Abstract
The pattern recognition receptor, RAGE, has been shown to be involved in adaptive immune responses but its role on the components of these responses is not well understood. We have studied the effects of a small molecule inhibitor of RAGE and the deletion of the receptor (RAGE−/− mice) on T cell responses involved in autoimmunity and allograft rejection. Syngeneic islet graft and islet allograft rejection was reduced in NOD and B6 mice treated with TTP488, a small molecule RAGE inhibitor (p < 0.001). RAGE−/− mice with streptozotocin-induced diabetes showed delayed rejection of islet allografts compared with wild type (WT) mice (p < 0.02). This response in vivo correlated with reduced proliferative responses of RAGE−/− T cells in MLRs and in WT T cells cultured with TTP488. Overall T cell proliferation following activation with anti-CD3 and anti-CD28 mAbs were similar in RAGE−/− and WT cells, but RAGE−/− T cells did not respond to co-stimulation with anti-CD28 mAb. Furthermore, culture supernatants from cultures with anti-CD3 and anti-CD28 mAbs showed higher levels of IL-10, IL-5, and TNF-α with RAGE−/− compared with WT T cells, and WT T cells showed reduced production of IFN-γ in the presence of TTP488, suggesting that RAGE may be important in the differentiation of T cell subjects. Indeed, by real-time PCR, we found higher levels of RAGE mRNA expression on clonal T cells activated under Th1 differentiating conditions. We conclude that activation of RAGE on T cells is involved in early events that lead to differentiation of Th1+ T cells.
Immune responses to foreign and autoantigens involve activation of both innate and adaptive immune responses. “Danger signals,” for example, those initiated by stressed or dying cells, may stimulate pathogen-associated molecular pattern receptors, such as TLR, that lead to expression of molecules that activate the adaptive immune responses (1). Activation of innate responses may also alter the way in which Ags are presented to T and other cells of the adaptive immune response. The link between innate and adaptive immune responses has led investigators to postulate a role of innate responses in regulating autoimmune diseases such as Type 1 diabetes (2, 3). In support of this hypothesis, deficiency of IL-1 receptor and treatment with IL-1 receptor antagonist attenuates the rate of diabetes in NOD mice (4, 5).
Innate immune responses have also been shown to be involved in the control of adaptive responses. Activation of NK T cells with α-gal-cer was shown to prevent diabetes in the NOD mouse, and a biased response of invariant Vα24JαQ T cells was identified in first degree relatives of patients who progressed to diabetes when compared with nonprogressors (6, 7). In contrast, blockade of NKG2D prevented diabetes in NOD mice (8). Clearly the progression to diabetes in animal models and in humans is primarily dependent on the adaptive responses of T cells, but the way in which the early innate responses lead to and shape adaptive immune responses is not clear.
The pattern recognition receptor, receptor for advanced glycation endproducts (RAGE),3 is a potential link between adaptive and innate responses (9). RAGE was originally identified as the receptor for ligands for molecules whose concentrations are increased in patients with diabetes, and a role for this molecule in the development of secondary end-organ complications has been postulated. However, there are other ligands for RAGE, including S100/calgranulins and HMGB1, products of cellular destruction that may be generated during inflammatory responses and released by inflammatory cells (10). A role for RAGE in innate responses has been identified. Liliensiek et al. (11) showed that mice deficient in RAGE (RAGE−/−) were protected from the lethal effects of septic shock following cecal ligation, and suggested that RAGE plays a role in propagating inflammation. Hofmann et al. (12) found that interaction of RAGE ligands such as members of the S100/calgranulin family with cellular RAGE on endothelium, mononuclear phagocytes, and lymphocytes triggered cellular activation, with generation of key proinflammatory mediators. Kokkola et al. (13) found that HMGB1 has the potential to induce a proinflammatory phenotype in macrophages following interactions with RAGE. More recently, Tian et al. (14) found that in systemic lupus erythematosus, DNA-containing immune complexes that bind HMGB1-bound RAGE, activating TLR-9, and caused plasma dendritic cells (DC) to secrete IFN-α.
It has also been suggested that RAGE ligation may affect adaptive immune responses, but the role of this pattern recognition receptor on T cells has not been well studied, and some investigations have failed to show a role of RAGE in adaptive responses (11). These studies and previous studies indicate a role of RAGE in adaptive responses: soluble RAGE (sRAGE) blocked delayed-type hypersensitivity and inflammatory colitis and a dominant-negative RAGE rendered an encephalogenic CD4+ T cell clone nonpathogenic (12, 15). We previously showed that transfer of diabetes by diabetogenic spleen cells could be inhibited with sRAGE (16). The previous studies generally used sRAGE to block ligation of RAGE by its ligands, but this approach did not allow us to directly identify RAGE as the cellular molecule involved in immune responses, and the specific role of RAGE on T cellular responses had not been addressed. Moreover, in our previous studies we found RAGE expressed on T cells, but the relative expression of RAGE was far greater on other immune cells, which raised a question, therefore, about the significance of the RAGE expression on T cells.
Therefore, to determine whether RAGE is involved in adaptive T cell responses, we studied islet allograft rejection in RAGE−/− mice and recurrent autoimmune diabetes in NOD mice following treatment with a specific small molecule RAGE inhibitor. Treatment with a small molecule RAGE inhibitor attenuated syngeneic islet destruction in an auto- and alloimmune setting, and RAGE−/− mice showed reduced rates of islet allograft rejection. Our studies also suggest a previously unrecognized role of RAGE in T cell activation that may account for the reduced responses. RAGE−/− T cells show reduced allo-reactive responses in vitro and a reduced response to CD28 costimulatory signals. Furthermore, RAGE activation appears to be involved in differentiation of T cell phenotypes since RAGE−/− T cells produce relatively greater amounts of IL-10 in response to TCR activation and there is increased expression of RAGE on clonal Th1 cells. These studies show a role of RAGE in modifying activation pathways during adaptive T cell responses.
