Abstract
Intracerebroventricular nociceptin/orphanin FQ (N/OFQ) produces cardiovascular depressor, diuretic, and renal sympathoinhibitory responses in conscious rats. These studies examined how a chronic high-NaCl intake alters these peptide-evoked responses and the activity of the endogenous central N/OFQ peptide (NOP) receptor system. In normotensive Sprague-Dawley rats fed a chronic (3-wk) high (8%)-NaCl diet, intracerebroventricular N/OFQ (5.5 nmol) produced prolonged bradycardic, hypotensive, and diuretic responses but failed to suppress renal sympathetic nerve activity. In a separate group of rats maintained on a high-NaCl diet, intracerebroventricular infusion of the NOP receptor antagonist UFP-101 significantly decreased urine output. At the tissue level, high-NaCl treatment of rats significantly increased NOP receptor density, without altering endogenous N/OFQ peptide levels in whole hypothalamus (control, 712 ± 35 fmol/mg vs. 8% NaCl, 883 ± 49 fmol/mg, P < 0.05) and paraventricular nucleus. Furthermore, in the hypothalamus, basal GTPγS binding was increased without altering the sensitivity of N/OFQ-stimulated G protein coupling. In contrast, in whole medulla and the ventrolateral medulla (VLM), high-NaCl treatment decreased NOP receptor density (medulla: control, 1,473 ± 131 fmol/mg vs. 8% NaCl, 327 ± 31 fmol/mg, P < 0.05) and endogenous N/OFQ peptide levels (medulla: control, 35.3 ± 2 fmol/mg vs. 8% NaCl, 11.9 ± 3 fmol/mg, P < 0.05), while increasing the sensitivity of G protein signaling pathways to N/OFQ stimulation. Together, these findings suggest that during a chronic high-salt intake, regional changes in the activity of the N/OFQ-NOP system in the brain may contribute to the tonic regulation of cardiovascular function and urine output and to the altered physiological responses to exogenous central N/OFQ.
Keywords: sodium-chloride diet, cardiovascular function, renal excretory function, central nervous system, renal sympathetic nerve activity
nociceptin/orphanin fq (N/OFQ) is an endogenous opioid-like peptide that selectively binds to the N/OFQ peptide (NOP) receptor (24, 28). The NOP receptor is a G protein-coupled receptor that elicits its physiological responses via pathways involving downstream Gαi1,2,3, GαoA,B (9, 32), Gαz (4, 13), and Gαq subunit proteins (33). The intracerebroventricular administration of N/OFQ (N/OFQ) to conscious rats evokes a unique pattern of changes in cardiovascular (hypotension, bradycardia, inhibition of central sympathetic outflow) and renal excretory function (water diuresis), which are independent from those produced by central nervous system (CNS) activation of μ-, δ-, or κ-opioid systems (16, 17, 19). Although the cardiovascular and renal responses produced by central administration of N/OFQ are well characterized, the endogenous role(s) of the N/OFQ-NOP receptor system remain unclear. Both N/OFQ and NOP receptors are highly expressed in CNS sites that regulate cardiovascular and renal homeostasis (25, 34). N/OFQ has been demonstrated to play a critical modulatory role in the adaptive behavioral fear responses to stressful stimuli (5, 12); therefore it is likely that the endogenous N/OFQ-NOP receptor system plays a physiologically important role in regulating cardiovascular and renal responses to acute/chronic stressors (18).
The stress of excessive dietary NaCl intake can have significant impact on cardiovascular and renal function and contribute to the development of hypertension (2, 24). However, the mechanisms by which high-salt intake produces or augments hypertension have yet to be clearly elucidated. Typically, in health, neural (renal sympathetic) and circulating humoral (angiotensin-aldosterone) sodium-retaining mechanisms are suppressed as a means to facilitate the renal excretion of a sodium load (2, 7). In this setting (e.g., healthy normotensive Sprague-Dawley rats) enhanced water intake (i.e., drinking) associated with consumption of a high-salt diet would be expected to occur without an increase in plasma vasopressin (AVP, antidiuretic hormone) levels as a means to facilitate an increase in urine output. This is in contrast to the increase in plasma AVP and water retention that occur in certain models of salt-sensitive hypertension (27, 30, 31). Together, in health, these regulatory mechanisms operate to help maintain total body water/sodium balance and normotension.
As noted above, central NOP receptor activation causes cardiovascular depressor and diuretic responses via inhibiting central sympathetic outflow (19) and AVP secretion (15, 33), respectively. The ability of central N/OFQ to influence these regulatory pathways is of potential physiological importance. Since a chronic high-NaCl intake can alter central sympathetic outflow (2, 8), basal plasma AVP levels (30) and urine output (26, 29), it is possible that the endogenous central N/OFQ system may act to oppose NaCl-induced changes in these parameters. On the basis of on this premise, it would be expected that a chronic high-NaCl intake would cause changes in the activity of the endogenous N/OFQ-NOP receptor system in specific brain regions involved in the control of cardiovascular function and the renal handling of water.
Therefore, the present studies were performed to determine how a chronic (3-wk) high (8%)-NaCl intake alters the cardiovascular and renal excretory responses to central administration of N/OFQ in conscious Sprague-Dawley rats. As a correlate, we examined how a chronic high-NaCl stress alters endogenous levels of NOP receptor expression and activity (GTPγS binding) in the hypothalamus and the medulla of the brain. In addition, high-salt-induced changes in N/OFQ peptide levels and NOP receptor expression were measured in the ventrolateral medulla (VLM) and the paraventricular nucleus (PVN), these being key brain regions involved in the central neural control of cardiovascular function and AVP synthesis/release, respectively. Finally, experiments were performed to investigate the premise that endogenous central N/OFQ systems are activated as an adaptive mechanism to counter the influence of chronic high-NaCl on cardiovascular function and/or urine output in rats. In these studies, intracerebroventricular administration of the selective NOP receptor antagonist UFP-101 (4, 34) was used to pharmacologically block a potential ongoing tonic influence of central N/OFQ on a given cardiovascular or renal excretory parameter.
METHODS
Animals
Male Sprague-Dawley rats (Harlan, Indianapolis, IN), 275–300 g, were housed individually under a 12:12-h light-dark cycle with free access to food and water. All procedures were conducted in accordance with the National Institutes of Health, and all experimental protocols were approved by the Louisiana State University Health Sciences Center Institutional Animal Care and Use Committee and met their Guidelines for the Care and Use of Animals.
Surgical and Experimental Methods
Sprague-Dawley rats were maintained for 3 wk on either a normal rodent diet (0.4% NaCl) plus tap drinking water, or as an experimental model of chronic high-salt intake (7), a high (8%)-NaCl diet (TestDiet, Richmond, IN). One week before experimentation, all rats were anesthetized (30 mg/kg im ketamine in combination with 2 mg/kg im xylazine; 17, 19), and stereotaxically implanted with a stainless steel cannula into the right lateral cerebral ventricle. Verification of cannula position was established via observation of cerebrospinal fluid and/or placement of dye following intracerebroventricular injection (17, 19). On the day of study, rats were anesthetized with sodium methohexital (20 mg/kg ip, supplemented with 10 mg/kg iv, as required; 16, 17, 19) and instrumented with catheters in the left femoral artery, left femoral vein, and bladder, as described previously, for measurement of arterial blood pressure, administration of drugs/saline, and collection of urine, respectively (17, 19, 20). In certain studies, rats (still anesthetized with methohexital sodium) were then implanted with a recording electrode on a renal nerve bundle for direct measurement of multifiber renal sympathetic nerve activity (RSNA; 19, 20). Following surgical preparation, rats were placed in a rat holder and an intravenous infusion of isotonic saline (55 μl/min) was started and continued for the duration of the experiment. The experimental protocol commenced after the animal regained full consciousness, and cardiovascular and renal excretory functions stabilized (4–6 h). Mean arterial pressure (MAP), heart rate (HR), and RSNA were continuously recorded using computer-driven BIOPAC data acquisition software (MP100 and AcqKnowledge version 3.8.2). Because of the limitations of comparing values for multifiber RSNA between animals, RSNA data was expressed as percentage of control with the control values for each animal taken as 100% (20). Urine volume was determined gravimetrically. Urine sodium concentration was measured by flame photometry (model 943; Instrumentation Laboratories, Lexington, MA) and expressed as urinary sodium excretion.
