Abstract
Introduction: Auditory hallucinations are a hallmark symptom of schizophrenia. The neural basis of auditory hallucinations was examined using data from a working memory task. Data were acquired within a multisite consortium and this unique dataset provided the opportunity to analyze data from a large number of subjects who had been tested on the same procedures across sites. We hypothesized that regions involved in verbal working memory and language processing would show activity that was associated with levels of hallucinations during a condition where subjects were rehearsing the stimuli. Methods: Data from the Sternberg Item Recognition Paradigm, a working memory task, were acquired during functional magnetic resonance imaging procedures. The data were collected and preprocessed by the functional imaging biomedical informatics research network consortium. Schizophrenic subjects were split into nonhallucinating and hallucinating subgroups and activity during the probe condition (in which subjects rehearsed stimuli) was examined. Levels of activation from contrast images for the probe phase (collapsed over levels of memory load) of the working memory task were also correlated with levels of auditory hallucinations from the Scale for the Assessment of Positive Symptoms scores. Results: Patients with auditory hallucinations (relative to nonhallucinating subjects) showed decreased activity during the probe condition in verbal working memory/language processing regions, including the superior temporal and inferior parietal regions. These regions also showed associations between activity and levels of hallucinations in a correlation analysis. Discussion: The association between activation and hallucinations scores in the left hemisphere language/working memory regions replicates the findings of previous studies and provides converging evidence for the association between superior temporal abnormalities and auditory hallucinations.
Keywords: auditory hallucinations, schizophrenia, temporal-occipital-parietal junction, superior temporal sulcus, superior temporal gyrus, functional magnetic resonance imaging, consortium
Introduction
Auditory hallucinations are a hallmark symptom of schizophrenia. It has been estimated that up to 74% of schizophrenic patients experience auditory verbal hallucinations (AVH) in the form of hearing a voice or voices that are attributed to an outside source.1 The neural basis of auditory hallucinations has been studied within a neuroimaging context in several ways. Some investigations require actively hallucinating schizophrenic subjects to respond when they are experiencing hallucinations; these studies examine neural activity during the experience of the hallucination in comparison to a period of time when the subject is not hallucinating. The presumption of these “symptom capture” studies is that during the experience of the hallucination, brain regions that participate in producing the experience will be active. Another technique is to measure the neural response to external stimulation (eg, presenting auditory stimuli) in patients who are prone to auditory hallucinations; we will refer to these studies as cognitive interference studies. In the cognitive interference studies, it is usually presumed that the hallucinatory activity will compete with or reduce activity in response to the external stimulation in those regions involved in the hallucinatory experience. Many studies of auditory hallucinations, especially the symptom capture studies, typically have small subject numbers (sometimes reporting only on a single subject); see Weiss and Heckers2 and Allen et al3 for reviews. The symptom capture studies consistently show increased activity within superior temporal auditory regions (although the lateralization reported differs). However, activity in several other regions (such as inferior parietal cortex, Broca's area, or the hippocampus) is sometimes reported, but the results have been inconsistent.2,3 The cognitive interference studies have fairly consistently reported decreased activity in response to auditory stimuli in schizophrenic subjects who are prone to auditory hallucinations, especially in superior temporal and middle temporal regions.2,3 Results of both symptom capture and cognitive interference studies in nonpsychotic subjects with visual hallucinations show corresponding increases of activity in visual regions during the experience of visual hallucinations and decreases in activity during visual perception in those subjects who have visual hallucinations.4 It has also been reported that subjects with visual hallucinations show tonic increases in activity within the visual system, even when they are not hallucinating (see discussion in reference5).
Because of the difficulties involved in understanding the brain bases of auditory hallucinations in schizophrenia, converging evidence is needed. For the current study, we assessed the relationship between levels of auditory hallucinations and activity within a large neuroimaging dataset collected from schizophrenic and control subjects that were recruited from a number of sites across the country. The data were collected and preprocessed by the functional imaging biomedical informatics research network (FBIRN) consortium. Subjects underwent functional magnetic resonance imaging (fMRI) during performance of the Sternberg Item Recognition Paradigm (SIRP) task, a continuous performance, choice reaction time task that required working memory (WM). The one used here was similar to the version used previously with schizophrenic subjects.6 This dataset provided the opportunity to analyze a large number of subjects that were collected across the United States and who were tested on the same (or similar) procedures in each laboratory.
