Abstract
Background: The Functional Imaging Biomedical Informatics Network is a consortium developing methods for multisite functional imaging studies. Both prefrontal hyper- or hypoactivity in chronic schizophrenia have been found in previous studies of working memory. Methods: In this functional magnetic resonance imaging (fMRI) study of working memory, 128 subjects with chronic schizophrenia and 128 age- and gender-matched controls were recruited from 10 universities around the United States. Subjects performed the Sternberg Item Recognition Paradigm1,2 with memory loads of 1, 3, or 5 items. A region of interest analysis examined the mean BOLD signal change in an atlas-based demarcation of the dorsolateral prefrontal cortex (DLPFC), in both groups, during both the encoding and retrieval phases of the experiment over the various memory loads. Results: Subjects with schizophrenia performed slightly but significantly worse than the healthy volunteers and showed a greater decrease in accuracy and increase in reaction time with increasing memory load. The mean BOLD signal in the DLPFC was significantly greater in the schizophrenic group than the healthy group, particularly in the intermediate load condition. A secondary analysis matched subjects for mean accuracy and found the same BOLD signal hyperresponse in schizophrenics. Conclusions: The increase in BOLD signal change from minimal to moderate memory loads was greater in the schizophrenic subjects than in controls. This effect remained when age, gender, run, hemisphere, and performance were considered, consistent with inefficient DLPFC function during working memory. These findings from a large multisite sample support the concept not of hyper- or hypofrontality in schizophrenia, but rather DLPFC inefficiency that may be manifested in either direction depending on task demands. This redirects the focus of research from direction of difference to neural mechanisms of inefficiency.
Keywords: fMRI, schizophrenia, working memory, dorsolateral prefrontal cortex, cortical inefficiency
Introduction
Several lines of evidence suggest working memory, and the dorsolateral prefrontal cortex (DLPFC) component in particular, as a critical domain of dysfunction in the pathophysiology of schizophrenia. In neuropsychological studies, patients with schizophrenia have been found to show performance deficits on nearly all measures of functioning, with working memory among the domains most severely affected.3,4 The working memory deficits are associated with the severity of negative symptoms and impairments in social and occupational functioning.5–9 Structural neuroimaging studies provide convergent evidence indicating that working memory anatomical correlates are affected in schizophrenia. Specifically, a relatively greater degree of reduction in frontal and temporal cortical volumes compared with posterior cortical volumes in patients with this disorder have been reported.10 After accounting for individual differences in gyral patterning and shape, the DLPFC is a key cortical region in which gray matter is reduced in volume in schizophrenic patients compared with their clinically unaffected MZ cotwins, changes that are correlated with the degree of cognitive dysfunction and negative symptom severity in the patients.11
Schizophrenia is characterized by longer reaction time and less accurate working memory performance, especially as the memory load increases. The brain activation patterns responsible for the poor performance remain controversial.2,12,13 Manoach et al observed greater activation in the DLPFC in the schizophrenic group compared with controls and a positive correlation between activation and task performance. DLPFC activation appears to be strongly affected by memory load12; therefore, performance changes with increasing memory load require consideration. Performance is a relevant issue as schizophrenic subjects have shown that peak activation of the working memory system may be reached at a lower memory load than in normal controls and that the decline in DLPFC activity observed in some studies of schizophrenia may be related to exceeding the performance capacities of the schizophrenic subjects.13,14 A difference in relative task difficulty between cases and controls may account for whether DLPFC activation is lower or higher than normal in schizophrenic patients in any given paradigm.15,16 In healthy volunteers (HV), BOLD signal in the DLPFC region appears to increase with increasing memory load12,17 but on some tasks activation appears to asymptote (and may decline) at the highest load levels.18 Patients appear to reach peak activation of the working memory system at a lower processing load than do healthy controls.14 Thus, at least on certain tasks, at low levels of difficulty, patients with schizophrenia may use greater prefrontal resources yet achieve lower accuracy compared with healthy subjects (ie, inefficiency), while at higher levels of difficulty, patients may fail to sustain the prefrontal network that processes the information, achieving even lower accuracy as a result.19–24 Kindermann et al,25 however, observed a different pattern of brain response even when schizophrenia subjects performed within the range of their performance capacities.
Further evidence for abnormal circuitry has been suggested by the Wolf et al26 finding of reduced functional connectivity between the DLPFC and the temporal lobe structures during the encode process, while demonstrating increased connectivity between the ventral lateral PFC and temporal lobe, that they hypothesized as compensatory activation. Zhou et al27 observed evidence for bilateral DLPFC functional increased connectivity in first-episode patients using resting fMRI BOLD measures. These and other studies13,28 suggest that schizophrenic subjects activate different brain networks in performing memory tasks. Scheuerecker et al29 pointed out that these different networks or patterns of compensatory circuitry are insufficient as memory task demands increase. Additional evidence of abnormal circuitry in the PFC and temporal lobe and in the uncinate and arcuate fasciculi that connect them has been observed in diffusion tensor-imaging studies (reviewed by Kubicki et al30 and Kanaan et al31).
While the degree of increased or decreased activity in the DLPFC during the working memory task appears to be a function of task difficulty, most studies average across fMRI runs. Esslinger et al32 in a mental maze task (nonworking memory) observed decreases in activation in patients between early and late trials in contrast to control subjects who showed an increased activation with time. The temporal aspects of brain activation patterns in working memory tasks have not been sufficiently studied.
The above findings of both hypo- and hyperactivation in schizophrenic patients compared with normal controls appear related to task, load, performance relative to capacity, ability to compensate, and perhaps temporal variation over time. One important limitation of the above referenced studies is their small sample size, which precludes disentangling these various influences. A meta-analysis by Van Snellenberg et al33 concluded that the magnitude of WM performance differences between schizophrenia subjects and HV was associated with the activation differences in the DLPFC. Meta-analyses address the small sample size by combining studies in the analysis, although they are necessarily limited by the assumptions inherent in equating subjects and task paradigms from different studies. This limitation can be addressed by large multicenter studies. Multicenter studies offer the possibility of developing datasets which are more representative of the population, including differences in health care, comorbidities, racial, and socioeconomic characteristics. To meaningfully combine fMRI data obtained at multiple sites requires methods for reducing intersite variance which, if left unchecked, mitigates the value of the increased sample size. To accomplish this goal, the Functional Imaging Biomedical Informatics Research Network (FBIRN) consortium develops common image acquisition procedures, and assessment tools, calibration, and QA methods to minimize intersite variance, and developed a common data storage and computational environment.
Methods
The participating institutions in this study were University of California: Irvine (UCI), Los Angeles; University of New Mexico, University of Iowa, University of Minnesota, Duke University/University of North Carolina, Brigham and Women's Hospital, Massachusetts General Hospital (MGH), and Yale University. Analyses were performed at UCSD, Yale, MGH, and UCI. All data are reported by site code rather than site name.
Subjects
All sites received local Institutional Review Board approval for this study. Healthy comparison subjects (HV) and schizophrenic/schizoaffective (SZ) male and female adults between the ages of 18 and 70 were recruited for this study. All subjects had regular hearing levels (no more than a 25-db loss in either ear), had sufficient eyesight or were correctable to be able to see the visual display, were fluent in English, and were able to perform the cognitive tasks in this study. No female subjects were pregnant; all subjects were screened for contraindications to MRI.
