Abstract
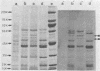
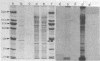
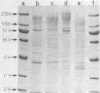
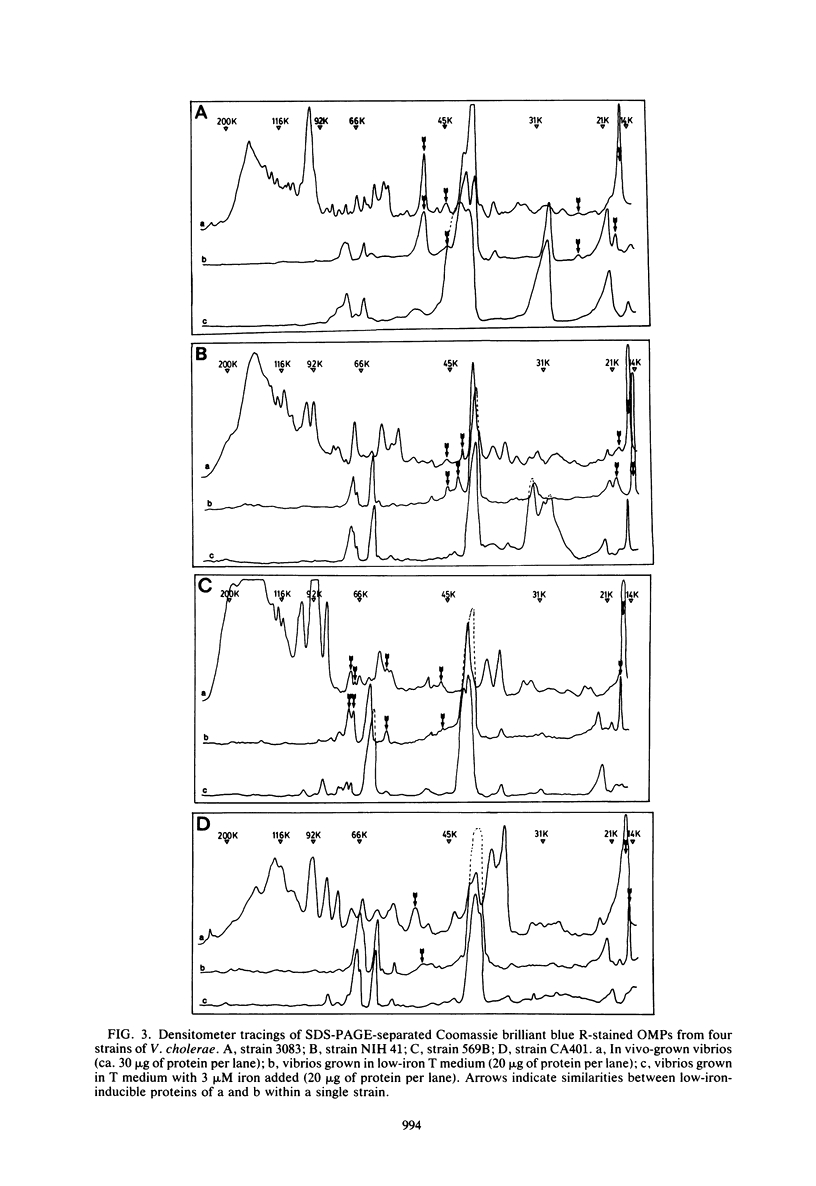
A comparison was made, using sodium dodecyl sulfate-polyacrylamide gel electrophoresis, of the outer membrane proteins of four strains of Vibrio cholerae grown in vivo in infant rabbits and in vitro in low-iron and iron-supplemented defined media. In vivo-grown V. cholerae expressed novel outer membrane-associated proteins which, in part, were similar to those observed on V. cholerae grown in vitro under conditions of iron deprivation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brener D., DeVoe I. W., Holbein B. E. Increased virulence of Neisseria meningitidis after in vitro iron-limited growth at low pH. Infect Immun. 1981 Jul;33(1):59–66. doi: 10.1128/iai.33.1.59-66.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J. The significance of iron in infection. Rev Infect Dis. 1981 Nov-Dec;3(6):1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- DE S. N., CHATTERJE D. N. An experimental study of the mechanism of action of Vibriod cholerae on the intestinal mucous membrane. J Pathol Bacteriol. 1953 Oct;66(2):559–562. doi: 10.1002/path.1700660228. [DOI] [PubMed] [Google Scholar]
- FINKELSTEIN R. A., NORRIS H. T., DUTTA N. K. PATHOGENESIS EXPERIMENTAL CHOLERA IN INFANT RABBITS. I. OBSERVATIONS ON THE INTRAINTESTINAL INFECTION AND EXPERIMENTAL CHOLERA PRODUCED WITH CELL-FREE PRODUCTS. J Infect Dis. 1964 Jun;114:203–216. doi: 10.1093/infdis/114.3.203. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Boesman M., Neoh S. H., LaRue M. K., Delaney R. Dissociation and recombination of the subunits of the cholera enterotoxin (choleragen). J Immunol. 1974 Jul;113(1):145–150. [PubMed] [Google Scholar]
- Finkelstein R. A., Fujita K., LoSpalluto J. J. Procholeragenoid: an aggregated intermediate in the formation of choleragenoid. J Immunol. 1971 Oct;107(4):1043–1051. [PubMed] [Google Scholar]
- Finkelstein R. A., Vasil M. L., Holmes R. K. Studies on toxinogenesis in Vibrio cholerae. I. Isolation of mutants with altered toxinogenicity. J Infect Dis. 1974 Feb;129(2):117–123. doi: 10.1093/infdis/129.2.117. [DOI] [PubMed] [Google Scholar]
- Finn T. M., Arbuthnott J. P., Dougan G. Properties of Escherichia coli grown in vivo using a chamber implant system. J Gen Microbiol. 1982 Dec;128(12):3083–3091. doi: 10.1099/00221287-128-12-3083. [DOI] [PubMed] [Google Scholar]
- Griffiths E., Humphreys J. Isolation of enterochelin from the peritoneal washings of guinea pigs lethally infected with Escherichia coli. Infect Immun. 1980 Apr;28(1):286–289. doi: 10.1128/iai.28.1.286-289.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981 May 21;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Honda T., Finkelstein R. A. Selection and characteristics of a Vibrio cholerae mutant lacking the A (ADP-ribosylating) portion of the cholera enterotoxin. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2052–2056. doi: 10.1073/pnas.76.4.