Abstract
Chronic intermittent hypoxia (CIH), as occurs in sleep apnea, impairs baroreflex-mediated reductions in heart rate (HR) and enhances HR responses to electrical stimulation of vagal efferent. We tested the hypotheses that HR responses to activation of α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) and N-methyl-d-aspartate (NMDA) receptors in the nucleus ambiguus (NA) are reduced in CIH-exposed rats and that this impairment is associated with degeneration of glutamate receptor (GluR)-immunoreactive NA neurons. Fischer 344 rats (3–4 mo) were exposed to room air (RA) or CIH for 35–50 days (n = 18/group). At the end of the exposures, AMPA (4 pmol, 20 nl) and NMDA (80 pmol, 20 nl) were microinjected into the same location of the left NA (−200 μm to +200 μm relative to caudal end of area postrema; n = 6/group), and HR and arterial blood pressure responses were measured. In addition, brain stem sections at the level of −800, −400, 0, +400, and +800 μm relative to obex were processed for AMPA and NMDA receptor immunohistochemistry. The number of NA neurons expressing AMPA receptors and NMDA receptors (NMDARs) was quantified. Compared with RA, we found that after CIH 1) HR responses to microinjection of AMPA into the left NA were reduced (RA −290 ± 30 vs. CIH −227 ± 15 beats/min, P < 0.05); 2) HR responses to microinjection of NMDA into the left NA were reduced (RA −302 ± 16 vs. CIH −238 ± 27 beats/min, P < 0.05); and 3) the number of NMDAR1, AMPA GluR1, and AMPA GluR2/3-immunoreactive cells in the NA was reduced (P < 0.05). These results suggest that degeneration of NA neurons expressing GluRs contributes to impaired baroreflex control of HR in rats exposed to CIH.
Keywords: brain stem, parasympathetic efferent, heart, baroreflex, sleep apnea
chronic intermittent hypoxia (CIH) during sleep is now routinely used as a representative model for sleep apnea syndromes (20, 36, 43). In patients with obstructive sleep apnea, baroreflex control of heart rate (HR) is reduced (3, 4). In CIH-exposed rats and mice, CIH significantly reduced baroreflex control of HR (25, 30, 33). In addition, CIH decreased the central mediation of baroreflex bradycardia and conversely augmented the baroreceptor afferent function and the HR responses to electrical stimulation of the vagus nerve (25). These findings suggest that the impairment with CIH occurs within the brain stem and not in sensory or efferent premotor fibers.
A substantial body of evidence indicates that the baroreflex control of HR is mediated through glutamate transmission in the nucleus ambiguus (NA) (46). Furthermore, the NA cardiac motoneurons express the ionotropic glutamate receptors α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) and N-methyl-d-aspartate (NMDA) (11), and electrical stimulation of the nucleus of the solitary tract (NTS) evokes fast and slow postsynaptic potentials in the NA cardiac neurons that are mediated by both non-NMDA (AMPA or kainate) and NMDA receptors (49). Furthermore, microinjection of l-glutamate into the NA induces bradycardic responses, and lesions of the NA almost completely abolish the baroreflex control of HR and largely reduce the HR response to l-glutamate injection into the NA (9). Therefore, glutamatergic transmission in the NA is critically important for baroreflex control of HR. In this study, we hypothesized that the HR response to AMPA and NMDA microinjections into the NA is attenuated by CIH, and that such attenuation would be accompanied by significant reductions in glutamate receptor-immunoreactive motoneurons in the NA. Since ionotropic glutamate receptors GluR1 and GluR2/3 (subunits of AMPA receptors) and NMDA receptor (NMDAR)1 (a subunit of NMDA receptors) are immunoreactive in the somata of cardiac motoneurons in the NA (11), we selected these receptor subunits for this study.
EXPERIMENTAL PROCEDURES
Fischer 344 (F344; 3–4 mo) rats were used. Procedures were approved by the University of Central Florida Animal Care and Use Committee and followed the guidelines established by the National Institutes of Health.
Intermittent hypoxia exposure.