Subjects were excluded if they had a current or past history of a major medical illness; previous head injury or prolonged unconsciousness; substance and/or alcohol dependence; IQ less than 75 (as measured by the North American Adult Reading Test [NAART]); or if they were using migraine treatments. Control volunteers were excluded if a first-degree family member had a diagnosis of a psychotic illness. Subjects with schizophrenia or schizoaffective disorder meeting Diagnostic Standard Manual-IV criteria were allowed in the study; schizophreniform subjects were excluded. Patients were also excluded if they currently had significant extrapyramidal symptom or tardive dyskinesia. Subjects were required to be clinically stable with no significant changes in their psychotropic medications in the previous 2 months.
Clinical measures
Prior to participating in scanning procedures, all subjects received extensive diagnostic evaluations by experienced raters. Subjects were diagnosed using the Structured Clinical Interview for Diagnosis (November 2002 Non-Patient34 and Patient35 version); demographic and other socioeconomic information was collected by interview. Other measures collected on all subjects included the Edinburgh Handedness Inventory,36 Fagerstom Test for Nicotine Dependence,37 and the NAART.38
In addition, all patients received the Scales for the Assessment of Positive39 and Negative Symptoms,40 the Calgary Depression Scale,41 Schedule for the Deficit Syndrome,42 and the InterSePT Scale for Suicidal Thinking.43 Movement disorders were measured with the Abnormal Involuntary Movement Scale,44 Barnes Akathisia Rating Scale,45 and the Simpson-Angus Scale.46 Rating methods for the symptom scales were standardized across sites through cross-site group training sessions by experienced clinical raters and by having the raters at each site rate videotapes of several subjects for comparison with expert assessments.
Imaging methods
There were 6 3T scanners, 1 4T scanner, and 2 1.5T scanners used in data collection. Both Siemens and GE scanners were included and 1 Marconi (Picker) scanner.
Scanning protocols
The scanning session consisted of a localizer scan as needed to identify the AC-PC axis; any shimming that a site used (higher order when possible); a 3D T1-weighted scan, (FSPGR on GE, MP-RAGE on Siemens scanners, 24 cm FOV, 1.2–1.5 mm slice thickness, 160–170 slices as needed to cover the entire head, sagittal orientation); a T2 scan which set the slice prescription for the remaining EPI scans (FOV 22 cm, 27 slices if possible, 4 mm thickness with a 1 mm gap, 256 × 192 matrix). B0 field mapping scans were acquired on Siemens sites only.
The functional scans were T2*-weighted gradient echo EPI sequences, with TR = 2, TE = 30 ms, flip angle 90°, acquisition matrix 64 × 64, 22 cm FOV, 27 slices when possible, 4 mm thick with 1 mm gap, and oblique axial AC-PC aligned.
Each scan session consisted of a brief training session to familiarize the subject with the paradigms and placement in the scanner for about 1.5 h during which structural and functional images were collected. At least 24 h later and no more than 3 weeks later, the subject repeated the entire session. Only the first visit is reported here. Subjects were to have a normal night's sleep the night before each scan, no more than one alcoholic drink the night before, and abstain from drinking coffee within 2 h prior to lying down in the scanner. Subjects who smoked refrained from smoking starting 40 min before lying down in the scanner.
Cognitive paradigm methods
The stimuli and responses were presented and collected using E-prime software, using an SRBox response device (Psychology Software Tools, Inc.). These E-Prime programs are now available at http://www.nbirn.net/.
“Visual stimuli” were delivered using various methods. Several sites in the consortium used an LCD projector, with the presentation focused onto a back-projection screen mounted in the magnet bore. Several sites were using projectors onto head coil–mounted mirrors; others used MRI-compatible goggles.
Prior to scanning, subjects completed at least one practice run without coaching; the behavioral analysis was run immediately to determine that the subjects were performing at greater than 75% correct.
At each site, subjects were scanned according to the same protocol. The Sternberg Item Recognition Paradigm (SIRP) was collected as part of the larger protocol. The order of the tasks was constant, insofar as possible. The stimulus generation computer and scanner were linked by a trigger signal. All scanning paradigms began with a 6-s (3 TR) countdown to allow for dummy acquisitions. To maintain motivation, subjects received an additional 5 cents for every correct response.
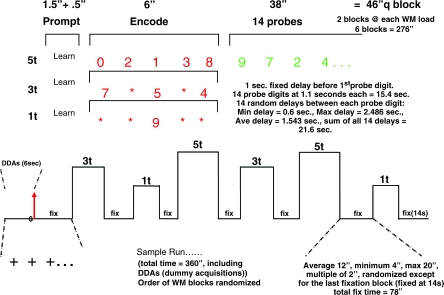
The SIRP timing and design are as follows (see figure 1, below). During the “encode” condition, subjects memorize a set of target digits. They are then presented with probes (single digits) and respond by indicating whether the probe is a target (a member of the memorized set) or a foil (not a member of the memorized set).
Fig. 1.
Time Course of the SIRP Design.
Fig. 2.
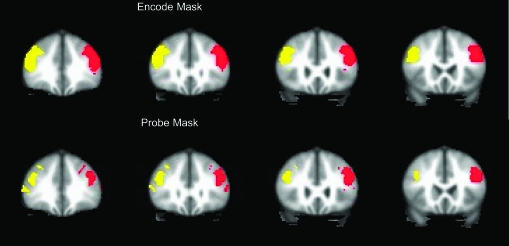
Image of the DLPFC Mask Shown in Standard MNI Space.
This version of the SIRP task consisted of 3 working memory loads, of 1, 3, or 5 target digits (in red), followed by a series of probe digits (in green). Two memory sets for each of the 3 loads were presented in each run of the paradigm. Each condition includes both an encode and probe epoch. Subjects were asked to learn the sets of red digits and instructed to press with their index finger if the green probe digit matches one of the targets and with their middle finger if it does not.
The order of the 3 memory load conditions was pseudorandom. In between memory sets, subjects fixated on a flickering cross. The flickering interval was 2 s: 1.85 s on and 0.15 s off (with the exception of the first interval, which lasts 2.8 s). During the encode epochs, red target digits were presented for 6 s. For the 1 and 3 target condition to match for visual stimulation, asterisks (*) were used in place of digits so that 5 items were on the screen during every encode epoch. Following the encode epoch, there was a 2.7-s “delay” and a longer “probe” epoch. During the probe epoch, subjects were shown 14 individual probes, serially, in green. Within each condition, half of the probes were targets, half foils, and each member of the target set was presented at least once. Probe digits are random integers between 0 and 9 and are not repeated within a single memory set; no more than 3 consecutive digits were targets within a memory set. The timing of the probe digits was 2.7 s, made up of random jitter around a 1.1 s display. The overall probe epoch lasted 38 s.
Analysis methods
Behavioral analysis
The mean accuracy for each load level was calculated for each subject, averaging over all blocks of the same condition in each run. Mean response times were calculated both for all trials and for correct trials only. A mixed-model design assessed the effects of memory load and run within subject and site and diagnosis across subjects on both measures.