The SIRP task did not use auditory stimuli, but the auditory verbal language system (inner voice) was expected to be used to rehearse the stimuli (visually presented numbers). Our general hypothesis was that subjects who had previously reported having hallucinations in clinical interviews would experience them in a probabilistic manner that was related to their hallucinations scores from the Scale for the Assessment of Positive Symptoms (SAPS).7 For the SIRP task, memoranda were displayed during the entire extent of the encode phase but had to be kept in mind and matched against numbers presented during the probe period. Hence, we expected the verbal rehearsal system to be used to a greater extent during the probe period relative to the encode period. Schizophrenic subjects reporting high levels of auditory hallucinations would be expected to be relatively more likely to experience them in the scanner and/or to possibly have tonically increased activity within regions subserving hallucinations (as in visual hallucinations). Verbal rehearsal in the probe condition was expected to (at least) activate the superior temporal region, especially the superior temporal sulcus and the sylvian-temporal-parietal region or (Spt) as well as inferior frontal regions (see Buchsbaum and D'Esposito8 and Postle9 for reviews). There have also been several recent reports that WM maintenance is associated with inferior parietal activation—especially within the intraparietal sulcus.10
Hence, as in the cognitive interference studies, we expected that schizophrenic subjects with auditory hallucinations would show decreased activity during the WM rehearsal or probe phase of the experiment. We tested this hypothesis in 2 different analyses. For the first analysis, we split the schizophrenic subjects into 2 demographically matched groups. One group of schizophrenics did not report having auditory hallucinations (SAPS hallucinations score = 0) and the other group was composed of subjects whose hallucinations scores were above zero. We analyzed activity during the probe condition for these 2 groups of subjects. We predicted that subjects with auditory hallucinations would show less activity during the probe condition compared with those without auditory hallucinations. For the second analysis, we took the group of all subjects with auditory hallucinations and correlated levels of hallucinations with levels of activity during the probe condition. We predicted that more hallucinations would be correlated with less activity in the probe condition. In other words, the auditory/verbal system is in use during the probe condition, and hence, hallucinatory activity within this system would be expected to result in decreased activation.
Methods
Detailed methods for subject recruitment/inclusion, task procedures, imaging procedures, and data analysis/preprocessing are available in this issue.11 Some aspects of the methods that are particularly important for the current study will also be briefly described below.
Subjects
Subjects were recruited at the various consortium sites including University of California: Irvine, Los Angeles, University of New Mexico, University of Iowa, University of Minnesota, Duke University/University of North Carolina, Brigham and Women's Hospital, Massachusetts General Hospital (MGH), and Yale University. Healthy comparison subjects and schizophrenic/schizoaffective male and female adults between the ages of 18 and 70 years were recruited for this study. Subjects with schizophrenia or schizoaffective disorder meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria were allowed in the study; schizophreniform subjects were excluded. Extensive training for agreement on clinical criteria was conducted for the study by Dr John Lauriello. Subjects were excluded if they had a current history of a major medical; previous head injury or prolonged unconsciousness; or substance and/or alcohol dependence. Patients were also excluded if they currently had an IQ less than 75 as measured by the North American Adult Reading Test, migraine treatments, significant extrapyramidal symptom or tardive dyskinesia (measured by the Global Section of the Abnormal Involuntary Movement Scale). Subjects were required to be clinically stable with no significant changes in their psychotropic medications in the previous 2 months. All subjects had regular hearing levels (no more than a 25 db loss in either ear), had sufficient eyesight or were correctable to be able to see visual display, were fluent in English, and were able to perform the cognitive tasks in this study and had no contraindications to MRI scanning.
Data from 74 schizophrenic subjects were included in the analyses (20 female and 54 male). The analyses were performed on data from all schizophrenia subjects who had hallucinations and whose data were of sufficient quality to be released (quality assurance was assessed for all data by University of California San Diego (UCSD)—G.G.B. and R.N., see below) and used in the SIRP data analyses. All sites received local Institutional Review Board approval for this study.
Clinical Evaluation
Patients were tested using several clinical rating scales. For the purposes of this study, hallucinations ratings were used from the SAPS that were collected within 7 days of the scanning session.
Scanning Protocols
Wherever possible, sites used the imaging protocols that provided the best results for their scanners as determined by their usual protocols. This led to some sites using spiral acquisitions while other used linear k-space trajectories. The scanning session is described in detail in Brown et al.11 The functional scans were T2*-weighted gradient echo planar images sequences, with repetition time = 2, echo time = 30 milliseconds, flip angle = 90°, acquisition matrix = 64 × 64, 22 cm field of view, 27 slices when possible, 4 mm thick with 1-mm gap, oblique axial anterior commissure-posterior commissure aligned. Six seconds (3 acquisitions) of scans were discarded at the beginning of each functional run. The data reported in this article were obtained from the first scanning session.
Paradigms
The stimuli and responses were presented and collected using E-prime software, using an SRBox response device (see Psychology Software Tools, Inc, http://www.pstnet.com/products/e-prime/). These E-Prime programs are now available at www.nbirn.net. Visual stimuli were delivered using various methods including back projection, projection onto head coil–mounted mirrors, and MRI-compatible goggles.
At each site, subjects were scanned according to the same protocol. The SIRP task was presented using a block design with 3 runs of 360 seconds each. Blocks of rest or fixation (flashing crosshair—average duration 12 s) alternated with the task block. Each task block consisted of 3 phases: the learn prompt (2 s), the encode phase (6 s), and the probe phase (38 s). During the task, subjects were asked to memorize digits presented in the encode phase. In a given encode epoch, subjects were shown an encode memory set of 1, 3, or 5 target digits in red (all at once). This was followed by a series of 14 probe digits (7 previously presented probes, 7 foils—randomly intermixed) in green presented sequentially for 1.1 seconds each with a jittered delay between probes (0.6- to 2.5-s delay). During the probe phase, subjects were required to respond with their dominant hand by indicating whether the probe was a target (a member of the memorized encode set) or a foil (not a member of the memorized set). Subjects were instructed to press with their index finger if the green probe digit matched one of the encode targets and with their middle finger if it did not. The order of the 3 conditions was pseudorandom. For each run, 2 memory sets for each of the 3 loads, or conditions, were presented. Each run included 2 blocks composed of memory set sizes at each of the 3 memory loads. Over the 6 blocks presented for each memory set size, participants responded to 42 positive and 42 negative probes, and task blocks were separated by a visual fixation period.