Central N/OFQ studies.
Experiments were performed to determine the changes in systemic cardiovascular and renal excretory function and RSNA produced by intracerebroventricular N/OFQ in conscious Sprague-Dawley rats that were chronically (3 wk) fed either a normal (0.4%) or high (8%)-NaCl diet. On the day of the experiment, cardiovascular function and urine output were initially measured in rats during a 20-min control period. Following this, N/OFQ (5.5 nmol; 16) or isotonic saline vehicle (5 μl) were injected intracerebroventricularly (n = 8 per group). The dose of N/OFQ used in these studies does not represent either the EC50 or maximally effective dose; instead the dose of 5.5 nmol has been previously demonstrated to produce consistent, significant, reproducible effects on the physiological parameters under investigation (16). Cardiovascular function was then measured, and urine samples were collected during a 90-min experimental period (consecutive 10-min periods).
Central UFP-101 antagonist studies.
Studies were performed to determine the changes in systemic cardiovascular and renal excretory function produced by acute blockade of central NOP receptors in conscious Sprague-Dawley rats maintained for 3 wk on either a normal (0.4%) or high (8%)-NaCl chow. On the day of the experiment, systemic cardiovascular function was measured, and urine was collected during a 20-min control period. Next, rats then received an intracerebroventricular infusion of isotonic saline vehicle (5 μl/h; n = 6/group) or the selective UFP-101 (18 nmol·5 μl−1·h−1; n = 6/group) (4, 34), which was continued for the duration of the study. Cardiovascular function was then measured, and urine samples were collected during a 90-min experimental period (consecutive 10-min periods).
AVP Measurement
Naïve Sprague-Dawley rats were fed a control diet (0.4% NaCl) or high-salt (8% NaCl) diet for 3 wk (n = 6 per group) (7). Animals were then decapitated, and plasma AVP was determined using an AVP ELISA kit as per manufacturers' instruction (Assay Designs, MI) and expressed as picograms per milliliter.
Brain Tissue Collection
Naïve Sprague-Dawley rats were fed a control diet (0.4% NaCl) or high-salt (8% NaCl) diet for 3 wk (7). Animals were then killed by decapitation and brain tissue was dissected on ice and stored at −80°C. Brain tissue was taken from the frontal cortex, the whole hypothalamus, and medulla as identified visually using morphological landmarks (28). In separate groups of rats, whole brains were removed, wrapped in aluminum foil, and frozen at −80°C. Brain cortex, PVN, and VLM tissue samples were then taken from frozen brain sections cut on a cryostat using a brain punch tool (Stoelting, IL). Brain cortex and PVN samples were taken using a punch diameter of 1.00 mm; VLM samples were taken using a punch diameter of 0.76 mm and were stored at −80°C. The location of the PVN and VLM was determined using visual landmarks (27) and by identification of neuron populations in sections examined under a light microscope.
N/OFQ RIA
Peptide extracts were prepared from brain regions (brain cortex, hypothalamus, medulla), and specific brain regions (PVN, VLM) (n = 6, 0.4% NaCl diet; n = 6, 8% NaCl diet), and quantified for protein content (21). NOP levels were quantified using a RIA kit (Phoenix Pharmaceuticals, Burlingame, CA), and expressed as femtomoles N/OFQ per milligram of protein.
[leucyl-3H]N/OFQ Saturation Binding Assay
Sprague-Dawley CNS tissue membrane preparations from brain cortex, whole hypothalamus, and medulla (n = 6, 0.4% NaCl diet; n = 6, 8% NaCl diet) were prepared and quantified for protein content (21). Samples were not prepared from PVN and VLM punches owing to tissue limitations of total protein content obtained from brain tissue punch samples. Tissue membranes (100 μg) were incubated with varying concentrations (∼ 0.002 pM–2 nM) of [leucyl-3H] N/OFQ for 60 min at room temperature in 500 μl of binding buffer supplemented with a 10 μM peptidase inhibitor cocktail (containing amastatin, bestatin, captopril, and phosphoramidon; 10 μM each) (23). Nonspecific binding was determined in the presence of 1 μM unlabeled N/OFQ. Reactions were terminated via vacuum filtration through polyethyleneimine (0.5%) presoaked Whatman GF/B filters using a Brandel-harvester (23). Radioactivity was determined following filter extraction using liquid scintillation spectroscopy. NOP receptor levels are expressed as femtomoles NOP per milligram of protein, concentration response curves were analyzed using computer-assisted curve fitting with Graphpad Prism 4, with data fitted to a one-site binding model, and subjected to nonlinear regression using a sigmoidal dose response (variable slope) model, to produce affinity (pKD) and receptor density (Bmax) values.
NOP Receptor Immunoblotting
Brain punch samples (BC, PVN, VLM), whole hypothalamus and medulla were taken from Sprague-Dawley rats maintained on control (0.4% NaCl) or high (8% NaCl)-salt diets for 3 wk (n = 6 per group). Tissue lysates, containing both membrane and cytoplasmic fractions, were then prepared, and protein levels were quantified (21). Lysates were resolved on SDS-PAGE gels and transferred to nitrocellulose membrane (GE Healthcare, Piscataway, NJ). NOP receptor levels were determined using anti-KOR3 antibody (1:1,000; Santa Cruz, CA), protein levels were normalized to GAPDH (1:1,000, anti-GAPDH; Abcam, MA; 34). Chemiluminescent immunoreactive bands were detected by horseradish peroxidase-conjugated secondary antibody. Data was imaged and analyzed using Bio-Rad Quantity One software.
GTPγ35S Assay
Sprague-Dawley brain tissue membrane preparations from frontal cortex, whole hypothalamus, and medulla were prepared (n = 6, 0.4% NaCl diet; n = 6, 8% NaCl diet) and quantified for protein content (21). Samples were not prepared from PVN and VLM punches owing to tissue limitations of total protein content of brain punch samples. A N/OFQ-stimulated GTPγ35S binding assay was performed using 20 μg of tissue membranes. Membranes were incubated for 1 h at 30°C with gentle shaking in 500 μl of incubation buffer (pH 7.4) containing 50 mM Tris, 0.2 mM EGTA, 100 mM NaCl, 1 mg/ml BSA, 0.15 mM bacitracin, 10 μM amastatin, 10 μM bestatin, 10 μM captopril, 10 μM phophoramidon, 100 μM GDP, and ∼150 pM GTPγ35S. Nonspecific binding was determined in the presence of 10 μM unlabeled GTPγS, and N/OFQ was added over the concentration range log 10−5–10−12 M. Reactions were terminated via vacuum filtration through Whatman GF/B filters using a Brandel-harvester (23). Radioactivity was determined following filter extraction using liquid scintillation spectroscopy. Concentration response curves were analyzed using computer-assisted curve fitting with Graphpad Prism 4, with data subjected to nonlinear regression analysis using a sigmoidal dose response (variable slope) model to produce functional potency (pEC50) and efficacy (stimulation factor). Data are presented as stimulation factor, which is the ratio between N/OFQ-stimulated GTPγ35S binding and basal-specific binding, and pEC50, which is the negative logarithm to base 10 of the agonist (N/OFQ) molar concentration that produces 50% of the maximal possible effect, and unstimulated basal binding, expressed as total disintegrations per minute, representing endogenous GTPγ35S binding (4, 23).