Imaging analysis
Errors in the imaging data coming from all the sites were identified by examining the content of the XCEDE XML47 files that accompanied the shared NIfTI images, by checking image header information and by visual inspection of the images.
Given our a priori hypothesis that activation in the DLPFC during the working memory task would distinguish schizophrenia subjects from control subjects, our primary analysis focused on the DLPFC as the region of interest (ROI). We describe the preprocessing and analysis steps that lead to the summary measure for the ROI.
Images were processed with a developmental version of the FBIRN Image Processing System (FIPS), an image analysis pipeline primarily using routines from the FMRIB Software Library (FSL).48 In the version of FIPS used here, preprocessing steps were separated from the remainder of the FIPS pipeline, with XML and related files developed to track provenance information. The data were corrected for head movement using FSL's MCFLIRT (usually aligning to the middle volume); PRELUDE and FUGUE were used to B0 correct images at sites where field maps were collected and “slicetimer” to correct images for slice-timing differences.49 To equilibrate images for potential site differences in the BOLD signal due to spatial smoothness, we smoothed all 3-D volumes to 8 mm FWHM.50
In the first-level analysis, for each subject's run, the functional time series was high-pass filtered, intensity normalized to 10 000 and spatially normalized by a 12-parameter affine transformation to MNI-152 atlas space.51 A linear model was fit to each subject's preprocessed functional time series for each SIRP run to estimate regression parameters for encode and probe conditions. The linear model also included temporal derivatives of the gamma function to account for specific temporal shifts of the BOLD response that might vary over load level, event type, and run. Both linear and quadratic terms were included in the model to account for baseline drift. Contrasts of parameter estimates—copes—were formed to test for load effects during encode and probe events. The magnitude of each cope, along with an estimate of its variability derived from model residuals, was passed to a second-level analysis to combine copes from separate runs, yielding a composite cope value for each contrast of interest for each subject. These composite cope values were formed by the weighted sum of copes from individual runs with the weights inversely proportional to the run-specific variation in each cope value.52 The composite copes were used to map functional contrast.
Following spatial normalization of the composite cope images to the MNI atlas, we calculated a Jaccard index53 between the base image from each functional time series and the MNI-152 atlas51 in order to identify images with poor geometric properties and assessed each run for temporal outliers using AFNI's 3dToutcount54 tool. Images with poor spatial geometry included spiral images where poor fat suppression appeared to produce flared image intensities at the edge of the head, images with cysts, images poorly rotated during spatial normalizing, and images with poor head placement within the limited FOV. A run was discarded if at least 34 of the 177 volumes from the functional time series were identified as having an outlier spike, if the base image displayed a visually obvious structural flaw, or if the Jaccard index flagged the fit of the base image to the MNI-152 atlas as being more than 1.5 interquartile ranges above the 75th percentile of the remaining values. Altogether 1.4% of runs were discarded due to poor temporal or geometric properties. The BOLD values for the ROIs for these runs (see below) were replaced with a mean value calculated from data collected for the subject's diagnostic group at the site where the subject was scanned.
ROI analysis
The ROI included all voxels within the mask for Brodmann areas 9 and 46 as defined by the WFU PickAtlas,55,56 without the medial wall, which were significantly activated in either the schizophrenic or healthy volunteer group analyses (p < .05, false discovery rate correction). After obtaining masks of the middle and frontal gyri from the PickAtlas that roughly corresponded to Brodmann areas 9 and 46, we formed the intersection of the PickAtlas areas with a gray matter mask derived from the MIN152 Atlas. The medial surface of the resulting mask was then removed. These steps were taken in order to limit the ROI to cortical areas and to limit the mask to lateral cortex. After labeling left and right hemispheres within the mask, we created voxel-wise activation maps for each group identifying voxels where the BOLD response was significantly related to increasing memory load. The false detection rate was set to .05 to correct for the multiple statistical tests performed within the ROI. We then formed the intersection of each within-group activation map with the anatomical DLPFC mask, producing DLPFC empirical masks for each group. Finally, we formed the union of these 2 group masks to produce the final DLPFC masks used in the study. The aim of this method was to restrict the activity of interest to the DLPFC without washing out group effects due to averaging over a large region—while allowing for the possibility of group differences in activation patterns across subregions of the DLPFC. Because the masks were in part determined empirically, they were created for both encode and probe conditions. The resulting ROIs for the encode and probe conditions are shown in Figure 2. Within the ROI, each subject's mean BOLD signal change was computed for encode and probe conditions separately, for each load. These mean BOLD signal changes were the input to the final analyses.
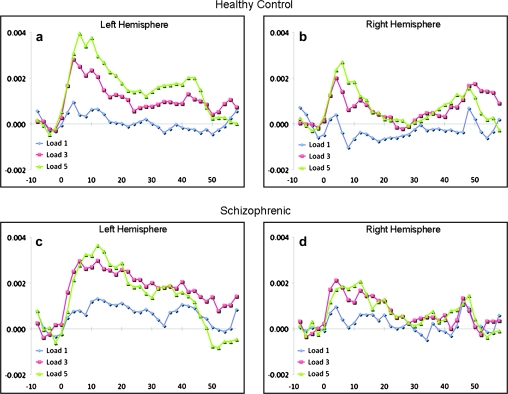
Fig. 4.
The Mean Time Courses of Activation Averaged Across All Voxels Within the Left (a, c) and Right (b, d) Hemisphere DLPFC Regions of Interest for Healthy Controls (a, b) and Schizophrenic (c, d) Subjects. The horizontal axis represents time in seconds relative to the onset of the memorandum at time zero (0) for memory loads of 1 (blue line), 3 (red line), and 5 (green line) items. The first memory probe was presented at 7 sec and the last memory probe occurred at 38 sec.
In the final analyses, the BOLD signal from the encode and probe conditions were analyzed in separate ANOVAs contrasting each memory load with fixation. Site and diagnosis were between-subject factors and hemisphere and run were within-subject factors. Significance thresholds were set at p < .05.
Results
Subjects
The clinical and demographic data for the 256 subjects are shown in tables 1 and 2. The distribution of male and female subjects, or right and left handed subjects, was not different by disease status; mean age was similar between the 2 groups. The Full-Scale IQ (FSIQ) estimate from the NAART was significantly higher in HV, as were educational levels.
Table 1.
Demographic Information
| Demographic Characteristics | Patients | Controls | Statistical Significance | % Reporting | |
| Number of subjects | 128 | 128 | — | 100 | |
| Race (% Caucasian) | 70.3 | 77.3 | ns | 99 | |
| Gender (% male) | 71.9 | 62.5 | ns | 100 | |
| Handedness (% right) | 89.8 | 89.8 | ns | 100 | |
| Mean age (SD) | Range: 18–65 | 38.0 (11.6) | 36.2 (11.9) | ns | 100 |
| Subject's mean years of education (SD) | Range: 5–24 | 13.3 (1.9) | 15.9 (2.3) | <0.001 | 90 |
| Mother's mean years of education (SD) | Range: 0–21 | 13.1 (3.1) | 14.0 (3.3) | ns | 82 |
| Father's mean years of education (SD) | Range: 0–22 | 13.9 (3.8) | 14.9 (3.5) | <0.05 | 81 |
| Mean premorbid FSIQa estimate (SD) | Range: 85–126 | 104.9 (9.6) | 112.7 (8.1) | <0.001 | 94 |
Full Scale Intelligence Quotient; derived from the NAART.38
Table 2.
