the reactivity to the prepulse-alone stimulus indexed as prepulse-elicited reactivity (PPER), and acoustic blink reflex excitability in terms of paired pulse suppression (PPS) within a single recording session in 13 unmedicated and 24 medicated (11 first break) schizophrenia patients in comparison to 43 healthy control subjects. The results showed that PPI was significantly reduced in unmedicated, but not in medicated schizophrenia patients. Furthermore, unmedicated patients could be distinguished from the medicated patients and control subjects in terms of PPER. In contrast to PPI, PPS did not differ between patients and control subjects. These findings are in line with the “protective hypothesis” of PPI and indicate that reduced sensorimotor gating in schizophrenia patients might be based on a reduced perception and/or processing of the prepulse stimulus. The extent to which PPER may or may not be causally associated with sensorimotor gating in schizophrenia has to be further investigated in human and animal studies.
Keywords: schizophrenia, prepulse inhibition, prepulse-elicited reaction, paired pulse suppression, reflex excitability, human
Introduction
It has been postulated that impaired cognition and positive symptoms of schizophrenia are related to deficient inhibition and/or filtering during early information processing, potentially leading to sensory overload, which is thought to be associated with psychotic symptom formation (for a review, see Braff et al1). Central inhibition or gating can be assessed by prepulse inhibition (PPI) of the acoustic startle response. PPI refers to the attenuation of the reflexive startle reaction elicited by an intense pulse stimulus when its presentation is shortly preceded (30–500 milliseconds) by a weak prepulse stimulus.2,3 According to the “protective hypothesis” of Graham,3–5 the inhibitory effect of the prepulse stimulus upon subsequent pulse stimulus processing reflects the protection of the ongoing processing of the antecedent prepulse against interference by the succeeding pulse. Deficient sensorimotor gating has been described in patients with schizophrenia by many different investigators worldwide (for a review, see Braff et al1).
On the basis of the theoretical accounts put forward by Graham3–5 and Braff et al,6 enhanced perception of the prepulse stimulus and therefore of its processing would be associated with an enhancement of PPI. Indeed, it is widely accepted that a more intense prepulse will generate a stronger PPI effect.7,8 Moreover, manipulations designed to influence the perception of the prepulse stimulus, such as verbal instructions to attend or ignore the prepulse stimulus in human subjects, can produce clear effects on the magnitude of PPI. Increased inhibition has been observed when subjects were instructed to explicitly attend to the prepulse stimulus.9–12 Similarly, it has been suggested that the disrupting effect of the dopamine agonist apomorphine on PPI in rats might be based on reduced detectability of weak prepulse stimuli.13 Furthermore, a positive correlation between prepulse-elicited reactivity (PPER) and PPI has been found recently in mice.14,15 Therefore, the speculation arises that reduced sensorimotor gating in schizophrenia patients could be related to a weakened perception of and/or reaction to the prepulse stimulus.
Although it has been generally assumed that a prepulse of a typical intensity used in human and animal PPI experiments (8–16 dB above background noise) does not per se elicit a measurable motor response,2,3,16,17 Blumenthal et al18,19 have shown over a decade ago that auditory stimuli of an intensity in the range of 50–60 dBA are indeed able to elicit a measurable motor response in the absence of a constant background noise. Yet this finding cannot be readily generalized to studies in which a background noise, partly masking the prepulse stimulus, is applied. Until recently,14,15,20–25 few attempts have been made to assess directly the reaction or the processing to the prepulse stimulus per se because it is not uncommon that trials in which only the prepulse stimulus is presented, thus allowing a quantification of PPER, are omitted in the testing procedures. However, a number of recent studies directly assessing PPER in human and animal experiments yield divergent results. Dahmen and Corr23 reported notable PPER that led them to classify it as a startle response, and we recently demonstrated that quantification of PPER, although of a magnitude much less than that reported by Dahmen and Corr,23 is reliable and reproducible in a series of experiments including healthy human volunteers20–22 and rodents.14,15 In contrast, Swerdlow et al24,25 failed to detect significant response to prepulse stimuli per se in healthy volunteers, schizophrenia patients,25 and rodents.24 One major discrepancy between the findings of Dahamen and Corr23 in humans and those of Yee et al14,15 based on experiments conducted in rodents lies in the nature of correlation. While there was a positive correlational relationship between PPI and PPER in rodents, Dahamen and Corr23 reported a negative correlation between the response probability to the prepulse and PPI.
As an alternative to the “protective hypothesis” of sensorimotor gating, Schicatano et al26 suggested that PPI might be more closely governed by reflex excitability. Based on their findings in rodents and patients with Parkinson disease, the authors proposed that the blink reflex modification by a prepulse (ie, PPI) reflects the intrinsic characteristics of the reflex circuit rather than an external adjustment of the reflex by the prepulse. The excitability of blink reflex circuits can be estimated using another form of startle modification, the so-called paired pulse suppression (PPS [Also often denoted as “paired pulse inhibition.” The term “paired pulse suppression” has been preferred in the present report as “paired pulse inhibition” and “prepulse inhibition” would share the same abbreviation.]) of the startle paradigm, in which pairs of identical blink-eliciting stimuli, separated typically by 500–2000 milliseconds, are presented. The ratio of the response magnitude to the second stimulus (S2) relative to the first stimulus (S1) serves as a measure of the blink reflex circuit excitability.27,28 Normally, upon perception of the startle-eliciting stimulus, excitatory processes initially dominate the reflex circuit, whereas inhibitory processes are prominent at a later phase. The inhibitory period initiated by S1 dominates usually for several hundreds of milliseconds, thus with lead intervals of less than 1500 milliseconds, the blink evoked by S2 is smaller than that elicited by S1.27,29 With each eyelid movement due to a blink, air races through the eyelashes and the lid rubs against the cornea. Presentation of either of these stimuli is sufficient to elicit a reflex blink. Thus, if a blink or the stimulus that evokes it fails to initiate such a period of inhibition, then each blink will elicit a reflex blink, starting blepharoclonus or a spasm of lid closure.27 The state of the blink reflex circuit is labeled as hyperexcitable, if the subject's response to S2 is even greater than the response magnitude elicited by S1. Abnormalities in blink reflex excitability has been observed in various neurological and psychiatric disorders like Parkinson disease,26,29 facial palsy30 and blepharsospasm,31,32 Tourette syndrome,33–35 and Huntington disease.36,37
Despite a number of operational and parametric differences between PPI and PPS, such as the low-intensity prepulse in PPI compared with the distinct startle-evoking stimuli used in PPS, and the much shorter lasting inhibitory phase initiated by the prepulse in PPI (<500 milliseconds) compared with the long lasting inhibitory period elicited by S1 in PPS (>1000 milliseconds), there are also many similarities: both PPI and PPS can be demonstrated across species using comparable stimulus parameters16,26 and both are disrupted by the dopamine agonist apomorphine in rodents.16 Moreover, PPI and PPS are known to be deficient in patients with Tourette syndrome33–35 and blepharospasm,31,32 and it has been shown that the 2 phenomena are highly correlated in humans as well as in rodents.16,26 However, it is noteworthy that most of the experiments investigating deficient inhibitory processes in various patients populations rarely make use of both PPI and PPS within the same study. For instance, neurological disorders like Parkinson disease and blepharospasm have been intensively investigated using PPS, while psychiatric disorders like schizophrenia have been characterized by the use of PPI. It is of great importance to understand the conceptual difference underlying these 2 paradigms of startle modification: while deficits characterized on the basis of PPS are discussed in relation to blink circuit excitability, results derived from PPI experiments are discussed in terms of sensorimotor gating. This has led Swerdlow et al16 to conclude that clinical research using PPI and PPS have run strikingly parallel, nonintersecting paths.