The procedure for CIH exposure was previously described in detail (21, 25). Briefly, animals (3–4 mo old) were housed in Plexiglas chambers (30 × 20 × 20 in3; Oxycycler model A44XO; BioSpherix Instruments, Redfield, NY) in a room in which light and dark cycles were set at 12 h:12 h (6:00 AM to 6:00 PM). O2 concentration in chambers was continuously measured by an O2 analyzer and was controlled by a computerized system through a gas valve outlet. O2 concentration in chambers was programmed and adjusted automatically based on the prescribed cycle of CIH. Any deviation from the desired concentration was corrected by adding pure N2 or O2 through solenoid valves. Ambient CO2 in the chambers was periodically monitored and maintained at 0.03% by adjusting the overall chamber basal ventilation. Humidity was measured and maintained at 40–50%. Temperature was kept at 22–24°C. The CIH profile consisted of alternating 21% (90 s) and 10% (90 s) O2 every 6 min for 12 h during the light cycle and maintaining O2 at 21% for the night period, with an overall exposure duration of 35–50 days. The room air (RA) control animals were housed in room air under the same conditions as in the Oxycycler chambers except for the concentration of O2, which was continuously maintained at 21%.
Cardiovascular responses to microinjection of AMPA and NMDA into NA.
Rats were initially anesthetized with pentobarbital sodium (50 mg/kg ip) and artificially ventilated with oxygen-enriched room air through the trachea. Supplemental doses (5 mg/kg iv) of anesthetics were administered as needed to prevent eyeblink and withdrawal reflexes and fluctuations in arterial blood pressure (AP). Body temperature was monitored by a rectal probe and maintained at 37 ± 1°C with a homeostatic blanket (Harvard, Holliston, MA). Polyethylene catheters (PE-50) were placed into the left femoral artery to monitor mean arterial pressure (MAP) and into the right vein to inject supplemental doses of pentobarbital sodium. AP was measured with a PowerLab Data Acquisition System (PowerLab/8 SP) that was connected to a pressure transducer (CB Sciences, BP100). HR was calculated from pulse pressure waves with the ratemeter function of Chart 5.2 software provided in the PowerLab System.
The surgical procedure to expose the brain stem was identical to that previously described (7, 9, 10). Briefly, animals were placed in a stereotaxic instrument equipped with a head holder adapted to permit the neck to be sharply flexed. A dorsal incision was made over the neck muscles, which were retracted to expose the atlantooccipital membrane. This membrane was opened with an incision, exposing the cisterna magna and the dorsal medulla. The caudal end of the area postrema was used as a rostrocaudal reference for stereotaxic coordinates. Using a multichannel injector (MDI, PM8000), we injected AMPA (A6816, Sigma-Aldrich) and NMDA (M3262, Sigma-Aldrich) into the left NA at the same sites and at the level of the area postrema. The volume of injection was monitored by the displacement of the meniscus in the pipette through a microscope fitted with an eyepiece graticule. Saline was injected at the same site to provide a vehicle control. Changes in HR and MAP relative to the prestimulus baselines were measured. We first experimentally determined the injection sites for the maximal responses by injecting the different sites along and around the NA at the level of the area postrema with a large dose (AMPA 10 pmol/20 nl or NMDA 160 pmol/20 nl). We then determined the saturation doses for AMPA and NMDA and higher doses that would not increase the amplitude of the responses further. The saturation doses of AMPA and NMDA were ultimately used for the rest of the experiments in both groups. Injection sites were comparable because similar coordinates were used in all animals. In three RA control rats, we first injected the tracer Fluoro-Gold (FG; 3 μl of 2 mg/ml) into the left nodose ganglion as described previously, to retrogradely label vagal motoneurons in the NA (Refs. 8, 9; also see below). Five days after FG injection, we injected the tracer DiI into the NA region to leave a marker for the injection site of AMPA and NMDA after maximal HR responses were elicited.
Identification of NA region in the brain stem.