Clinical Summary
| Range | Mean | SD | % Reporting | |
| SANS (global measures sum) | 0–19 | 8.87 | 4.4 | 93 |
| SAPS (global measures sum) | 0–16 | 6.12 | 3.3 | 95 |
| InterSePT suicidality scale (sum of 11 items) | 0–13 | 1.49 | 2.7 | 76 |
| Calgary depression scale (total score) | 0–20 | 4.78 | 4.6 | 91 |
Of these 256 subjects, imaging data were missing from 24 subjects. A further 13 subjects were removed because they responded to fewer than 80% of the trials or they responded at lower than chance levels on any of the 3 runs. The final sample of 217 subjects consisted of 111 HV (43 female) and 106 subjects (40 female) with SZ. This group did not differ from the overall group in mean age, handedness, gender or racial distribution, NAART, clinical symptom ratings, or education levels. All behavioral and imaging results presented below are from these 217 subjects.
Behavioral data
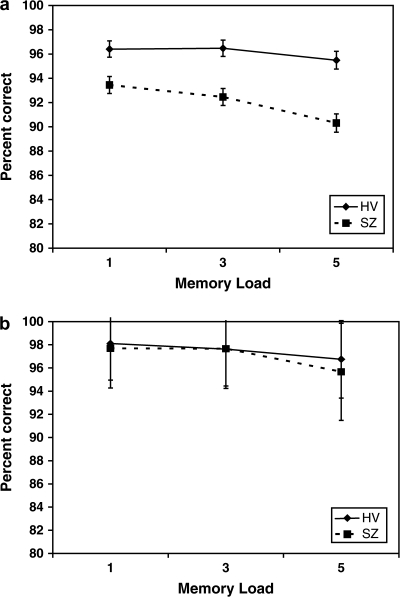
The mean accuracy by memory load and diagnostic group are shown in figure 3. An ANOVA with diagnosis as a between-subject variable and load level as within-subject variables showed the effect of memory load was as expected ((F(2, 416) = 19.8, p < .0001), with increased load leading to reduced accuracy. The effect of diagnosis was significant (HV = 96.1 ± .64, SZ = 92.1 ± .66; F(1,208) = 19.3, p < .0001) and interacted with memory load (F(2, 416) = 5.4, p < .005) as shown in the figure. An ANOVA also showed the effect of memory load on reaction time as expected, with increasing memory load leading to increased reaction time for all trials and correct trials (F(2,2.1) = 741, p < .0001 for the overall RT, similar for RT on correct trials only). Reaction time was approximately 100 ms slower in the SZ subjects at each memory load (F(1,20) = 24, p < .0001); the load by diagnosis interaction (F(2,2.1) = 9.1, p < .0001) showed the difference between SZ and HV increased slightly with increasing memory load (73 ms at 1 item, 109 ms at 5 items).
Fig. 3.
Mean Accuracy at Each Memory Load by Diagnostic Group. (a) Mean accuracy levels in the entire 217 subject dataset. Error bars equal 1 SD. (b) Mean accuracy levels in the behaviorally matched subsample (65 SZ, 65 HV). Error bars indicate 1 SD.
Imaging results by ROI
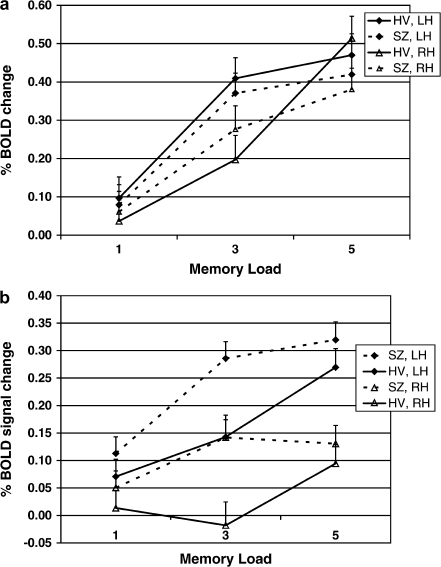
In figure 4, the average BOLD signal changes over time from the DLPFC region are shown by memory load and diagnostic group. Relative to fixation, the BOLD signal increases at the onset of the items to be remembered and is maintained (particularly in the higher load conditions) during retrieval. The major difference between the schizophrenic subjects and the controls is the degree of activation at memory load of 3 items relative to memory load of 1.
Encode results
There were significant effects of site, load, run, and hemisphere on the mean DLPFC BOLD signal change in the encode condition, as well as interactions between run and hemisphere and hemisphere and load. However, there was no significant effect of diagnosis and no interactions with diagnosis. The results are summarized in table 3. The effect of load by hemisphere for the 2 diagnostic groups can be seen in figure 4 and is summarized in figure 5a.
Table 3.
Effects of Diagnosis, Load, Hemisphere, Run, and Site on BOLD signal change in the DLPFC in the Encode and Probe analyses
| Source | df | df(error) | F | Encode Significance | F | Probe Significance |
| Diagnosis | 1 | 199 | 0.23 | 0.64 | 5.54 | 0.02 |
| Site | 8 | 199 | 2.05 | 0.04 | 1.45 | 0.18 |
| Diagnosis × site | 8 | 199 | 1.51 | 0.15 | 0.93 | 0.49 |
| Load | 2 | 398 | 42.11 | 0.00 | 26.94 | 0.00 |
| Hemi | 1 | 199 | 3.71 | 0.06 | 37.42 | 0.00 |
| Hemi × load | 2 | 398 | 4.09 | 0.02 | 11.41 | 0.00 |
| Load × diagnosis | 2 | 398 | 0.93 | 0.39 | 5.41 | 0.00 |
| Run × hemi × load | 4 | 796 | 1.23 | 0.30 | 3.65 | 0.01 |
| Run | 2 | 398 | 8.59 | 0.00 | 5.11 | 0.01 |
| Run × hemi | 2 | 398 | 5.45 | 0.00 | 2.86 | 0.06 |
| Run × load × diagnosis | 4 | 796 | 1.00 | 0.41 | 1.76 | 0.13 |
| Run × hemi × site | 16 | 398 | 2.43 | 0.00 | 1.36 | 0.16 |
| Run × hemi × load × diagnosis | 4 | 796 | 0.36 | 0.84 | 1.64 | 0.16 |
| Run × load | 4 | 796 | 0.50 | 0.74 | 1.43 | 0.22 |
| Load × diagnosis × site | 16 | 398 | 1.40 | 0.14 | 1.10 | 0.35 |
| Run × diagnosis × site | 16 | 398 | 0.89 | 0.58 | 1.10 | 0.36 |
| Run × load × diagnosis × site | 32 | 796 | 0.61 | 0.96 | 1.00 | 0.46 |
| Run × hemi × load × diagnosis × site | 32 | 796 | 0.90 | 0.63 | 0.91 | 0.62 |
| Run × site | 16 | 398 | 1.55 | 0.08 | 0.80 | 0.69 |
| Run × diagnosis | 2 | 398 | 0.09 | 0.92 | 0.32 | 0.72 |
| Run × load × site | 32 | 796 | 1.54 | 0.03 | 0.84 | 0.72 |
| Run × hemi × diagnosis × site | 16 | 398 | 0.87 | 0.60 | 0.72 | 0.78 |
| Run × hemi × diagnosis | 2 | 398 | 0.25 | 0.78 | 0.19 | 0.83 |
| Hemi × load × diagnosis | 2 | 398 | 1.42 | 0.24 | 0.17 | 0.84 |
| Hemi × site | 8 | 199 | 1.21 | 0.29 | 0.50 | 0.85 |
| Hemi × load × diagnosis × site | 16 | 398 | 0.60 | 0.88 | 0.64 | 0.85 |
| Hemi × diagnosis × site | 8 | 199 | 0.27 | 0.98 | 0.40 | 0.92 |
| Load × site | 16 | 398 | 1.13 | 0.33 | 0.55 | 0.92 |
| Hemi × diagnosis | 1 | 199 | 0.21 | 0.65 | 0.00 | 0.99 |
| Hemi × load × site | 16 | 398 | 0.72 | 0.78 | 0.35 | 0.99 |
Hemi, hemisphere; load = 1, 3, or 5 memory item condition; run = 1st, 2nd, or 3rd run of the task within the same scanning session; diagnosis = SZ or HV
Fig. 5.