The primary aim of the present study was to investigate the 2 conceptual divergent approaches underlying the regulation of PPI. Namely, if deficient sensorimotor gating in schizophrenia patients is related to weakened reaction to or detection of the prepulse stimulus or alternatively if deficient PPI is associated with the excitatory state of the blink reflex circuit and therefore regulated by intrinsic characteristics of the reflex circuit. To this end, sensorimotor gating as indexed by PPI, the reactivity to the prepulse-alone stimulus as indexed by PPER, and acoustic blink reflex excitability in terms of PPS were assessed within a single recording session in unmedicated and medicated schizophrenia patients in comparison to healthy control subjects. We hypothesize that PPI would be impaired in unmedicated schizophrenia patients, but such impairments might be limited or absent in the patients treated with atypical antipsychotic medication. Furthermore, we speculate that if PPI is at least partly regulated by the degree of the perception or detection of the prepulse stimulus, PPER would be reduced in patients exhibiting deficient PPI. This speculation is based on an expected positive relationship between PPER and PPI that has been observed in mice,14,15 although an equivalent relationship has not been reported in human subjects (but also see Dahmen and Corr23). On the other hand, if PPI is mainly determined by intrinsic characteristics of the blink reflex circuit, diminished PPI in the patients should be associated with abnormalities in PPS.
Materials and Methods
Subjects
Thirty-nine patients suffering from schizophrenia according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, diagnostic criteria and 46 healthy control subjects participated in the study. Fourteen patients were never medicated, first break schizophrenia patients. Of the 25 medicated patients (all were treated with atypical antipsychotic medication, see table 1), 11 had their first psychotic episode within the last 4 weeks before the time of testing. All patients were inpatients, except 5 medicated patients who were stable outpatients. Patients were recruited through the Psychiatric University Hospital Zurich and the Psychiatric Services of Aargau Canton. The control subjects were recruited through local advertisements. The exclusion criteria in schizophrenia patients and control subjects included substance dependence, major medical or neurological disorder, mental retardation, and hearing defects as determined by a standard computerized whispered voice test (for a review, see Pirozzo et al38). Furthermore, patients were excluded for evidence of any additional psychiatric illnesses other than schizophrenia. In addition, the control group was also screened by the DIA-X39 diagnostic expert system to exclude those subjects with personal or family (first-degree relatives) histories of major psychiatric disorders. The data of 3 control subjects and 2 patients (one medicated, one unmedicated) were rejected because no distinct startle reaction could be elicited (nonresponders, mean startle amplitude on pulse (S1) trials <10 μV).
Table 1.
Demographic and Clinical Characteristics
| Control subjects (mean ± SE) | Unmedicated patients (mean ± SE) | Medictated patientsa (mean ± SE) | |
| Age [y] | 27.3 ± 1.1 | 24.3 ± 1.7 | 30.2 ± 1.5 |
| Sex [m/f] | 35/8 | 12/1 | 19/5 |
| Smokers | 24 | 7 | 15 |
| PANSS, positive symptoms | 12.9 ± 2.0 | 13.1 ± 1.2 | |
| PANSS, negative symptoms | 14.3 ± 2.8 | 16.4 ± 1.6 | |
| PANSS, general psychopathology | 26.1 ± 2.3 | 28.8 ± 1.6 |
Note: SE, standard error; PANSS, Positive and Negative Syndrome Scale.
Medication: clozapine (n = 4), quetiapine (n = 6), risperidone (n = 6), olanzapine (n = 6), aripirazole (n = 2), one patient receiving clozapine and one patient receiving quetiapine additionally received haloperidol.
After receiving a complete description of the study (orally and written), subjects gave written informed consent to participate in the study. The study protocol and consent forms were approved by the Ethical Committee of the Zurich and Aargau cantons. On the day of testing, patients’ symptoms were rated with the Positive and Negative Syndrome Scale (PANSS40). Duration of illness was determined based on the first appearance of psychotic symptoms as reported in the case records.41,42 All participants were instructed to abstain from drinking alcohol for at least 24 hours before each test session, not to drink any caffeine-containing beverages on the day of testing, and to keep their usual smoking habits. Smoking was not allowed from 1 hour prior to the recording session. In a subgroup of patients receiving low dose of sedative medication (lorazepam) before sleeping, the medication was not administered from the evening before data assessment. Demographic and clinical relevant characteristics are summarized in table 1.
Session Definition
The test session was composed of a mixture of the following types of trials that were presented against a constant 70 dBA background noise: (a) trials in which 2 startle-eliciting stimuli (S1 and S2), separated by a stimulus-onset asynchrony (SOA) of either 500 (pulse-pulseSOA500ms) or 1000 milliseconds (pulse-pulseSOA1000ms), (b) trials in which a prepulse stimulus preceded a startle-eliciting pulse stimulus with an SOA of 60 (prepulse-pulseSOA60ms) or 120 milliseconds (prepulse-pulseSOA120ms), (c) trials in which only the prepulse stimulus was presented (prepulse-alone), and (d) trials in which no discrete stimulus other than the constant background noise was presented (ns [no-stimulus] trials). All stimuli and background noise employed in the experiment consisted of broadband white noise. The stimulus intensity of S1 and S2 in pulse-pulse trials and of the pulse stimulus in prepulse-pulse trials was set at 115 dBA and was of a duration of 40 milliseconds. The intensity of the prepulse stimulus in prepulse-pulse and prepulse-alone trials was 86 dBA, and was of a duration of 20 milliseconds. Rise and fall time of all stimuli was less than 1 millisecond. The SOAs of 60 and 120 milliseconds had been chosen because a majority of studies with schizophrenia patients show deficient PPI at these SOAs (for a review see Braff et al1 and Hamm et al43). Moreover, the 2 SOAs produce very reliable PPI occurring in over 90% of normal human volunteers who exhibit a normal startle eyeblink response.44 After a 3-minute period of acclimatization to the background noise, 70 discrete trials were presented according to a variable intertrial interval with a mean of 14 seconds (ranged from 9–17 seconds). The first (block 1) and last block (block 3) consisted of 5 consecutive pulse-pulse trials. The middle block (block 2) consisted of 60 trials, ie, 12 trials of each of the 5 conditions (pulse-pulseSOA500/1000ms, prepulse-pulseSOA60ms, prepulse-pulseSOA120ms, prepulse-alone, and ns trial), presented in a pseudorandomized order. The 2 different SOAs (500 and 1000 milliseconds) employed in the pulse-pulse trials alternated throughout the whole session. The test session lasted approximately 20 minutes.
Apparatus, Data Recording, and Data Processing
Acoustic startle stimuli were generated by EMG-SR (San Diego Instruments, San Diego, CA) and presented binaurally through headphones (TDH-39-P, Maico, Minneapolis, MN). The orbicularis oculi electromyography (EMG) was measured using the ActiveTwo system (Biosemi, Amsterdam, The Netherlands). All electrodes were active silver/silver chloride electrodes. Two electrodes were placed below the right eye over the orbicularis oculi muscle to measure eye-blink activity. The system recorded continuously during the whole session, using a sampling rate of 4096 Hz. BrainVision Analyzer (Brainproducts, Munich, Germany) was used to preprocess the recorded data. The two electrodes located over the orbicularis oculi muscle were referenced bipolarly, then the data were band-pass filtered (30–500 Hz), downsampled to 1000 Hz, and rectified. Segmentation was performed from 50 milliseconds prior to the onset of the relevant stimulus (the prepulse in prepulse-pulse trials, S1 in pulse-pulse, and the prepulse in prepulse-alone trials) to 1450 miliseconds after stimulus onset. The segmented data were exported for quantitative analysis. The EMG record of each and every trial was separately scored using the Windows-based software emgBLINK version 1.2 (CST, Zurich, Switzerland). Before scoring, the EMG was smoothed with a time constant of 5 milliseconds. Baseline amplitude was calculated by the mean response amplitude of the first 50 milliseconds before any stimulus onset. Stimulus response amplitudes were assessed as peak response minus baseline value of the respective trial. Peak response was defined as the highest reaction in the time window between stimulus onset to 150 milliseconds after stimulus onset. Response amplitudes on ns trials were scored as peak response sample between 51 and 201 milliseconds minus baseline value of the respective trial. Every trial was examined for signs of spontaneous eye blinks in the scoring windows, and other possible signs of corrupted EMG signal, and if present the trial was excluded. A total of 193 trials out of a grand total of 5600 trials (<3.5%) were therefore excluded. Moreover, for 3 subjects (2 controls and 1 medicated patient), peak latencies on prepulse-pulse trials were not included because the very high PPI in these subjects resulted in the absence of a distinct startle reaction and consequently the peak latency could not be reliably determined. In each of these cases, subjects exhibited more than 94% PPI. Analogously, peak latencies of 3 subjects (1 control subject, 2 medicated patients) to S2 in pulse-pulse trials were rejected.