To identify the NA region, we injected tetramethylrhodamine dextran (TMR-D, 7%, 0.5 μl, mol wt 3,000; catalog no. D-3308, Molecular Probes) into the nodose ganglion to label vagal motoneurons in the NA. Twelve animals (6 for left and 6 for right) of the same age and size were anesthetized with pentobarbital sodium (50 mg/kg ip). A midline incision was made along the neck, and ventral neck muscles were gently separated by blunt dissection to expose the nodose ganglion medial to the internal carotid artery (8, 9). Multiple injections of TMR-D (total dose of 0.5 μl) were made into the left or right nodose ganglion through a micropipette that was connected to a picospritzer. After each injection, the micropipette was left in place for 1 min before being withdrawn to reduce dye leakage of the micropipette track. After completion of all injections, the surgical wound was closed with sutures and the animal was returned to its cage. After a survival period of 7 days to allow for tracer transport to the brain stem, each animal was anesthetized with an overdose of pentobarbital sodium (100 mg/kg ip) and perfused through the heart with warm 0.9% saline (300 ml) and phosphate-buffered (pH 7.4, 600 ml) 10% formalin. The brain stem and the nodose-petrosal ganglion complex were then removed. Each brain stem, containing the entire NA, NTS, and dorsal motor nucleus of the vagus (DmnX), was stored in 15% sucrose formalin overnight and sectioned transversely at 40 μm with a cryostat. All tissues were then dehydrated through a graded series of ethanol rinses (70%, 2 min; 90%, 2 min; and 2 × 100%, 1.5 min each). Finally, the tissue was mounted and coverslipped in Cytoseal XYL. Serial brain stem sections containing the NA were examined systematically and completely, using a confocal microscope to determine the locations of NA neurons. Brain stem sections at −800, −400, 0, +400, and +800 μm relative to obex were scanned. With the Neurolucida system, the brain stems (6 left and 6 right) were scaled and superimposed on top of each other, respectively. The TMR-D-labeled NA neurons in the corresponding coordinates in six animals were superimposed to mark the location and the boundary of the NA.
Immunocytochemistry.
After the rats were deeply anesthetized with an overdose of pentobarbital sodium (100 mg/kg ip), rats (RA and CIH, n = 6/group) were perfused via the ascending aorta and the blood was quickly flushed out with 300 ml of warm saline (0.9%, 40°C), immediately followed by 500 ml of 4% cold (4°C) paraformaldehyde in 0.1 M phosphate-buffered saline (PBS, pH 7.4). Brain stems were removed and postfixed by immersion in the above fixative for 24 h. They were then stored overnight in a 30% sucrose paraformaldehyde solution and sectioned transversely at 40 μm with a cryostat. Brain stem slices at the level of −800, −400, 0, +400 and +800 μm relative to obex were collected for staining.
Brain stem sections were processed free floating (22, 51) and incubated in 0.3% H2O2 for 30 min. After extensive washing with PBS, sections were treated for 1 h with 0.5% blocking reagent (NEL702, PerkinElmer LAS)-10% goat normal serum (Vector, Burlingame, CA)-0.4% Triton X-100 in 0.1 M PBS (pH 7.4) to block nonspecific binding sites. The sections were then separately incubated at 4°C for 24 h with the primary antibodies diluted at the appropriate concentrations in 0.5% blocking reagent-10% goat serum solution. The dilutions were 1:100 for rabbit anti-GluR1 (AB 1504, Chemicon), 1:100 for rabbit anti-GluR2/3 (AB 1506, Chemicon), and 1:200 for rabbit anti-NMDAR1 (AB 1516, Chemicon). After being thoroughly washed with PBS, sections were incubated for 1 h with the secondary antibody, goat anti-rabbit IgG (1:600, Vector). After these washes, sections were incubated with streptavidin-horseradish peroxidase (1:100, NEL702, PerkinElmer LAS) for 30 min. Sections were then washed in fluorophore tyramide (1:50, NEL702, PerkinElmer LAS) for 3 min. Finally, sections were washed with PBS, transferred to slides, air dried, and coverslipped. Control experiments were performed to determine whether the primary or secondary antibodies produced false positive results.
The sections were examined with a Nikon Eclipse 80i microscope. The numbers of AMPA- and NMDA-immunoreactive neurons in left and right NA were counted for each brain stem section (−800, −400, 0, +400, +800 μm). For counting AMPA- and NMDA-immunoreactive motoneurons, two criteria were used: 1) neurons located in the region defined by the NA region reconstructed by the TMR-D labeling at each level and 2) neurons with a minimum diameter of 20 μm (53). This method permitted correct counting and discrimination of both glia and smaller neurons with somata diameters 10–15 μm.
