Abstract
Regulation of body water homeostasis is critically dependent on the kidney and under the control of AVP, which is released from the neurohypophysis. In the collecting duct (CD) of the kidney, AVP activates adenylyl cyclase via vasopressin V2 receptors. cAMP-dependent activation of protein kinase A phosphorylates the water channel aquaporin-2 and increases water permeability by insertion of aquaporin-2 into the apical cell membrane. However, local factors modulate the effects of AVP to fine tune its effects, accelerate responses, and potentially protect the integrity of CD cells. Nucleotides like ATP belong to these local factors and act in an autocrine and paracrine way to activate P2Y2 receptors on CD cells. Extracellular breakdown of ATP and cAMP forms adenosine, the latter also induces specific effects on the CD by activation of adenosine A1 receptors. Activation of both receptor types can inhibit the cAMP-triggered activation of protein kinase A and reduce water permeability and transport. This review focuses on the role and potential interactions of the ATP and adenosine system with regard to the regulation of water transport in the CD. We address the potential stimuli and mechanisms involved in nucleotide release and adenosine formation, and discuss the corresponding signaling cascades that are activated. Potential interactions between the ATP and adenosine system, as well as other factors involved in the regulation of CD function, are outlined. Data from pharmacological studies and gene-targeted mouse models are presented to demonstrate the in vivo relevance to water transport and homeostasis.
Keywords: aquaporin-2, cAMP, collecting duct, vasopressin, cell volume
water is the most abundant molecule in the human body. The maintenance of water balance is dependent on water intake, initiated by the sensation of thirst, and the regulation of water excretion by the kidneys. Whereas all nephron segments contribute to various extents to water homeostasis, it is primarily the collecting duct (CD) system in which the reabsorption of ∼5–10% of the filtered water is regulated and adjusted to meet bodily needs. The antidiuretic hormone AVP is the primary regulator of water reabsorption in the CD system and critically involved in the regulation of water balance and stabilization of plasma osmolality (52). AVP is released from the neurohypophysis in response to small increases in plasma osmolality or greater reductions in circulating volume (52). As illustrated in Fig. 1, AVP acts on the CD via the Gs protein-coupled vasopressin V2 receptor (V2R) to stimulate adenylyl cyclase (AC) and thus the synthesis of cAMP. Increases in cAMP activate PKA, which phosphorylates the water channel aquaporin-2 (AQP2), with subsequent insertion of the channel into the apical plasma membrane. This allows water to be reabsorbed from the CD lumen into the cell and via basolateral AQP3 and AQP4 into the interstitium down its osmotic gradients. In addition, PKA-mediated phosphorylation of a cAMP-response element binding protein (CREB protein) promotes its binding to DNA and increases the transcription of the AQP2 gene in the longer term (for a review, see Ref. 50).
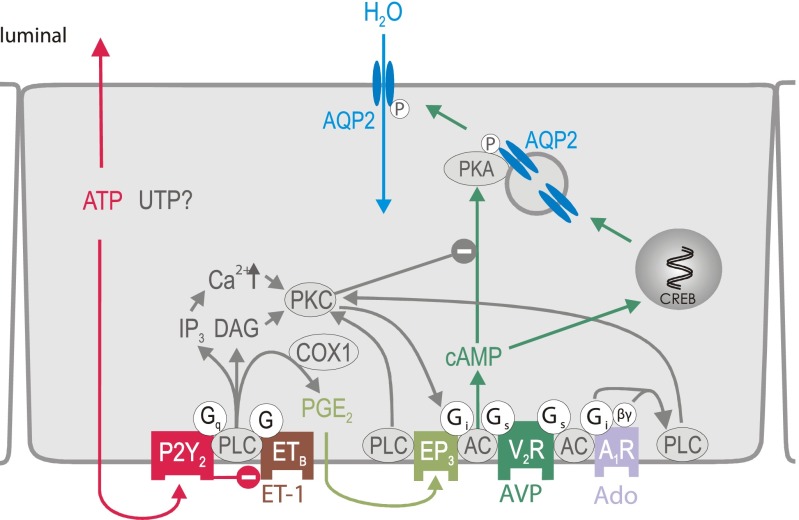
Fig. 1.
Proposed model for signaling mechanisms involved in aquaporin-2 (AQP-2) regulation in inner medullary collecting duct principal cells. See text for details. A1R, adenosine A1 receptor; AC, adenylyl cyclase; COX-1, cyclooxygenase 1; CREB, cAMP responsive element-binding protein; DAG, diacylglycerol; EP3, PGE2 receptor subtype 3; ET-1, endothelin-1; ETB, endothelin B receptor; Gi, inhibitory G protein; Gq, phospholipase C stimulatory G protein; Gs, stimulatory G protein; IP, inositol triphosphate; βγ, G protein βγ subunit; P, phosphorylation; PKA, protein kinase A; PKC, protein kinase C; PLC, phospholipase C; V2R, vasopressin V2 receptor.
Importantly, the AVP-mediated effects on water reabsorption are modulated by local factors. The functional importance of local factors may, in part, relate to the relatively long AVP half-life of 10–35 min (15). Since AVP-mediated free water reabsorption in the kidney is an important determinant of plasma osmolality, and thus the water distribution between extracellular and intracellular spaces, rapid adjustments in renal free water handling in response to changes in plasma osmolality are necessary. Local mechanisms may thus serve to tune CD water transport to bodily needs until responses in AVP levels can take over, as well as to fine adjust the effects induced by AVP. In addition, local factors may modulate AVP actions to protect CD cells from excessive perturbations in cell volume as a consequence of rapid changes in extracellular osmolality in the inner renal medulla. Increases in CD cell volume may release local factors and thus serve as a sensor of changes in plasma osmolality to accelerate AVP responses as well to maintain cell volume and integrity (see below).
As illustrated in Fig. 1, local AVP-counterregulatory factors in the CD principal cell include endothelin-1 (ET-1) (44) and PGE2 (10). ET-1 is formed and released from CD cells in response to changes in osmolality (45). However, the exact mechanisms are still under investigation. ET-1 activates ETB receptors in an autocrine/paracrine fashion to reduce PKA activation (see Fig. 1). Supporting this concept, inner medullary CD (IMCD) suspensions of CD-specific ET-1 knockout mice have enhanced AVP- and forskolin-stimulated cAMP formation and reduced plasma AVP levels (26). ETB receptor activation also stimulates cyclooxygenase-1 (COX-1) and PGE2 formation and release (46). The most abundant PGE2 receptor in the CD is the EP3 receptor subtype (38), which is a Gi protein-coupled receptor that inhibits AVP-induced cAMP formation in the CD (9, 10). In addition, EP3 receptor activation inhibits PKA via PLC-mediated activation of PKC (30). Of note, ETB receptors were found to be mainly localized to the basolateral membrane (46), whereas V2R (51) and EP3 receptors (65) were localized to both the luminal and basolateral membrane of CD cells, which is expected to further enhance the regulatory versatility.
Over the last years, evidence is accumulating that the renal tubular and CD system releases nucleotides like ATP in response to physiological stimuli and that these nucleotides act locally to modulate transport processes (for review, see Refs. 48, 74, 75). ATP has a short extracellular half-life of <5 min and can be broken down to form adenosine, another well-established local regulator of renal vascular and tubular function (for a review, see Ref. 76). The regulation of CD water transport by ATP and adenosine is the focus of this review.