Startle reactivity was indexed by the peak amplitude reaction of the startle reaction elicited by S1. For the calculation of PPI, the ratios of the startle reaction elicited by S1 and the startle reaction amplitude elicited by the pulse in prepulse-pulse trials were calculated separately for both SOAs (60 and 120 milliseconds) and expressed as percent suppression (%PPI) by the formula [1 – (amplitudeprepulse-pulse)/(amplitudeS1(block2))] × 100%. Similarly, percent PPS (%PPS) was calculated according to the formula [1 – (amplitudeS2(block2))/(amplitudeS1(block2))] × 100%, separately for each of the 2 pulse-pulse SOAs (500 and 1000 miliseconds). Percent habituation of the startle reaction was calculated according to the formula [1 – (amplitudeS1(block3))/(amplitudeS1(block1)] × 100%.
Statistical Analysis
All statistical analyses were conducted using the statistical software Statistica 7 for Windows (Statsoft Inc, Tulsa, OK). Startle amplitudes were ln transformed because of the highly skewed distribution and the resulting deviation from the normal distribution (PShapiro-Wilk W < .001 for all 3 groups), which was accompanied by a significant heterogeneity of variance among the 3 groups (Levene test for homogeneity; P < .05). Even though parametric analysis of variance (ANOVA) can tolerate deviations from the normality assumption, enhanced compliance to it, which often also results in homogeneity of variance, improves considerably the power of the statistical tests.45,46 After ln transformation, startle amplitudes did not deviate significantly from normality (PShapiro-Wilk W > .05 for all three groups) and homogenity of variance (Levene test for homogeneity; P > .05). Previous examination of nontransformed data further confirmed that the patterns of results were largely in agreement with those obtained using the ln-transformed data. The same ln transformation was also applied to the prepulse-alone amplitudes. Percent calculations were performed on the basis of nontransformed startle data, and both the %PPI and %PPS data sets conformed to a normal distribution.
Startle amplitudes were analyzed using repeated-measures ANOVA with blocks (1–3) as within-subject factor and group (unmedicated patients vs medicated patients vs control subjects) as between-subject factor. A 2-way ANOVA (stimulus condition as repeated measures and group as between-subject factor) for PPER was performed including the prepulse-alone and ns conditions. Startle amplitude latencies were similarly analyzed with stimulus conditions S1, prepulse-pulseSOA60ms and prepulse-pulseSOA120ms with respect to PPI, and with the stimulus conditions S1, S2SOA500, and S2SOA1000 with respect to PPS as within-subject factors and group as between-subject factor. The %PPI and %PPS values were subjected to a 2 × 3 (SOA × group) repeated-measures ANOVA. Percent habituation was analyzed using 1-way ANOVA. Pearson correlation was used to assess potential linear relationship between %PPI and PPER and between %PPI and %PPS. Duration of illness was not taken into account in the final analysis because our data did not reveal any significant difference between medicated first break and medicated chronic patients in any electrophysiological or psychometrical parameters assessed, which is in line with previous literature.42 The significance level of all statistical tests was set at P < .05. Fisher's LSD post hoc pair-wise comparison was used to examine the patterns of significant between-subjects factors.
Results
Clinical and Demographic Characteristics
Unmedicated patients and control subjects did not differ significantly in age or smoking habits. Medicated patients were slightly, but significantly older than unmedicated patients (Pposthoc < .05). However, as seen in table 1, the mean age differences between these groups were minimal. Unmedicated and medicated patients did not differ in the symptoms rated by the PANSS. Moreover, there were no significant correlations between PANSS scores and any startle measures.
Startle Reactivity and Habituation
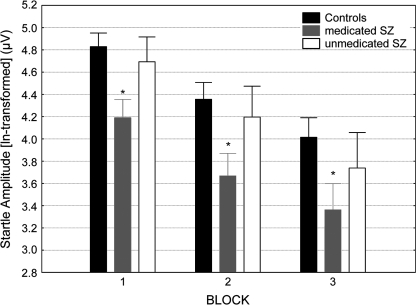
Analysis of the startle amplitudes elicited by S1 attained a significant group effect (F2,77 = 3.7, P < .05), indicating reduced startle reaction in the medicated patients (Pposthoc < .01), independent of the factor blocks because there was no group × blocks interaction. The unmedicated patients and control subjects did not differ from each other in their startle reaction (figure 1). The main effect of blocks was highly significant (F2,154 = 104.7, P < .0001), reflecting the process of habituation. Because the analysis of the startle reaction revealed no group × blocks interaction, it can be concluded that the habituation of the startle reaction across the 3 blocks did not differ significantly between patients and control subjects. This is in agreement with the absence of a significant difference in %habituation among the 3 groups (meancontrols = 48.23 ± 4.19% SE; meanunmedicated = 52.85 ± 9.03% SE; meanmedicated = 49.25 ± 5.78% SE).
Fig. 1.
Startle Reactivity (ln transformed) of the Control Subjects, Medicated and Unmedicated Schizophrenia Patients Across the 3 Blocks of the Test Session. Asterisk indicates significant (P < .05) reduction in startle reactivity compared with the control subjects and unmedicated patients. Error bars refer to ± standard error of the mean.
To facilitate comparison with other studies reporting nontransformed startle data, the respective raw amplitude data are also provided here: Block 1 (meancontrols = 157.47 ± 14.34 μV SE; meanunmedicated = 138.84 ± 29.24 μV SE; meanmedicated = 92.59 ± 14.80 μV SE); Block 2 (meancontrols = 112.34 ± 13.01 μV SE; meanunmedicated = 108.22 ± 34.32 μV SE; meanmedicated = 61.98 ± 12.18 μV SE); Block 3 (meancontrols = 89.86 ± 12.29 μV SE; meanunmedicated = 89.16 ± 35.63 μV SE; meanmedicated = 52.88 ± 12.87 μV SE).
Prepulse Inhibition
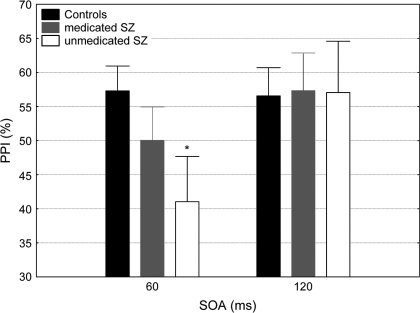
Analysis of %PPI revealed a significant main effect of SOA (F1,77 = 7.45, P < .01) and a significant SOA × group interaction (F2,77 = 3.2, P < .05). Post hoc analysis showed that unmedicated patients exhibited significantly reduced %PPI in the SOA60ms condition (P < .05) compared with the control subjects. Medicated patients exhibited an intermediate level of PPI in the SOA60ms condition, between that of unmedicated patients and control subjects, but not significantly different from either group. %PPI at the SOA120ms condition did not differ among the 3 groups (figure 2).
Fig. 2.
Percent Prepulse Inhibition (PPI) at the 2 Prepulse–Pulse Conditions (SOA: 60 and 120 milliseconds) in the Control Subjects, Medicated ,and Unmedicated Schizophrenia Patients. Asterisk indicates significant (P < .01) difference in %PPI compared with the control subjects. Error bars refer to ± standard error of the mean.