Materials and Methods
Mice and reagents
NOD/LtJ, C57BL/6, B10.A.5R, B10.BR, and BALB/c were purchased from The Jackson Laboratory. RAGE−/− mice, described previously, were crossed into C57BL/6 for >10 generations (11, 17). B10.BR-TCR mice (heterozygous animals carrying the transgenic AND TCR) were bred in our facility. All the mice were housed under pathogen-free conditions at our facility. All experiments were approved by the Institutional Animal Care and Use Committees of Columbia and Yale Universities.
The small molecule RAGE antagonist, TTP488, was developed by Trans-Tech Pharma. The TTP488 or control peptide was administered i.p. daily at a dose of 100 mcg/d.
Syngeneic and allogeneic islet transplantation
Diabetes was diagnosed when two random glucose levels, measured by a hand-held meter (Glucometer Elite XL; Bayer), were >250 mg/dl. Diabetic recipient mice received islet grafts within 2 days of the diagnosis. Islets from nondiabetic donor mice were isolated and transplanted into the subcapsular space of the right kidneys of recipient mice as previously described (16). In each experiment, control mice (that received placebo) or wild-type (WT) mice received islets from the same pool as TTP488-treated or RAGE−/− mice. Islet graft function was monitored by serial blood glucose measurements daily for the first 2 wks after islet transplantation, followed by every other day thereafter. The immediate graft function after transplantation was confirmed by finding glucose levels <200 mg/dl. Graft loss or recurrent diabetes was determined when blood glucose exceeded 250 mg/dl on two measurements.
In syngeneic islet transplantation experiments, islets were isolated from young prediabetic NOD/LtJ (6–7 wk old) mice and transplanted into NOD mice with spontaneous diabetes. The mice were treated with TTP488 or control peptide as described above. For allogeneic islet transplantation experiments, islets were isolated from WT BALB/c mice (8–10 wk old) and transplanted into B6 mice that were rendered diabetic by treatment with a single i.v. injection of streptozotocin (STZ) (Sigma-Aldrich) (200 mg/kg).
T cell activation in vitro
Mixed lymphocyte reactions
To study the responses of T cells to alloantigens, MLRs were performed with purified T cells from RAGE−/− or WT B6 mice as responders and irradiated (3000 rad) T-depleted BALB/c splenocytes as stimulators. T cells were isolated using Pan T Cell Isolation Kit (Miltenyi Biotec) following the manufacturer’s methods. Stimulator cells (T-depleted BALB/c splenocytes) were cultured with responder cells at a 1:1 ratio (at 1 × 106/ml) in triplicate cultures in RPMI 1640 (Invitrogen) medium supplemented with 10% heat-inactivated FCS (Sigma-Aldrich), 25 mM HEPES (Invitrogen), 10 mM L-glutamine (Invitrogen), 100 μg/ml penicillin/streptomycin, and 2-ME (Invitrogen) in 96-well round-bottom plates at 37°C with 5% CO2 for 4 days. Supernatants from the cultures were harvested for measurement of cytokines. One μCi of [3H]thymidine was then added to the cultures which were harvested 18 h later.
T cell activation with anti-CD3 and anti-CD28 mAbs
To study the role of RAGE in differentiation and activation of T cells, splenic T lymphocytes were isolated to 99% purity. Splenocytes from WT or RAGE−/− B6 mice were cultured in complete media on plastic tissue culture plates for 4 h and the non-adherent cells were then placed on T Cell Isolation columns (Miltenyi Biotec) twice. The cells purified in this manner were found to be 99% CD3+ by flow cytometry. The purified T cells were cultured (1 × 105/well, in triplicate) for 72 h in U-bottom tissue culture wells coated with graded concentrations of anti-CD3 mAb with our without anti-CD28 mAb (mAbs 145–2C11 and Pv-1, both from BD Pharmingen). The culture supernatants were harvested for measurement of cytokines and [3H]thymidine was added for 18 h. The cells were harvested after 72 h in culture, and the uptake of [3H]thymidine by the cells was measured as described above. Cytokines IL-2, IL-4, IL-5, IL-10, IFN-γ, and TNF-α were measured in the supernatants of these cultures with Cytokine Multiplex Kit (BioSource International). The T cell proliferative data are presented as both the cpm from the cultures as well as the stimulation index of the cells in response to anti-CD3 mAb with anti-CD28 mAb to determine the effects of T cell costimulation with anti-CD28 mAb. For this analysis, the cpm in the presence of anti-CD3 and the indicated doses of anti-CD28 mAb were divided by the cpm in cultures of the same cells in the absence of anti-CD28 mAb.
In certain MLR’s, TTP488 4 nm was added to cultures of WT or RAGE−/− mice. The percent inhibition was calculated as: 1 − (cpm in cultured with stimulators and TTP488 − cpm without stimulators/cpm with stimulators − cpm without stimulators). In addition, responders from the MLR’s of WT cells that were cultured with or without TTP488 were activated with PMA/ionomycin (50 ng/ml and 500 ng/ml). The culture supernatants were harvested after 16 h and the concentrations of IL-2, IFN-γ, IL-4, and IL-10 were measured with a Luminex cytometer with cytokine specific beads.
Flow cytometry
The following mAbs were used for T cell analysis: PE-conjugated rat anti-mouse CD4, PE-conjugated rat anti-mouse CD8a, PerCP-conjugated rat anti-mouse CD4, allophycocyanin-conjugated rat anti-mouse CD3e, allophycocyanin-conjugated rat anti-mouse CD25, and PE-conjugated anti-CD28 (all from BD Pharmingen).