Data analysis.
Data are presented as means ± SE. Student's t-tests were used to compare the differences in HR and MAP responses to AMPA and NMDA injections and the number of AMPA and NMDA receptor-immunoreactive neurons between groups. Statistical significance was considered at the level of P < 0.05.
RESULTS
Injection sites and doses of AMPA and NMDA.
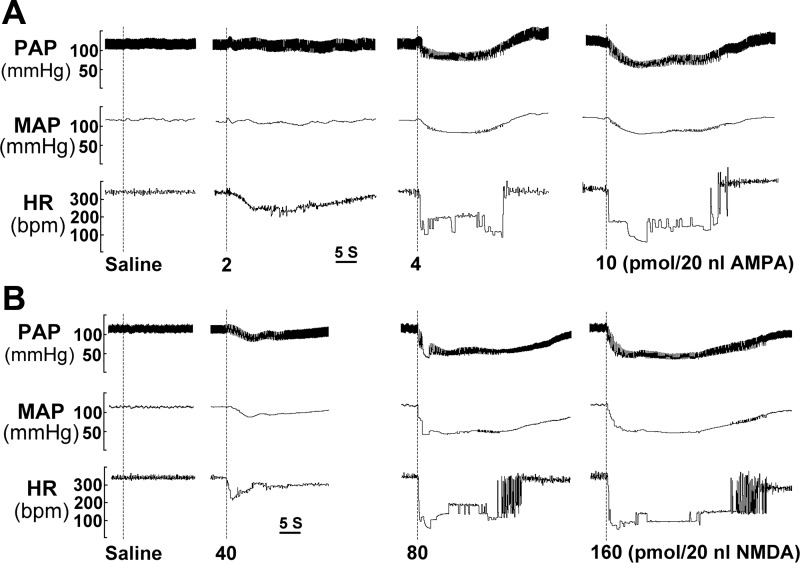
Consistent with the previous experiments in which we injected tracers (DiI or DiA) or l-glutamate into the NA (7, 9, 10), we reliably delivered AMPA and NMDA to the NA and evoked HR responses. The amplitudes of HR and MAP responses depended on both the dose and injection sites of AMPA and NMDA, as illustrated in Fig. 1. Figure 1A shows an injection site of AMPA (the illustration also applies for NMDA injection). Figure 1B represents a drawing of six representative AMPA (or NMDA) injection sites. Six representative HR responses to a 4-pmol AMPA injection at these locations in a RA animal are shown in Fig. 1C. Among these responses, we selected the maximum response, which exhibited the characteristics of a fast descending phase and a slow recovery phase. At the site where we could maximally evoke the response, we injected 0 (saline), 2, 4, and 10 pmol of AMPA (20 nl) to test the dose-dependent effect (Fig. 2A). Saline injections (0 mM) into the NA did not induce any response. Injection of AMPA at the dose of 4 pmol into the NA induced fast and large HR and AP responses. However, the amplitude of HR and MAP responses did not appear to be any larger at the higher dose (10 pmol). Thus we selected 4 pmol as the saturation dose for AMPA, and this dose was then used in all subsequent experiments. Of note, repeated injections within the same site with this dose evoked similar and maximal HR responses, indicating that the challenges were reproducible. Thus peak amplitudes were measured and compared between groups. Similarly, we injected 0 (saline), 40, 80, and 160 pmol of NMDA (20 nl, Fig. 2B). Injection of NMDA at 80 pmol could evoke the maximal HR response, and thus it was used in the remainder of the experiments.