Peak Latency
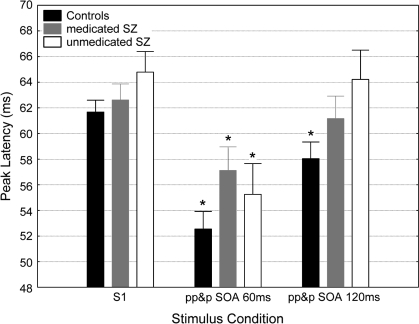
Analysis of peak latency revealed a significant effect of group (F2,77 = 3.14, P < .05) and of stimulus condition (F2,144 = 29.18, P < .0001) but not of their interaction (F4,144 = 1.21, P = .31). To test whether peak latency facilitation (ie, the reduction of peak latency caused by the prepulse stimulus) differed between the 3 groups, post hoc pairwise comparisons were performed. This analysis revealed that S1 peak latencies did not differ across the 3 groups. However, while there was significant peak latency facilitation in regard to the prepulse-pulseSOA60 condition across all the groups (P < .01, for all groups), only the control subjects exhibited the peak latency facilitation in the prepulse-pulseSOA120 condition (P < .01) (figure 3). In contrast to these results associated with PPI, neither significant main effects nor an interaction between the factors SOA and group was observed in regard to PPS.
Fig. 3.
Peak Latencies at the S1 (pulse) Condition and the 2 Prepulse-Pulse Conditions (SOA: 60 and 120 milliseconds) in the Control Subjects, Medicated, and Unmedicated Schizophrenia Patients. Asterisk indicates significant (P < .01) latency reduction in respect to the S1 condition. Error bars refer to ± standard error of the mean.
Prepulse-Elicited Reactivity
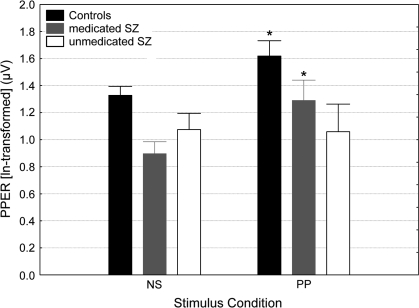
Results of the ANOVA revealed significant main effects group (F2,77 = 5.53, P < .01) and of stimulus condition (F1,77 = 8.7, P < .01) and a nonsignificant group × stimulus condition interaction (F2,77 = 2.00, P = .14). Nevertheless, based on our a priori hypothesis that the level of PPER differed in schizophrenia patients exhibiting deficient PPI in comparison to the healthy volunteers, post hoc pairwise comparisons were performed. This analysis revealed that the control subjects and medicated patients exhibited distinct PPER significantly above the baseline reactivity obtained in the ns trials. Interestingly, this was not the case for the unmedicated patients, whose reactivity to the prepulse stimulus was not statistically distinguishable from that obtained in the ns trials (figure 4). Reactivity on ns trials did not differ between unmedicated patients and control subjects, but medicated patients had a significantly reduced baseline activity compared with control subjects (Pposthoc < .01). Pearson correlation analysis revealed a significant negative correlation between PPER and %PPI in the SOA60ms condition in the control subjects (R = −0.45, P < .01) and in the unmedicated patients (R = −0.64, P < .05). There was no significant correlation between PPER and %PPI in the SOA120ms condition for any of the 3 groups.
Fig. 4.
Prepulse-Elicited Reactivity (ln Transformed) at the No Stimulus (NS) and Prepulse-alone (PP) Conditions in the Control Subjects, Medicated and Unmedicated Schizophrenia Patients. Asterisk indicates significant prepulse-elicited reaction above baseline (NS). Error bars refer to ± standard error of the mean.
To facilitate comparison with other studies reporting nontransformed startle data, the respective raw amplitude data are also provided here: ns condition: (meancontrols = 4.13 ± 0.27 μV SE; meanunmedicated = 3.16 ± 0.39 μV SE; meanmedicated = 2.71 ± 0.28 μV SE); prepulse condition: (meancontrols=6.83 ± 0.98 μV SE; meanunmedicated = 3.34 ± 0.64 μV SE; meanmedicated = 5.53 ± 1.53 μV SE).
Paired Pulse Suppression
Analysis of %PPS revealed neither a significant effect of group nor an interaction between SOA and group. %PPS was higher in the SOA500ms condition (meancontrols = 72.29 ± 2.07% SE; meanunmedicated = 66.75 ± 6.93% SE; meanmedicated = 74.39 ± 3.91% SE) than in the SOA1000ms condition (meancontrols = 46.35 ± 3.68% SE; meanunmedicated = 47.61 ± 8.07% SE; meanmedicated = 57.57 ± 4.62% SE) (main effect of SOA: F1,77 = 63.28, P < .0001). As shown in table 2, %PPS in the SOA500ms condition correlated positively with %PPI in SOA60ms condition in all 3 groups. A similar positive correlation was also found between %PPS in the SOA1000ms and %PPI in SOA60ms condition, but this correlation was only significant in the medicated patients, while only a trend in the same direction was observed in the unmedicated patients and control subjects. There were no significant correlations between any of the 2 %PPS conditions and %PPI in the SOA60ms condition among the medicated patients. In contrast, %PPS in SOA500ms condition correlated positively with %PPI in SOA120ms condition in both the unmedicated patients and control subjects (table 2). Hyperexcitability was observed only in 2 cases; one unmedicated patient and one control subject exhibited negative %PPS values that were limited to the SOA1000ms condition.
Table 2.
Pearson Correlation Coefficient (r) From the Diverse Correlations Between %PPI and %PPS
| %PPISOA60ms | %PPISOA120ms | |
| Controls: | ||
| %PPSSOA500ms | 0.48a | 0.65b |
| %PPSSOA1000ms | 0.27 | 0.24 |
| Unmedicated SZ: | ||
| %PPSSOA500ms | 0.75b | 0.68b |
| %PPSSOA1000ms | 0.52 | 0.22 |
| Medicated SZ: | ||
| %PPSSOA500ms | 0.44c | 0.31 |
| %PPSSOA1000ms | 0.49c | 0.25 |
| All subjects | ||
| %PPSSOA500ms | 0.54a | 0.54a |
| PPSSOA1000ms | 0.35a | 0.54c |
Note: PPI, prepulse inhibition; PPS, paired pulsse suppression; SOA, stimulus-onset asynchrony.
P < .001.
P < .01.
P < .05.
Discussion
To our knowledge, this is the first study assessing sensorimotor gating as indexed by PPI, reflex excitability in terms of PPS, and the direct reaction to the prepulse stimulus (PPER) within a single recording session in schizophrenia patients in comparison to healthy control subjects. This therefore allowed us to investigate possible relationships between PPI, PPER, and PPS and to address specifically if deficient sensorimotor gating in schizophrenia patients is linked to weakened PPER or alternatively related to reflex excitability.
The unmedicated schizophrenia patients exhibited deficient %PPI at the SOA60ms condition relative to the healthy control subjects. There was no difference in sensorimotor gating at the lead interval of 120 milliseconds between patients and control subjects. This result is in line with the findings of several recent studies reporting a selective PPI impairment at shorter lead intervals (30 or 60 milliseconds) but not at the 120-millisecond lead condition in schizophrenia patients.42,47–56 This adds evidence that short lead intervals might be more sensitive for the detection of deficient sensorimotor gating in schizophrenia patients. In this regard, it is worth noting that PPI is sensitive to attentional modulation for lead intervals above 100 milliseconds, whereas there is no evidence for such a modulation at shorter SOAs.9–12 Although, PPI deficits at the 120-millisecond lead interval in schizophrenia patients relative to healthy control subjects have been reported previously,57–62 the present result underlines the importance of including short SOA conditions in PPI experiments to enhance the likelihood for the detection of potential sensorimotor gating deficits in schizophrenia-spectrum patients.
Here, the medicated patients showed only a nonsignificant deficiency in sensorimotor gating, but with increased statistical power (eg, larger sample size), a significant PPI deficit is likely to emerge at the SOA60ms condition. Our finding that patients receiving atypical antipsychotics treatment exhibited no statistically significant PPI deficit is in line with the results of previous studies.42,47,48,54,55,58,59,63 In this connection, it is worth noting that the atypical antipsychotics, clozapine64 and quetiapine,65 but not the typical antipsychotic haloperidol,22 have been shown to enhance PPI in healthy volunteers, with low baseline levels of sensorimotor gating. Despite the fact that several other studies have failed to show such PPI-elevating effects of atypical antipsychotics in medicated patients,57,66–68 the present results lend support to the suggestion that atypical antipsychotics have some PPI-enhancing properties in schizophrenia patients.