Data Analyses
The data were initially analyzed at UCSD (headed by G.G.B.) in collaboration with MGH (headed by D. Greve), and contrast images were provided for the correlation analyses. A detailed account of the preprocessing resulting in the contrast images can be found in Brown et al11 and will be briefly described.
Functional images were processed using the FBIRN Image Processing System, a pipeline utilizing the FMRIB Software Library of FSL. Functions used from FSL included MCFLIRT for motion correction, PRELUDE and FUGUE for B0 image correction, and “slicetimer” for slicetiming correction; the smooth-to script within the “betfunc” program was used for skull stripping. The Freesurfer “mri_fwhm” program and FSL's “ip” program were subsequently used to do spatial smoothing so that all datasets were smoothed to 8-mm field width half maximum. Geometric and temporal outliers were identified and discarded.
The functional imaging data were high-pass filtered, intensity normalized to 10 000, and spatially normalized using a 12-parameter affine transformation to Montreal Neurological Institute-152 atlas space. A linear model was fitted to each subjects preprocessed functional time series, and each contrast was estimated in a series of steps. Because of the close temporal spacing between phases or conditions, an event-related analysis was used (even though a block design was used for the experiment) and a composite regression parameter was estimated for all 14-probe events following a stimulus set. Given the task design, encode events were assumed to last for 6 seconds. The indicator coding (1 vs 0) of encode and probe explanatory variables was convolved with a single gamma density distribution (σ = 3 s, delay to peak = 6 s or, equivalently, shape = 5.8284, scale = 1.2426) to produce predictors of MR signal magnitude associated with each of the 3 encode and probe loads. Runs for each subject were combined to produce the contrasts of interest. For the purposes of the analyses in this article, we used contrasts that were collapsed over all levels of load and only examined data from the probe condition for which we had the most straightforward predictions.
Schizophrenic subjects were split into 2 groups for the t test analyses of the probe condition for subjects with vs without auditory hallucinations. We used contrasts for all nonhallucinating subjects with usable data. We then took the hallucinating group (defined as SAPS auditory hallucinations score greater than zero) and eliminated subjects to achieve equal numbers of subjects in the 2 subgroups. Subjects with lower auditory hallucinations scores were eliminated first until the group numbers were equal. Only the probe conditions were analyzed (collapsed over the 3 levels of load). t tests were computed using SPM5 for main effects of probe in hallucinators, and nonhallucinators vs hallucinators.
Scripts for the correlation analyses were originally written by B.J.R. in collaboration with and within the laboratory of D.H.M. and J.M.F. We modified those scripts for the SIRP—auditory hallucinations correlation analyses. These analyses were run using SPM5. Activation from all available brain voxels for the probe contrast (collapsed over all load levels) was correlated with levels of auditory hallucinations using the multiple regression module in SPM5. Subject site was used as a covariate, and the SAPS auditory hallucinations score for each subject was correlated with levels of activation for the probe contrasts at each voxel. Although we did have a priori predictions based on previous results,12,13 we did not limit the analysis to any particular region. Results were subsequently thresholded at P < .005, uncorrected, and islands of less than 10 voxels were removed (20 voxels for the t test results).
Results
Clinical, Demographic, and Behavioral Results
Table 1 lists the values for demographic and performance variables as well as levels of auditory hallucinations (means and SDs) for the subjects in the matched groups of hallucinators and nonhallucinators. There were no significant differences between demographic or performance variables for the 2 subgroups (determined by 2-sample t tests; P < .05 significance level). Table 2 lists the values for demographic and performance variables as well as levels of auditory hallucinations for the subjects used in the correlation analyses. Only subjects with SAPS hallucination scores greater than zero were included in the correlation analyses. Note that for some variables, there were missing values; hence, we also report the number of subjects included in each mean value.
Table 1.
Demographics, Performance, and Clinical Information for the Matched Groups of Schizophrenic Subjects With and Without Auditory Hallucinations
| Variable | Average (SD) |
Number of Subjects Used in Calculation |
||
| Hallucinators | Nonhallucinators | Hallucinators | Nonhallucinators | |
| Age (Years) | 36.13 (10.34) | 39.06 (12.67) | 48 | 48 |
| Education (Years) | 12.98 (1.84) | 13.72 (2.05) | 41 | 43 |
| SES | 8.69 (2.03) | 7.98 (2.41) | 45 | 45 |
| Handedness | 0.76 (0.36) | 0.67 (0.53) | 45 | 46 |
| Length of illness (Years) | 13.73 (10.42) | 17.00 (12.46) | 30 | 32 |
| Age of onset (Years) | 20.23 (4.45) | 23.38 (8.6) | 30 | 32 |
| Response time (Seconds) | 0.78 (0.14) | 0.83 (0.15) | 43 | 46 |
| Response accuracy (Percent correct) | 0.87 (0.14) | 0.86 (0.19) | 43 | 46 |
| Auditory hallucinations score (SAPS) | 4.15 (0.77) | 0.00 | 48 | 48 |
| Voices commenting | 2.50 (1.94) | 0.00 | 48 | 48 |
| Voices conversing | 2.27 (1.85) | 0.00 | 48 | 48 |
SES, socioeconomic status; SAPS, Scale for the Assessment of Positive Symptoms.