Statistical Analysis
All data are expressed as means ± SE. The magnitude of the changes in cardiovascular and renal excretory parameters at different time points after intracerebroventricular injection of drugs were compared with respective group control values by a one-way repeated-measures ANOVA with subsequent Dunnett's test. Differences occurring between treatment groups (e.g., 0.4% and 8% NaCl) were assessed using two-way repeated-measures ANOVA with treatment being one fixed effect and time the other, with the interaction included. The time (minutes) was then the repeated factor. Post hoc analysis was performed using Bonferroni's test. Where appropriate, a Student's t-test was also used to compare means between two groups. In each case, statistical significance was defined as P < 0.05.
RESULTS
Cardiovascular and Renal Excretory Responses to Intracerebroventricular N/OFQ
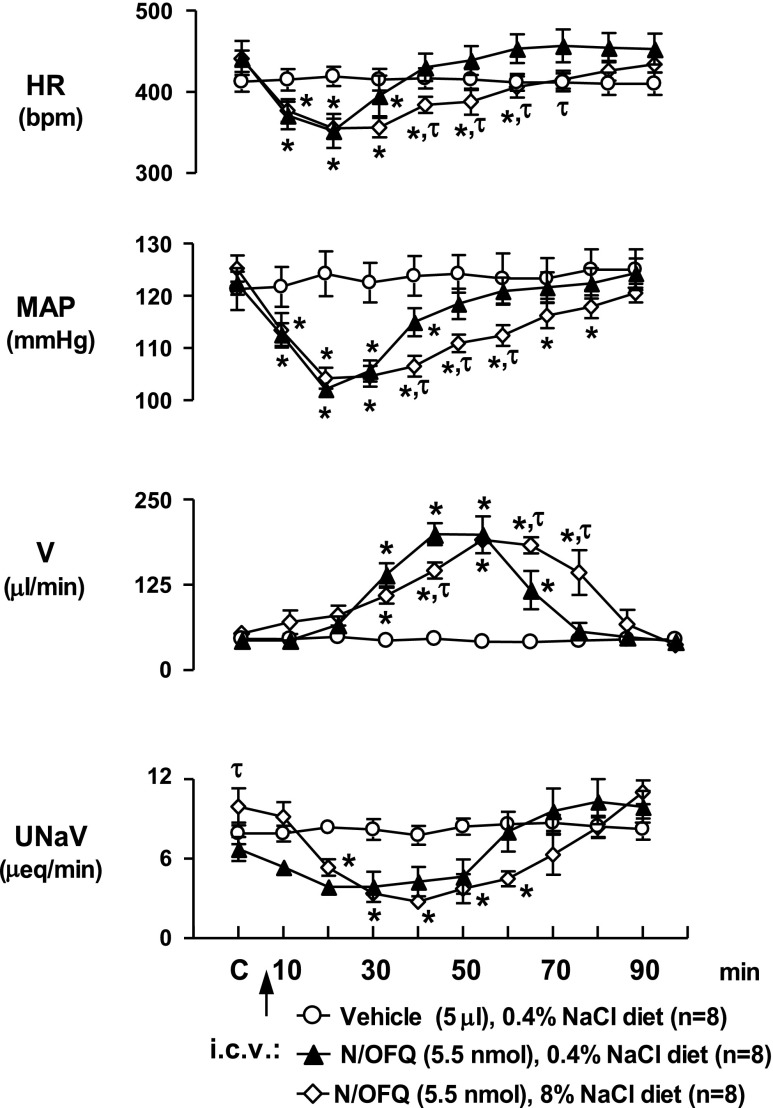
In control rats fed a normal diet (0.4% NaCl), intracerebroventricular N/OFQ (5.5 nmol), but not saline vehicle (5 μl icv), produced characteristic reductions in HR, MAP, and urinary sodium excretion and an increase in urine flow rate (Fig. 1). To determine whether chronic salt loading alters these responses, changes in cardiovascular and renal excretory function produced by intracerebroventricular N/OFQ were examined in rats maintained for 3 wk on a high-salt diet (8% NaCl chow plus tap drinking water). In these studies, levels for baseline systemic hemodynamics and urine output were not statistically different from those in rats maintained on a normal NaCl intake. However, basal urinary sodium excretion was significantly increased in rats that consumed a high-salt diet (Fig. 1). As depicted (Fig. 1), in high-NaCl-treated rats the duration of cardiovascular depressor responses (bradycardia, hypotension) to intracerebroventricular N/OFQ were significantly prolonged. In contrast to control diet animals in which HR and MAP returned to predrug levels by 40 min, the bradycardia and hypotension persisted for 60 and 80 min, respectively, in the 8% NaCl group (Fig. 1). There was a delayed diuresis in response to intracerebroventricular N/OFQ in both control and high-NaCl groups, with increased urinary flow detectable within 30-min. However, the diuretic response was significantly prolonged in animals fed an 8% NaCl diet (70-min urine flow rate: control diet, 56 ± 12 μl/min vs. 8% NaCl diet, 123 ± 32 μl/min, P < 0.05) resulting in significantly greater cumulative urine output (control diet, 4,869 ± 324 μl; 8% NaCl diet, 5,900 ± 226 μl; P < 0.05). Similarly, the antinatriuresis to intracerebroventricular N/OFQ tended to be of longer duration than that obtained in the control diet group.
Fig. 1.
Cardiovascular and renal responses produced by intracerebroventricular nociceptin/orphanin FQ (N/OFQ; 5.5 nmol/5 μl) (n = 8) or intracerebroventricular isotonic saline vehicle (5 μl; n = 8) in conscious male Sprague-Dawley rats maintained on a control diet (0.4% NaCl) or a high (8%)-NaCl diet for 3 wk (n = 8). HR, heart rate; MAP, mean arterial pressure; V, urine flow rate; UNaV, urinary sodium excretion. Results are means ± SE. *P < 0.05, compared with control value at respective time points, τP < 0.05, between control diet and 8% NaCl content diet groups at respective time points.
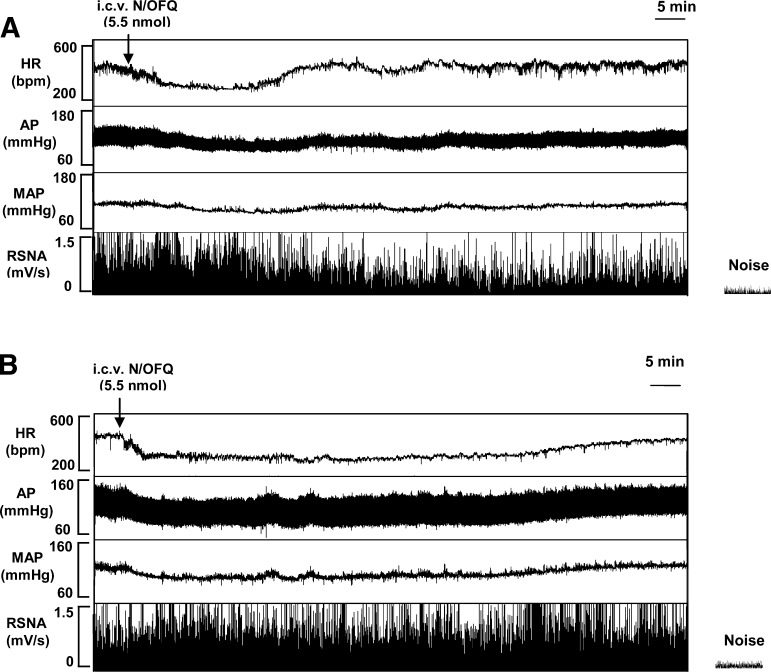
Additional experiments were performed in rats chronically maintained (3 wk) on a normal (0.4%; n = 8) or high (8%; n = 8)-NaCl diet to examine the influence of high-salt intake on N/OFQ-induced changes in RSNA. In control diet animals, RSNA did not significantly change over the first 20 min following intracerebroventricular N/OFQ, despite a drug-induced reduction in MAP (Fig. 2). However, by 30 min post-drug injection (a time in which MAP tended to return to control level) RSNA was significantly (P < 0.05) decreased (30-min RSNA, 76 ± 6% predrug control level; n = 8) and remained depressed for the duration of the experimental protocol (90-min RSNA, 70 ± 9% predrug control level; n = 8). In contrast, in rats chronically maintained on an 8% NaCl diet, intracerebroventricular N/OFQ caused a more prolonged hypotensive response but did not significantly alter RSNA throughout the protocol (30-min RSNA, 85 ± 6% predrug control level; n = 8; 90-min RSNA, 95 ± 7% predrug control level; n = 8; and Fig. 2).