Although the unmedicated patients exhibited deficient PPI in the 60-millisecond lead interval condition, they showed a significant peak latency facilitation effect similar to that seen in the control subjects. This finding is in line with the results of several studies showing impaired PPI, as indexed by deficient amplitude suppression of the startle reflex caused by a prepulse stimulus, in the absence of deficient latency facilitation.6,52,57,61,62,69,70 Moreover, while PPI at the SOA120ms condition was not reduced in any of the 2 patient groups, both unmedicated and medicated patients did not, in contrast to the control subjects, exhibit significant latency facilitation. Therefore, it can be concluded that deficient PPI with respect to startle amplitude suppression and reduced latency facilitation are not directly associated in schizophrenia patients.
Medicated patients exhibited diminished startle reactivity in the present study. Although the majority of previous studies investigating sensorimotor gating have not found a difference in startle reactivity in medicated42,47,49–51,57,59–61,66 or unmedicated57,60,67 schizophrenia patients in comparison to the healthy control subjects, some studies reported an enhancement62,63 or a reduction55,58,70,71 of startle reactivity in patients predominantly receiving atypical medication. In this context, it has been demonstrated that atypical antipsychotics significantly reduces startle reactivity in healthy volunteers64,72,73 and rodents.74–76 Importantly, the quantification of PPI offers a special challenge, if groups differ in their baseline startle reactivity.1,77 To address this in the present study, an additional examination utilizing analysis of covariance (ANCOVA) using startle amplitude as a covariate to compare %PPI in the 60-millisecond lead interval condition among 3 groups has been conducted. This analysis yield no fundamental different results; medicated patients exhibited still an intermediate level of PPI between that of unmedicated patients and control subjects, not significantly different from the 2 latter groups. However, it has to be noted that the use of ANCOVA is not unproblematic when the groups under investigation differ significantly in the covariate.78–82
Unlike the control subjects and medicated patients, the unmedicated patients failed to show any detectable reaction to the prepulse stimulus, ie, their PPER was statistically indistinguishable from their baseline reactivity recorded in ns trials. This finding of deficient PPER cannot be attributed to a general reduction in sensitivity to acoustic stimulation because unmedicated patients and control subjects did not differ in their startle reactivity. The finding that healthy control subjects and medicated schizophrenia patients exhibited significant PPER, while unmedicated patients did not, seems to be at odds with that of Swerdlow et al25 who could neither find PPER in healthy volunteers nor in schizophrenia patients using a wide range of prepulse stimulus intensities. Nevertheless, these authors' results indicated a trend for reduced PPER in patients relative to the control subjetcs at the prepulse intensity of 86 dB (16 dB above background noise). However, because Swerdlow et al25 did not employ prepulse-pulse trials in their test session, they were not able to measure PPI at the same time and therefore preventing a direct evaluation of any possible relationship between PPI and PPER. In contrast, the present study is the first to investigate PPER and PPI in schizophrenia patients, with and without antipsychotic treatment.
The pattern of %PPI at the SOA60ms condition indicates deficient sensorimotor gating in the unmedicated patients, whereas medicated patients exhibited PPI levels in-between that of unmedicated patients and the control subjects. Interestingly, a parallel pattern of outcome was observed with respect to PPER. The combination of deficient PPI and the absence of PPER in the unmedicated patients is consistent with the hypothesis that impaired sensorimotor gating in schizophrenia patients is related to weakened detection of the prepulse stimulus.
However, this view is inconsistent with the negative correlation between PPI and PPER observed in control subjects as well as in the unmedicated patients. It should be noted that the lack of a significant PPER above reactivity obtained on ns trials in the unmedicated patients may pose an interpretative problem for the correlation between PPER and %PPI revealed in this group. However, although the PPER levels in unmedicated patients did not significantly exceed the baseline reactivity on ns trials, this lack of difference was based on comparison of the group's average. Individual variability in the PPER could therefore allow a correlation with %PPI to emerge. The possibility that the observed correlation was solely attributed to EMG noise can be excluded on the grounds that no correlation between %PPI and baseline reactivity on ns trials was observed in any group and on any SOA conditions. Of relevance here is that a similar association between these 2 variables was also identified in the control subjects. Moreover, Dahmen and Corr23 also reported a negative correlation between the response probability to prepulse stimuli, ranging from 80–90 dBA (in the presence of 70 dBA background noise) and PPI for some but not all prepulse conditions. Indeed, the relationship between PPI and PPER seems to underlie a complex pattern, especially in the light of the fact that Yee et al14,15 found an opposite (positive) correlation between PPI and PPER in mice. These conflicting results between mice and humans might be attributed to a number of differences in experimental design (eg, whole-body startle in mice vs eye blink startle in human), or it might reflect a genuine species difference. The latter may imply a serious limitation to the cross-species translational power of the PPI paradigm. Therefore, this issue certainly warrants further investigation.
A positive correlation between the 2 measures would be in accordance with the theoretical accounts on sensorimotor gating formulated by Graham.3–5 Moreover, it would fit the observations of PPI enhancement by directing the subjects’ attention toward the prepulse stimulus,9–12 and the disruption of PPI by the dopamine agonist apomorphine in rats might be attributed to a reduced detectability of prepulse stimuli,13 although Yee et al14,15 found that apomorphine enhanced PPER while disrupted PPI in mice. However, it is known that PPER directly detectable by the means of EMG recording is not a prerequisite for the detection of PPI.21,24,83 Nevertheless, unmedicated patients could be distinguished from the medicated patients and control subjects in terms of PPER. The degree to which PPER is causally linked to the expression of sensorimotor gating in the form of PPI has to be further investigated in humans, including patients, and animals in conjunction with specific pharmacological challenge.
The present study is also the first to compare PPS and PPI within a single test session in schizophrenia patients and healthy controls. Although, %PPS and %PPI were positively correlated, at least for some lead interval conditions; %PPS was, unlike %PPI, not deficient in either unmedicated or medicated schizophrenia patients. The correlation between the 2 measures is in agreement with the findings in humans and rodents.16,26 Hyperexcitability was not a prominent characteristic of the blink reflex circuit in the schizophrenia patients because only one patient (and one control subject) exhibited negative %PPS values. The present data do not support the view that deficient PPI in schizophrenia is due to enhanced or abnormal blink reflex excitability. In contrast, it has been proposed by Schicatano et al26 that the reflex modification by a prepulse (ie, PPI) reflects the intrinsic characteristics of the reflex circuit rather than an external adjustment of the reflex by the prepulse. If this would have been the case, parallel deficient PPS in unmedicated patients would have emerged. Similarly, it has been shown that impaired PPI in patients with Parkinson and Huntington disease did not depend on blink reflex excitability.37
The degree to which PPI and PPS may reflect similar or different (independent) brain processes is currently not well understood. Given that the main difference between the 2 paradigms lies mainly in the intensity of the prestimulus, it is highly unlikely that the concepts of sensorimotor gating with respect to PPI and reflex excitability in terms of PPS can be easily separated. Indeed, there is evidence that the 2 forms of startle modification share a common ground. In addition to the correlative nature between PPI and PPS, Swerdlow et al16 demonstrated that a weak prepulse stimulus shortly preceding S1 attenuated the startle response elicited by S1 to a great extent without affecting PPS per se. This led the authors to conclude that PPS is independent of the motoric response elicited by S1. However, the inhibitory phase initiated by intense prestimulation in PPS exceeds the inhibitory phase caused by a much weaker prepulse in PPI by more than 1000 milliseconds, thus reflecting an apparent difference between the 2 paradigms. The influence of different stimulus intensities and lead intervals has been studied extensively in humans and animals. It is widely accepted that a more intense prepulse will generate a stronger PPI effect7,8 and that PPI decreases with increasing lead intervals beyond 100–150 milliseconds, resulting in an absence or even facilitation at very long SOAs (ie, 2000 milliseconds).84,85 Consequently, with sufficiently high prepulse stimulus intensities appreciable levels of PPI can be measured even at long SOAs, at which minimal inhibition or facilitation would normally be observed with low prestimulus intensities.85 From this point of view, it can be concluded that PPS and PPI indeed engage, at least to a great extent, the same neural mechanisms, and thus to completely seperate the concepts of PPS (reflex circuit excitability) and PPI (sensorimotor gating) may not be sustainable. However, the extent to which this conclusion can be generalized needs to be further tested using auditory stimulation for both PPI and PPS because most existing data from PPS experiments are based on trigeminal rather than auditory evoked blink reflexes. Nevertheless, the present results add further evidence to the conclusion by Powers et al27 that low intensity prepulses as used in PPI and blink-evoking “prepulses” as used in PPS might be 2 ends of a continuum.