P2Y Receptors and CD Water Transport
P2Y receptors are heptahelical receptors, which can be subdivided into five Gq-coupled subtypes (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11) and three Gi-coupled subtypes (P2Y12, P2Y13, P2Y14) (1). Whereas the Gq-coupled receptors activate PLC and increase [Ca2+]i, the Gi-coupled subtypes inhibit AC and lower cAMP levels. With different techniques P2Y1, P2Y2, P2Y4, and P2Y6 have been identified in the CD (4, 5, 43, 72, 73). Depending on the receptor subtype, P2Y receptor can be activated by ADP, ATP, UDP, UTP, or UDP sugars. P2Y2 receptors are typically activated nearly equipotent by ATP and UTP (1). Several groups have described an inhibitory effect of ATP on AVP-stimulated osmotic water permeability (Pf) in the cortical CD (CCD) and IMCD. On the basis of a pharmacological approach, many of these studies proposed a role of a P2Y2-like receptor as outlined below.
Rouse et al. (64) (Table 1) were the first to report that ATP can modulate the AVP-stimulated Pf in rabbit CCD by activation of the PLC/Ca2+ signaling pathway, consistent with the expression of a functional purinergic receptor. Ecelbarger et al. (19) provided evidence for the existence of a P2Y2-like receptor in rat terminal IMCD using ATP, UTP, and ATPγS (a stable analog of ATP), which all increased [Ca2+]i. Cha et al. (13) showed in isolated rat outer medullary CD the possible involvement of P2Y1 and/or P2Y2 receptors using 2-methylthio-ATP (2-MeS-ATP, P2Y receptor agonist), suramin and reactive blue 2 (both P2Y receptor antagonists). Kishore et al. (42) demonstrated in the isolated perfused terminal IMCD of the rat that ATP decreases AVP-stimulated cAMP formation by ∼30% in a PKC-dependent manner, most likely resulting from activation of the phosphoinositide signaling pathway; this was associated with inhibition of AVP-stimulated Pf by ∼40%. Supporting this concept, in rat IMCD cells, activation of PKC resulted in inhibition of AVP-stimulated cAMP formation (69, 70) (Fig. 1).
Table 1.
Effects of P2Y and adenosine A1 receptor activation in native renal tissue
| Receptor | Segment | Effect | Method (Site of Effect) Criterion for Receptor Subtype |
|---|---|---|---|
| P2Y2 | CD | AVP-stimulated cAMP formation and water reabsorption↓ | Evidence in P2Y2−/− mice (59) |
| P2Y2-like | CD, diverse | PLC↑, [Ca2+]i↑, PKC↑, cAMP↓, AVP-stimulated Pf↓ | Isolated, perfused CCD, OMCD, or IMCD of rabbit or rat (B) agonist profile (13, 19, 21, 42, 64) |
| P2Y2-like | CCD | PLC↑, [Ca2+]i↑ | Isolated, perfused rat or rabbit CCD, principal and intercalated cells (A and B) agonist profile (17, 64, 84) |
| P2Y2-like | IMCD | COX-1-dependent PGE2 release↑, hydrated > dehydrated rats, endothelin-1 release↓ | Rat IMCD suspension (B) agonist profile (33, 67, 81) |
| A1R | CD | Basal cAMP formation↓ | Isolated, cultured rabbit CCD (2, 3), rat IMCD primary culture (85, 86) and freshly isolated IMCD of mice with A1R agonist (60) |
| A1R | CD | AVP-stimulated cAMP formation↓ | Evidence in A1R−/− mice (60) |
| A1R | CCD, IMCD | AVP-stimulated cAMP formation↓ dependent on a functional Gi protein | Cultured rabbit CCD with A1R agonist (2, 3), rat IMCD primary culture with A1R agonist (85) |
| A1R | IMCD | AVP-induced increases in Pf and cAMP formation↓ | Rat IMCD (B) A1R agonist (20) |
| A1R | IMCD | AVP-stimulated cAMP formation↓ | Rat IMCD primary culture with A1R agonist (A, B) (85, 86) |
| A1R | IMCD | IP3↑, [Ca2+]i↑ | Isolated, superfused terminal IMCD of rat (19) and cultured rabbit CCD cells with A1R agonist (2) |
A, apical; AVP, arginine-vasopressin; B, basolateral; [Ca2+]i, intracellular Ca2+; IP, inositol phosphates; Pf, osmotic water permeability; PLC, phospholipase C; PKC, protein kinase C; COX-1, cyclooxygenase 1; CD, collecting duct; CCD, cortical collecting duct; IMCD, inner medullary collecting duct, OMCD, outer medullary collecting duct.
Ecelbarger et al. (19) demonstrated that prior exposure of rat IMCD to indomethacin, an unselective COX inhibitor, attenuates [Ca2+]i responses to ATP, suggesting that PGE2 is facilitating or mediating the response in [Ca2+]i. Moreover, ATPγS stimulated PGE2 release in freshly isolated IMCD preparations of hydrated rats, whereas the response was blunted in dehydrated rats (67). Finally, hypervolemic conditions are associated with greater P2Y2 receptor abundance in the renal medulla than hypovolemia (68). The purinergic-prostanoid system was speculated to represent a vasopressin-independent regulatory mechanism of IMCD function (68).
To further substantiate the above pharmacological evidence, we studied aspects of renal water transport in mice lacking P2Y2 receptors (P2Y2−/−) (59). Studies in freshly isolated IMCD showed that ATPγS enhanced the EC50 for the stimulation of cAMP formation by the V2R agonist, 1-desamino-8-d-arginine vasopressin (dDAVP) in wild-type (WT) mice, but not in P2Y2−/− mice (59). To determine the ambient contribution of V2R activation on water transport in WT and P2Y2−/− mice, acute responses to the V2R antagonist SR121463 were assessed. In WT animals, SR121463 increased urinary flow rate and electrolyte free water clearance (Cle-H2O) (Fig. 2, A and C). These changes in WT were associated with an increase in urinary PGE2 (Fig. 2B) but a reduction in urinary ATP (Fig. 3A), implying a tonic inhibition of PGE2 formation but stimulation of ATP release by V2R activation (the regulation of ATP release in CD is discussed in more detail below). In P2Y2−/−, SR121463 elicited a significantly greater diuresis and Cle-H2O compared with WT (Fig. 2, A and C), indicating greater basal reabsorption of fluid in the CD of P2Y2−/−, which was associated with greater renal AQP2 expression in the latter animals. SR121463 induced similar increases in urinary PGE2 in P2Y2−/− and WT (Fig. 3A). Basal urinary excretion of fluid and Cle-H2O were not different between P2Y2−/− and WT mice. This is proposed to be the consequence of a greater delivery of a more hypotonic fluid to the distal nephron segments in P2Y2−/−, which is the consequence of the integrated renal and blood pressure phenotype of these mice (59, 75). Under basal conditions, the hyperactivity of the V2R-AQP2 system reabsorbs the excess water resulting in normal net fluid excretion in P2Y2−/−. Together, the data are consistent with the concept that P2Y2 receptor activation inhibits the cAMP-mediated effects of AVP on water transport in the CD (Fig. 1) (59).
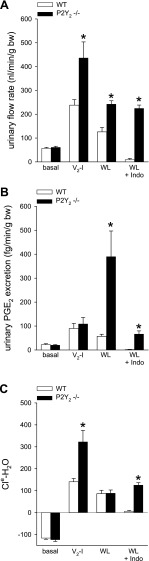
Fig. 2.