Fig. 2.
Representative tracing obtained from the BIOPAC data acquisition system illustrating typical HR, arterial pressure (AP), MAP, and integrated renal sympathetic nerve activity (RSNA), responses produced by injection of N/OFQ (5.5 nmol/5 μl icv) in a conscious Sprague-Dawley rat maintained on a control diet (0.4% NaCl; A) and a conscious Sprague-Dawley rat maintained chronically (3-wk) on an 8% NaCl diet (B).
UFP-101 Antagonist Studies
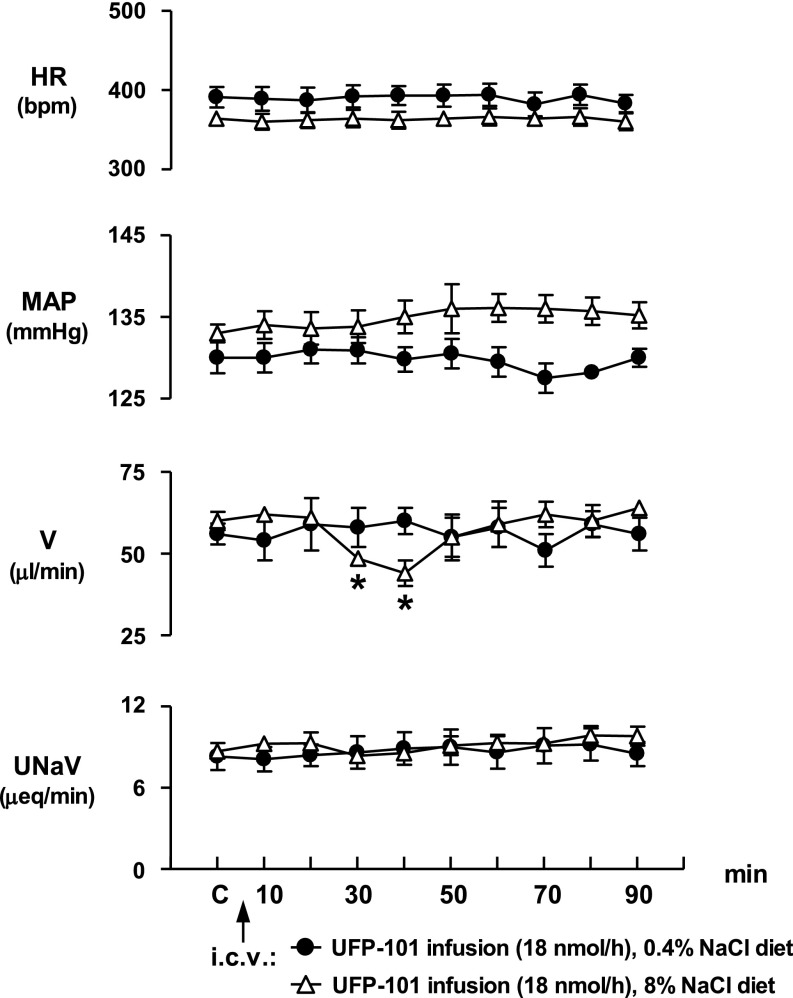
The continuous central infusion of UFP-101 (18 nmol·h−1·5 μl−1) did not alter cardiovascular or renal excretory function in Sprague-Dawley rats maintained on a 0.4% NaCl diet (Fig. 3). In this control group, HR, MAP, urine output, and urinary sodium excretion remained constant over the duration of the experimental period in which UFP-101 was continuously infused. In contrast to the lack of response observed in rats maintained on a normal salt diet, intracerebroventricular infusion of UFP-101 significantly altered urine output in animals chronically maintained on a high-NaCl intake (Fig. 3). In particular, urine flow rate significantly decreased 30 and 40 min after starting the UFP-101 infusion (control urine flow rate, 60 ± 2.8 μl/min vs. 40-min urine flow rate, 44 ± 3.9 μ/min, P < 0.05), after which levels for urine flow rate returned to predrug, baseline control levels. In rats maintained on a high-NaCl intake, intracerebroventricular infusion of UFP-101 also produced a small increase in MAP, which occurred ∼50 min after start of intracerebroventricular antagonist infusion which persisted for the duration of the experimental protocol (Fig. 3). However, this central UFP-101-evoked increase in MAP did not achieve statistical significance. Intracerebroventricular UFP-101 infusion did not alter HR or urinary sodium excretion over the course of the 90-min experimental protocol.
Fig. 3.
Effect of continuous N/OFQ peptide (NOP) receptor antagonist UFP-101 infusion (18 nmol·5 μl−1·h−1 icv) on cardiovascular and renal excretory function in conscious male Sprague-Dawley rats maintained on either a control (0.4%) NaCl diet or a high (8%)-NaCl diet for 3-wk (n = 8/group). Values are means ± SE and illustrate the cardiovascular and renal effects of central UFP-101 infusion in 6 conscious rats/group. *P < 0.05 compared with respective group control value (C). τP < 0.05 significantly different. Compared with control diet group at respective time points.
Plasma AVP Levels
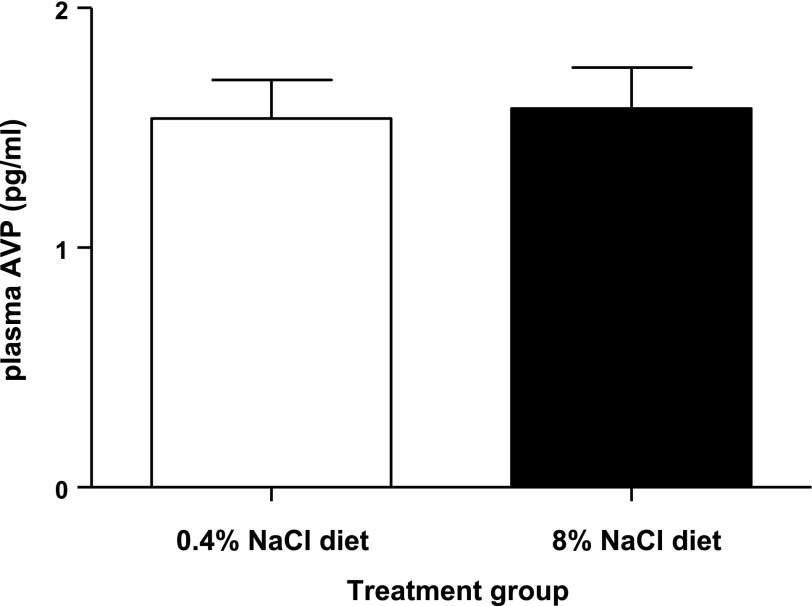
In control male Sprague-Dawley rats maintained on a normal (0.4%) NaCl diet, the level of endogenous plasma AVP was 1.54 ± 0.16 pg/ml. Maintenance of rats on a chronic (3-wk) high (8%)-NaCl diet did not alter the endogenous level of plasma AVP (1.58 ± 0.17 pg/ml; Fig. 4).