BOLD Signal Change in DLPFC, by Load and Hemisphere, for HV and Schizophrenic Patients. (a) Encode results by diagnostic group. (b) Probe results by diagnostic group. Error bars indicate one standard deviation.
BOLD signal response during encoding increased for both groups with increasing memory load. The hemispheric differences are minimal, only significant in the 3-item condition (left > right), and there is no interaction with diagnosis. The effect of run within the scanning session was significant, similarly for both diagnostic groups; BOLD signal response decreased significantly in the third run (p < .008).
The effect of site was statistically significant and primarily due to site #18 that had significantly lower mean BOLD than 5 other sites. There was one additional pair-wise site difference. However, there was no interaction with diagnosis.
Probe results
There were significant effects of diagnosis on the probe response. Significant effects were also observed for load, hemisphere and run, and for the interactions between diagnosis and load and hemisphere and load. The effect of site was not significant. See table 3 and figure 5b for all results.
As can be seen in figure 5b, the interaction between diagnosis and load was not linear (F = 0), but the quadratic term was significant (F = 10.2, p < .002). The effect of increasing memory load is to increase the BOLD signal change for both groups (all loads are significantly different from each other, p < .001). The difference between schizophrenic subjects and HV is significant at the memory load of 3 items. Schizophrenic subjects dramatically increase their BOLD response as the memory load increases from 1 to 3 items, and then the response flattens out with the memory load of 5 items. Normal subjects in contrast do not increase dramatically from 1 to 3 items, but do show a significant increase in the highest memory load condition. At the highest memory load conditions, the groups are not significantly different.
The effect of run within the scanning session was significant; BOLD signal response decreased significantly in the third run (p < .003), for both diagnostic groups. The left hemisphere response was overall stronger than the right, significantly so in the 3- and 5-item memory conditions; it was not different by diagnosis.
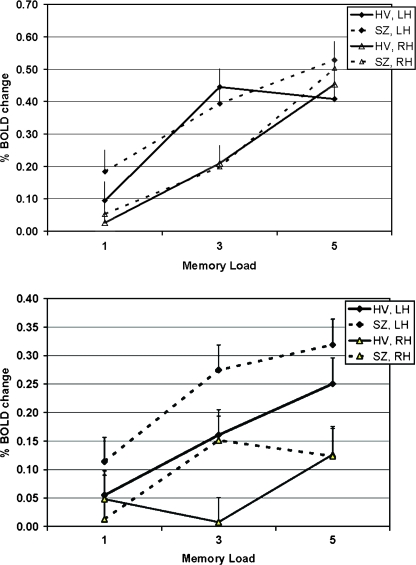
Given the differences in performance between groups and the possibility that the observed differences BOLD activation in the DLPFC could merely reflect those performance differences, we censored our data to match subjects from both groups on performance. Subjects were matched on performance accuracy (±1%) (see figure 3b), scanner field strength, age within 7 years, gender, and handedness (the latter when possible). This resulted in a dataset of 65 schizophrenic subjects and 65 controls, with a mean accuracy of 98% in the 1-item condition, 98% in the 3-item condition, and 96% in the 5-item condition, with no significant differences between diagnostic groups at any level (see figure 3b). There was no difference in movement measures (absolute and relative movement from centroid) between groups. The mean BOLD signal differences during the encode and probe conditions by load and hemisphere for this censored, matched subset, are shown in figure 6. The results were similar to the overall group for the probe condition—left hemisphere was more active than right hemisphere in both groups, the same effect of run was seen, and importantly, the same effect of diagnosis and interaction between diagnosis and increasing memory load was seen, even when accuracy was matched between groups. (This effect was not seen for the encode condition.) The correlation between accuracy and IQ as measured by the NAART FSIQ was weakly significant only for the 5-item condition (r = 0.2 with an r2 = 0.04, p < .05). Nevertheless, we covaried for NAART score, and the diagnosis by load remains significant.
Fig. 6.
As in figure 5 but limited to the behaviorally matched sample.
Discussion
In spite of evidence of greater complexity in prefrontal dysfunction in schizophrenia, the notion of task-related “hypofrontality” remains at the crux of many theories of cognitive deficits and symptoms. The present findings, using a large, multisite sample, strengthen, replicate, and extend previous work that used small, fairly homogenous samples. Here we report significantly greater DLPFC activation in patients that varied as a function of working memory load, even when groups were matched for performance accuracy. These findings support the concept not of task-related hyper- or hypofrontality in schizophrenia, but rather inefficient DLPFC function in schizophrenia that may be manifested in either direction depending on task demands. They also support the a priori hypothesis of an inverted U–shaped function describing the relation of working memory load to DLPFC activation in both patients and HV, with a leftward shift of the curve in patients.57
One factor influencing where schizophrenic subject DLPFC activation on the inverted U during working memory tasks is task performance. Using the N-back working memory task, initial studies reporting hypofrontality may have reflected poorer performance in schizophrenic subjects.58,59 Increased DLPFC activation interpreted as “inefficiency” has been observed when subjects were matched to healthy controls for performance.60 Perlstein et al61 observed a drop in activation at the highest load in schizophrenia patients. Activation increased with increasing memory load despite poor memory performance, until “capacity” was increased when activation decreased below control activation levels.14 Interpretation of these studies is hampered by the levels of difficulty inherent in the N-back task: level 1 is very easy and level 3 is too difficult for many patients.