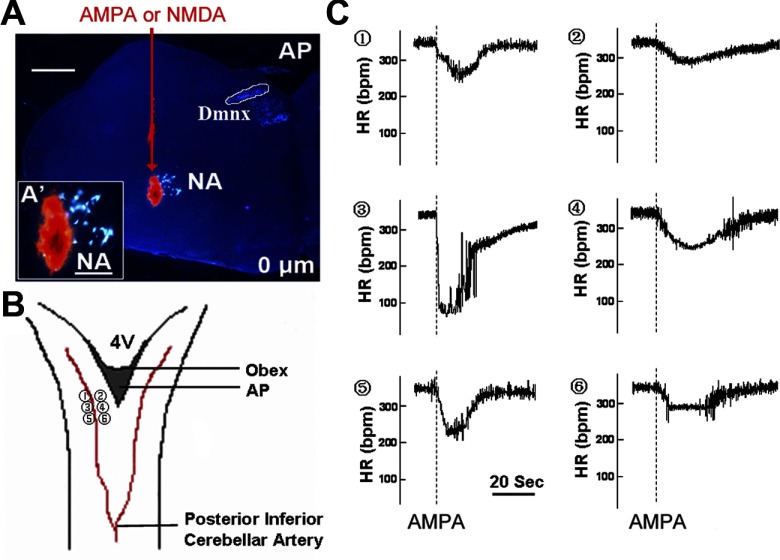
Fig. 1.
Injection sites of α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) and N-methyl-d-aspartate (NMDA) in the left nucleus ambiguus (NA). A: microphotograph of a brain stem slice at 0 μm relative to obex in a room air (RA) rat (coronal section) shows the injection site of AMPA and NMDA. Red arrow indicates the track that the injection pipette went through from the dorsal brain stem and advanced to the NA motoneurons. Motoneurons in the NA were retrogradely labeled by Fluoro-Gold (FG) injection into the left nodose ganglion and are in gold-yellow color. The AMPA and NMDA injection site was marked by the tracer DiI in orange-red color. AP, area postrema; DmnX, dorsal motor nucleus of vagus. A′: NA region at high magnification shows FG-labeled NA motoneurons (gold-yellow) and DiI injection (orange-red) in the NA region. B: dorsal view of the brain stem showing locations 1–6 where the pipette loaded with AMPA and NMDA penetrated through to reach the NA as shown in A. C: 1–6, heart rate (HR) responses induced by microinjections of AMPA into the left NA at those locations in a RA rat. The maximal response is at location 3. bpm, Beats/min. Scale bars: 600 μm for A, 200 μm for A′.
Fig. 2.
HR and arterial pressure responses to AMPA and NMDA injections into the left NA of a RA rat. A: AMPA was injected at 0 (saline), 2, 4, and 10 pmol; 4 pmol AMPA/20 nl was determined as a saturation dose. B: NMDA was injected at 0 (saline), 40, 80, and 160 pmol; 80 pmol NMDA/20 nl was determined as a saturation dose. MAP, mean arterial pressure; PAP, pulse arterial pressure.