Aspects of renal water transport in P2Y2 knockout mice (P2Y2−/−) under basal conditions (basal), in response to acute vasopressin V2 receptor inhibition (V2-I), acute water loading (WL) or WL plus pretreatment with indomethacin (WL+Indo). A, C: compared with basal measurements, V2-I increased urinary flow rate and electrolyte free-water clearance (Cle-H2O) to a greater extent in P2Y2−/− vs. wild-type (WT) mice (determined over a 2-h period after intraperitoneal application of the receptor inhibitor). These findings indicated greater water reabsorption but also the basal delivery of greater amounts of more hypotonic fluid to the distal nephron in P2Y2−/−. This could explain the facilitated Cle-H2O in response to water loading in P2Y2−/−, i.e., less suppression of vasopressin and/or cAMP is sufficient to increase Cle-H2O to the same extent as in WT (not shown). B: Increases in urinary PGE2 after oral water loading (3% of body weight) were much greater in P2Y2−/−; treatment with indomethacin reduced urinary PGE2 excretion in water-loaded P2Y2−/− to levels of WT mice without indomethacin treatment; urinary flow rate and electrolyte-free water clearance (Cle-H2O) were unaffected in P2Y2−/− mice compared with untreated. *P < 0.05 vs. WT; data for WL+Indo are unpublished; the other data are from Ref. 59.
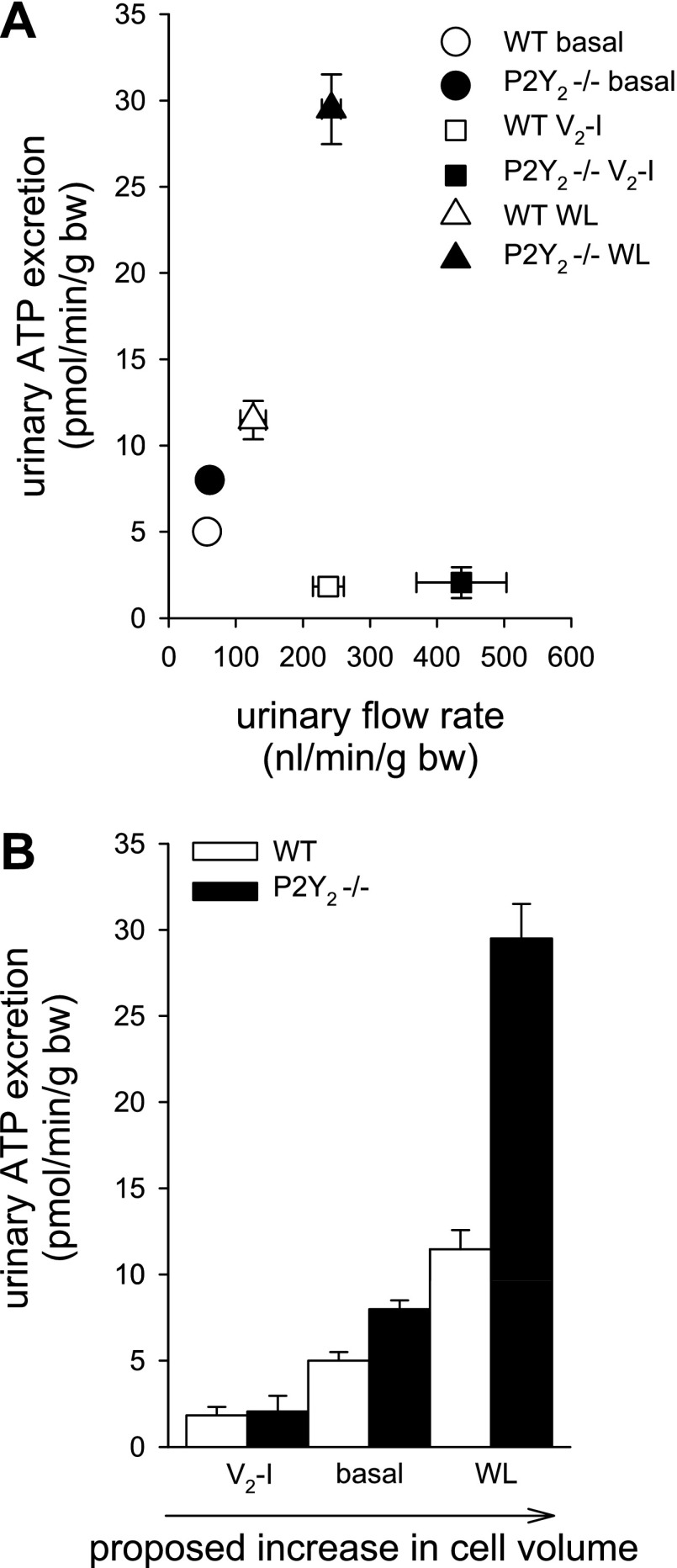
Fig. 3.
A: relationship between urinary ATP excretion and urinary flow rate under different maneuvers. Urinary ATP was reduced by acute vasopressin V2 receptor inhibition (V2-I) but increased by acute WL. Increasing urinary flow rate alone is not a good predictor of urinary ATP excretion. For further details, see text. B: a proposed positive relationship between the cell volume, manipulated by different maneuvers in WT and P2Y2−/− mice and urinary ATP excretion. We propose that feedback regulation of cell volume via cell volume-regulated ATP release is absent/reduced in P2Y2−/− mice resulting in greater ATP release. The influence is minimized by reducing water uptake by V2-I and maximized by water loading-induced cell swelling. All data are from Ref. 59.
How does the absence of P2Y2 receptors affect the response to acute water loading? Acute water loading induced similar increases in urine flow rate and Cle-H20 in WT mice as observed in response to V2R blockade (Fig. 2, A and C). Likewise, water loading increased urinary PGE2 excretion (Fig. 2B). In contrast to V2R blockade, however, water loading enhanced urinary ATP excretion (Fig. 3A). In P2Y2−/−, the water load-induced increase in Cle-H2O was similar to that in WT. However, a lesser suppression of the vasopressin and cAMP system was sufficient in P2Y2−/− to increase Cle-H2O to the same extent as in WT, indicating that free water excretion was actually facilitated in the P2Y2−/− (59). Whereas this may relate to the delivery of greater amounts of hypotonic fluid to the distal nephron in these mice, they also presented greater urinary excretion of both PGE2 (Fig. 2A) and ATP in response to acute water loading (Fig. 3A).
Our unpublished work showed that treatment of WT mice with indomethacin (5 mg/kg ip) 30 min before an acute oral water load (3% of body weight) almost completely inhibited urinary PGE2 excretion and blunted the urine flow response in the 2-h experimental period (Fig. 2, A and B). Remarkably, urinary PGE2 excretion in P2Y2−/− was reduced to levels observed in WT mice without indomethacin treatment, and the responses in urinary flow rate and Cle-H2O were unaffected compared with untreated P2Y2−/− mice (Fig. 2, A and C). Whether this is due to incomplete COX inhibition in P2Y2−/− or involves indomethacin-insensitive PGE2 formation, needs to be determined, but it stresses potential differences in the quantitative or qualitative role of PGE2 in P2Y2−/−.
P2X Receptors and CD Water Transport
P2X receptors are ligand-activated ion channels with permeability to Na+, K+, Ca2+ and in a few cases Cl−, and P2X1, P2X2, P2X3, P2X4, P2X5, P2X6, and P2X7 channels have been described (40, 53). Studies in native rat tissue have immunolocalized P2X4, P2X5, and P2X6 in CD principal cells (74) and protein expression of P2X1, P2X4, and P2X6 was confirmed by mRNA expression of these receptors in microdissected CD (72). Preliminary studies in the Xenopus oocyte expression system proposed a functional interaction between AQP2 and P2X2 (82, 83), but the in vivo relevance remains to be determined. In conclusion, very little is known about the physiological relevance of P2X receptors for water transport in the kidney. The use of knockout mouse models should be helpful to unravel their functions.