Table 2.
Demographics, Performance, and Clinical Information for the Schizophrenic Subjects Used in the Analysis of the Correlation Between Auditory Hallucinations and Activity During the Probe Condition
| Variable | Average | Number of Subjects Used in Calculation |
| Age | 36.93 (10.61) | 73 |
| Education | 13.14 (1.83) | 61 |
| SES | 8.55 (2.05) | 71 |
| Handedness | 0.64 (0.52) | 70 |
| Length of illness | 12.11 (9.35) | 45 |
| Age of onset | 22.98 (7.71) | 46 |
| Response time | 0.81 (0.16) | 66 |
| Response accuracy | 0.87 (0.14) | 66 |
| Auditory hallucinations score (SAPS) | 3.28 (1.38) | 74 |
| Voices commenting | 1.86 (1.84) | 74 |
| Voices conversing | 1.58 (1.8) | 74 |
SES, socioeconomic status; SAPS, Scale for the Assessment of Positive Symptoms.
Main Effects of Task Conditions—SIRP Task Activation
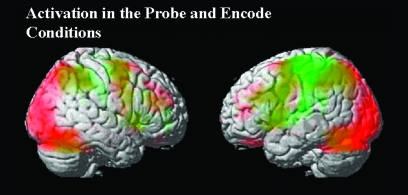
Note that orbital and cerebellar activation (as well as activations at the very top of the brain) should be considered to be of questionable reliability because of signal fallout and possible lack of consistent coverage across sites. In order to present a context for the interpretation of subsequent results, we show the main effects of the encode (red) and probe (green) conditions in figure 1. The activation seen in figure 1 is comprised of the group average using all the contrast images from the schizophrenic subjects whose data were used in the auditory hallucinations correlation analyses. Inferior parietal, visual, and prefrontal regions were activated bilaterally during encoding. Inferior parietal and left superior temporal and prefrontal regions were predominately activated during the probe condition.
Fig. 1.
Activation: Probe and Encode. Red: Activation during performance of the encoding block (collapsed over all loads) of the sternberg item recognition paradigm task for those schizophrenic subjects used in the correlation analyses. green: activation during performance of the probe block (collapsed over all loads). Images theresholded at P < .005, uncorrected.
For the purposes of the present study, the important result to note is the relative increased involvement of the left superior temporal, inferior parietal and frontal articulatory regions in the probe condition relative to the encode condition—presumably related to rehearsal/WM of the numbers.
Group Analyses of the Probe Condition for Hallucinators and Nonhallucinators
Schizophrenic subjects were split into hallucinating and nonhallucinating groups (for auditory hallucinations); figure 2 shows the resulting analyses of activation during the probe condition within these 2 subgroups. Both hallucinators and nonhallucinators showed significant activation of the right and left parietal and left superior temporal regions during the probe condition. Schizophrenic subjects who did not report having auditory hallucinations showed greater activity during the probe condition, including greater activation of bilateral inferior parietal and left superior temporal regions. Tables 3 and 4 show the coordinates and significance levels for activity. No regions showed significantly greater activity in hallucinators than in nonhallucinators for this analysis.
Fig. 2.
Activation: Probe Condition for Matched Schizophrenic Groups. Activation during the probe condition for the group of schizophrenic subjects with auditory hallucinations (top panel) and a matched group of schizophrenic subjects without auditory hallucinations (middle panel). The bottom panel shows activity that is greater in nonhallucinating schizophrenic subjects when compared with those with auditory hallucinations (all images thresholded at P < .005, uncorrected). The contrast showing greater activity for hallucinators than nonhallucinators did not show any significant voxels (not shown).
Table 3.