CIH significantly reduced HR responses to AMPA and NMDA microinjections into NA.
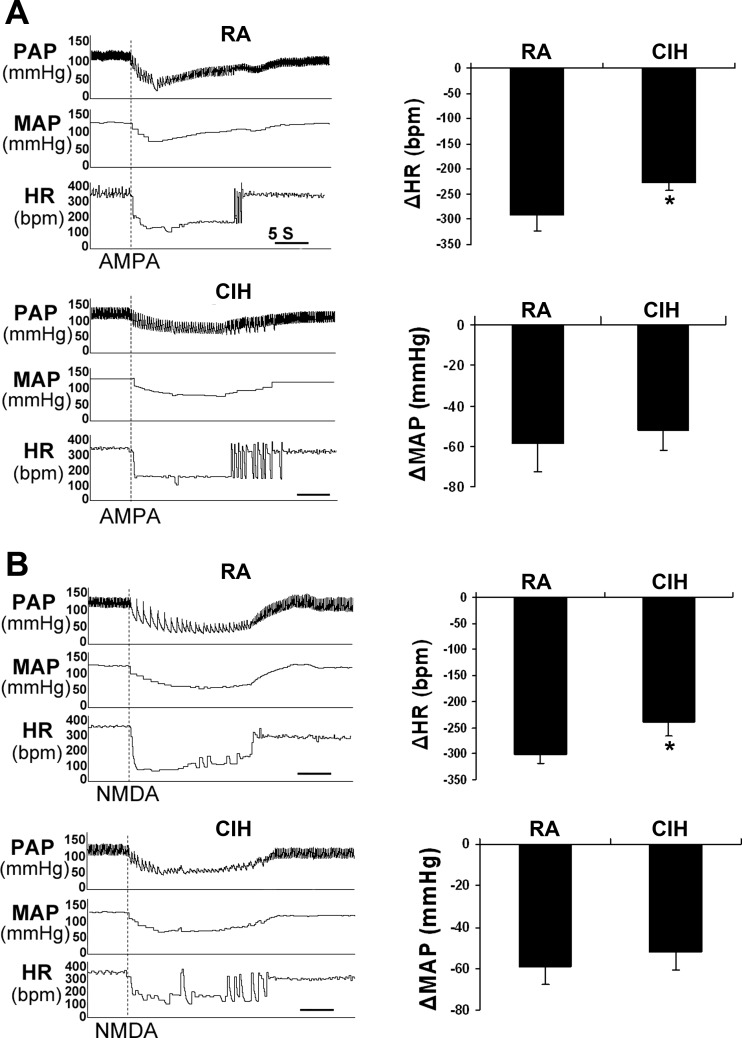
The baseline values of HR and MAP were not significantly different in RA and CIH rats [RA 360.2 ± 15.1 beats/min (bpm), CIH 348.4 ± 25.0 bpm; RA 116.6 ± 2.7 mmHg, CIH 122.3 ± 6.8 mmHg; n = 6/group, P > 0.10]. Since the baseline MAP and HR values were comparable, we could examine the HR and MAP changes in response to the microinjection of AMPA and NMDA in RA and CIH rats. CIH significantly reduced the maximal HR responses to AMPA injection (RA −290.3 ± 26.8 bpm, CIH −227.6 ± 15.2 bpm; n = 6/group, P < 0.05; Fig. 3A) but did not affect the MAP responses (RA −59.1 ± 14.2 mmHg, CIH −51.5 ± 9.8 mmHg; n = 6/group, P > 0.05). Similarly, CIH significantly reduced the maximal HR responses to NMDA injection (RA −302.1 ± 16.7 bpm, CIH −238.8.6 ± 24.5 bpm; n = 6/group, P < 0.05; Fig. 3B) and did not modify the MAP responses (RA −58.3 ± 8.7 mmHg, CIH −52.6 ± 8.6 mmHg; n = 6/group, P > 0.05). Of note, anesthesia might attenuate the difference of resting HR and MAP in RA and CIH.
Fig. 3.
HR and MAP responses to microinjections of AMPA and NMDA into the left NA. A: representative responses to AMPA injection in RA and CIH rats (left) and average number of HR and MAP responses (right). B: representative responses to NMDA injection in RA and CIH rats (left) and average number of HR and MAP responses (right). In both cases, CIH significantly reduces the HR responses (*P < 0.05) but not the MAP responses (P > 0.05). n = 6/group.
Heart rate responses to AMPA and NMDA injections: specificity.