Identification of RAGE transcripts in Th1 and Th2 cells
T cell-depleted APC were prepared by Ab-mediated complement lysis of B10.A (5R) splenocytes and treated with treated with 50 μg/ml mitomycin C (Sigma-Aldrich) before use. CD4 T cells from lymph nodes and spleens of TCR-transgenic (AND) mice were isolated by immunomagnetic negative selection using Abs against CD8, CD32/CD16, B220, MHC class II, and NK cells, followed by incubation with anti-mouse and anti-rat Ig-coated magnetic beads (Poly-sciences). Purity of the recovered Vα11CD4 T cells was 85–95% as determined by staining with anti-CD4 and anti-Vα11 mAbs (18).
Induction of naive T cell differentiation was performed by incubating mitomycin C-treated APC (1 × 106/ml) with CD4 T cells (5 × 105/ml) from AND TCR transgenic mice in the presence of peptide (moth cytochrome C at 5 μg/ml l), rIL-2 (25 U/ml), and anti-IFN-γAb (XMG1.2) for Th2 or anti-IL4 Ab (11B11) for Th1 (19, 20).
Total RNA was prepared using TRIzol (Invitrogen) and 1–10 μg RNA aliquots were further cleaned using the Rneasy Mini kit (Qiagen). RNA samples were subjected to reverse transcriptase reactions (Superscript II; Invitrogen) in the presence of oligo(dT) and hexamers. Approximately, 2 ng cDNA template were used for one PCR. All experiments were performed using gene-specific primer pairs and SYBR green I (Molecular Probes) fluorescence detection in an MX3000P instrument (Stratagene). The sequence of the gene-specific and reference gene (actin) primers selected is Rage-F: GTCATTCTCACAGGAAGGCAGAAAG; Rage-R: TTCTTGTTCACAGCAGCACACTTC (228 bp product); Actin-F: GCT GTGCTGTCCCTGTATGCCTCT; Actin-R: CCTCTCAGCTGTGGT GGTGAAGC (206 bp product). All reactions were done using 40 cycles of 20 s/94°C denaturing, 10 s/61°C annealing and 30 s/74°C extension (17). For every primer pair, the quality of the PCR amplification was assessed by visualization of the specific amplification product on a 2.5% agarose gel. Quantitative PCR of reference gene (β-actin) was use to normalize the amount of cDNA in each sample tested. For quantitative evaluation of amplification efficiency, a standard curve of fluorescence vs template amount was recorded for each primer pair, using decreasing concentrations of T cell cDNA as template (40; 4; 1; 0.25; 0.0625; 0.015 ng/reaction). At the end of each quantitative PCR, a melting curve analysis was performed to analyze product identity after the run and avoid recording false positives. For relative quantitation, the threshold cycle values of the gene tested and of the reference gene (β-actin) were exported into the Q-gene software.
Statistical analysis
Group data are expressed as mean ± SEM. An unpaired Student’s t test was used to compare average values between groups. Graft survival was compared between recipient groups by Kaplan-Meier analysis. Cytokine data was log transformed before performing statistical tests. Values of p < 0.05 were considered significant.
Results
Blockade of RAGE by treatment with the small molecule RAGE antagonist (TTP488) prolongs islet auto and allograft survival
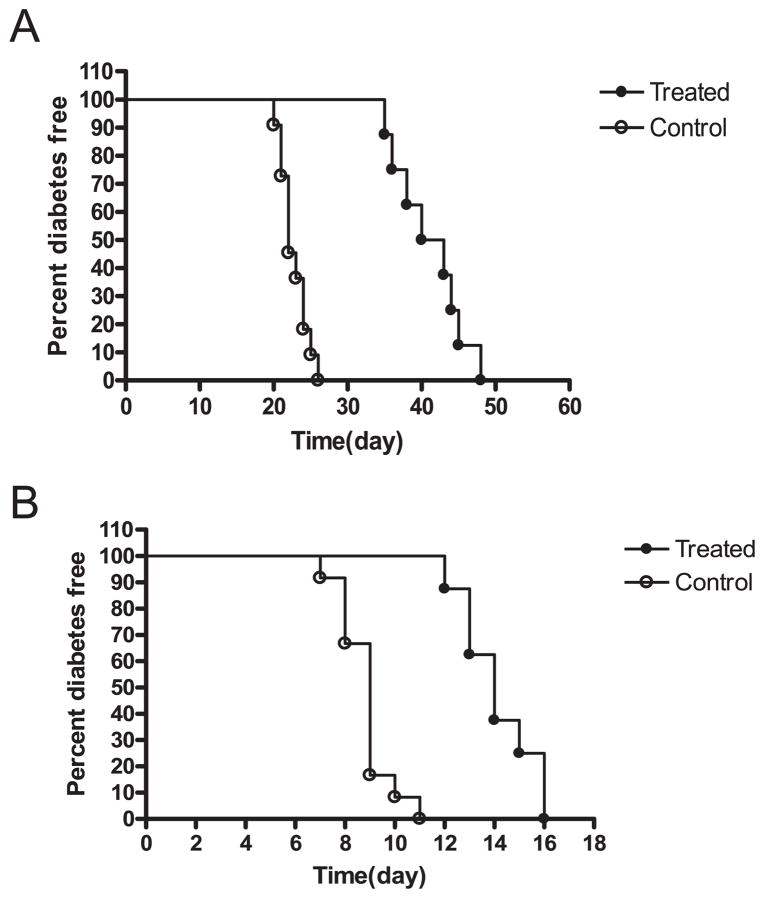
We used two model systems to study the role of RAGE in auto- or alloimmune adaptive immune responses. We first tested the effects of a small molecule RAGE antagonist (TTP488) on the survival of syngeneic islet grafts transplanted into NOD mice with autoimmune diabetes (Fig. 1A) or BALB/c allografts transplanted into B6 mice with chemically induced diabetes (Fig. 1B). When diabetic NOD mice were transplanted with syngeneic islets and treated daily with TTP488, the median survival time increased from 22.7 ± 0.56 days (n = 11) to 41.1 ± 1.6 days (n = 8; p < 0.001) (Fig. 1A). In a similar manner, treatment with TTP488 increased the median survival of BALB/c allografts from 8.8 ± 0.3 days (n = 8) to 14.1 ± 0.5 days (n = 8; p < 0.001) (Fig. 1B).