Probe Condition Activation for Hallucinating Group and Nonhallucinating Group
| Brain Region | T (Number of Activated Voxels) | Z | P Uncorrected | x, y, z Coordinates |
| Probe condition: hallucinating group | ||||
| Left intraparietal sulcus | 9.76 (12229) | Inf | .000 | −34, −52, 48 |
| 8.95 | 7.58 | .000 | −44, −26, 52 | |
| 8.69 | 7.42 | .000 | 0, 8, 50 | |
| Right cerebellum | 9.44 (4130) | Inf | .000 | 32, −58, −28 |
| 8.76 | 7.46 | .000 | 20, −58, −24 | |
| 8.48 | 7.28 | .000 | 10, −76, −42 | |
| Right inferior/middle frontal | 5.63 (581) | 5.21 | .000 | 36, 26, 22 |
| 4.59 | 4.35 | .000 | 38, 34, 16 | |
| 4.57 | 4.33 | .000 | 38, 36, 24 | |
| Left posterior thalamus | 5.58 (289) | 5.17 | .000 | −10, −20, 2 |
| 3.56 | 3.44 | .000 | −12, −14, 18 | |
| 3.44 | 3.33 | .000 | −12, −28, 14 | |
| Right intraparietal sulcus | 5.43 (1246) | 5.05 | .000 | 34, −52, 38 |
| 4.95 | 4.65 | .000 | 42, −54, 46 | |
| 4.49 | 4.26 | .000 | 46, −34, 38 | |
| Left cerebellum | 4.71 (214) | 4.45 | .000 | −38, −60, −28 |
| 3.13 | 3.04 | .001 | −26, −62, −34 | |
| Right fusiform | 4.25 (91) | 4.05 | .000 | 44, −28, −18 |
| Right posterior superior temporal sulcus | 3.51 (23) | 3.39 | .000 | 52, −40, −6 |
| Right inferior temporal | 3.16 (35) | 3.07 | .001 | 64, −42, −18 |
| Left frontal pole | 3.09 (28) | 3.01 | .001 | −28, 50, 10 |
| 3.03 | 2.95 | .002 | −28, 44, 18 | |
| Probe condition: nonhallucinators | ||||
| Left intraparietal sulcus/parietal/sensory and motor cortex | 11.62 (25760) | Inf | .000 | −30, −52, 44 |
| 10.85 | Inf | .000 | −46, −26, 50 | |
| 10.44 | Inf | .000 | −40, 40, 40 | |
| Right cerebellum/fusiform (right and left) | 9.61 (10204) | Inf | .000 | 28, −64, −30 |
| 9.39 | 7.83 | .000 | 20, −54, −26 | |
| 8.32 | 7.17 | .000 | 6, −74, −38 | |
| Right intraparietal sulcus | 8.35 (2901) | 7.19 | .000 | 46, −42, 46 |
| 7.66 | 6.73 | .000 | 40, −38, 40 | |
| 5.87 | 5.40 | .000 | 32, −66, 46 | |
| Left hippocampus | 4.07 (49) | 3.89 | .000 | −40, −26, −10 |
| Left anterior calcarine sulcus/precuneus | 3.53 (60) | 3.41 | .000 | −18, −50, 10 |
| 2.84 | 2.77 | .003 | −22, −44, 2 | |
| Right fusiform gyrus | 3.00 (21) | 2.93 | .002 | 46, −40, −24 |
Note: Z is the Z-score at the peak voxel; inf indicates a Z>8.2.
Table 4.
Probe Condition—Regions Showing Greater Activity in Nonhallucinators Vs Hallucinators
| Brain Region | T (Number of Activated Voxels) | Z | P Uncorrected | x, y, z Coordinates |
| Right intraparietal sulcus | 4.60 (585) | 4.36 | .000 | 44, −42, 46 |
| 4.51 | 4.28 | .000 | 40, −34, 48 | |
| 2.98 | 2.91 | .002 | 32, −30, 56 | |
| Right cerebellum | 4.48 (194) | 4.25 | .000 | 16, −56, −44 |
| 3.86 | 3.71 | .000 | 8, −58, −40 | |
| Left posterior infraparietal sulcus | 4.35 (1431) | 4.14 | .000 | −12, −72, 46 |
| 4.24 | 4.04 | .000 | −20, −60, 32 | |
| 3.61 | 3.48 | .000 | −28, −70, 44 | |
| Left cerebellum/fusiform | 4.23 (1740) | 4.04 | .000 | −24, −60, −50 |
| 3.89 | 3.73 | .000 | −24, −76, −12 | |
| 3.87 | 3.71 | .000 | −12, −62, −14 | |
| Left inferior temporal gyrus | 4.10 (44) | 3.92 | .000 | 48, −52, −12 |
| Left pre-SMA | 3.80 (205) | 3.66 | .000 | −6, −2, 64 |
| 3.24 | 3.15 | .001 | −8, −4, 52 | |
| Left inferior parietal | 3.75 (1849) | 3.61 | .000 | −50, −36, 48 |
| 3.74 | 3.60 | .000 | −52, −24, 30 | |
| 3.70 | 3.56 | .000 | −28, −30, 46 | |
| Right intraparietal sulcus/superior parietal | 3.74 (166) | 3.60 | .000 | 26, −66, 44 |
| 3.19 | 3.10 | .001 | 26, −64, 36 | |
| Right lateral occipital sulcus/posterior superior temporal sulcus | 3.74 (101) | 3.60 | .000 | 44, −78, 8 |
| 3.08 | 3.00 | .001 | 38, −74, 14 | |
| 2.69 | 2.63 | .004 | 38, −76, 22 | |
| Right cerebellum | 3.73 (113) | 3.59 | .000 | 10, −68, −18 |
| Right pre-supplementary motor area | 3.70 (137) | 3.56 | .000 | −6, −22, 56 |
| Right midcingulate | 3.42 | 3.31 | .000 | −8, −28, 46 |
| Right thalamus | 3.69 (352) | 3.55 | .000 | 12, −18, 8 |
| Left thalamus | 3.39 | 3.29 | .001 | −10, −20, 4 |
| 3.30 | 3.20 | .001 | 0, −14, 6 | |
| Left cerebellum | 3.68 (46) | 3.55 | .000 | −56, −52, −26 |
| Left caudate | 3.64 (57) | 3.51 | .000 | −20, −2, 20 |
| Left superior temporal plane | 3.64 (161) | 3.51 | .000 | −60, −20, 12 |
| Left superior temporal gyrus | 3.24 | 3.14 | .001 | −58, −12, −2 |
| Right inferior posterior cingulate/precuneus | 3.60 (36) | 3.47 | .000 | 10, −50, 12 |
| Right precentral | 3.35 (41) | 3.25 | .001 | 14, −30, 72 |
| 2.94 | 2.87 | .002 | 14, −38, 70 | |
| Left anterior superior temporal gyrus | 3.30 (115) | 3.20 | .001 | −62, 10, −2 |
| 3.24 | 3.14 | .001 | −62, 0, 4 | |
| Right cuneus | 3.30 (77) | 3.20 | .001 | 4, −70, 26 |
| Right calcarine sulcus | 3.21 (58) | 3.12 | .001 | 6, −86, 4 |
| 2.97 | 2.90 | .002 | 16, −80, 4 | |
| Right basal ganglia/putamen—globus pallidus | 3.19 (123) | 3.10 | .001 | 30, −12, −8 |
| 3.16 | 3.07 | .001 | 32, −4, −2 | |
| 3.12 | 3.04 | .001 | 22, −4, −4 | |