Nucleotide Release in CD
Cellular ATP release is an essential and physiological relevant part of purinergic signaling in the kidney with the released ATP acting on P2 receptors in an autocrine/paracrine way (47, 59). Possible sources of extracellular ATP in the kidney include perivascular and peritubular nerve terminals, circulating erythrocytes, aggregating platelets, and renal endothelial and epithelial cells (8, 14). In human renal cortex, adrenergic stimulation was shown to release ATP from neuronal and nonneuronal sources (79). Under basal conditions, intracellular ATP concentrations are in the range of 3–5 mM (27). Thus, there is a big pool of intracellular ATP and just a fraction (resulting in values 100-fold lower than those inside the cell) needs to be released to mediate purinergic autocrine/paracrine signaling (1, 58). Once outside the cell, ATP has a half-life of minutes due to ecto-nucleotidases and other hydrolytic activities (23).
With regard to ATP release mechanisms in the kidney, evidence has been provided for a role of a maxi-anion channel for the basolateral release of ATP from the macula densa, but the molecular nature of this channel is unknown (7). The released ATP is converted by ecto-ATPase (54) and ecto-5′-nucleotidase (12, 32) to adenosine, the final mediator of the tubuloglomerular feedback (71), illustrating the close link between the ATP and adenosine system. The mechanisms and proteins involved in ATP release in the tubule and CD system are also largely unknown. Connexin (Cx) hemichannels have been proposed to contribute to ATP release, and recent studies by McCulloch et al. (49) identified continuous Cx30 hemichannel expression from the medullary thick ascending limb to the CD system. In the mouse CCD, the expression of Cx30.3 appeared to be restricted to the cytosol and the apical membrane of intercalated cells (29). A physiological role in ATP release remains to be established.
The presence of ATP in tubular fluid and its release by epithelial cells in the rat was described by means of micropuncture (77). In this study, the half-life of ATP in proximal tubular fluid was ∼3.4 min with concentrations between 100 and 300 nM. Concentrations closer to the plasma membrane are expected to be significantly greater. Measurement of ATP in distal tubules showed 3.5-fold lower ATP values compared with the proximal tubule. However, ATP concentrations in the distal tubule may have been underestimated because of the need for longer collection times and the presence of soluble nucleotidase. ATP in tubular fluid of CD has not been measured yet.
ATP release in the CD is triggered by changes in tubular flow rate, as well as cell volume (31, 39). In the kidney, flow-induced ATP release was shown to be at least partially dependent on the presence of the primary cilium (57). Cilia have important roles in sensory physiology in response to flow and osmotic stimuli (reviewed in Refs. 57 and 66). Supporting this concept, Hovater et al. (31) showed that ATP release is impaired when the cilium is malformed. This conclusion was drawn from studies in CD principal cells derived from an Oak Ridge polycystic kidney (Tg737orpk) mouse model of autosomal recessive polycystic kidney disease, which lack a well-formed apical cilium: principal cell monolayers with normally formed apical cilia responded with 3- to 5-fold greater ATP release to hypotonicity than mutant monolayers lacking cilia. The observed cilium-derived Ca2+ transient required an underlying paracrine/autocrine ATP signal that is likely transduced by P2X and P2Y on or near the cilium (31). Notably, Woda et al. (84) showed that a flow-induced rise in [Ca2+]i can also be triggered in intercalated cells from perfused rabbit CD, which do not have cilia.
IMCD were shown to respond to AVP-stimulation with an increase in cell volume (25). Cells of various organs respond to changes in cell volume with a release of ATP (23, 80). Measuring urinary ATP excretion, our studies in P2Y2−/− mice provided indirect evidence for a volume-dependent ATP release in CD in vivo (59). Urinary ATP excretion was similar in P2Y2−/− compared with WT during inhibition of water transport by V2R blockade but modestly greater under basal conditions and much greater in response to acute water loading in P2Y2−/− compared with WT. A summary of these findings is illustrated in Fig. 3A. V2R blockade and acute water loading both increased urinary flow rate but had opposite effects on ATP excretion. We propose that 1) urinary flow rate alone is not a good predictor of urinary ATP excretion, 2) acute water loading increases ATP release, which may reflect increases in CD cell volume due to a reduction in extracellular tonicity, 3) acute pharmacological blockade of V2R reduces cell volume by blocking the apical water entry, thus offsetting the basal, AVP- and cell volume-induced ATP release, and 4) urothelial cells of the lower urinary tract, which were exposed to similar increases in flow rate and therefore similar distension, and hypotonicity may not play a dominant role for urinary ATP excretion. A graph illustrating the proposed relationship between CD cell volume and ATP release and thus urinary ATP excretion is shown in Fig. 3B.
Adenosine Receptors and CD Water Transport
Consistent with a prominent role of adenosine A1 receptor (A1R) in the regulation of CD function, studies in rats and mice revealed a strong corticomedullary gradient for the A1R expression, with the highest density in CD and, in particular, IMCD (56, 73, 76, 78, 87, 90). In fact, stimulation of A1R by adenosine and other agonists inhibits AVP-induced cAMP formation in cultured rabbit CCD cells (2, 3), as well as rat medullary and IMCD cells (85, 86). Basal, nonstimulated, cAMP formation is reduced in rabbits, rats, and mice by selective A1R activation (3, 60, 85). Activation of A1R inhibits AC activity through activation of pertussis toxin-sensitive Gi proteins, as well as through activation of PLC via Gβγ subunits (55) (Fig. 1). The selective A1R agonist, N6-2-phenylethyladenosine (NPEA), mobilized [Ca2+]i in IMCD, a response that was significantly inhibited by the selective A1R antagonist, 8-phenyltheophylline (8-PT) (19). This would suggest that A1R, like P2Y2 receptors, are linked to a signaling pathway capable of mobilizing [Ca2+]i in the IMCD (Fig. 1).
Studies by Yagil (85, 86) showed the dose dependence of AVP-stimulated cAMP in primary cultures of rat IMCD. Interestingly, AVP applied from either side increased cAMP formation, although lower concentrations (100 pM and 1 nM) were more effective when applied from the basolateral side. When adenosine was applied from the basolateral side, 1 μM was sufficient to inhibit AVP-stimulated cAMP formation, whereas 100 μM were necessary to inhibit AVP-stimulated cAMP formation from the apical side. In this regard, it may be relevant that concentrative nucleotide transporters (CNT) expressed in the apical membrane (16, 28) could reduce adenosine availability on the apical surface and increase basolateral adenosine because of passive adenosine efflux via equilibrative nucleoside transporters (ENT1 and ENT2) expressed in the basolateral membrane (16, 28, 63) (Fig. 4).
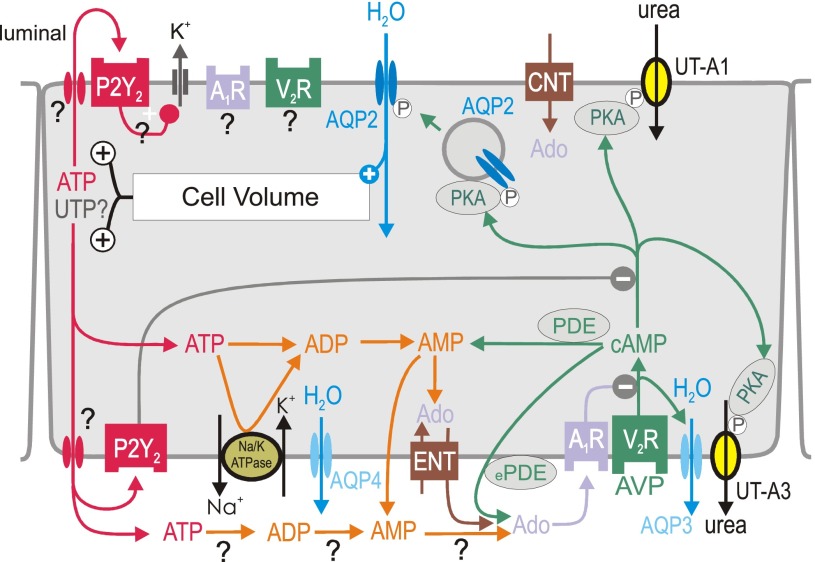
Fig. 4.