FIGURE 1.
Survival of syngeneic or allogeneic islet grafts in mice with diabetes. A, NOD mice with hyperglycemia received a transplant of syngeneic islet grafts and were treated with TTP488 (n = 8) or control peptide (n = 11) as indicated in the Materials and Methods. The percentage of nondiabetic mice is shown. There was a significant prolongation of the survival of islets in mice treated with TTP488 (p < 0.001). B, C57BL/6 mice with STZ-induced diabetes were transplanted with BALB/c islets and treated with TTP488 (n = 8) or control protein (n = 8). There was a significant prolongation of graft survival with the RAGE antagonist (p < 0.001).
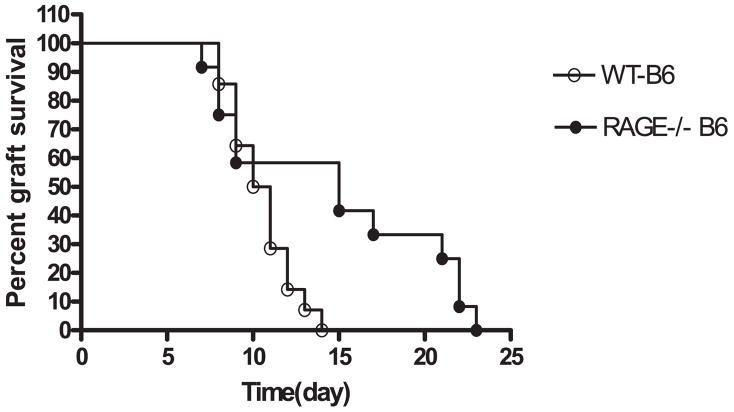
We next studied the survival of allogeneic (BALB/c) islets that were transplanted into WT or RAGE−/− B6 mice with diabetes induced with a single dose of STZ (Fig. 2). There was a significant prolongation of allograft survival of the allogeneic islets in RAGE−/− mice (median survival time = 15 days, n = 12) compared with RAGE+/+ recipients (median survival time = 10.5 days, n = 14, p = 0.02). The reduced responses were also not the result of deficiency of T cells in the RAGE−/− mice because the percentage of CD4+ and CD8+ T cells was similar in RAGE−/− (19.3 ± 0.68 and 13.3 ± 1.1%, n = 5) and WT (18.8 ± 0.9 and 10.9 ± 1.2%, n = 4) splenocytes respectively.
FIGURE 2.
Survival of allogeneic islets in WT and RAGE−/− mice. Diabetes was induced with a single dose of STZ in WT (n = 14) or RAGE−/− (n = 12) B6 mice, which then received a transplant of BALB/c islets. There was a significant delay in rejection of the allogeneic (BALB/c) islets in RAGE−/− mice (p < 0.02).
Proliferative responses in vitro are reduced in the absence of RAGE
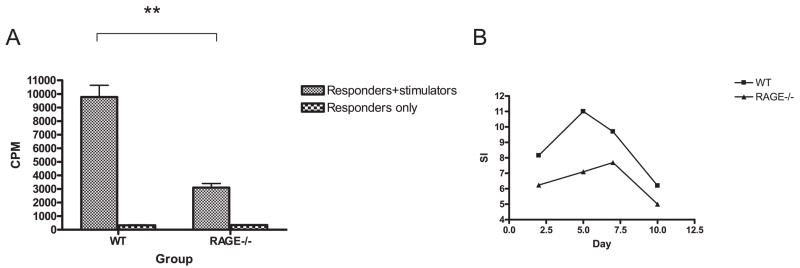
To determine the basis for the reduced responses in vivo, we compared the responses of WT and RAGE−/− B6 splenocytes to irradiated BALB/c stimulator cells in primary MLRs (WT or RAGE−/− B6 vs BALB/c) (Fig. 3A). The proliferative responses were significantly reduced in the RAGE−/− B6 mice compared with WT B6 mice in response to T-depleted BALB/c stimulator cells (n = 4; p < 0.01 vs WT). The reduced responses that we had seen on the 5th day of the primary MLR were not simply a difference in the kinetics of the responses of the RAGE−/− T cells. The RAGE−/− T cells showed a lower proliferative response after 3 days in culture, and the difference with the WT cells was persistent even after 10 days when the responses of both the WT and RAGE−/− T cells were waning (Fig. 3B).
FIGURE 3.
Reduced MLR responses in RAGE−/− mice. A, The proliferative responses of WT or RAGE−/− T cells to BALB/c stimulators were studied in primary mixed lymphocyte reactions after 5 days. The data represent the mean values of four separate experiments. There was a significant reduction (**, p < 0.01) in the proliferative response by RAGE−/− T cells when the actual cpm or the stimulation index (cpm with stimulators/cpm without stimulators) were compared (31.0 ± 1.6 vs 9.0 ± 0.67, p < 0.001). B, The kinetics of the responses of a single experiment (representative of two) comparing WT and RAGE−/− cells are shown.
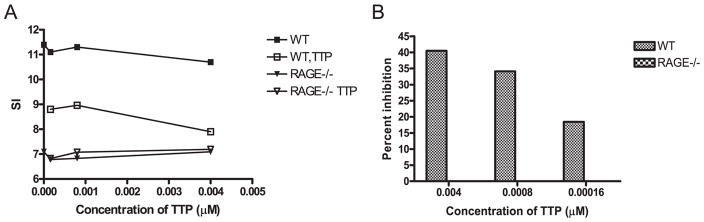
We also studied the effects of the small molecule inhibitor of RAGE, TTP488, on the proliferative responses of purified WT T cells in a MLR. The responses to BALB/c stimulators were reduced when the TTP488 was added to cultures of WT but not RAGE−/− T cells (Fig. 4).