| Left fusiform | 3.15 (105) | 3.06 | .001 | −32, −32, −24 |
| 3.15 | 3.06 | .001 | −34, −40, −26 | |
| 3.10 | 3.02 | .001 | −36, −48, −24 | |
| Right temporal pole | 3.11 (41) | 3.02 | .001 | 30, 10, −34 |
| 2.74 | 2.68 | .004 | 34, 0, −40 | |
| Left thalamus | 3.08 (22) | 3.00 | .001 | −20, −30, 8 |
| Right superior temporal sulcus | 3.06 (24) | 2.98 | .001 | 50, −48, 16 |
| Right inferior posterior frontal | 3.04 (50) | 2.96 | .002 | 46, −12, 18 |
| Left putamen/globus pallidus | 2.88 (33) | 2.81 | .002 | −22, 4, 10 |
| 2.75 | 2.69 | .004 | −24, −4, 6 |
Correlations Between Probe Activity and Levels of Auditory Hallucinations
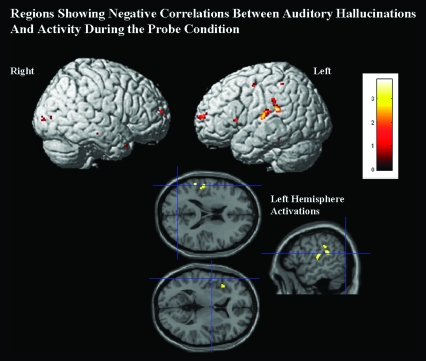
Activity during the probe condition was negatively correlated with levels of auditory hallucinations in schizophrenic subjects. Left hemisphere activations were found in language regions including the inferior parietal, superior temporal, a posterior inferior frontal region, and bilateral anterior insula (see figure 3). See table 5 for coordinates and statistics for significantly activated regions. A very small region (14 voxels) in visual cortex showed a positive correlation between auditory hallucinations and probe activity (x = −20, y = −88, 0), but no other regions showed a significant positive correlation between probe activity and levels of auditory hallucinations.
Fig. 3.
Activation: Correlation Between Activity in the Probe Condition and Auditory Hallucinations. Activation negatively correlated with levels of hallucinations during the probe condition (collapsed over all levels of load); we predicted that this condition would have maximally involved rehearsal and the use of left hemisphere language systems and that these systems would show decrements in subjects with high levels of hallucinations. Left hemisphere superior temporal and inferior parietal language regions (shown in the right figure) showed negative correlations with levels of hallucinations (P < .005, uncorrected).
Table 5.
Activated Voxels Showing Significant Negative Correlations Between the Probe Condition and Levels of Auditory Hallucinations
| Brain Region | T (Number of Activated Voxels) | Z | P Uncorrected | x, y, z Coordinates |
| Left posterior superior temporal gyrus | 3.82 (59) | 3.61 | .000 | −56, −38, 20 |
| Left superior temporal plane (Heschl's gyrus) | 3.56 (73) | 3.38 | .000 | −58, −18, 12 |
| Right anterior insula | 3.17 (38) | 3.05 | .001 | 32, 26, 2 |
| 3.01 | 2.90 | .002 | 36, 34, −2 | |
| Right temporal pole | 3.14 (10) | 3.02 | .001 | 34, 14, −32 |
| Left frontal pole | 3.04 (35) | 2.92 | .002 | −28, 60, 12 |
| 2.96 | 2.85 | .002 | −24, 68, 12 | |
| Right cerebellum | 3.00 (10) | 2.89 | .002 | 32, −46, −24 |
| Left anterior insula | 2.97 (13) | 2.86 | .002 | −40, 16, 6 |
| Left inferior parietal | 2.90 (11) | 2.80 | .003 | −54, −32, 32 |
| Left SMA | 2.86 (13) | 2.77 | .003 | 0, 8, 58 |
Discussion
During the encode condition of the SIRP task, the memoranda were displayed; however, during the probe condition, these memoranda were rehearsed and compared with those on the screen in order to make a response. Hence, we focused on activity during the probe condition for our analyses of auditory hallucinations because this condition should emphasize verbal WM, rehearsal, and use of the inner voice. As expected, the probe condition of the SIRP task showed activity in regions that were previously shown to be active in verbal WM such as inferior parietal, superior temporal, and posterior inferior frontal (Broca's area) regions.9,8 Schizophrenic subjects were split into groups who previously endorsed having auditory hallucinations and those who did not. As predicted, we found decreased activity during the probe condition in schizophrenic subjects who had previously reported having auditory hallucinations. In a separate analysis, we examined the correlation between levels of activity during the probe condition and levels of auditory hallucinations. As predicted, we found significant negative correlations between regions that have been previously demonstrated to be involved in verbal WM and in language production/perception.8 Left superior temporal, inferior parietal, and posterior inferior frontal regions showed significant negative correlations with levels of auditory hallucinations, as did bilateral anterior insula. The findings show that higher hallucinations scores were especially associated with reduced activity within the portion of the WM circuit that has been demonstrated to be especially involved in verbal WM and language production and perception. These results are consistent with previous neuroimaging studies of hallucinating subjects. This result could arise from tonically elevated activity within these regions as has been seen in nonpsychotic individuals with visual hallucinations,5 or because of activity from auditory hallucinations within these regions, or from both sources.