Proposed integrated model for local water transport inhibition by activation of P2Y2 and adenosine A1 receptors (A1R) in inner medullary collecting duct principal cell. Activation of vasopressin V2 receptors (V2R) by arginine-vasopressin (AVP) stimulates aquaporin-2 (AQP2)-mediated water flux, which increases cell volume. The latter increases basolateral and apical release of ATP (and potentially UTP?) by unknown mechanism. Basolateral water exit is mediated by constitutively expressed aquaporin-4 (AQP4) and AVP-regulated aquaporin-3 (AQP3) water channels. Urea transporters UT-A1 and UT-A3 play key roles in the urinary concentrating mechanism and fluid homeostasis; both are regulated by AVP. P2Y2 receptor activation may inhibit water reabsorption by (I) stimulation of volume-regulatory K+ channels, and (II) inhibition of protein kinase A (PKA). ATP may also be broken down to adenosine (Ado). Alternative sources of extracellular Ado are the extracellular cAMP-Ado pathway (conversion by extracellular phosphodiesterase, ePDE, and ecto-5′-nucleotidase) or transport mediated by equilibrative nucleoside transporter (ENT). To which extent extracellular ATP is degraded by ectonucleotidases to ADP, AMP, and Ado at the luminal (not shown) and basolateral surface is unclear. Activation of A1R and P2Y2 receptors inhibits AVP-stimulated water flux, which helps to normalize cell volume and accelerates the excretion of free water in response to water loading before circulating AVP levels fall. AC, adenylyl cyclase; AQP-3, -4, aquaporin-3, -4; CNT, concentrative nucleoside transporter; COX-1, cyclooxygenase 1; DAG, diacylglycerol; P, phosphorylation. See also text for details.
Using freshly isolated IMCD, we showed that the A1R agonist N6-cyclohexyladenosine (CHA, 1 μM) reduced basal, as well as forskolin-stimulated cAMP formation in WT, but was without effect in A1R−/− (60). dDAVP-induced increases in cAMP formation were about twofold greater in A1R−/− compared with WT mice, and, in contrast to WT mice, A1R−/− mice were not inhibited by CHA. Thus, A1R−/− mice show an absence of CHA-mediated inhibition of cAMP formation in IMCD cells, as well as an enhanced response to dDAVP, consistent with the loss of a A1R-mediated inhibition of this response.
With regard to functional consequences, Dillingham and Anderson (18) showed, using an in vitro CCD perfusion system, a stimulatory effect of high concentrations of adenosine on hydraulic conductivity and net volume flux. In rat IMCD, Edwards and Spielman (20) found that adenosine, acting from the basolateral side, inhibits AVP-induced increases in cAMP formation and Pf.
Little has been known about the net in vivo role of A1R for water transport in the intact organism. We observed that A1R−/− mice have higher urinary flow rates and greater fluid intake compared with WT mice, which may relate to the proposed basal antidiuretic tone of A1R activation in the proximal tubule (11, 60–62) but argues against a prominent role of A1R to offset AVP effects on water reabsorption in the CD. Moreover, A1R−/− and WT mice had similar basal urinary excretion of AVP and expression of AQP2 protein in renal cortex and medulla. They also increased urinary flow rate and Cle-H2O in response to the V2R antagonist SR121463 or acute water loading to the same extent. Finally, the dose dependence of dDAVP-induced antidiuresis after acute water loading was not different between genotypes (60). The lack of a clear phenotype of A1R in AVP control of renal water reabsorption in vivo was surprising considering the very high receptor abundance in the CD system and the demonstrated ability of A1R to inhibit AVP-induced cAMP formation in isolated IMCD. These findings indicate effective compensation for the absence of A1R in CD in vivo. This is discussed in more detail below.
Adenosine Generation in CD
In the CD, extracellular adenosine can be generated by different mechanisms. Some are more closely linked to ATP metabolism and, therefore, discussed below in the section on ATP-adenosine interactions. Another mechanism relates to the so-called extracellular cAMP-adenosine pathway (34, 35). The rate of cellular cAMP efflux is proportional to intracellular levels (6, 41) and is increased following stimulation of AC (22). In the CD, AVP-mediated V2R activation triggers intracellular cAMP synthesis. The latter is released and extracellular cAMP converted to adenosine by the sequential actions of ecto-phosphodiesterase [in the kidney possibly involving PDE8 (37)] and ecto-5′-nucleotidase (36). As illustrated in Figs. 1 and 4, this autocrine/paracrine pathway may constitute a mechanism for feedback inhibition when water transport in the CD is stimulated by AVP-induced cAMP formation (20, 34, 85, 86). Further studies are necessary to define the quantitative contribution of this pathway in the CD, e.g., by studies on AVP-induced cAMP formation in IMCDs of mice lacking PDE8.
Interactions Between the ATP and Adenosine System in the CD
Extracellular adenosine can derive from extracellular breakdown of ATP, AMP, or cAMP. Vekaria et al. (77) provided evidence for the breakdown of exogenous and endogenous ATP in tubular fluid by soluble nucleotidases. In addition, various ecto-ATPases and ecto-5′-nucleotidase are differentially expressed along the tubular and collecting duct epithelia (for a review, see Ref. 75). As a consequence, the cellular release of ATP can inhibit water transport by activation of P2Y2 receptors and via A1R after ATP breakdown to adenosine (Figs. 1 and 4). Both pathways converge and suppress AVP-induced cAMP formation and, thus, reduce water transport and potentially cell volume.
Intracellular breakdown of cAMP and ATP can also generate adenosine. Adenosine itself can exit the cell by ENT1 and ENT2 primarily localized to basolateral membranes, where they mediate bidirectional facilitated diffusion of adenosine (16, 28, 63). In comparison, CNT1-CNT3 are mainly localized to the apical membrane, where they mediate unilateral, cellular uptake of nucleosides (Fig. 4) (36). The role of ecto-nucleotidases and of the asymmetric expression of these transport pathways for basolateral vs. luminal effects of ATP and adenosine remain to be determined.
The fact that A1R−/− presented no in vivo phenotype after V2R activation or blockade prompted us to assess under basal conditions and after acute water loading the expression level of several local factors that may compensate. Acute water loading upregulated inner medullary mRNA expression of ET-1 in WT (60), confirming previous studies (26). Notably, CD-specific ET-1 knockout mice have a reduced ability to excrete an acute water load (26). Moreover, acute water loading also increased inner medullary expression of A1R mRNA in WT (60). Although the mechanism(s) that contribute(s) to the upregulation of A1R in WT under these conditions remain to be determined, this response could help to facilitate water excretion. Under basal conditions A1R−/− had greater inner medullary expression of COX-1, and acute water loading increased P2Y2 and EP3 receptor expression more in A1R−/− than in WT mice (60), responses that may have compensated for the absence of A1R and support the functional interaction between these systems. Unpublished observations showed that urinary nitric oxide metabolite excretion (NOx) was not significantly different between genotypes in response to acute water loading (WT: 8.3 ± 3 vs. A1R−/−: 9.2 ± 2 nmol·min−1·g body weight−1), consistent with similar endothelial nitric oxide synthase expression between genotypes under basal conditions or after acute water loading (60). The up-regulation of ET-1 on the mRNA level by water loading was not altered in A1R−/−.