FIGURE 4.
Inhibition of WT but not RAGE−/− T cells by TTP488. A, Purified WT or RAGE−/− T cells were added to irradiated BALB/c stimulator cells with TTP488 at the indicated concentrations or a similar dilution of DMSO. The incorporation of [3H]thymidine was measured after 5 days. The data are expressed as the stimulation index (cpm at the indicated concentration of TTP488 or DMSO/cpm of the responder cells only). The cpm of the responder cells were WT: 11884, RAGE−/−: 12895). B, The percent inhibition (1−cpm with TTP/cpm with equivalent dilution of DMSO *100) of the WT and RAGE−/− T cells was calculated. The data shown are from a single experiment representative of three independent experiments.
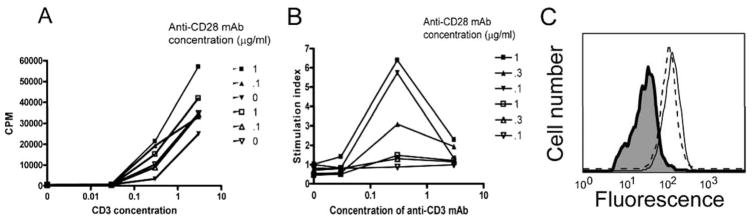
The reduced proliferative responses we observed in the MLR’s could be due to differences in RAGE−/− T or non-T cells. Therefore, we purified splenic T cells and tested their proliferative and cytokine responses to anti-CD3 and anti-CD28 mAbs (Fig. 5). Overall, the responses of the RAGE−/− T cells in response to activation with maximal doses of anti-CD3 (3 μg/ml) and anti-CD28 (1 μg/ml) mAbs were only marginally different in RAGE−/− and WT mice (Fig. 5A). However, the proliferative responses of RAGE−/− T cells to anti-CD3 mAb in the absence of anti-CD28 mAb were generally higher (31%) when compared with WT T cells. We found a striking difference in the T cell responses to costimulation with anti-CD28 mAb (Fig. 5B). There was little enhancement of the proliferative responses of RAGE−/− T cells by costimulation with anti-CD28 mAb above proliferation that was induced with anti-CD3 mAb alone. At a concentration of 0.3 μg/ml of immobilized anti-CD3 mAb, anti-CD28 mAb (@1 μg/ml) increased the proliferative responses by 5.71 ± 0.52-fold over the responses to anti-CD3 mAb alone in WT, but only 1.50 ± 0.08-fold in RAGE−/− mice (n = 3 independent experiments, p = 0.001). Likewise, the stimulation index was 1.25 ± 0.08 in WT cells stimulated with anti-CD3 @ 0.03 μg/ml with anti-CD28 @ 1 μg/ml but the RAGE−/− T cells the responses were not higher than those without anti-CD28 mAb (p = 0.03). These findings indicate that RAGE−/− T cells are unresponsive to costimulation with anti-CD28 mAb. The unresponsiveness to anti-CD28 mAb was not due to reduced expression of CD28 on RAGE−/− T cells on T cells. The expression of CD28 on CD4+ cells was similar in WT and RAGE−/− splenocytes (Fig. 5C).
FIGURE 5.
Diminished responsiveness to CD28 costimulation by RAGE−/− T cells. A, T cells were isolated from B6 (filled symbols) or RAGE−/− (open symbols) mice and added to tissue culture plates coated with the indicated concentrations of anti-CD3 mAb without or with anti-CD28 mAb at the indicated concentrations. B, The effects of CD28 costimulation were analyzed by dividing the cpm from cultures of T cells from WT or RAGE−/− T cells in the presence of the indicated concentrations of anti-CD28 mAb by the cpm from cultures without CD28. There was markedly reduced response to CD28 costimulation by the RAGE−/− T cells. The data shown is from a single experiment that is representative of three independent studies. C, To exclude differences in CD28 expression as the basis for the findings in B, the expression of CD28 was analyzed on WT (dashed line) and RAGE−/− (solid line) CD4+ T cells by flow cytometry (the isotype control is shaded).
RAGE is associated with differentiation of T cells
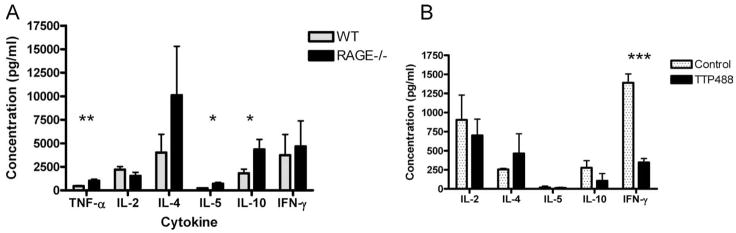
To determine the mechanisms by which RAGE deficiency affects the development of T cell effector responses, we measured the levels of cytokines in the supernatants of purified T cells from WT or RAGE−/− mice that were activated with anti-CD3 and anti-CD28 mAbs (Fig. 6A). The levels of IL-10 and IL-5 (p < 0.05) and TNF-α (p < 0.01) were significantly increased in the supernatants from T cells from RAGE−/− compared with WT mice. The levels of IL-4 were also higher on average in the cultures with RAGE −/− cells but the difference with the WT cells was not statistically significant (p = 0.24).
FIGURE 6.
Cytokine concentrations in the supernatants of activated T cells. A, Purified RAGE−/− (solid bars) and WT (stippled bars) T cells were activated with anti-CD3 and anti-CD28 mAbs and the culture supernatants were harvested for measurement of cytokine concentrations (mean ± SEM, n = 4/group). There was a significant increase in the concentration of TNF-α, IL-5, and IL-10 in the cultures from RAGE−/− mice compared with WT mice (*, p < 0.05; **, p < 0.01). B, Primary MLR’s with purified WT T cells were cultured in the presence of TTP488 or the excipient DMSO as described. The responders were harvested after 5 days and activated with PMA/ionomycin overnight. The concentrations in the supernatants were measured (n = 3/group). There was a significant reduction in the production of IFN-γ in responders that had been cultured with TTP488.