Fig. 4.
Effect of chronic high-NaCl intake in conscious male Sprague-Dawley rats maintained on either a control (0.4%) NaCl diet or a high (8%)-NaCl diet for 3-wk (n = 6/group), on endogenous plasma AVP levels. Values are means ± SE.
Brain N/OFQ Peptide Levels
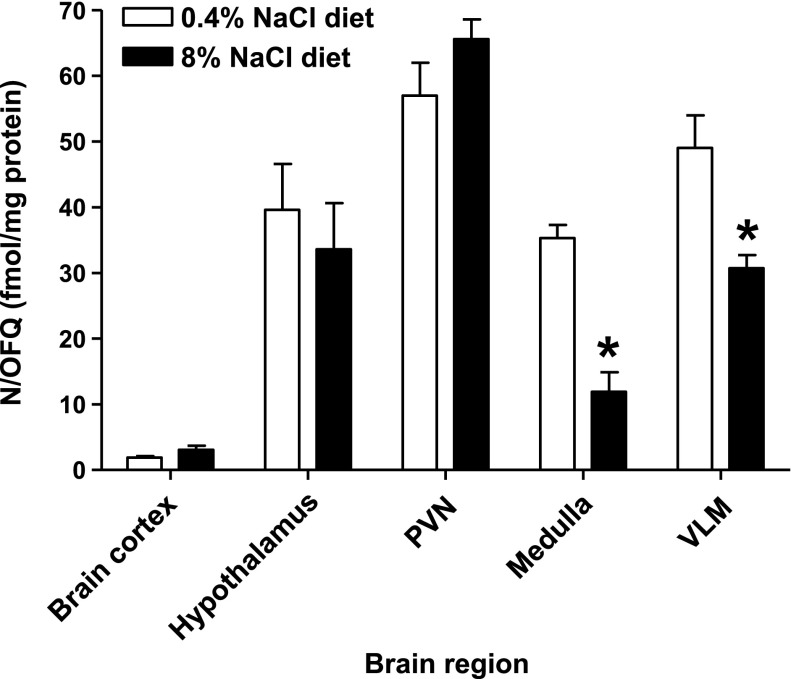
In control male Sprague-Dawley rats maintained on a normal (0.4%) NaCl diet, N/OFQ peptide levels in brain cortex tissue (1.9 ± 0.4 fmol/mg) were significantly lower than that observed in the hypothalamus and medulla (40 ± 7 and 35 ± 2 fmol/mg, respectively). Tissue samples from the PVN and VLM had higher endogenous levels of N/OFQ (57 ± 4 and 49 ± 6 fmol/mg, respectively) compared with peptide levels present in hypothalamic and medulla samples (Fig. 5). Compared with animals on a normal chow, maintenance of rats on a chronic (3-wk) high (8%)-NaCl diet did not significantly alter endogenous N/OFQ levels in the brain cortex, hypothalamic, or PVN samples. However, in high-NaCl-treated rats (Fig. 5), there was a significant reduction in N/OFQ peptide levels in both the whole medulla and VLM (medulla: control diet, 35.3 ± 2 fmol N/OFQ/mg protein vs. 8% NaCl diet, 11.9 ± 3 fmol N/OFQ/mg protein, P < 0.05; VLM: control diet, 49 ± 6 fmol N/OFQ/mg protein vs. 8% NaCl diet, 32 ± 2 fmol N/OFQ/mg protein, P < 0.05).
Fig. 5.
Endogenous N/OFQ peptide levels in brain cortex, whole hypothalamus, whole medulla, paraventricular nucleus (PVN), and ventrolateral medulla (VLM) tissue isolated from male Sprague-Dawley rats maintained 3-wk on either a control diet (0.4% NaCl) or an 8% NaCl diet (n = 6/group). Results are means ± SE. *P < 0.05, statistically different from normal respective chow value.
Brain NOP Receptor Expression
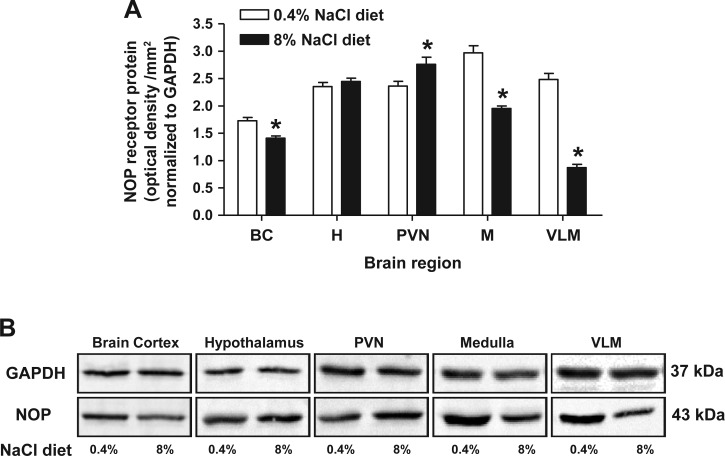
NOP receptor expression was determined via radioligand binding using membrane preparations and immunoblotting using tissue homogenates containing both membrane and cytoplasmic fractions. Owing to tissue limitations, NOP receptor expression was not determined via radioligand binding in brain punch samples and was instead examined through immunoblotting. In control rats fed a normal (0.4%) NaCl diet, the highest NOP receptor levels were observed in the medulla and VLM, followed by hypothalamic and PVN tissue. Lower expression levels were detected in brain cortex samples (Table 1, Fig. 6A). Chronic (3-wk) intake of high (8%)-NaCl produced regional changes in NOP receptor density within the brain of Sprague-Dawley rats (Table 1, Fig. 6). Data from radioligand binding studies revealed no significant difference between pKD values across brain regions (Table 1) following high-salt intake, indicating chronic high-salt intake does not alter the affinity of N/OFQ for the NOP receptor (Table 1). Following high-salt intake, NOP receptor levels were elevated in both the hypothalamus and PVN; when assessed by immunoblotting these changes were significant in the PVN (Fig. 6A). When assessed by the more sensitive method of radioligand binding, a statistically significant increase in NOP receptor expression was observed in the whole hypothalamus (Table 1). In contrast to the increase in NOP expression in the hypothalamus and PVN, endogenous NOP receptor levels were significantly reduced in animals maintained on a high-salt diet in the brain cortex and medulla (medulla: control diet, 1,473 ± 131 fmol NOP/mg of protein vs. 8% NaCl diet, 327 ± 31 fmol NOP/mg of protein, P < 0.05) as assessed by radioligand binding (Table 1) and immunoblotting (Fig. 6A). Additionally, correlating with a reduced level of NOP receptor expression in the whole medulla, NOP receptor levels were significantly reduced in the VLM (VLM: NOP control diet, 2.5 ± 0.1 optical density units/mm2 normalized to GAPDH vs. 8% NaCl diet, 0.87 ± 0.06 optical density units/mm2 normalized to GAPDH, P < 0.05) (Fig. 6).
Table 1.