The Sternberg Item Recognition Paradigm has a considerably less steep memorandum difficulty curve. Using the Sternberg paradigm and matching for performance by comparing across different levels of working memory load, Manoach et al62 found no difference in DLPFC mean activation between schizophrenia patients and controls; however, her sample size was only 9 subjects per group. Johnson et al63 matched groups (n = 18) of subjects for performance by comparing schizophrenics at memory loads of 4, 5, 6 with controls at 6, 7, 8; they found left DLPFC activation greater in controls during encoding, but less activation during retrieval in the bilateral DLPFC. When they limited their analysis to epochs with perfect performance, these differences were not observed. However, only analyzing trials with perfect performance may miss the very trials that are associated with schizophrenia, a deficit in working memory. They concluded that “patients’ activation pattern appeared more ‘normal’ during encode than during retrieval.” Their findings are consistent with our observations; the major difference between schizophrenia subjects and controls is in the retrieval condition. Our study design however, could not distinguish any encode effects carrying into the retrieval period.
Our large sample allowed us to match for performance accuracy with the identical level of memory load. The lack of differences in the encoding condition suggests that schizophrenia subjects are able to store the memoranda (over a range of 1–5 items) similarly to controls but require greater activation of the DLPFC to achieve the same level of performance accuracy as controls. The major DLPFC activation difference that we observed in the retrieval conditions is present at memory load of 3, despite the subjects performing on average at the same level of accuracy in the censored analysis. Schizophrenia subjects activate the DLPFC to a greater degree than healthy controls to achieve the same level of performance. This is consistent with the inefficiency hypothesis first put forward by Callicott et al19 and Manoach et al.2,15
It is possible that the “inefficient” brain activation observed in the schizophrenia subjects was related to IQ as indexed by lower NAART scores in the patients rather than by schizophrenia per se. The lack of a significant correlation between performance and NAART and between accuracy and reaction time and BOLD activation are not supportive of this possibility. We did not find a decrease in activation in the schizophrenic subjects at the highest working memory load (5 items) to reflect the expected disengagement as performance fails as suggested by Callicott et al.19 The use of reward related to performance may have kept our subjects involved despite increasing task difficulty. We did see a smaller increment in DLPFC activation as patients moved from the intermediate to high working memory load compared with the increment from low to intermediate. Controls, in contrast, showed comparable increases from low to intermediate to high levels of working memory load. This is consistent with our prediction of an inverted U–shaped relation of DLPFC activation to working memory load. In patients, for whom we hypothesized, the curve is shifted to the left (ie, the DLPFC reaches capacity at a lower level of load), their DLPFC response from low to medium may have reflected that they were at the flat part of the curve (ie, nearing or reaching capacity), while controls were still on the upslope. Our major interest is the DLPFC activation in schizophrenia as memory load increases within the subjects’ capacity.
An advantage of a task design parametrically varying memory load is that physiological activity can be evaluated across a number of levels of task difficulty in the same sample. The 3 levels of memory load revealed a quadratic effect that would have been obscured had we used only 2 memory loads. We did not study higher memory loads and cannot address the relationship between performance and activation at more demanding memory loads in either HV or schizophrenic subjects. The prediction in patients is that they would have shown DLPFC hypoactivity relative to controls as working memory capacity was exceeded.
There were significant hemisphere by load effect for both the encode and probe conditions with left hemisphere response overall stronger than the right; however, these interactions were not different by diagnosis. Given inconsistencies in prior studies regarding lateralized DLPFC activation during SIRP performance and its differences between groups,2,62 we did not have clear a priori hypotheses regarding laterality. In the present study, both groups showed greater left than right DLPFC activation. A possible interpretation of this is that one strategy by which digits are actively maintained in working memory is covert verbal rehearsal as was suggested by Sternberg in his original work.1 This strategy may have engaged left DLPFC more strongly and been more critical at higher loads, accounting for the interaction of load by hemisphere. It should be noted that the literature concerning DLPFC specialization by material type is controversial (for review see D'Esposito et al64), and other aspects of performance may have accounted for hemispheric differences. Finally, hemisphere differences are difficult to interpret as hemispheric function is influenced by and may partially reflect activity from the opposite hemisphere.
Figure 5 suggests there may be a difference between schizophrenics and normals across all memory loads. The 3-item condition was statistically significant, while the 1-item and 5-item conditions were not. With the sample variance that we observed, the differences at 1- and 5-item loads would have to be at least twice what we observed to achieve statistical significance. We had 99% power to detect statistical differences at memory loads 1 and 5, if they were as large as shown in figure 5.
One of the limitations of this analysis is that we confined it to the DLPFC and did not include other brain areas related to working memory or attention. The parietal lobe and several subcortical structures eg thalamus and basal ganglia, amygdala, and hippocampus have been suggested to play a role in the compensatory response in schizophrenic subjects as they possibly recruit a more extensive network to perform the working memory task.62,63,65,66 Another limitation of our analysis is that DLPFC encompasses a fairly broad and functionally heterogeneous territory. DLPFC subregions may have differed in their pattern of activation during task performance. Given the need to smooth the data for the group analysis, and the likelihood that distinct DLPFC functional subregions will not be aligned in group averages given the heterogeneity of their localization, particularly in schizophrenia, the best way to evaluate the possibility of functionally distinct subregions is with an ROI analysis based on individual anatomy. This approach avoids signal loss due to morphological and functional variability between participants and increases statistical power due to signal averaging within participants.15,62,67 A voxel-based analysis can identify intergroup differences within the ROI as well as explore nonhypothesized regions. This is beyond the scope of the present work, but planned for future, finer-grained analyses. While our focus was on the DLPFC, these finer-grained and connectivity analyses are the focus of other papers, and these datasets are being made publicly available through the BIRN Data Repository (www.nbirn.net), so that a wide variety of analyses can be performed on them by the research community.
In order to use multisite data optimally, site effects need consideration. The FBIRN efforts in multisite studies focused on reducing scanner differences as much as possible and have been largely effective. In this analysis, the 1.5T scanners tended to show lower mean signal changes than the 3T scanners, but the differences were not significant. The site effects we observed were limited to the encode condition. The site differences in the encode condition could reflect a systematic difference in data acquisition or a true difference in populations. The cognitive paradigm and instructions to the subjects were standardized. The stimulus equipment, however, was not standardized; a variety of display equipment was used, perhaps accounting for the difference observed during stimulus presentation. A site by diagnosis effect was not observed for either the encode or the retrieval condition, indicating the between-group effects were similar regardless of the source of the data. A full discussion of site effects will be addressed in a separate manuscript.
All our schizophrenic subjects were medicated. While we cannot exclude medication effects as a potential confound in our findings of group differences, aberrant DLPFC activation during working memory has been observed in medication-naive patients,68 and current antipsychotics have not been shown to substantially affect cognitive deficits in schizophrenia.69 Karlsgodt et al13 recently reported that medicated patients with schizophrenia and non-ill, medication-naive cotwins of patients with schizophrenia show working memory performance deficits and a similar positive association between working memory performance and DLPFC activation, with DLPFC hyperactivity in the patients and the nonill, unmedicated cotwins compared with controls at higher performance levels. In addition, the fact that patients were medicated does not detract from the clinical relevance because the vast majority of patients with schizophrenia are medicated.
The advantages of our study include our ability to separate encode from retrieval activations, to match subjects for performance accuracy, to include a sufficiently large number of subjects from multiple centers, to enhance the generalizability of our findings as well as to be able to maintain sufficient power to support subanalyses.