We also studied the cytokine responses of responders of a MLR in the presence or absence of TTP488. WT C57BL/6 T cells were stimulated with T-depleted BALB/c splenocytes in the presence or absence of TTP488 and the production of cytokines by the responders after activation with PMA/ionomycin was measured. The levels of IFN-γ were significantly lower in the presence of the TTP488 (n = 3 mice/group, p < 0.01) (Fig. 6B). There was also a reduction in the concentration of IL-2 and an increase in IL-4 in the supernatants from the cells cultured in the presence of TTP488 compared with those cultured in its absence but the differences did not reach statistical significance.
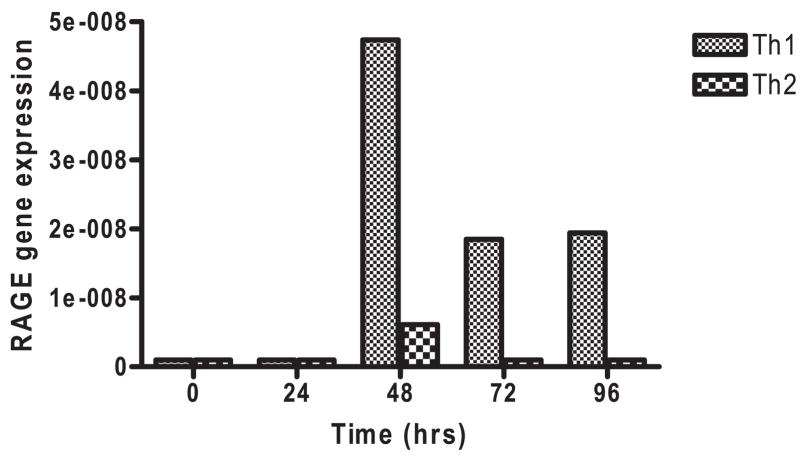
These findings suggested that RAGE may be associated with differentiation of Th1 cells. We therefore studied RAGE expression in clonal T cells differentiated under conditions leading to Th1 or Th2 phenotypes. We studied the expression of RAGE by real-time PCR in AND transgenic T cells that were activated with Ag (mouse cytochrome C) and mitomycin C-treated APC’s under conditions leading to Th1 or Th2 phenotypes (Fig. 7). The expression of RAGE mRNA was increased on cells that were cultured with peptide, IL-2, IL-12, and anti-IL-4 mAb compared with the same cells that were cultured with peptide, IL-4, and anti-IFN-γ mAb. The RAGE mRNA expression was greatest on T cells during primary cell activation. RAGE mRNA expression was negligible in cells during secondary activation with Ag (data not shown).
FIGURE 7.
Expression of RAGE transcripts in AND transgenic T cells cultured under Th1 and Th2 differentiating conditions. RAGE transcripts were measured by real-time PCR and compared with actin in naive AND transgenic T cells cultured with mitomycin C-treated APC’s and Ag under Th1 (fine hatched bar) and Th2 (coarse hatched bar) skewing conditions as described in Materials and Methods. The data are from a single experiment that is representative of two independent experiments.
Discussion
We have shown a role for RAGE in adaptive immune responses to allo- and autoantigens. A small molecule inhibitor of RAGE reduced the destruction of syngeneic islets that were transplanted into NOD mice with spontaneous diabetes, and RAGE-deficient B6 mice rejected allogeneic BALB/c islet grafts at a reduced rate compared with WT B6 mice. RAGE−/− T cells were not anergic. Their response in a MLR was reduced, but they showed the same proliferative responses to activation with full doses of anti-CD3 and anti-CD28 mAbs as WT T cells. However, there were markedly reduced responses of the RAGE−/− T cells to CD28 co-stimulatory signals with anti-CD3 mAb that was not explained by differences in the expression of CD28 on the surfaces of RAGE−/− T cells. In response to anti-CD3 and anti-CD28 mAbs, RAGE−/− T cells produce relatively greater amounts of IL-10, IL-5, and TNF-α in response to TCR ligation, which is similar to our findings previously in which there was higher levels of IL-10 in the pancreata of NOD/SCID mice that were treated with sRAGE and showed reduced development of diabetes after receiving diabetogenic splenocytes (16). The small molecule RAGE inhibitor TTP488 also inhibited responses of T cells in a MLR, and inhibition was not seen with RAGE−/− cells. Moreover, the responders from MLR’s that had been cultured with TTP488 secreted reduced levels of IFN-γ in response to activation with PMA/ionomycin consistent with a role of RAGE in differentiation of Th1 cells. The discrepancies between the cytokine pattern in the RAGE−/− T cells activated with anti-CD3 and anti-CD28 and responders in a MLR in the presence of the RAGE inhibitor are likely due to the different culture systems used to activate the cells – one involving the direct activation of naive cells and the other the secondary activation after differentiation in the presence of the RAGE inhibitor. Indeed, in a previous report, we found that OTII cells on a RAGE−/− background produced reduced levels of IFN-γ and IL-2 when activated with PMA/ionomycin after priming with peptide pulsed DC’s (21). Finally, our finding of increased RAGE expression on activated clonal populations of cells under conditions that promote development of Th1 compared with Th2 cells is consistent with the role of this receptor in early events that lead to development of T cell phenotypes, specifically polarization toward a Th1 phenotype.