Conclusion
Our results showed that diminished PPI was accompanied by reduced PPER in unmedicated schizophrenia patients. The unmedicated patients could therefore be distinguished from the medicated patients and control subjects in terms of PPER. These findings would support that reduced sensorimotor gating in schizophrenia patients might be linked to a reduced perception and/or processing of the prepulse stimulus. In contrast, although in some conditions correlated with PPI, PPS was neither reduced compared with the control subjects nor were the patients’ reflex circuit state hyper-excitable. The degree to which PPER is associated with sensorimotor gating has to be further investigated in human and animal studies, including patient studies covering a wide range of psychiatric and neurological diseases and by the use of pharmacological manipulation in healthy volunteers and animals.
Funding
Zurich Psychiatric University Hospital; ETH Zurich; Stiftung für Klinische Neuro-Psychiatrische Forschung, Berne, Switzerland (to P.A.C.); The Mental Health Research Association (NARSAD Independent Investigator Award to F.X.V.).
Acknowledgments
Disclosure: None of the authors has any competing interest to declare, and the work was not supported by pharmaceutical industry grants.
References
- 1.Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev. 1980;87:175–189. [PubMed] [Google Scholar]
- 3.Graham FK. The more or less startling effects of weak prestimuli. Psychophysiology. 1975;12:238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 4.Graham FK. Neural mechanism of goal-directed behaviour learning. In: Thompson RF, Hicks VB, Shvyrkov VB, editors. Control of Blink Reflex Excitability. New York, NY: Academic Press; 1980. pp. 511–519. [Google Scholar]
- 5.Graham FK. Attention and information processing in infants and adults: perspectives from human and animal research. In: Campell BA, Hayne H, Richardson R, editors. Attention: The Heartbeat, the Blink, and the Brain. Hillsdale, NJ: Lawrence Erlbaum Associates; 1992. pp. 3–29. [Google Scholar]
- 6.Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- 7.Blumenthal TD. Prepulse inhibition of the startle eyeblink as an indicator of temporal summation. Percept Psychophys. 1995;57:487–494. doi: 10.3758/bf03213074. [DOI] [PubMed] [Google Scholar]
- 8.Graham FK, Murray GM. Discordant effects of weak prestimulation on magnitude and latency of the blink reflex. Physiol Psychol. 1977;5:108–114. [Google Scholar]
- 9.Elden A, Flaten MA. Similar effects of attention directed to acoustic and tactile stimuli on prepulse inhibition of acoustic startle. Scand J Psychol. 2003;44:363–372. doi: 10.1111/1467-9450.00356. [DOI] [PubMed] [Google Scholar]
- 10.Filion DL, Poje AB. Selective and nonselective attention effects on prepulse inhibition of startle: a comparison of task and no-task protocols. Biol Psychol. 2003;64:283–296. doi: 10.1016/s0301-0511(03)00077-2. [DOI] [PubMed] [Google Scholar]
- 11.Hazlett EA, Levine J, Buchsbaum MS, et al. Deficient attentional modulation of the startle response in patients with schizotypal personality disorder. Am J Psychiatry. 2003;160:1621–1626. doi: 10.1176/appi.ajp.160.9.1621. [DOI] [PubMed] [Google Scholar]
- 12.Heekeren K, Meincke U, Geyer MA, Gouzoulis-Mayfrank E. Attentional modulation of prepulse inhibition: a new startle paradigm. Neuropsychobiology. 2004;49:88–93. doi: 10.1159/000076416. [DOI] [PubMed] [Google Scholar]
- 13.Davis M, Mansbach RS, Swerdlow NR, Campeau S, Braff DL, Geyer MA. Apomorphine disrupts the inhibition of acoustic startle induced by weak prepulses in rats. Psychopharmacology. 1990;102:1–4. doi: 10.1007/BF02245735. [DOI] [PubMed] [Google Scholar]
- 14.Yee BK, Chang DT, Feldon J. The effects of dizocilpine and phencyclidine on prepulse inhibition of the acoustic startle reflex and on prepulse-elicited reactivity in C57BL6 mice. Neuropsychopharmacology. 2004;10:1865–1877. doi: 10.1038/sj.npp.1300480. [DOI] [PubMed] [Google Scholar]
- 15.Yee BK, Russig H, Feldon J. Apomorphine-induced prepulse inhibition disruption is associated with a paradoxical enhancement of prepulse stimulus reactivity. Neuropsychopharmacology. 2004;29:240–248. doi: 10.1038/sj.npp.1300323. [DOI] [PubMed] [Google Scholar]
- 16.Swerdlow NR, Shoemaker JM, Stephany N, Wasserman L, Ro HJ, Geyer MA. Prestimulus effects on startle magnitude: sensory or motor? Behav Neurosci. 2002;116:672–681. doi: 10.1037//0735-7044.116.4.672. [DOI] [PubMed] [Google Scholar]
- 17.Ison JR, Sanes JN, Foss JA, Pinckney LA. Facilitation and inhibition of the human startle blink reflexes by stimulus anticipation. Behav Neurosci. 1990;104:418–429. doi: 10.1037//0735-7044.104.3.418. [DOI] [PubMed] [Google Scholar]
- 18.Blumenthal TD, Goode CT. The startle eyeblink response to low intensity acoustic stimuli. Psychophysiology. 1991;28:296–306. doi: 10.1111/j.1469-8986.1991.tb02198.x. [DOI] [PubMed] [Google Scholar]
- 19.Blumenthal TD. The startle response to acoustic stimuli near startle threshold: effects of stimulus rise and fall time, duration, and intensity. Psychophysiology. 1988;25:607–611. doi: 10.1111/j.1469-8986.1988.tb01897.x. [DOI] [PubMed] [Google Scholar]
- 20.Csomor PA, Vollenweider FX, Feldon J, Yee BK. On the feasibility to detect and to quantify prepulse-elicited reaction in prepulse inhibition of the acoustic startle reflex in humans. Behav Brain Res. 2005;162:256–263. doi: 10.1016/j.bbr.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Csomor PA, Yee BK, Quednow BB, Stadler RR, Feldon J, Vollenweider FX. The monotonic dependency of prepulse inhibition of the acoustic startle reflex on the intensity of the startle-eliciting stimulus. Behav Brain Res. 2006;174:143–150. doi: 10.1016/j.bbr.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Csomor PA, Stadler RR, Feldon J, Yee BK, Geyer MA, Vollenweider FX. Haloperidol differentially modulates prepulse inhibition and P50 suppression in healthy humans stratified for low and high gatin levels. Neuropsychopharmacology. 2007:1–16. doi: 10.1038/sj.npp.1301421. [DOI] [PubMed] [Google Scholar]
- 23.Dahmen JC, Corr PJ. Prepulse-elicited startle in prepulse inhibition. Biol Psychiatry. 2004;55:98–101. doi: 10.1016/s0006-3223(03)00638-3. [DOI] [PubMed] [Google Scholar]
- 24.Swerdlow NR, Talledo J, Shoemaker JM, Codon K, Goins J, Auerbach PP. Weak prepulses inhibit but do not elicit startle in rats and humans. Biol Psychiatry. 2004;55:1195–1198. doi: 10.1016/j.biopsych.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 25.Swerdlow NR, Sprock J, Braff DL. Prepulse-elicited motor reactions do not differ between schizophrenia patients and control subjects. Behav Neurosci. 2006;120:224–227. doi: 10.1037/0735-7044.120.1.224. [DOI] [PubMed] [Google Scholar]