In summary, we have shown that successful working memory performance in schizophrenia is associated with inefficient DLPFC function compared with healthy normal controls, even at the same level of accuracy. The findings support the concept not of hyper- or hypofrontality in schizophrenia, but rather reduced efficiency of prefrontal function in schizophrenia that may be manifested in either direction depending on task demands. This reframes the problem and redirects the focus of research from direction of difference to mechanisms of inefficiency.
Funding
U24-RR021992 to the Functional Imaging Biomedical Informatics Research Network (FBIRN, http://www.fbirn.org) that is funded by the National Center for Research Resources at the National Institutes of Health.
Acknowledgments
We gratefully acknowledge Aaron Kemp and Jerod Rasmussen for assistance with additional statistical analyses and also the editorial support of Liv McMillan and Divya Rajpoot. A special thank you and acknowledgment must also go to the FBIRN subjects for their participation, patience, and cooperation; without their help this project could not have been accomplished. Authorship contributions: the inclusion of the Functional Imaging Biomedical Informatics Research Network as an author represents the efforts of many otherwise unlisted researchers over the years who also had explicit input into the conception, design, and implementation of the work. The following authors conceived, designed, and/or implemented the Sternberg Item Recognition Paradigm and experiment; and/or facilitated data acquisition or data sharing for that experiment: D.S.M., R.G., G.M., C.G.W., G.G.B., A.B, J.M.F., D.N.G., T.G.M.van E., A.W.T., J.L., D.O'L., G.H.G, J.A.T., and S.G.P. These authors contributed to the analysis of the imaging or clinical data presented in this article: G.G.B., J.A.T., D.N.G., D.H.M., A.P., G.M., and S.G.P. These authors contributed to interpretation of the analyses and writing the manuscript: S.G.P., J.A.T., A.P., G.M., G.G.B., D.S.M., and J.M.F.
References
- 1.Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- 2.Manoach DS, Press DZ, Thangaraj V, et al. Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol Psychiatry. 1999;45:1128–1137. doi: 10.1016/s0006-3223(98)00318-7. [DOI] [PubMed] [Google Scholar]
- 3.Saykin AJ, Gur RC, Gur RE, et al. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch Gen Psychiatry. 1991;48:618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- 4.Saykin AJ, Shtasel DL, Gur RE, et al. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994;51(2):124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- 5.Bowen L, Wallace CJ, Glynn SM, Nuechterlein KH. Schizophrenic individuals’ cognitive functioning and performance in interpersonal interactions and skills training procedures. J Psychiatr Res. 1994;28(3):289–301. doi: 10.1016/0022-3956(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 6.Corrigan PW, Wallace CJ, Schade ML, Green MF. Learning medication self-management skills in schizophrenia: relationships with cognitive deficits and psychiatric symptoms. Behav Ther. 1994;25(1):5–15. [Google Scholar]
- 7.Mueser KT, Bellack AS, Douglas MS, Wade JH. Prediction of social skill acquisition in schizophrenic and major affective disorder patients from memory and symptomatology. Psychiatry Res. 1991;37:281–296. doi: 10.1016/0165-1781(91)90064-v. [DOI] [PubMed] [Google Scholar]
- 8.Penn DL, Spaulding W, Reed D, Sullivan M. The relationship of social cognition to ward behavior in chronic schizophrenia. Schizophr Res. 1996;20:327–335. doi: 10.1016/0920-9964(96)00010-2. [DOI] [PubMed] [Google Scholar]
- 9.Kern RS, Green MF, Satz P. Neuropsychological predictors of skills training for chronic psychiatric patients. Psychiatry Res. 1992;43:223–230. doi: 10.1016/0165-1781(92)90055-8. [DOI] [PubMed] [Google Scholar]
- 10.Cannon TD, van Erp TG, Huttunen M, et al. Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry. 1998;55:1084–1091. doi: 10.1001/archpsyc.55.12.1084. [DOI] [PubMed] [Google Scholar]
- 11.Cannon TD, Thompson PM, van Erp TG, et al. Cortex mapping reveals regionally specific patterns of genetic and disease-specific gray-matter deficits in twins discordant for schizophrenia. Proc Natl Acad Sci U S A. 2002;99:3228–3233. doi: 10.1073/pnas.052023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altamura M, Elvevag B, Blasi G, et al. Dissociating the effects of Sternberg working memory demands in prefrontal cortex. Psychiatry Res. 2007;154(2):103–114. doi: 10.1016/j.pscychresns.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Karlsgodt KH, Glahn DC, van Erp TG, et al. The relationship between performance and fMRI signal during working memory in patients with schizophrenia, unaffected co-twins, and control subjects. Schizophr Res. 2007;89:191–197. doi: 10.1016/j.schres.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Jansma JM, Ramsey NF, van der Wee NJ, Kahn RS. Working memory capacity in schizophrenia: a parametric fMRI study. Schizophr Res. 2004;68(2–3):159–171. doi: 10.1016/S0920-9964(03)00127-0. [DOI] [PubMed] [Google Scholar]
- 15.Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- 16.Callicott JH, Egan MF, Mattay VS, et al. Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am J Psychiatry. 2003;160:709–719. doi: 10.1176/appi.ajp.160.4.709. [DOI] [PubMed] [Google Scholar]
- 17.Jaeggi SM, Seewer R, Nirkko AC, et al. Does excessive memory load attenuate activation in the prefrontal cortex? Load-dependent processing in single and dual tasks: functional magnetic resonance imaging study. Neuroimage. 2003;19(2 Pt 1):210–225. doi: 10.1016/s1053-8119(03)00098-3. [DOI] [PubMed] [Google Scholar]
- 18.Callicott JH, Mattay VS, Bertolino A, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9(1):20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- 19.Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- 20.Ragland JD, Gur RC, Glahn DC, et al. Frontotemporal cerebral blood flow change during executive and declarative memory tasks in schizophrenia: a positron emission tomography study. Neuropsychology. 1998;12(3):399–413. doi: 10.1037//0894-4105.12.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- 22.Callicott JH, Bertolino A, Mattay VS, et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- 23.Ramsey NF, Koning HA, Welles P, Cahn W, van der Linden JA, Kahn RS. Excessive recruitment of neural systems subserving logical reasoning in schizophrenia. Brain. 2002;125(Pt 8):1793–1807. doi: 10.1093/brain/awf188. [DOI] [PubMed] [Google Scholar]
- 24.Cannon TD, Glahn DC, Kim J, et al. Dorsolateral prefrontal cortex activity during maintenance and manipulation of information in working memory in patients with schizophrenia. Arch Gen Psychiatry. 2005;62(10):1071–1080. doi: 10.1001/archpsyc.62.10.1071. [DOI] [PubMed] [Google Scholar]
- 25.Kindermann SS, Brown GG, Zorrilla LE, Olsen RK, Jeste DV. Spatial working memory among middle-aged and older patients with schizophrenia and volunteers using fMRI. Schizophr Res. 2004;68:203–216. doi: 10.1016/j.schres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Wolf DH, Gur RC, Valdez JN, et al. Alterations of fronto-temporal connectivity during word encoding in schizophrenia. Psychiatry Res. 2007;154:221–232. doi: 10.1016/j.pscychresns.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Y, Liang M, Jiang T, et al. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci Lett. 2007;417:297–302. doi: 10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]