A number of recent studies have highlighted the role of RAGE on the activation and maturation of DCs and other APCs, but few have addressed the role of RAGE on T cells. Exposure of neutrophils, monocytes, or macrophages to the RAGE ligand HMGB1 enhances expression of proinflammatory cytokines by these cells, but RAGE was shown to play only a minor role in activation of these cells by HMGB1 (22). Likewise, these same investigators found that HMGB1 interacts with TLRs 2 and 4 but they were unable to show binding of HMGB1 to RAGE by fluorescence resonance energy transfer and immunoprecipitation. However, Tian et al. (14) recently reported that DNA-containing immune complexes in systemic lupus erythematosus bind HMGB1 and RAGE activating TLR9 on plasma DCs causing them to secrete IFNα. A role for RAGE in T cell activation was suggested by previous studies by Yan et al. (15) in which a dominant-negative RAGE, expressed on CD4+ T cells, blocked induction of experimental allergic encephalomyelitis. We previously showed that sRAGE attenuated the adoptive transfer of diabetes by diabetogenic T cells into NOD/SCID recipients (16). However, sRAGE did not prevent diabetes induced with a clonal population of CD4+ T cells, which raised the possibility that the role of RAGE was indirect, rather than directly on T cells, or alternatively that RAGE played a role in earlier stages of T cell differentiation. These new studies, however, establish a role of RAGE signaling on the early stages of T cell differentiation. Moreover, in other studies, we have also found that RAGE−/− OVA-reactive OTII cells show diminished proliferative responses to Ag when transferred into WT recipients (21).
Studies in RAGE null mice have indicated that RAGE is involved in perpetuation of cellular responses (9). This conclusion is consistent with our previous studies in autoimmune diabetes and the present studies of recurrent diabetes in diabetic NOD recipients of syngeneic islet grafts in which the responses of previously activated effector cells were inhibited. However, in the current studies, involving responses to allografts, alloantigens, and TCR signaling, the differences in cytokine production and RAGE expression on T cell phenotypes were in primary responses. There was negligible expression of RAGE on T cells studied after secondary activation (not shown).
CD28 expression is required for the formation of the mature immunologic synapse – central supramolecular activation cluster localization of PKC θ that is found in CD28+ T cells is absent in CD28− T cells (23). CD28 engagement leads to the redistribution and clustering of membrane and intracellular kinase-rich raft microdomains at the site of TCR engagements. This results in higher rates and more stable tyrosine phosphorylation of several substrates and higher consumption of Lck (24). These important events in TCR signaling may be absent in the RAGE−/− mice, but further studies of TCR signaling will help to define the basis for the loss of CD28 responsiveness in the absence of RAGE. RAGE ligands, such as HMGB1, have previously been shown to affect differentiation of DCs and HMGB1 released by DCs is needed for clonal expansion, survival, and functional polarization of naive T cells (25). Release of HMGB1 by human DCs was necessary for up-regulation of CD80 and CD86, ligands for CD28. RAGE was required for this effect of HMGB1 on DCs, suggesting that the innate HMGB1/RAGE signaling pathway results in adaptive immune responses (25).
Our findings differ from those of Liliensiek et al. (11) who found that deletion of RAGE provides protection from the lethal effects of septic shock caused by cecal ligation and puncture, but their studies did not support a role of RAGE in adaptive immune responses. However, these previous studies were done in mice on a mixed genetic background, whereas our studies were done on mice in which the RAGE mutation was backcrossed to B6 for more than 10 generations so that differences between WT and KO mice could be identified more clearly (11). Consistent with our findings, Dumitriu et al. (25) found that HMGB1 signaling through RAGE was needed for clonal expansion, survival, and functional polarization of naive T cells. These findings also support a direct effect of RAGE on T cell activation and differentiation rather than just T cell migration or localization as has been suggested in other model systems (15, 17). Likewise, we have also found that clonal populations of RAGE−/− T cells show reduced responses when adoptively transferred into WT recipients, indicating that the role of RAGE on T cell activation is primary (21).
Our findings suggest a novel testable hypothesis for the relationship between metabolic control of Type 1 diabetes and the immunologic progression of disease in patients. Studies from the Diabetes Control and Complications Study showed that individuals with new onset Type 1 diabetes, who maintained intensive glycemic control, had reduced loss of C-peptide responses compared with individuals with conventional metabolic treatment (26). Reduced availability of RAGE ligands with strict glycemic control would be expected to modulate adaptive responses that may be responsible for destruction of β cells. Indeed, the results in these experimental settings indicate that RAGE activation can affect even previously primed responses.
In summary, we have demonstrated a role of RAGE in the activation of adaptive immune responses to auto- and alloantigens. RAGE expression on T cells affects the early events its absence is manifest by reduced activation of T cells to alloantigens in vivo and in vitro. RAGE is involved in the differentiation of T cells along a Th1 phenotype and RAGE mRNA is more abundant in Th1 compared with Th2 cells. The reduced rate of recurrent diabetes and prolonged allograft survival by RAGE blockade and in RAGE-deficient mice suggests that RAGE may be an important new target for therapeutic strategies to prevent adaptive immune responses. The activation of RAGE by ligands, such as HMGB1, released from dying cells implies a broad role for this pathway in immune homeostasis. Moreover, the increased availability of RAGE ligands during hyperglycemia suggests a hypothesis for the amplification of autoimmune responses in Type 1 diabetes that may be tested in future clinical settings.
Footnotes
This work was supported by Grant 2004-808 from the Juvenile Diabetes Research Foundation.