[leucyl-3H]N/OFQ binding in CNS tissues isolated from male Sprague-Dawley rats maintained on either a chronic (3-week) a normal (0.4%) or high (8%)-NaCl chow
|
PKD |
Bmax, fmol NOP Receptor/mg Protein | |||
|---|---|---|---|---|
| Normal Diet, 0.4% NaCl | High-Salt Diet, 8% NaCl | Normal Diet, 0.4% NaCl | High-Salt Diet, 8% NaCl | |
| Brain cortex | 9.09±0.03 | 9.43±0.07 | 416±45 | 218.1±22* |
| Hypothalamus | 9.43±0.04 | 9.27±0.14 | 712.1±35 | 883.2±49* |
| Medulla | 9.14±0.12 | 9.07±0.04 | 1473±131 | 326.5±31† |
Data are means ± SE from 6 individual experiments. N/OFQ, nociceptin/orphanin FQ; NOP, N/OFQ peptide; CNS, central nervous system; Bmax, maximum binding capacity;
P < 0.05,
P < 0.01, statistically different from normal chow value.
Fig. 6.
A: NOP receptor protein expression normalized to GAPDH in brain cortex, whole hypothalamus, whole medulla, PVN, and VLM tissue from male Sprague-Dawley rats maintained for 3 wk on a control diet or an 8% NaCl diet (n = 6/group). Results are means ± SE. *P < 0.05 control vs. 8% NaCl diet. B: representative immunoblots of GAPDH and NOP receptor protein expression in brain regions from male Sprague-Dawley rats maintained for 3 wk on a control diet or an 8% NaCl diet. Samples were loaded as tissue lysates at a concentration of 20 μg total protein.
Brain N/OFQ-Stimulated GTPγ35S Binding Activity
N/OFQ evoked GTPγ35S binding activity (an indicator of G protein activation) was determined in brain regions using radioligand binding to investigate whether chronic high-salt intake alters N/OFQ-stimulated G protein activity. Brain tissue punches were not used in these studies owing to insufficient levels of total protein. In rats fed a normal (0.4% NaCl) diet, maximal N/OFQ-stimulated GTPγS activity was present in the hypothalamus and medulla. Medullary tissue was the least sensitive to N/OFQ stimulation (medulla: pEC50 7.48 ± 0.26) with brain cortex displaying the greatest sensitivity (brain cortex: pEC50 9.49 ± 0.25) (Table 2). Maintenance of rats on a chronic (3-wk) high (8%)-NaCl diet, significantly and differentially, altered the sensitivity of brain tissues to N/OFQ stimulation (Table 2). Medullary tissue exhibited increased sensitivity (medulla: control diet, pEC50 7.48 ± 0.26 vs. 8% NaCl diet, 8.33 ± 0.26, P < 0.05) in contrast to a reduction in sensitivity to N/OFQ stimulation in brain cortical tissue (brain cortex: control diet, pEC50 9.49 ± 0.25 vs. 8% NaCl diet, 8.21 ± 0.09, P < 0.05). Chronic high-salt intake significantly increased basal GTPγS binding ∼2.5-fold in the hypothalamus, with no change in basal binding observed in the medulla or brain cortex (Table 2). Maximal N/OFQ stimulation of GTPγS binding was unaltered by high-salt intake in the brain cortex and medulla; in contrast, a significant reduction in maximal stimulation was observed in the hypothalamus (hypothalamus stimulation factor: control diet, 2.14 ± 0.16 vs. 8% NaCl diet, 1.43 ± 0.08, P < 0.05, Table 2).
Table 2.
GTPγ35S activity in CNS tissues isolated from male Sprague-Dawley rats maintained on either a chronic (3-wk) normal (0.4%) or high (8%)-NaCl chow
| pEC50 |
Stimulation Factor | Unstimulated Basal Binding, Total dpm | ||||
|---|---|---|---|---|---|---|
| Normal Diet, 0.4% NaCl | High-Salt Diet, 8% NaCl | Normal Diet, 0.4% NaCl | High-Salt Diet, 8% NaCl | Normal Diet, 0.4% NaCl | High-Salt Diet, 8% NaCl | |
| Brain cortex | 9.49±0.25 | 8.21±0.09* | 1.16±0.03 | 1.30±0.03 | 3816±59 | 4899±390 |
| Hypothalamus | 8.18±0.47 | 8.83±0.14 | 2.14±0.16 | 1.43±0.08* | 1774±156 | 4164±100* |
| Medulla | 7.48±0.16 | 8.33±0.26* | 1.67±0.06 | 1.50±0.11 | 6318±348 | 5871±398 |
Data are means ± SE from 6 individual experiments. Data are presented as stimulation factor, which is the ratio between N/OFQ-stimulated GTPγ35S binding and basal specific binding, pEC50, which is the negative logarithm to base 10 of the agonist (N/OFQ) molar concentration that produces 50% of the maximal possible effect, and unstimulated basal binding, expressed as total disintegrations per minute, representing endogenous GTPγ35S binding.
P < 0.05, statistically different from normal chow value.
DISCUSSION
The central administration of N/OFQ to conscious rats significantly decreases HR and MAP and produces a water diuresis via central pathways that involve inhibition of central sympathetic outflow and AVP secretion, respectively (17, 18, 19). The findings of the present studies demonstrate that a chronic high-NaCl diet significantly increases the duration of the cardiovascular depressor responses and the magnitude and duration of diuresis evoked by central N/OFQ in Sprague-Dawley rats. In particular, in chronic high-NaCl-treated rats, the central N/OFQ-evoked bradycardia and hypotension persisted for ∼60 and 80 min, respectively. This is in contrast to a typical recovery time of 30 to 40 min that is observed in Sprague-Dawley rats fed a control NaCl diet (present study and Refs. 17 and 19). While a high-NaCl diet affected the duration of the cardiovascular depressor responses, the peak bradycardic and hypotensive responses were not significantly different from those produced in rats maintained on a normal NaCl diet. In addition to sustained cardiovascular depressor responses, the chronic treatment of rats with a high-NaCl diet also altered the pattern of diuresis elicited by central N/OFQ. Compared with rats fed a normal chow, animals fed a chronic high-NaCl diet displayed a diuresis that was significantly delayed in onset, of greater duration (70 min), and of greater cumulative output. Together, these findings clearly demonstrate that chronic high-salt intake can markedly modify the cardiovascular and diuretic responses to the exogenous central administration of N/OFQ.
In conscious Sprague-Dawley rats, intracerebroventricular N/OFQ produces cardiovascular depressor responses and an inhibition of RSNA (19, 32). Moreover, the bradycardia to intracerebroventricular N/OFQ is blocked by systemic pretreatment of rats with propranolol, but not atropine (32). Since these findings indicate that central N/OFQ elicits cardiovascular depressor responses primarily by decreasing central sympathetic outflow (19, 32), we also examined whether a chronic high-NaCl diet would alter the ability of central N/OFQ to inhibit RSNA. In the present investigations, intracerebroventricular injection of N/OFQ to rats maintained on a chronic high-NaCl diet failed to suppress RSNA over the entire time course studied (90-min post-drug injection). This finding is of considerable interest since our laboratory and others have demonstrated a temporal correlation between the cardiovascular and renal nerve responses produced by central N/OFQ (19, 32). More specifically, while central N/OFQ can directly (and rapidly) inhibit RSNA (e.g., as demonstrated in sinoaortic denervated rats; 19), in intact animals the renal sympathoinhibitory response is delayed (30 min) and was only observed after the decrease in MAP produced by this peptide returns to pre-drug injection levels (present study and Refs. 19 and 32). These findings indicate that during the peptide-evoked hypotension observed in intact rats, central N/OFQ selectively blocks the baroreflex-induced sympathoexcitatory effect to the heart (and possibly blood vessels) but not to the kidneys (18, 19, 32). Analogous to these findings, our present results strongly suggest that in high-salt-treated rats, the prolonged hypotension produced by central N/OFQ counters/masks the characteristic decrease in RSNA by a pathway that involves the baroreflex. Although RSNA can have significant impact on the renal excretion of water and sodium (16, 17, 20) our previous studies have shown the diuretic (and antinaturetic) response to central N/OFQ can occur via a renal nerve-independent pathway (19) involving the suppression of AVP release into the systemic circulation (15, 34). This may explain why intracerebroventricular N/OFQ continued to produce a diuretic response in animals maintained on a chronic high-NaCl diet.