Why, with regard to AVP-induced cAMP formation and antidiuresis, do A1R−/− mice have a phenotype in isolated IMCD, but not in vivo, whereas P2Y2−/−mice show a phenotype in vivo, but not in freshly isolated inner medulla. In experiments in isolated IMCD, application of the V2R agonist is expected to trigger cAMP formation, perhaps followed by extracellular adenosine formation, activation of A1R, and inhibition of AC activity, an effect that is blunted in mice lacking A1R. In the absence of osmotic gradients, however, the in vitro application of the V2R agonist may not alter cell volume and thus may not generate a stimulus for ATP release. To unmask a defect in isolated IMCD of mice lacking P2Y2 receptors, we had to apply ATPγS. In vivo, lesser compensation in the absence of P2Y2 receptors than of A1R may indicate that the feedback inhibition via cell volume-regulated ATP release and activation of P2Y2 receptors is a more potent system than the extracellular formation of adenosine (from cellular release of cAMP and extracellular breakdown of ATP) and the subsequent activation of A1R.
Perspectives and Significance
Multiple P2X, P2Y, and adenosine receptors are expressed in renal epithelial cells, often in the same membrane domain. There is accumulating evidence for an important role for both ATP, via P2Y2 receptors, and adenosine, via A1R, to inhibit AVP-stimulated water transport in the CD. Interactions between ATP and the adenosine system support the concept of sequentially organized feedback loops. The complexity of the ATP-adenosine interaction is further enhanced by the existence of receptor heterodimers (1, 24, 40). Heterodimeric associations between P2Y2 and A1R were described in transfected cells (88, 89), but their relevance in the kidney is unknown. Important issues to be clarified in future studies also include the identification and regulation of transport pathways involved in ATP release, as well as the variation of ATP and adenosine concentrations close to the receptor under physiological conditions. The role of apical versus basolateral signaling deserves further consideration, including the conversion of ATP to adenosine, and transport of the latter by asymmetrically expressed concentrative and equilibrative nucleoside transporters. The use of gene knockout mice will be helpful, but compensatory changes have to be considered, as shown in our studies using the A1R−/−. Finally, a potential role of the ATP and adenosine system in the pathophysiology of CD water transport needs to be explored.
GRANTS
This research was supported by the National Institutes of Health Grants DK56248, DK28602, and GM66232, the O'Brien Kidney Center (Grant P30DK079337), the American Heart Association (Grant 0655232Y), and the Research Service of the Department of Veterans Affairs (all to V. Vallon). T. Rieg was supported by the German Research Foundation (RI 1535/3-1 and 3-2) and a National Kidney Foundation Fellowship.
REFERENCES
- 1.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: Update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58: 281–341, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arend LJ, Burnatowska-Hledin MA, Spielman WS. Adenosine receptor-mediated calcium mobilization in cortical collecting tubule cells. Am J Physiol Cell Physiol 255: C581–C588, 1988. [DOI] [PubMed] [Google Scholar]
- 3.Arend LJ, Sonnenburg WK, Smith WL, Spielman WS. A1 and A2 adenosine receptors in rabbit cortical collecting tubule cells. Modulation of hormone-stimulated cAMP. J Clin Invest 79: 710–714, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey MA, Imbert-Teboul M, Turner C, Marsy S, Srai K, Burnstock G, Unwin RJ. Axial distribution and characterization of basolateral P2Y receptors along the rat renal tubule. Kidney Int 58: 1893–1901, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Bailey MA, Imbert-Teboul M, Turner C, Srai SK, Burnstock G, Unwin RJ. Evidence for basolateral P2Y(6) receptors along the rat proximal tubule: functional and molecular characterization. J Am Soc Nephrol 12: 1640–1647, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Barber R, Butcher RW. The quantitative relationship between intracellular concentration and egress of cyclic AMP from cultured cells. Mol Pharmacol 19: 38–43, 1981. [PubMed] [Google Scholar]
- 7.Bell PD, Lapointe JY, Sabirov R, Hayashi S, Peti-Peterdi J, Manabe K, Kovacs G, Okada Y. Macula densa cell signaling involves ATP release through a maxi anion channel. Proc Natl Acad Sci USA 100: 4322–4327, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res 26: 959–969, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Breyer MD, Jacobson HR, Hebert RL. Cellular mechanisms of prostaglandin E2 and vasopressin interactions in the collecting duct. Kidney Int 38: 618–624, 1990. [DOI] [PubMed] [Google Scholar]
- 10.Breyer MD, Breyer RM. Prostaglandin E receptors and the kidney. Am J Physiol Renal Physiol 279: F12–F23, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Brown RD, Thoren P, Steege A, Mrowka R, Sallstrom J, Skott O, Fredholm BB, Persson AE. Influence of the adenosine A1 receptor on blood pressure regulation and renin release. Am J Physiol Regul Integr Comp Physiol 290: R1324–R1329, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Castrop H, Huang Y, Hashimoto S, Mizel D, Hansen P, Theilig F, Bachmann S, Deng C, Briggs J, Schnermann J. Impairment of tubuloglomerular feedback regulation of GFR in ecto-5′-nucleotidase/CD73-deficient mice. J Clin Invest 114: 634–642, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cha SH, Sekine T, Endou H. P2 purinoceptor localization along rat nephron and evidence suggesting existence of subtypes P2Y1 and P2Y2. Am J Physiol Renal Physiol 274: F1006–F1014, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Chan CM, Unwin RJ, Burnstock G. Potential functional roles of extracellular ATP in kidney and urinary tract. Exp Nephrol 6: 200–207, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Czaczkes JW, Kleeman CR, Koenig M. Physiologic studies of antidiuretic hormone by its direct measurement in human plasma. J Clin Invest 43: 1625–1640, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damaraju VL, Elwi AN, Hunter C, Carpenter P, Santos C, Barron GM, Sun X, Baldwin SA, Young JD, Mackey JR, Sawyer MB, Cass CE. Localization of broadly selective equilibrative and concentrative nucleoside transporters, hENT1 and hCNT3, in human kidney. Am J Physiol Renal Physiol 293: F200–F211, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Deetjen P, Thommas J, Lehrmann H, Kim SJ, Leipziger J. The luminal P2Y receptor in the isolated perfused mouse cortical collecting duct. J Am Soc Nephrol 11: 1798–1806, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Dillingham MA, Anderson RJ. Purinergic regulation of basal and arginine vasopressin-stimulated hydraulic conductivity in rabbit cortical collecting tubule. J Membr Biol 88: 277–281, 1985. [DOI] [PubMed] [Google Scholar]
- 19.Ecelbarger CA, Maeda Y, Gibson CC, Knepper MA. Extracellular ATP increases intracellular calcium in rat terminal collecting duct via a nucleotide receptor. Am J Physiol Renal Fluid Electrolyte Physiol 267: F998–F1006, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Edwards RM, Spielman WS. Adenosine A1 receptor-mediated inhibition of vasopressin action in inner medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 266: F791–F796, 1994. [DOI] [PubMed] [Google Scholar]