Abbreviations used in this paper: RAGE, receptor for advanced glycation endproduct; sRAGE, soluble RAGE; WT, wild type; DC, dendritic cell.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Zanin-Zhorov A, Bruck R, Tal G, Oren S, Aeed H, Hershkoviz R, Cohen IR, Lider O. Heat shock protein 60 inhibits Th1-mediated hepatitis model via innate regulation of Th1/Th2 transcription factors and cytokines. J Immunol. 2005;174:3227–3236. doi: 10.4049/jimmunol.174.6.3227. [DOI] [PubMed] [Google Scholar]
- 2.Alyanakian MA, Grela F, Aumeunier A, Chiavaroli C, Gouarin C, Bardel E, Normier G, Chatenoud L, Thieblemont N, Bach JF. Transforming growth factor-β and natural killer T-cells are involved in the protective effect of a bacterial extract on type 1 diabetes. Diabetes. 2006;55:179–185. [PubMed] [Google Scholar]
- 3.Zipris D, Lien E, Nair A, Xie JX, Greiner DL, Mordes JP, Rossini AA. TLR9-signaling pathways are involved in Kilham rat virus-induced autoimmune diabetes in the biobreeding diabetes-resistant rat. J Immunol. 2007;178:693–701. doi: 10.4049/jimmunol.178.2.693. [DOI] [PubMed] [Google Scholar]
- 4.Thomas HE, Irawaty W, Darwiche R, Brodnicki TC, Santamaria P, Allison J, Kay TW. IL-1 receptor deficiency slows progression to diabetes in the NOD mouse. Diabetes. 2004;53:113–121. doi: 10.2337/diabetes.53.1.113. [DOI] [PubMed] [Google Scholar]
- 5.Sandberg JO, Eizirik DL, Sandler S. IL-1 receptor antagonist inhibits recurrence of disease after syngeneic pancreatic islet transplantation to spontaneously diabetic non-obese diabetic (NOD) mice. Clin Exp Immunol. 1997;108:314–317. doi: 10.1046/j.1365-2249.1997.3771275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson SB, Kent SC, Patton KT, Orban T, Jackson RA, Exley M, Porcelli S, Schatz DA, Atkinson MA, Balk SP, et al. Extreme Th1 bias of invariant Vα24JαQ T cells in type 1 diabetes. Nature. 1998;391:177–181. doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]
- 7.Wang B, Geng YB, Wang CR. CD1-restricted NK T cells protect nonobese diabetic mice from developing diabetes. J Exp Med. 2001;194:313–320. doi: 10.1084/jem.194.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogasawara K, Hamerman JA, Ehrlich LR, Bour-Jordan H, Santamaria P, Bluestone JA, Lanier LL. NKG2D blockade prevents autoimmune diabetes in NOD mice. Immunity. 2004;20:757–767. doi: 10.1016/j.immuni.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta. 2000;1498:99–111. doi: 10.1016/s0167-4889(00)00087-2. [DOI] [PubMed] [Google Scholar]
- 11.Liliensiek B, Weigand MA, Bierhaus A, Nicklas W, Kasper M, Hofer S, Plachky J, Grone HJ, Kurschus FC, Schmidt AM, et al. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest. 2004;113:1641–1650. doi: 10.1172/JCI18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 13.Kokkola R, Andersson A, Mullins G, Ostberg T, Treutiger CJ, Arnold B, Nawroth P, Andersson U, Harris RA, Harris HE. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol. 2005;61:1–9. doi: 10.1111/j.0300-9475.2005.01534.x. [DOI] [PubMed] [Google Scholar]
- 14.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 15.Yan SS, Wu ZY, Zhang HP, Furtado G, Chen X, Yan SF, Schmidt AM, Brown C, Stern A, LaFaille J, et al. Suppression of experimental autoimmune encephalomyelitis by selective blockade of encephalitogenic T-cell infiltration of the central nervous system. Nat Med. 2003;9:287–293. doi: 10.1038/nm831. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Yan SS, Colgan J, Zhang HP, Luban J, Schmidt AM, Stern D, Herold KC. Blockade of late stages of autoimmune diabetes by inhibition of the receptor for advanced glycation end products. J Immunol. 2004;173:1399–1405. doi: 10.4049/jimmunol.173.2.1399. [DOI] [PubMed] [Google Scholar]
- 17.Chavakis T, Bierhaus A, Al-Fakhri N, Schneider D, Witte S, Linn T, Nagashima M, Morser J, Arnold B, Preissner KT, et al. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med. 2003;198:1507–1515. doi: 10.1084/jem.20030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaye J, Hsu ML, Sauron ME, Jameson SC, Gascoigne NR, Hedrick SM. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature. 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- 19.Levin D, Constant S, Pasqualini T, Flavell R, Bottomly K. Role of dendritic cells in the priming of CD4+ T lymphocytes to peptide antigen in vivo. J Immunol. 1993;151:6742–6750. [PubMed] [Google Scholar]
- 20.Balamuth F, Brogdon JL, Bottomly K. CD4 raft association and signaling regulate molecular clustering at the immunological synapse site. J Immunol. 2004;172:5887–5892. doi: 10.4049/jimmunol.172.10.5887. [DOI] [PubMed] [Google Scholar]
- 21.Moser B, Desai DD, Downie MP, Chen Y, Yan SF, Herold K, Schmidt AM, Clynes R. Receptor for advanced glycation end products expression on T cells contributes to antigen-specific cellular expansion in vivo. J Immunol. 2007;179:8051–8058. doi: 10.4049/jimmunol.179.12.8051. [DOI] [PubMed] [Google Scholar]
- 22.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 23.Huang J, Lo PF, Zal T, Gascoigne NR, Smith BA, Levin SD, Grey HM. CD28 plays a critical role in the segregation of PKC θ within the immunologic synapse. Proc Natl Acad Sci USA. 2002;99:9369–9373. doi: 10.1073/pnas.142298399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 25.Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, Arnold B, Bianchi ME, Manfredi AA, Rovere-Querini P. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol. 2005;174:7506–7515. doi: 10.4049/jimmunol.174.12.7506. [DOI] [PubMed] [Google Scholar]
- 26.The Diabetes Control and Complications Trial Research Group. Effect of intensive therapy on residual β-cell function in patients with type 1 diabetes in the diabetes control and complications trial: a randomized, controlled trial. Ann Intern Med. 1998;128:517–523. doi: 10.7326/0003-4819-128-7-199804010-00001. [DOI] [PubMed] [Google Scholar]