As noted above, a chronic high-NaCl diet significantly altered the pattern of cardiovascular, renal excretory, and renal nerve responses to intracerebroventricular N/OFQ in conscious rats. Based on these findings, it may be speculated that a high-salt intake may alter the activity of the endogenous N/OFQ-NOP receptor system as a mechanism(s) to counter the effects of high salt on systemic cardiovascular and renal excretory regulatory systems. In studies performed in Sprague-Dawley rats maintained on a normal salt intake, the continuous intracerebroventricular infusion of the selective NOP receptor antagonist UFP-101 (34) failed to produce a change in any systemic cardiovascular or renal excretory parameter. This suggests that under conditions of normal NaCl intake the endogenous N/OFQ-NOP system does not appear to play a major role in the control of cardiovascular function or urine output. In contrast, the present studies demonstrated that when Sprague-Dawley rats were maintained on a chronic high-NaCl intake, the pharmacological blockade of the central NOP receptor system with UFP-101 resulted in a significant, but transient reduction in urine output, suggesting that this antagonist blocked an ongoing inhibitory influence of endogenous N/OFQ on AVP secretion. This is of interest since in the present studies we observed that the chronic treatment of a high-NaCl diet to Sprague-Dawley rats did not alter plasma AVP levels; presumably this is mediated in part by activation of a pathway involving the endogenous central N/OFQ system. This is in contrast to the increase in plasma AVP that occurs in several hypertensive models (2, 9). These data provide evidence for an endogenous role of the N/OFQ-NOP system, likely at the level of the hypothalamic PVN, in inhibiting the secretion of AVP and thus enhancing urine output when animals are faced with the physiological stress of a high-salt challenge. The involvement of the native N/OFQ-NOP system in the excretion of water during stressful conditions has also been demonstrated in NOP receptor knockout mice, which exhibited an impaired ability to excrete urine following an acute water load compared with wild-type littermates (3).
In addition to changes in urine output, the continuous intracerebroventricular infusion of UFP-101 produced a slight, but persistent elevation in MAP in Sprague-Dawley rats maintained on a chronic NaCl diet. Although these changes were not statistically significant, these data suggest that the NOP receptor system may, under certain conditions, play a contributory role in countering the hypertensive effects of high salt on MAP.
Based on evidence from in vivo studies, we also examined how a chronic high-NaCl intake alters the central N/OFQ-NOP receptor system at the tissue level. Chronic high-salt loading did not alter endogenous N/OFQ peptide levels nor N/OFQ-stimulated G protein signaling sensitivity in the hypothalamus. Instead there was a significant increase in NOP receptor expression in the whole hypothalamus and PVN, coupled with a significant increase in basal hypothalamic GTPγS binding and a reduction in hypothalamic GTPγS stimulation factor. These findings are of particular interest since activation of NOP receptors within the hypothalamus, particularly in the PVN, results in a water diuresis by inhibiting the release of AVP (15, 20). During high-salt challenge, the modified N/OFQ system would favor greater endogenous hypothalamic and PVN N/OFQ signal transduction via an increased number of NOP receptors. Furthermore, the observed decrease in GTPγS stimulation factor, which is likely due to the observed increase in basal hypothalamic GTPγS activity, is hypothesized to reflect increased basal hypothalamic signaling pathways that are acting to suppress the release of AVP during the stress of high-salt challenge. Together, these salt-induced changes in the N/OFQ-NOP system reflect an endogenous mechanism functioning at the whole animal level to counter the effects of high-salt intake on increased AVP release and subsequently, water retention.
In contrast to the changes observed in the hypothalamus, Sprague-Dawley rats treated with a chronic high-salt intake demonstrated reduced levels of both the N/OFQ peptide and NOP receptor in the medulla and VLM, while increasing the sensitivity of the medulla to N/OFQ stimulated G protein activation. These salt-induced changes likely reflect an altered influence of endogenous N/OFQ activity on central cardiovascular regulatory mechanisms in the medulla/VLM, which contribute to maintaining normotension in the face of high-NaCl stress. The data obtained indicate that, despite a reduction in both the endogenous N/OFQ peptide and NOP receptor in the whole medulla and VLM, a given concentration of N/OFQ (exogenous or endogenously released) is able to stimulate greater G protein activity and, presumably, downstream signaling. The summation of these effects is likely to translate at the whole animal level to the prolonged bradycardic and hypotensive responses to intracerebroventricular N/OFQ observed in high-salt-treated Sprague-Dawley rats. These findings are of merit considering that increased dietary NaCl intake is known to increase neuronal activity in CNS sites that regulate sympathetic control of cardiovascular function, including the medullary nucleus tractus solitarius (1, 22) and the rostral VLM (11, 12). High-NaCl intake increases the sensitivity and, subsequently, the cellular properties of medullary tissue (rostal VLM sympathoexcitatory neurons), which may explain how several classes of compounds including glutamate (11, 12), GABAA and GABAB antagonists (7), and N/OFQ (present study) are able to exert either greater or more prolonged cardiovascular depressor effects in animals maintained on a high-NaCl diet. Finally, the tendency for MAP to increase in high-NaCl-treated rats during central UFP-101 infusion may reflect an ongoing activity of endogenous N/OFQ pathways in the medulla that are contributing to maintain constant MAP in the face of the stress of high-salt intake.
Perspectives and Significance
Together, these data demonstrate that the stress of a chronic high-NaCl diet alters the pattern in which central N/OFQ (native or exogenous) influences cardiovascular function and the renal excretion of water, presumably, in part, because central N/OFQ inhibits the same central neural (sympathetic) and humoral (AVP) regulatory pathways that are influenced by high NaCl. Additionally, this study provides evidence that endogenous N/OFQ peptide levels, NOP receptor density, and the sensitivity of N/OFQ-stimulated G protein activation are selectively and differentially altered by high NaCl in brain regions/sites involved in the regulation of systemic cardiovascular function and the renal handling of water. Together, these studies link altered physiological responses to central N/OFQ observed at the whole animal level during high-salt stress to modifications in the endogenous N/OFQ-NOP receptor system that occur at the brain tissue level. Finally, studies with UFP-101 revealed that endogenous central N/OFQ-NOP receptor pathways contribute, presumably via inhibiting AVP secretion, to enhancing urine output in Sprague-Dawley rats maintained on a chronic high-NaCl diet. On the basis of these findings, it may be speculated that the endogenous N/OFQ-NOP receptor system in the VLM and/or PVN (and potentially other brain regions) may be involved in the long-term regulation of cardiovascular function and body fluid homeostasis in the face of chronic high-salt challenge.
GRANTS
The research presented in this manuscript was funded by the National Institute of Health Grants DK-43337, HL-071212, and P20-RR-018766 (to D. R. Kapusta).