- 26.Schicatano EJ, Peshori KR, Gopalaswamy R, Sahay E, Evinger C. Reflex excitability regulates prepulse inhibition. J Neurosci. 2000;20:4240–4247. doi: 10.1523/JNEUROSCI.20-11-04240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powers AS, Schicatano EJ, Basso MA, Evinger C. To blink or not to blink: inhibition and facilitation of reflex blinks. Exp Brain Res. 1997;113:283–290. doi: 10.1007/BF02450326. [DOI] [PubMed] [Google Scholar]
- 28.Kimura J. Clinical uses of the electrically elicited blink reflex. Adv Neurol. 1983;39:773–786. [PubMed] [Google Scholar]
- 29.Kimura J. Disorder of interneurons in Parkinsonism. The orbicularis oculi reflex to paired stimuli. Brain. 1973;96:87–96. doi: 10.1093/brain/96.1.87. [DOI] [PubMed] [Google Scholar]
- 30.Toda N, Nakamura K, Takeda N. Blink reflex R2 recovery curves in patients with facial palsy within ten days after onset. ORL J Otorhinolaryngol Relat Spec. 2005;67:16–22. doi: 10.1159/000083009. [DOI] [PubMed] [Google Scholar]
- 31.Grandas F, Traba A, Alonso F, Esteban A. Blink reflex recovery cycle in patients with blepharospasm unilaterally treated with botulinum toxin. Clin Neuropharmacol. 1998;21:307–311. [PubMed] [Google Scholar]
- 32.Katayama M, Kohara N, Kaji R, Kojima Y, Shibasaki H, Kimura J. Effect of photic conditioning on blink reflex recovery function in blepharospasm. Electroencephalogr Clin Neurophysiol. 1996;101:446–452. [PubMed] [Google Scholar]
- 33.Smith SJ, Lees AJ. Abnormalities of the blink reflex in Gilles de la Tourette syndrome. J Neurol Neurosurg Psychiatry. 1989;52:895–898. doi: 10.1136/jnnp.52.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castellanos FX, Fine EJ, Kaysen DL, et al. Sensorimotor gating in boys with Tourette's syndrome and ADHD: preliminary results. Biol Psychiatry. 1996;39:33–41. doi: 10.1016/0006-3223(95)00101-8. [DOI] [PubMed] [Google Scholar]
- 35.Swerdlow NR, Karban B, Ploum Y, Sharp R, Geyer MA, Eastvold A. Tactile prepuff inhibition of startle in children with Tourette's syndrome: in search of an “fMRI-friendly” startle paradigm. Biol Psychiatry. 2001;50:578–585. doi: 10.1016/s0006-3223(01)01164-7. [DOI] [PubMed] [Google Scholar]
- 36.Agostino R, Berardelli A, Cruccu G, Pauletti G, Stocchi F, Manfredi M. Correlation between facial involuntary movements and abnormalities of blink and corneal reflexes in Huntington's chorea. Mov Disord. 1988;3:281–289. doi: 10.1002/mds.870030401. [DOI] [PubMed] [Google Scholar]
- 37.Valls-Sole J, Munoz JE, Valldeoriola F. Abnormalities of prepulse inhibition do not depend on blink reflex excitability: a study in Parkinson's disease and Huntington's disease. Clin Neurophysiol. 2004;115:1527–1536. doi: 10.1016/j.clinph.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 38.Pirozzo S, Papinczak T, Glasziou P. Whispered voice test for screening for hearing impairment in adults and children: systematic review. BMJ. 2003;327:967. doi: 10.1136/bmj.327.7421.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wittchen HU, Pfister H. DIA-X-Interview. Frankfurt, Hesse: Swets Test Services; 1997. [Google Scholar]
- 40.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;1987:261–71. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 41.Kendler KS, Tsuang MT, Hays P. Age at onset in schizophrenia. A familial perspective. Arch Gen Psychiatry. 1987;44:881–890. doi: 10.1001/archpsyc.1987.01800220047008. [DOI] [PubMed] [Google Scholar]
- 42.Kumari V, Soni W, Mathew VM, Sharma T. Prepulse inhibition of the startle response in men with schizophrenia: effects of age of onset of illness, symptoms, and medication. Arch Gen Psychiatry. 2000;57:609–614. doi: 10.1001/archpsyc.57.6.609. [DOI] [PubMed] [Google Scholar]
- 43.Hamm AO, Weike AI, Schupp HT. The effect of neuroleptic medication on prepulse inhibition in schizophrenia patients: current status and future issues. Psychopharmacology. 2001;156:259–265. doi: 10.1007/s002130100827. [DOI] [PubMed] [Google Scholar]
- 44.Graham FK. Distinguishing among orienting, defense, and startle reflexes. In: Kimmel HD, Van Olst EH, Orlebeke JF, editors. The Orienting Reflex in Humans. Hillsdale, NJ: Lawrence Erlbaum Associates; 1979. pp. 137–167. [Google Scholar]
- 45.Douglas W Levine, William P Dunlap. Power of the F test with skewed data: should one transform or not? Psychol Bull. 1982;92:272–280. [Google Scholar]
- 46.Bland JM, Altman DG. Transforming data. BMJ. 1996;312:770. doi: 10.1136/bmj.312.7033.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumari V, Soni W, Sharma T. Prepulse inhibition of the startle response in risperidone-treated patients: comparison with typical antipsychotics. Schizophr Res. 2002;55:139–46. doi: 10.1016/s0920-9964(01)00276-6. [DOI] [PubMed] [Google Scholar]
- 48.Kumari V, Soni W, Sharma T. Normalization of information processing deficits in schizophrenia with clozapine. Am J Psychiatry. 1999;156:1046–1051. doi: 10.1176/ajp.156.7.1046. [DOI] [PubMed] [Google Scholar]
- 49.Cadenhead KS, Swerdlow NR, Shafer KM, Diaz M, Braff DL. Modulation of the startle response and startle laterality in relatives of schizophrenic patients and in subjects with schizotypal personality disorder: evidence of inhibitory deficits. Am J Psychiatry. 2000;157:1660–1668. doi: 10.1176/appi.ajp.157.10.1660. [DOI] [PubMed] [Google Scholar]
- 50.Ludewig K, Geyer MA, Etzensberger M, Vollenweider FX. Stability of the acoustic startle reflex, prepulse inhibition, and habituation in schizophrenia. Schizophr Res. 2002;55:129–137. doi: 10.1016/s0920-9964(01)00198-0. [DOI] [PubMed] [Google Scholar]
- 51.Ludewig K, Vollenweider FX. Impaired sensorimotor gating in schizophrenia with deficit and with nondeficit syndrome. Swiss Med Wkly. 2002;132:159–165. doi: 10.4414/smw.2002.09873. [DOI] [PubMed] [Google Scholar]
- 52.Ludewig K, Geyer MA, Vollenweider FX. Deficits in prepulse inhibition and habituation in never-medicated, first-episode Schizophrenia. Biol Psychiatry. 2003;54:121–128. doi: 10.1016/s0006-3223(02)01925-x. [DOI] [PubMed] [Google Scholar]
- 53.Wynn JK, Dawson ME, Schell AM, McGee M, Salveson D, Green MF. Prepulse facilitation and prepulse inhibition in schizophrenia patients and their unaffected siblings. Biol Psychiatry. 2004;55:518–523. doi: 10.1016/j.biopsych.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 54.Swerdlow NR, Light GA, Cadenhead KS, Sprock J, Hsieh MH, Braff DL. Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Arch Gen Psychiatry. 2006;63:1325–1335. doi: 10.1001/archpsyc.63.12.1325. [DOI] [PubMed] [Google Scholar]
- 55.Leumann L, Feldon J, Vollenweider FX, Ludewig K. Effects of typical and atypical antipsychotics on prepulse inhibition and latent inhibition in chronic schizophrenia. Biol Psychiatry. 2002;52:729–739. doi: 10.1016/s0006-3223(02)01344-6. [DOI] [PubMed] [Google Scholar]
- 56.Kumari V, Fannon D, Sumich AL, Sharma T. Startle gating in antipsychotic-naive first episode schizophrenia patients: one ear is better than two. Psychiatry Res. 2007;151:21–28. doi: 10.1016/j.psychres.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 57.Mackeprang T, Kristiansen KT, Glenthoj BY. Effects of antipsychotics on prepulse inhibition of the startle response in drug-naive schizophrenic patients. Biol Psychiatry. 2002;52:863–873. doi: 10.1016/s0006-3223(02)01409-9. [DOI] [PubMed] [Google Scholar]