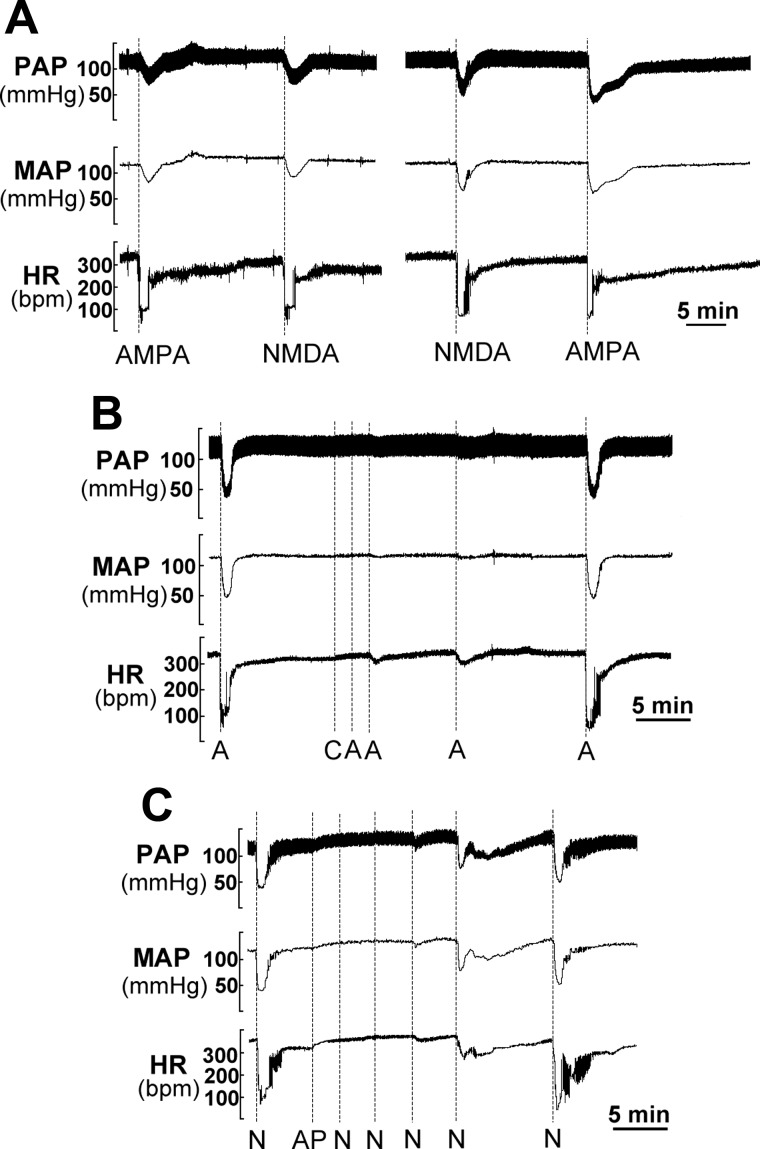
Injection of AMPA and NMDA into the same location of the NA using the respective saturation doses induced very comparable HR and AP responses, independent of the injection sequence (Fig. 4A). To test whether injections of AMPA or NMDA activated the counterpart receptor, we used the non-NMDA receptor blocker 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; C127, Sigma-Aldrich) and the NMDA receptor-specific blocker dl-2-amino-5-phosphonopentanoic acid (AP-5; M5282, Sigma-Aldrich) to block the HR responses to AMPA and NMDA, respectively. With CNQX the HR and AP responses were completely blocked (Fig. 4B), and such responses fully recovered within 20–25 min after CNQX injection. Similarly, with AP-5 the HR and AP responses were completely blocked (Fig. 4C) and fully recovered within 20–30 min after AP-5 injection. Therefore, AMPA or NMDA injections were receptor selective and did not activate the other receptor.
Fig. 4.
HR and arterial pressure responses to AMPA and NMDA injection: specificity. A: injection of AMPA and NMDA into the same location of the NA with the saturation doses induced very comparable HR and arterial pressure responses, indicating that the responses were independent of the injection sequence. B: within 3 min of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), HR and arterial pressure responses were completely blocked. Responses were fully recovered 20 min after CNQX. C: similarly, within 8 min after dl-2-amino-5-phosphonopentanoic acid (AP-5), HR and arterial pressure responses were completely blocked. Responses were fully recovered 20 min after AP-5 injection. Notably, injection of CNQX and AP-5 induced a significant increase of HR although small, possibly due to tonic glutamatergic drive to the NA cardiac motoneurons (40). A, AMPA; C, CNQX; N, NMDA; AP, AP-5.
CIH reduces AMPA and NMDA receptor-immunoreactive motoneurons in NA.