- 21.Edwards RM Basolateral, but not apical, ATP inhibits vasopressin action in rat inner medullary collecting duct. Eur J Pharmacol 438: 179–181, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Fehr TF, Dickinson ES, Goldman SJ, Slakey LL. Cyclic AMP efflux is regulated by occupancy of the adenosine receptor in pig aortic smooth muscle cells. J Biol Chem 265: 10974–10980, 1990. [PubMed] [Google Scholar]
- 23.Fitz JG Regulation of cellular ATP release. Trans Am Clin Climatol Assoc 118: 199–208, 2007. [PMC free article] [PubMed] [Google Scholar]
- 24.Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV Nomenclature and Classification of Adenosine Receptors. Pharmacol Rev 53: 527–552, 2001. [PMC free article] [PubMed] [Google Scholar]
- 25.Ganote CE, Grantham JJ, Moses HL, Burg MB, Orloff J. Ultrastructural studies of vasopressin effect on isolated perfused renal collecting tubules of the rabbit. J Cell Biol 36: 355–367, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ge Y, Ahn D, Stricklett PK, Hughes AK, Yanagisawa M, Verbalis JG, Kohan DE. Collecting duct-specific knockout of endothelin-1 alters vasopressin regulation of urine osmolality. Am J Physiol Renal Physiol 288: F912–F920, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Gordon JL Extracellular ATP: effects, sources and fate. Biochem J 233: 309–319, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Govindarajan R, Bakken AH, Hudkins KL, Lai Y, Casado FJ, Pastor-Anglada M, Tse CM, Hayashi J, Unadkat JD. In situ hybridization and immunolocalization of concentrative and equilibrative nucleoside transporters in the human intestine, liver, kidneys, and placenta. Am J Physiol Regul Integr Comp Physiol 293: R1809–R1822, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Hanner F, Schnichels M, Zheng-Fischhofer Q, Yang LE, Toma I, Willecke K, McDonough AA, Peti-Peterdi J. Connexin 303 is expressed in the kidney but not regulated by dietary salt or high blood pressure. Cell Commun Adhes 15: 219–230, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Hebert RL, Jacobson HR, Breyer MD. PGE2 inhibits AVP-induced water flow in cortical collecting ducts by protein kinase C activation. Am J Physiol Renal Fluid Electrolyte Physiol 259: F318–F325, 1990. [DOI] [PubMed] [Google Scholar]
- 31.Hovater MB, Olteanu D, Hanson EL, Cheng NL, Siroky B, Fintha A, Komlosi P, Liu W, Satlin LM, Bell PD, Yoder BK, Schwiebert EM. Loss of apical monocilia on collecting duct principal cells impairs ATP secretion across the apical cell surface and ATP-dependent and flow-induced calcium signals. Purinergic Signal 4: 155–170, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang DY, Vallon V, Zimmermann H, Koszalka P, Schrader J, Osswald H. Ecto-5′-nucleotidase (cd73)-dependent and -independent generation of adenosine participates in the mediation of tubuloglomerular feedback in vivo. Am J Physiol Renal Physiol 291: F282–F288, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Hughes AK, Stricklett PK, Kishore BK, Kohan DE. Adenosine triphosphate inhibits endothelin-1 production by rat inner medullary collecting duct cells. Exp Biol Med 231: 1006–1009, 2006. [PubMed] [Google Scholar]
- 34.Jackson EK, Mi Z, Zhu C, Dubey RK. Adenosine biosynthesis in the collecting duct. J Pharmacol Exp Ther 307: 888–896, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Jackson EK, Dubey RK. Role of the extracellular cAMP-adenosine pathway in renal physiology. Am J Physiol Renal Physiol 281: F597–F612, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Jackson EK, Raghvendra DK. The extracellular cyclic AMP-adenosine pathway in renal physiology. Annu Rev Physiol 66: 571–599, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Jackson EK, Ren J, Zacharia LC, Mi Z. Characterization of renal ecto-phosphodiesterase. J Pharmacol Exp Ther 321: 810–815, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Jensen BL, Mann B, SkOtt O, Kurtz A. Differential regulation of renal prostaglandin receptor mRNAs by dietary salt intake in the rat. Kidney Int 56: 528–537, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Jensen MEJ, Odgaard E, Christensen MH, Praetorius HA, Leipziger J. Flow-induced [Ca2+]i increase depends on nucleotide release and subsequent purinergic signaling in the intact nephron. J Am Soc Nephrol 18: 2062–2070, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PPA. International Union of Pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev 53: 107–118, 2001. [PubMed] [Google Scholar]
- 41.King CD, Mayer SE. Inhibition of egress of adenosine 3′, 5′-monophosphate from pigeon erythrocytes. Mol Pharmacol 10: 941–953, 1974. [Google Scholar]
- 42.Kishore BK, Chou CL, Knepper MA. Extracellular nucleotide receptor inhibits AVP-stimulated water permeability in inner medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 269: F863–F869, 1995. [DOI] [PubMed] [Google Scholar]
- 43.Kishore BK, Ginns SM, Krane CM, Nielsen S, Knepper MA. Cellular localization of P2Y2 purinoceptor in rat renal inner medulla and lung. Am J Physiol Renal Physiol 278: F43–F51, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Kohan DE The renal medullary endothelin system in control of sodium and water excretion and systemic blood pressure. Curr Opin Nephrol Hypertens 15: 34–40, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Kohan DE, Padilla E. Osmolar regulation of endothelin-1 production by rat inner medullary collecting duct. J Clin Invest 91: 1235–1240, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kohan DE, Padilla E, Hughes AK. Endothelin B receptor mediates ET-1 effects on cAMP and PGE2 accumulation in rat IMCD. Am J Physiol Renal Fluid Electrolyte Physiol 265: F670–F676, 1993. [DOI] [PubMed] [Google Scholar]
- 47.Komlosi P, Fintha A, Bell PD. Renal cell-to-cell communication via extracellular ATP. Physiology 20: 86–90, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Leipziger J Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol 284: F419–F432, 2003. [DOI] [PubMed] [Google Scholar]
- 49.McCulloch F, Chambrey R, Eladari D, and Peti-Peterdi J. Localization of connexin 30 in the luminal membrane of cells in the distal nephron. Am J Physiol Renal Physiol 289: F1304–F1312, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Nielsen S, Frokiar J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82: 205–244, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Nonoguchi H, Owada A, Kobayashi N, Takayama M, Terada Y, Koike J, Ujiie K, Marumo F, Sakai T, Tomita K. Immunohistochemical localization of V2 vasopressin receptor along the nephron and functional role of luminal V2 receptor in terminal inner medullary collecting ducts. J Clin Invest 96: 1768–1778, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Norsk P Role of arginine vasopressin in the regulation of extracellular fluid volume. Med Sci Sports Exerc 28: S36–S41, 1996. [DOI] [PubMed] [Google Scholar]
- 53.North RA Molecular physiology of P2X receptors. Physiol Rev 82: 1013–1067, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Oppermann M, Friedman DJ, Faulhaber-Walter R, Mizel D, Castrop H, Enjyoji K, Robson SC, Schnermann J. Tubuloglomerular feedback and renin secretion in NTPDase1/CD39-deficient mice. Am J Physiol Renal Physiol 294: F965–F970, 2008. [DOI] [PubMed] [Google Scholar]
- 55.Palmer TM, Stiles GL. Structure-function analysis of inhibitory adenosine receptor regulation. Neuropharmacology 36: 1141–1147, 1997. [DOI] [PubMed] [Google Scholar]