- 28.Mendrek A, Kiehl KA, Smith AM, Irwin D, Forster BB, Liddle PF. Dysfunction of a distributed neural circuitry in schizophrenia patients during a working-memory performance. Psychol Med. 2005;35(2):187–196. doi: 10.1017/s0033291704003228. [DOI] [PubMed] [Google Scholar]
- 29.Scheuerecker J, Frodl T, Koutsouleris N, et al. Cerebral differences in explicit and implicit emotional processing—an fMRI study. Neuropsychobiology. 2007;56(1):32–39. doi: 10.1159/000110726. [DOI] [PubMed] [Google Scholar]
- 30.Kubicki M, McCarley R, Westin CF, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41(1–2):15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanaan RA, Kim JS, Kaufmann WE, Pearlson GD, Barker GJ, McGuire PK. Diffusion tensor imaging in schizophrenia. Biol Psychiatry. 2005;58:921–929. doi: 10.1016/j.biopsych.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Esslinger C, Gruppe H, Danos P, et al. Influence of vigilance and learning on prefrontal activation in schizophrenia. Neuropsychobiology. 2007;55(3–4):194–202. doi: 10.1159/000108378. [DOI] [PubMed] [Google Scholar]
- 33.Van Snellenberg JX, Torres IJ, Thornton AE. Functional neuroimaging of working memory in schizophrenia: task performance as a moderating variable. Neuropsychology. 2006;20:497–510. doi: 10.1037/0894-4105.20.5.497. [DOI] [PubMed] [Google Scholar]
- 34.First MB, Spitzer RL, Gibbon M, Williams JBW. New York: Biometrics Research, New York State Psychiatric Institute; 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) [Google Scholar]
- 35.First MB, Spitzer RL, Gibbon M, Williams JBW. New York: Biometrics Research, New York State Psychiatric Institute; 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) [Google Scholar]
- 36.McFarland K, Anderson J. Factor stability of the Edinburgh Handedness Inventory as a function of test-retest performance, age and sex. Br J Psychol. 1980;71(1):135–142. [Google Scholar]
- 37.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 38.Blair J, Spreen O. Predicting premorbid IQ: a revision of the National Adult Reading Test. Clin Neuropsychol. 1989;3:129–136. [Google Scholar]
- 39.Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- 40.Andreasen NC. Modified Scale for the Assessment of Negative Symptoms (SANS) Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- 41.Addington D, Addington J, Maticka-Tyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry. 1993;(22):39–44. [PubMed] [Google Scholar]
- 42.Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT., Jr The Schedule for the Deficit syndrome: an instrument for research in schizophrenia. Psychiatry Res. 1989;30(2):119–123. doi: 10.1016/0165-1781(89)90153-4. [DOI] [PubMed] [Google Scholar]
- 43.Lindenmayer JP, Czobor P, Alphs L, et al. The InterSePT scale for suicidal thinking reliability and validity. Schizophr Res. 2003;63(1–2):161–170. doi: 10.1016/s0920-9964(02)00335-3. [DOI] [PubMed] [Google Scholar]
- 44.Abnormal Involuntary Movement Scale (AIMS) Psychopharmacol Bull. 1988;24:781–783. [PubMed] [Google Scholar]
- 45.Barnes TR. The Barnes Akathisia Rating Scale—revisited. J Psychopharmacol. 2003;17:365–370. doi: 10.1177/0269881103174013. [DOI] [PubMed] [Google Scholar]
- 46.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 47.Keator DB, Gadde S, Grethe JS, Taylor DV, Potkin SG. A general XML schema and SPM toolbox for storage of neuro-imaging results and anatomical labels. Neuroinformatics. 2006;4:199–212. doi: 10.1385/ni:4:2:199. [DOI] [PubMed] [Google Scholar]
- 48.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 49.Available at: www.fmrib.ox.ac.uk [Google Scholar]
- 50.Friedman L, Glover GH. Reducing interscanner variability of activation in a multicenter fMRI study: controlling for signal-to-fluctuation-noise-ratio (SFNR) differences. Neuroimage. 2006;33:471–481. doi: 10.1016/j.neuroimage.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 51.Carmack PS, Spence J, Gunst RF, Schucany WR, Woodward WA, Haley RW. Improved agreement between Talairach and MNI coordinate spaces in deep brain regions. Neuroimage. 2004;22:367–371. doi: 10.1016/j.neuroimage.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 52.Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- 53.Jaccard P. Étude comparative de la distribution florale dans une portion des Alpes et des Jura. Bull Soc Vaudoise Sci Nat. 1901;37:547–579. [Google Scholar]
- 54.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Bio Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 55.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 56.Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 57.Manoach DS, Greve DN, Lindgren KA, Dale AM. Identifying regional activity associated with temporally separated components of working memory using event-related functional MRI. Neuroimage. 2003;20:1670–1684. doi: 10.1016/j.neuroimage.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 58.Callicott JH, Ramsey NF, Tallent K, et al. Functional magnetic resonance imaging brain mapping in psychiatry: methodological issues illustrated in a study of working memory in schizophrenia. Neuropsychopharmacology. 1998;18(3):186–196. doi: 10.1016/S0893-133X(97)00096-1. [DOI] [PubMed] [Google Scholar]
- 59.Menon V, Anagnoson R, Glover G, Pfefferbaum A. Functional magnetic resonance imaging evidence for disrupted basal ganglia function in schizophrenia. Am J Psychiatry. 2001;158:646–649. doi: 10.1176/appi.ajp.158.4.646. [DOI] [PubMed] [Google Scholar]
- 60.Callicott JH. An expanded role for functional neuroimaging in schizophrenia. Curr Opin Neurobiol. 2003;13:256–260. doi: 10.1016/s0959-4388(03)00041-2. [DOI] [PubMed] [Google Scholar]
- 61.Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- 62.Manoach DS, Gollub RL, Benson ES, et al. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry. 2000;48(2):99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- 63.Johnson MR, Morris NA, Astur RS, et al. A functional magnetic resonance imaging study of working memory abnormalities in schizophrenia. Biol Psychiatry. 2006;60(1):11–21. doi: 10.1016/j.biopsych.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 64.D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn Brain Res. 1998;7(1):1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- 65.Tura E, Turner JA, Fallon JH, Kennedy JL, Potkin SG. Multivariate analyses suggest genetic impacts on neurocircuitry in schizophrenia. Neuroreport. 2008;19:603–607. doi: 10.1097/WNR.0b013e3282fa6d8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008;11(1):103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- 67.Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nat Rev Neurosci. 2002;3:243–249. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- 68.Barch DM, Carter CS, Braver TS, et al. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- 69.Sergi MJ, Green MF, Widmark C, et al. Social cognition [corrected] and neurocognition: effects of risperidone, olanzapine, and haloperidol. Am J Psychiatry. 2007;164:1585–1592. doi: 10.1176/appi.ajp.2007.06091515. [DOI] [PubMed] [Google Scholar]