Cognitive interference studies have consistently reported decreased activity in auditory/language regions in response to auditory stimuli in schizophrenic subjects who are prone to auditory hallucinations. Hence, our finding of superior temporal involvement replicates a frequent finding of decreased responsivity of the superior temporal region in schizophrenic subjects who have auditory hallucinations.2,3 In addition, activation of the superior temporal region was a consistent finding in over half of the existing neuroimaging studies of actively hallucinating schizophrenic subjects. These studies have also identified auditory hallucination related activity in the inferior parietal or temporal-parietal-occipital junction (TPJ) region and in posterior inferior frontal regions and insular cortex as was found in the current study. Studies of actively hallucinating patients have also found activity in the posterior middle/inferior temporal, inferior, and middle frontal regions; the hippocampus and parahippocampal gyrus; and the cingulated.14–19 See Allen et al3 for an extensive recent review of auditory hallucinations and brain abnormalities related to hallucinations.
The involvement of the superior temporal and inferior parietal regions in AVH has also been confirmed in volumetric and diffusion tensor imaging (DTI) studies. Superior temporal volume has been consistently reported to be decreased in schizophrenia and to be related to levels of auditory hallucinations (see Shenton20 for a review). The lateral aspect of the left temporoparietal section of the arcuate fasciculus showed evidence of having stronger connections in patients with auditory hallucinations vs patients without auditory hallucinations.21 Right temporoparietal stroke, followed by epileptic seizures, was found to produce psychotic symptoms (1 mo to 11 y later in 8 reported cases22–24). Left temporoparietal transcranial magnetic stimulation was found to ameliorate auditory hallucinations.25 These findings yield converging evidence that the TPJ and superior temporal regions play a prominent role in psychosis.
There are several factors to keep in mind while interpreting the data in the current study. First, schizophrenic subjects with auditory hallucinations also have other, highly correlated symptoms. It is impossible to disentangle the relative contribution of other symptoms to the results shown here based on the analyses presented. We have predicted symptom-activity correlations for other symptoms that also generally involve the superior temporal and inferior parietal regions bilaterally. These predictions will be explored in subsequent publications.22 In addition, it would also be difficult to disentangle several aspects of the hallucinatory experience and to relate them to activity within the context of our analyses for this study. These other aspects include the emotional response to the ensuing hallucination, attentional orienting, startle, and other factors. The extent of activation of language-related regions within the probe condition is also dependent on baseline use of these regions during normal inner thought. Any estimate of regions involved in active hallucinations would probably underestimate the extent of activation within language regions because they may be in constant use. For this and other reasons, we previously predicted that, especially for generative symptoms such as hallucinations, symptom-activation correlations should be more evident during baseline or no-task conditions,22 and hence, this will be explored in subsequent studies.
In summary, our findings provide converging evidence that regions involved in verbal WM and voice production/perception are involved in producing hallucinations. This study of large numbers of schizophrenic patients collected from sites around the country replicates previous findings in studies of actively hallucinating subjects and in challenge studies where subjects with hallucinations are asked to process auditory stimuli.
Funding
Biomedical Informatics Research Network (U24RR021992); National Institute of Mental Health (1 R01 MH067080-01A2 to C.G.W.); Harvard NeuroDiscovery Center (formally HCNR).
Acknowledgments
The inclusion of the FBIRN as an author represents the efforts of many otherwise unlisted researchers over the years who also had explicit input into the conception, design, and implementation of the work. For a listing of these investigators, please visit our website www.nbirn.net.
The following authors conceived, designed, and/or implemented the SIRP and experiment; and/or facilitated data acquisition or data sharing for that experiment: McCarthy, Wible, Lee, Preus, Hashimoto, Brown, Belger, Ford, Greve, Lauriello, Mathalon, O'Leary, Potkin, Roach, Turner, Diaz, and Voyvodic.
These authors contributed to the analysis of the data presented in this article: Greve, Notestine, Brown, Roach, Mathalon, Ford, Molina, Wible, and Hashimoto.