Acknowledgments
We would like to extend our appreciation to Dr. D. Lambert for technical consultation regarding the experimental procedures for the [leucyl-3H] N/OFQ saturation binding assay and the GTPγ35S assay.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bealer SL, Metcalf CS. Increased dietary sodium enhances activation of neurones in the medullary cardiovascular pathway during acute sodium loading in the rat. Auton Neurosci 117: 33–40, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Brooks VL, Haywood JR, Johnson AK. Neural, hormonal and renal interactions in long-term blood pressure control. Translation of salt retention to central activation of the sympathetic nervous system in hypertension. Clin Exp Pharmacol Physiol 32: 426–432, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Burmeister MA, Anosoff MA, Pintar JE, Kapusta DR. Transgenic nocieptin/orphanin FQ (N/OFQ) peptide (NOP) receptor knockout (−/−) mice exhibit an impaired ability to excrete urine following an acute water load (Abstract). FASEB J 19: A1133, 2005. [Google Scholar]
- 4.Calo G, Guerrini R, Rizzi A, Salvadori S, Burmeister M, Kapusta DR, Lambert DG, Regoli D. UFP-101, a peptide antagonist selective for the nociceptin/orphanin FQ receptor. CNS Drug Rev 11: 97–112, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan JSC, Yung LY, Lee JWM, Wi YL, Pei G, Wong YH. Pertussis toxin-insensitive signaling of the ORL1 receptor: coupling to Gz and G16 proteins. J Neurochem 71: 2203–2210, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Devine DP, Hoversten MT, Ueda Y, Akil H. Nociceptin/orphanin FQ content is decreased in forebrain neurones during acute stress. J Neuroendocrinol 15: 69–74, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Gao S, Tanaka K, Gotoh TM, Morita H. Effects of high NaCl diet on arterial pressure in Sprague-Dawley rats with hepatic and sinoaortic denervation. Jpn J Physiol 55: 229–234, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Hall JE, Granger JP, Hester RL, Montani JP. Mechanisms of sodium balance in hypertension: role of pressure naturesis. J Hypertens Suppl 4: 557–565, 1986. [PubMed] [Google Scholar]
- 9.Haywood JR, Brennan TJ, Hinojosa C. Neurohumoral mechanisms of sodium-dependent hypertension. Fed Proc 44: 2393–2399, 1985. [PubMed] [Google Scholar]
- 10.Hawes BE, Fried S, Yao X, Weig B, Graziano MP. Nociceptin (ORL-1) and μ-opioid receptors mediate mitogen-activated protein kinase activation in CHO cells through a Gi-coupled signaling pathway: Evidence for distinct mechanisms of agonist-mediated desensitization. J Neurochem 71: 1024–1033, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Isogai O, Tsukamoto K, Masubuchi Y, Tomioka S, Suzuki T, Kawato H, Yajima Y, Kasamaki Y, Ito S, Kanmatsuse K. High salt diet enhances cardiovascular responses from the nucleus tractus solitarius and ventrolateral medulla of Sprague-Dawley rats. Clin Exp Hypertens 27: 33–44, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Ito S, Gordon FJ, Sved AF. Dietary salt intake alters cardiovascular responses evoked from the rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 276: R1600–R1607, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Jenck F, Moreau JL, Martin JR, Kilpatrick GJ, Reinscheid RK, Monsma FJ Jr, Nothacker HP, Civelli O. Orphanin FQ acts as an anxiolytic to attenuate behavioural responses to stress. Proc Natl Acad Sci USA 94: 14854–14858, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong SW, Ikeda SRG. Protein α-subunit Gαz couples neurotransmitter receptors to ion channels in sympathetic neurons. Neuron 21: 1201–1212, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Kakiya S, Murase T, Arima H, Yokoi H, Iwasaki Y, Miura Y, Oiso Y. Role of endogenous nociceptin in the regulation of arginine vasopressin release in conscious rats. Endocrinology 141: 4466–4471, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Kapusta DR, Sezen SF, Chang JK, Lippton H, Kenigs VA. Diuretic and antinaturetic responses produced by the endogenous opioid-like peptide, nociceptin (orphanin FQ). Life Sci 60: PL15–PL21, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Kapusta DR, Chang JK, Kenigs VA. Central administration of [Phe1Ψ (CH2-NH)Gly2]nociceptin(1–13)-NH2 and orphanin FQ/nociceptin (OFQ/N) produce similar cardiovascular and renal responses in conscious rats. J Pharmacol Exp Ther 289: 173–180, 1999. [PubMed] [Google Scholar]
- 18.Kapusta DR, Dayan LA, Kenigs VA. Nociceptin/orphanin FQ modulates the cardiovascular, but not renal, responses to stress in spontaneously hypertensive rats. Clin Exp Pharmacol Physiol 29: 254–259, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Kapusta DR, Kenigs VA. Cardiovascular and renal responses produced by central orphanin FQ/nociceptin occur independent of renal nerves. Am J Physiol Regul Integr Comp Physiol 277: R987–R995, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Krowicki ZK, Kapusta DR. Tonic nociceptinergic inputs to neurons in the hypothalamic paraventricular nucleus contribute to sympathetic vasomotor tone and water and electrolyte homeostasis in conscious rats. J Pharmacol Exp Ther 317: 446–453, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 193: 265–275, 1951. [PubMed] [Google Scholar]
- 22.Masubuchi Y, Tsukamoto K, Isogai O, Yajima Y, Ito S, Saito S, Uchiyama Y. Effect of a high-salt diet on γ-aminobutyric acid-mediated responses in the nucleus tractus solitarius of Sprague-Dawley rats. Brain Res Bull 64: 221–226, 2004. [DOI] [PubMed] [Google Scholar]
- 23.McDonald J, Barnes TA, Okawa H, Williams J, Calo G, Rowbotham DJ, Lambert DG. Partial agonist behaviour depends upon the level of nociceptin/orphanin FQ expression: studies using the ecdysone-inducible mammalian expression system. Br J Pharmacol 140: 61–70, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev 85: 679–715, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, Mazarguil H, Vassart G, Parmentier M, Costentin J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 377: 532–535, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Mollereau C, Mouledous L. Tissue distribution of the opioid receptor-like (ORL1) receptor. Peptides 21: 907–917, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura K, Cowley AW Jr. Sequential changes of cerebrospinal fluid sodium during the development of hypertension in Dahl rats. Hypertension 13: 243–249, 1989. [DOI] [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (4th ed.), New York: Academic, 1998.
- 29.Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ Jr, Civelli O. Orphanin FQ: a neuropeptide that activates an opioid like G protein-coupled receptor. Science 270: 792–794, 1995. [DOI] [PubMed] [Google Scholar]
- 30.Roman RJ, Osborn JL. Renal function and sodium balance in conscious Dahl S and R rats. Am J Physiol Regul Integr Comp Physiol 252: R833–R841, 1987. [DOI] [PubMed] [Google Scholar]
- 31.Serino R, Ueta Y, Hanamiya M, Nomura M, Yamamoto Y, Yamaguchi KI, Nakashima Y, Yamashita H. Increased levels of hypothalamic neuronal nitric oxide synthetase and vasopressin in salt-loaded Dahl rat. Auton Neurosci 87: 225–235, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Shirasaka T, Kunitake T, Kato K, Takasaki M, Kannan H. Nociceptin modulates renal sympathetic nerve activity through a central action in conscious rats. Am J Physiol Regul Integr Comp Physiol 277: R1025–R1032, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Tso W, Wong YH. Opioid receptor-like (ORL1) receptor utilizes both GoA and GoB for signal transduction. Prot Pep Lett 13: 437–441, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Wainford RD, Kurtz K, Kapusta DR. Central G-α subunit protein mediated control of cardiovascular function, urine output and vasopressin (AVP) secretion in conscious Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 295: R535–R542, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witta J, Palkovits M, Rosenberger J, Cox BM. Distribution of nociceptin/orphanin FQ in adult human brain. Brain Res 997: 24–29, 2004. [DOI] [PubMed] [Google Scholar]