- 56.Pawelczyk T, Grden M, Rzepko R, Sakowicz M, Szutowicz A. Region-specific alterations of adenosine receptors expression level in kidney of diabetic rat. Am J Pathol 167: 315–325, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Praetorius HA, Spring KR. A physiological view of the primary cilium. Annu Rev Physiol 67: 515–529, 2005. [DOI] [PubMed] [Google Scholar]
- 58.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998. [PubMed] [Google Scholar]
- 59.Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, Vallon V. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J 21: 3717–3726, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Rieg T, Pothula K, Schroth J, Satriano J, Osswald H, Schnermann J, Insel PA, Bundey RA, Vallon V. Vasopressin regulation of inner medullary collecting ducts and compensatory changes in mice lacking adenosine A1 receptors. Am J Physiol Renal Physiol 294: F638–F644, 2008. [DOI] [PubMed] [Google Scholar]
- 61.Rieg T, Schnermann J, Vallon V. Adenosine A1 receptors determine effects of caffeine on total fluid intake but not caffeine appetite. Eur J Pharmacol 555: 174–177, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Rieg T, Steigele H, Schnermann J, Richter K, Osswald H, Vallon V. Requirement of intact adenosine A1 receptors for the diuretic and natriuretic action of the methylxanthines theophylline and caffeine. J Pharmacol Exp Ther 313: 403–409, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Rose JB, Coe IR. Physiology of nucleoside transporters: back to the future. Physiology 23: 41–48, 2008. [DOI] [PubMed] [Google Scholar]
- 64.Rouse D, Leite M, Suki WN. ATP inhibits the hydrosmotic effect of AVP in rabbit CCT: evidence for a nucleotide P2u receptor. Am J Physiol Renal Fluid Electrolyte Physiol 267: F289–F295, 1994. [DOI] [PubMed] [Google Scholar]
- 65.Sakairi Y, Jacobson HR, Noland TD, Breyer MD. Luminal prostaglandin E receptors regulate salt and water transport in rabbit cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 269: F257–F265, 1995. [DOI] [PubMed] [Google Scholar]
- 66.Singla V, Reiter JF. The Primary cilium as the cell's antenna: signaling at a sensory organelle. Science 313: 629–633, 2006. [DOI] [PubMed] [Google Scholar]
- 67.Sun R, Carlson NG, Hemmert AC, Kishore BK. P2Y2 receptor-mediated release of prostaglandin E2 by IMCD is altered in hydrated and dehydrated rats: relevance to AVP-independent regulation of IMCD function. Am J Physiol Renal Physiol 289: F585–F592, 2005. [DOI] [PubMed] [Google Scholar]
- 68.Sun R, Miller RL, Hemmert AC, Zhang P, Shi H, Nelson RD, Kishore BK. Chronic dDAVP infusion in rats decreases the expression of P2Y2 receptor in inner medulla and P2Y2 receptor-mediated PGE2 release by IMCD. Am J Physiol Renal Physiol 289: F768–F776, 2005. [DOI] [PubMed] [Google Scholar]
- 69.Teitelbaum I Protein kinase C inhibits arginine vasopressin-stimulated cAMP accumulation via a Gi-dependent mechanism. Am J Physiol Renal Fluid Electrolyte Physiol 264: F216–F220, 1993. [DOI] [PubMed] [Google Scholar]
- 70.Teitelbaum I, Berl T. Increased cytosolic Ca2+ inhibits AVP-stimulated adenylyl cyclase activity in rat IMCT cells by activation of PKC. Am J Physiol Renal Fluid Electrolyte Physiol 266: F486–F490, 1994. [DOI] [PubMed] [Google Scholar]
- 71.Thomson S, Bao D, Deng A, Vallon V. Adenosine formed by 5′-nucleotidase mediates tubuloglomerular feedback. J Clin Invest 106: 289–298, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Turner CM, Vonend O, Chan C, Burnstock G, Unwin RJ. The pattern of distribution of selected ATP-sensitive P2 receptor subtypes in normal rat kidney: an immunohistological study. Cells Tissues Organs 175: 105–117, 2003. [DOI] [PubMed] [Google Scholar]
- 73.Uawithya P, Pisitkun T, Ruttenberg BE, Knepper MA. Transcriptional profiling of native inner medullary collecting duct cells from rat kidney. Physiol Genomics 32: 229–253, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Unwin RJ, Bailey MA, Burnstock G. Purinergic signaling along the renal tubule: the current state of play. News Physiol Sci 18: 237–241, 2003. [DOI] [PubMed] [Google Scholar]
- 75.Vallon V P2 receptors in the regulation of renal transport mechanisms. Am J Physiol Renal Physiol 294: F10–F27, 2008. [DOI] [PubMed] [Google Scholar]
- 76.Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev 86: 901–940, 2006. [DOI] [PubMed] [Google Scholar]
- 77.Vekaria RM, Unwin RJ, Shirley DG. Intraluminal ATP concentrations in rat renal tubules. J Am Soc Nephrol 17: 1841–1847, 2006. [DOI] [PubMed] [Google Scholar]
- 78.Vitzthum H, Weiss B, Bachleitner W, Kramer BK, Kurtz A. Gene expression of adenosine receptors along the nephron. Kidney Int 65: 1180–1190, 2004. [DOI] [PubMed] [Google Scholar]
- 79.Vonend O, Oberhauser V, Von Kugelgen I, Apel TW, Amann K, Ritz E, Rump LC. ATP release in human kidney cortex and its mitogenic effects in visceral glomerular epithelial cells. Kidney Int 61: 1617–1626, 2002. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y, Roman R, Lidofsky SD, Fitz JG. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc Natl Acad Sci USA 93: 12020–12025, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Welch BD, Carlson NG, Shi H, Myatt L, Kishore BK. P2Y2 receptor-stimulated release of prostaglandin E2 by rat inner medullary collecting duct preparations. Am J Physiol Renal Physiol 285: F711–F721, 2003. [DOI] [PubMed] [Google Scholar]
- 82.Wildman SS, Boone M, Peppiatt CM, Churchill LJ, Shirley DG, King BF, Deen PMT, Unwin RJ. Immunofluorescent labelling reveals dDAVP-dependent P2 receptor expression and apical P2 receptor-mediated inhibition of AQP2 expression in mpkCCD(cl4) cultures (Abstract). Proc Physiol Soc 3: C3, 2006. [Google Scholar]
- 83.Wildman SS, Peppiatt CM, Boone M, Konings I, Marks J, Churchill LJ, Turner CM, Shirley DG, King BF, Deen PMT, Unwin RJ. Potential role of apical P2 receptors in modulating aquaporin-2-mediated water reabsorption in the collecting duct (Abstract). Proc Physiol Soc 2: PC3, 2006. [Google Scholar]
- 84.Woda CB, Leite M Jr, Rohatgi R, Satlin LM. Effects of luminal flow and nucleotides on [Ca2+]i in rabbit cortical collecting duct. Am J Physiol Renal Physiol 283: F437–F446, 2002. [DOI] [PubMed] [Google Scholar]
- 85.Yagil Y Interaction of adenosine with vasopressin in the inner medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 259: F679–F687, 1990. [DOI] [PubMed] [Google Scholar]
- 86.Yagil Y Differential effect of basolateral and apical adenosine on AVP-stimulated cAMP formation in primary culture of IMCD. Am J Physiol Renal Fluid Electrolyte Physiol 263: F268–F276, 1992. [DOI] [PubMed] [Google Scholar]
- 87.Yamaguchi S, Umemura S, Tamura K, Iwamoto T, Nyui N, Ishigami T, Ishii M. Adenosine A1 receptor mRNA in microdissected rat nephron segments. Hypertension 26: 1181–1185, 1995. [DOI] [PubMed] [Google Scholar]
- 88.Yoshioka K, Saitoh O, Nakata H. Heteromeric association creates a P2Y-like adenosine receptor. Proc Natl Acad Sci USA 98: 7617–7622, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoshioka K, Saitoh O, Nakata H. Agonist-promoted heteromeric oligomerization between adenosine A(1) and P2Y(1) receptors in living cells. FEBS Lett 523: 147–151, 2002. [DOI] [PubMed] [Google Scholar]
- 90.Zou AP, Wu F, Li PL, Cowley AW Jr. Effect of chronic salt loading on adenosine metabolism and receptor expression in renal cortex and medulla in rats. Hypertension 33: 511–516, 1999. [DOI] [PubMed] [Google Scholar]