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir S. Composition and immunochemical properties of outer membrane proteins of Vibrio cholerae. J Bacteriol. 1980 Oct;144(1):382–389. doi: 10.1128/jb.144.1.382-389.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley J. T., Parker C. D. Identification and preliminary characterization of Vibrio cholerae outer membrane proteins. J Bacteriol. 1981 Feb;145(2):1018–1024. doi: 10.1128/jb.145.2.1018-1024.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Black R. E., Clements M. L., Cisneros L., Nalin D. R., Young C. R. Duration of infection-derived immunity to cholera. J Infect Dis. 1981 Jun;143(6):818–820. doi: 10.1093/infdis/143.6.818. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Black R. E., Clements M. L., Young C. R., Lanata C., Sears S., Honda T., Finkelstein R. Texas Star-SR: attenuated "Vibrio cholerae" oral vaccine candidate. Dev Biol Stand. 1983;53:59–65. [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McIntosh M. A., Earhart C. F. Effect of iron of the relative abundance of two large polypeptides of the Escherichia coli outer membrane. Biochem Biophys Res Commun. 1976 May 3;70(1):315–322. doi: 10.1016/0006-291x(76)91144-x. [DOI] [PubMed] [Google Scholar]
- Moore D. G., Earhart C. F. Specific inhibition of Escherichia coli ferrienterochelin uptake by a normal human serum immunoglobulin. Infect Immun. 1981 Feb;31(2):631–635. doi: 10.1128/iai.31.2.631-635.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. Siderophore production by Vibrio cholerae. Infect Immun. 1978 Apr;20(1):310–311. doi: 10.1128/iai.20.1.310-311.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. The critical role of iron in host-bacterial interactions. J Clin Invest. 1978 Jun;61(6):1428–1440. doi: 10.1172/JCI109062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMON E. H., TESSMAN I. THYMIDINE-REQUIRING MUTANTS OF PHAGE T4. Proc Natl Acad Sci U S A. 1963 Sep;50:526–532. doi: 10.1073/pnas.50.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel S. P., Payne S. M. Effect of iron limitation on growth, siderophore production, and expression of outer membrane proteins of Vibrio cholerae. J Bacteriol. 1982 Apr;150(1):148–155. doi: 10.1128/jb.150.1.148-155.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K. H., Williams R. P. Use of iodo-gen and iodine-125 to label the outer membrane proteins of whole cells of Neisseria gonorrhoeae. Anal Biochem. 1982 Mar 1;120(2):254–258. doi: 10.1016/0003-2697(82)90344-x. [DOI] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward W. E., Gilman R. H., Hornick R. B., Libonati J. P., Cash R. A. Efficacy of a live oral cholera vaccine in human volunteers. Dev Biol Stand. 1976;33:108–112. [PubMed] [Google Scholar]
- Yancey R. J., Breeding S. A., Lankford C. E. Enterochelin (enterobactin): virulence factor for Salmonella typhimurium. Infect Immun. 1979 Apr;24(1):174–180. doi: 10.1128/iai.24.1.174-180.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]