- 58.Quednow BB, Wagner M, Westheide J, et al. Sensorimotor gating and habituation of the startle response in schizophrenic patients randomly treated with amisulpride or olanzapine. Biol Psychiatry. 2006;59:536–545. doi: 10.1016/j.biopsych.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 59.Oranje B, Van Oel CJ, Gispen-De Wied CC, Verbaten MN, Kahn RS. Effects of typical and atypical antipsychotics on the prepulse inhibition of the startle reflex in patients with schizophrenia. J Clin Psychopharmacol. 2002;22:359–365. doi: 10.1097/00004714-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 60.Weike AI, Bauer U, Hamm AO. Effective neuroleptic medication removes prepulse inhibition deficits in schizophrenia patients. Biol Psychiatry. 2000;47:61–70. doi: 10.1016/s0006-3223(99)00229-2. [DOI] [PubMed] [Google Scholar]
- 61.Braff DL, Light GA, Ellwanger J, Sprock J, Swerdlow NR. Female schizophrenia patients have prepulse inhibition deficits. Biol Psychiatry. 2005;57:817–820. doi: 10.1016/j.biopsych.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 62.Parwani A, Duncan EJ, Bartlett E, et al. Impaired prepulse inhibition of acoustic startle in schizophrenia. Biol Psychiatry. 2000;47:662–669. doi: 10.1016/s0006-3223(99)00148-1. [DOI] [PubMed] [Google Scholar]
- 63.Minassian A, Feifel D, Perry W. The relationship between sensorimotor gating and clinical improvement in acutely ill schizophrenia patients. Schizophr Res. 2007;89:225–231. doi: 10.1016/j.schres.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vollenweider FX, Barro M, Csomor PA, Feldon J. Clozapine enhances prepulse inhibition in healthy humans with low but not with high prepulse inhibition levels. Biol Psychiatry. 2006;60:597–603. doi: 10.1016/j.biopsych.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 65.Swerdlow NR, Talledo J, Sutherland AN, Nagy D, Shoemaker JM. Antipsychotic effects on prepulse inhibition in normal ‘low gating’ humans and rats. Neuropsychopharmacology. 2006;31:2011–2021. doi: 10.1038/sj.npp.1301043. [DOI] [PubMed] [Google Scholar]
- 66.Duncan E, Szilagyi S, Schwartz M, et al. Prepulse inhibition of acoustic startle in subjects with schizophrenia treated with olanzapine or haloperidol. Psychiatry Res. 2003;120:1–12. doi: 10.1016/s0165-1781(03)00161-6. [DOI] [PubMed] [Google Scholar]
- 67.Duncan EJ, Szilagyi S, Efferen TR, et al. Effect of treatment status on prepulse inhibition of acoustic startle in schizoprenia. Psychopharmacology. 2003;167:63–71. doi: 10.1007/s00213-002-1372-z. [DOI] [PubMed] [Google Scholar]
- 68.Perry W, Feifel D, Minassian A, Bhattacharjie I, Braff DL. Information processing deficits in acutely psychotic schizophrenia patients medicated and unmedicated at the time of admission. Am J Psychiatry. 2002;159:1375–1381. doi: 10.1176/appi.ajp.159.8.1375. [DOI] [PubMed] [Google Scholar]
- 69.Braff DL, Swerdlow NR, Geyer MA. Symptom correlates of prepulse inhibition deficits in male schizophrenic patients. Am J Psychiatry. 1999;156:596–602. doi: 10.1176/ajp.156.4.596. [DOI] [PubMed] [Google Scholar]
- 70.Meincke U, Mörth D, Voss T, Thelen B, Geyer MA, Gouzoulis-Mayfrank E. Prepulse inhibition of the acoustically evoked startle reflex in patients with an acute schizophrenic psychosis - a longitudinal study. Eur Arch Psychiatry Clin Neuosci. 2004;254:415–421. doi: 10.1007/s00406-004-0523-0. [DOI] [PubMed] [Google Scholar]
- 71.Kunugi H, Tanaka M, Hori H, Hashimoto R, Saitoh O, Hironaka N. Prepulse inhibition of acoustic startle in Japanese patients with chronic schizophrenia. Neurosci Res. 2007;59:23–28. doi: 10.1016/j.neures.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 72.Graham SJ, Langley RW, Bradshaw CM, Szabadi E. Effects of haloperidol and clozapine on prepulse inhibition of the acoustic startle response and the N1/P2 auditory evoked potential in man. J Psychopharmacol. 2001;15:243–250. doi: 10.1177/026988110101500411. [DOI] [PubMed] [Google Scholar]
- 73.Graham SJ, Scaife JC, Balboa Verduzco AM, Langley RW, Bradshaw CM, Szabadi E. Effects of quetiapine and haloperidol on prepulse inhibition of the acoustic startle (eyeblink) response and the N1/P2 auditory evoked response in man. J Psychopharmacol. 2004;18:173–180. doi: 10.1177/0269881104042615. [DOI] [PubMed] [Google Scholar]
- 74.Swerdlow NR, Geyer MA. Clozapine and haloperidol in an animal model of sensorimotor gating deficits in schizophrenia. Pharmacol Biochem Behav. 1993;44:741–744. doi: 10.1016/0091-3057(93)90193-w. [DOI] [PubMed] [Google Scholar]
- 75.Depoortere R, Perrault Gh, Sanger DJ. Some, but not all, antipsychotic drugs potentiate a low level of prepulse inhibition shown by rats of the wistar strain. Behav Pharmacol. 1997;8:364–372. doi: 10.1097/00008877-199708000-00009. [DOI] [PubMed] [Google Scholar]
- 76.Ouagazzal AM, Jenck F, Moreau JL. Drug-induced potentiation of prepulse inhibition of acoustic startle reflex in mice: a model for detecting antipsychotic activity? Psychopharmacology (Berl) 2001;156:273–283. doi: 10.1007/s002130100763. [DOI] [PubMed] [Google Scholar]
- 77.Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- 78.Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- 79.Owen SV, Froman RD. Uses and abuses of the analysis of covariance. Res Nurs Health. 1998;21:557–562. doi: 10.1002/(sici)1098-240x(199812)21:6<557::aid-nur9>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 80.Wainer H. Adjusting for differential base rates: lord's paradox again. Psychol Bull. 1991;109:147–151. doi: 10.1037/0033-2909.109.1.147. [DOI] [PubMed] [Google Scholar]
- 81.Jamieson J. Dealing with baseline differences: two principles and two dilemmas. Int J Psychophysiol. 1999;31:155–161. doi: 10.1016/s0167-8760(98)00048-8. [DOI] [PubMed] [Google Scholar]
- 82.Van Breukelen GJ. ANCOVA versus change from baseline: more power in randomized studies, more bias in nonrandomized studies [corrected] J Clin Epidemiol. 2006;59:920–925. doi: 10.1016/j.jclinepi.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 83.Yee BK, Feldon J. Prepulse lost and regained: a commentary on “Weak prepulses inhibit but do not elicit startle in rats and humans” Biological Psychiatry 55:98–101. Psychopharmacology (Berl) 2005;179:891–892. doi: 10.1007/s00213-004-2112-3. [DOI] [PubMed] [Google Scholar]
- 84.Aubert L, Reiss D, Ouagazzal AM. Auditory and visual prepulse inhibition in mice: parametric analysis and strain comparisons. Genes Brain Behav. 2006;5:423–431. doi: 10.1111/j.1601-183X.2005.00178.x. [DOI] [PubMed] [Google Scholar]
- 85.Plappert CF, Pilz PK, Schnitzler HU. Factors governing prepulse inhibition and prepulse facilitation of the acoustic startle response in mice. Behav Brain Res. 2004;152:403–412. doi: 10.1016/j.bbr.2003.10.025. [DOI] [PubMed] [Google Scholar]