These authors contributed to writing of the manuscript: Wible, Preus, Molina, Ford, Turner, Brown, Lauriello, O'Leary, Mathalon, and Belger.
References
- 1.Silbersweig D, Stern E. Functional neuroimaging of hallucinations in schizophrenia: toward an integration of bottom-up and top-down approaches. Mol Psychiatry. 1996;1:367–375. [PubMed] [Google Scholar]
- 2.Weiss AP, Heckers S. Neuroimaging of hallucinations: a review of the literature. Psychiatry Res. 1999;92:61–74. doi: 10.1016/s0925-4927(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 3.Allen P, Laroi F, McGuire PK, Aleman A. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neurosci Biobehav Rev. 2008;32:175–191. doi: 10.1016/j.neubiorev.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Ffytche DH, Howard RJ, Brammer MJ, David A, Woodruff P, Williams S. The anatomy of conscious vision: an fMRI study of visual hallucinations. Nat Neurosci. 1998;1:738–742. doi: 10.1038/3738. [DOI] [PubMed] [Google Scholar]
- 5.Santhouse AM, Howard RJ, Ffytche DH. Visual hallucinatory syndromes and the anatomy of the visual brain. Brain. 2000;123(pt 10):2055–2064. doi: 10.1093/brain/123.10.2055. [DOI] [PubMed] [Google Scholar]
- 6.Manoach DS, Press DZ, Thangaraj V, et al. Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol Psychiatry. 1999;45:1128–1137. doi: 10.1016/s0006-3223(98)00318-7. [DOI] [PubMed] [Google Scholar]
- 7.Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: University of Iowa College of Medicine; 1984. [Google Scholar]
- 8.Buchsbaum BR, D'Esposito M. The search for the phonological store: from loop to convolution. J Cogn Neurosci. 2008;20:762–778. doi: 10.1162/jocn.2008.20501. [DOI] [PubMed] [Google Scholar]
- 9.Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience. 2006;139:23–38. doi: 10.1016/j.neuroscience.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008;11:103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- 11.Brown GG, McCarthy G, Greve D, et al. Brain-performance correlates of working memory retrieval in schizophrenia: a cognitive modeling approach. Schizophr Bull. doi: 10.1093/schbul/sbn149. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han SD, Nestor PG, Hale-Spencer M, et al. Functional neuroimaging of word priming in males with chronic schizophrenia. Neuroimage. 2007;35:273–282. doi: 10.1016/j.neuroimage.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wible CG, Preus AP, Hashimoto R. A cognitive neuroscience view of schizophrenic symptoms: abnormal activation of the language corridor. Brain Imaging Behav. doi: 10.1007/s11682-008-9052-1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bentaleb LA, Beauregard M, Liddle P, Stip E. Cerebral activity associated with auditory verbal hallucinations: a functional magnetic resonance imaging case study. J Psychiatry Neurosci. 2002;27:110–115. [PMC free article] [PubMed] [Google Scholar]
- 15.Dierks T, Linden DE, Jandl M, et al. Activation of Heschl's gyrus during auditory hallucinations. Neuron. 1999;22:615–621. doi: 10.1016/s0896-6273(00)80715-1. [DOI] [PubMed] [Google Scholar]
- 16.Lennox BR, Park SB, Medley I, Morris PG, Jones PB. The functional anatomy of auditory hallucinations in schizophrenia. Psychiatry Res. 2000;100:13–20. doi: 10.1016/s0925-4927(00)00068-8. [DOI] [PubMed] [Google Scholar]
- 17.Shergill SS, Brammer MJ, Williams SC, Murray RM, McGuire PK. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry. 2000;57:1033–1038. doi: 10.1001/archpsyc.57.11.1033. [DOI] [PubMed] [Google Scholar]
- 18.Silbersweig DA, Stern E, Frith C, et al. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378:176–179. doi: 10.1038/378176a0. [DOI] [PubMed] [Google Scholar]
- 19.van de Ven VG, Formisano E, Roder CH, et al. The spatiotemporal pattern of auditory cortical responses during verbal hallucinations. Neuroimage. 2005;27:644–655. doi: 10.1016/j.neuroimage.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 20.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubl D, Koenig T, Strik W, et al. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. 2004;61:658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- 22.Levine DN, Finklestein S. Delayed psychosis after right temporoparietal stroke or trauma: relation to epilepsy. Neurology. 1982;32:267–273. doi: 10.1212/wnl.32.3.267. [DOI] [PubMed] [Google Scholar]
- 23.Pakalnis A, Drake ME, Jr, John K, Kellum JB. Forced normalization. Acute psychosis after seizure control in seven patients. Arch Neurol. 1987;44:289–292. doi: 10.1001/archneur.1987.00520150039018. [DOI] [PubMed] [Google Scholar]
- 24.Critchley M. The neurology of psychotic speech. Br J Psychiatry. 1964;110:353–364. doi: 10.1192/bjp.110.466.353. [DOI] [PubMed] [Google Scholar]
- 25.Jardri R, Lucas B, Delevoye-Turrell Y, et al. An 11-year-old boy with drug-resistant schizophrenia treated with temporo-parietal rTMS. Mol Psychiatry. 2007;12:320. doi: 10.1038/sj.mp.4001968. [DOI] [PubMed] [Google Scholar]