To reliably count the motoneurons within the NA, a virtual map of the brain slice at each level was made. Figure 5A shows five brain stem sections at different levels (−800 to + 800 μm relative to obex) of an animal. NA motoneurons were labeled by TMR-D (DmnX motoneurons and vagal afferent terminals were also labeled). The NA in Fig. 5A consists of the subdivisions of the nucleus including the external formation, which is related to cardiac control (2). Six confocal montages of the brain stem images at each level were then obtained from six different animals of similar size and were scaled and superimposed upon each other with Neurolucida System software (Neurolucida 7.0). Figure 5B shows the Neurolucida drawings of the boundaries of the NA at each level as outlined in red. The outlined NA boundaries were subsequently used as the outward frames within the confines of which AMPA and NMDA receptor-immunoreactive neurons were counted. Figure 6, A, B, and C, show the NA neurons expressing NMDAR1, AMPA GluR1, and AMPA GluR2/3, respectively, in the left NA at +400 μm in RA and CIH rats. CIH significantly reduced the number of AMPA GluR1-, AMPA GluR2/3-, and NMDAR1-immunoreactive neurons on both left and right sides (Table 1). Since the external formation is cardiac related (2), we further counted the immunoreactive neurons in the external formation. Our data indicate that CIH reduces the number of AMPA and NMDA receptor-immunoreactive neurons in the external formation (Table 2).
Fig. 5.
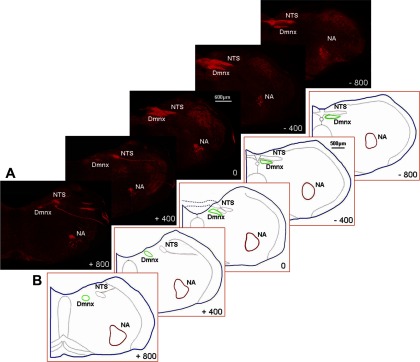
Nucleus ambiguus. A: montages of confocal projections of right brain stem sections showing NA motoneurons (−800 to + 800 μm relative to obex) that were retrogradely labeled by tetramethylrhodamine dextran (TMR-D) injection into the right nodose ganglion in a RA rat. B: brain stems were traced, scaled, and superimposed, using a Neurolucida tracing system to define the boundaries of the NA at each level, as enclosed in the red contours (n = 6). Note: vagal afferent terminals in the nucleus of the solitary tract (NTS) and vagal motoneurons in the DmnX were also labeled by TMR-D.
Fig. 6.
Photomicrographs of brain stem sections at the level +400 μm, showing NMDA- and AMPA-immunoreactive neurons in NA in RA and CIH rats. A: NMDAR1-immunoreactive neurons. B: AMPA GluR1-immunoreactive neurons. C: AMPA GluR2/3-immunoreactive neurons. CIH significantly reduced NMDA and AMPA receptor-immunoreactive neurons in NA (Table 1). Scale bars: 100 μm.
Table 1.
AMPA- and NMDA-immunoreactive neurons in NA
| −800 μm |
−400 μm | 0 μm | +400 μm | +800 μm | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | ||
| AMPA GluR1 | |||||||||||
| RA | 46.8±2.9 | 45.7±3.1 | 48.2±3.1 | 47.5±2.8 | 45.4±3.3 | 47.6±2.9 | 51.2±3.2 | 52.3±3.1 | 53.7±3.6 | 51.5±3.0 | 490.3±13.4 |
| CIH | 34.7±2.3* | 33.2±2.6* | 35.2±2.2* | 36.3±2.5* | 34.7±2.8* | 35.5±2.1* | 40.0±3.0* | 39.2±2.5* | 39.5±2.6* | 41.8±2.9* | 370.5±10.1* |
| AMPA GluR2/3 | |||||||||||
| RA | 49.3±3.4 | 50.9±3.5 | 48.3±3.8 | 47.6±3.2 | 45.3±3.1 | 48.7±3.7 | 55.5±3.6 | 54.6±4.0 | 56.3±3.7 | 57.2±3.3 | 514.2±13.1 |
| CIH | 32.7±2.0† | 33.4±2.1† | 31.8±2.9† | 32.5±2.6* | 34.3±2.7* | 36.4±2.4* | 39.2±2.6† | 40.6±3.0* | 39.8±2.9† | 41.4±3.2† | 362.5±8.9† |
| NMDAR1 | |||||||||||
| RA | 59.3±3.5 | 60.5±3.3 | 57.3±2.9 | 56.9±4.1 | 51.3±3.1 | 49.2±2.7 | 63.1±3.9 | 64.3±3.6 | 63.7±4.2 | 65.9±3.8 | 591.9±12.5 |
| CIH | 42.0±2.6† | 44.7±2.5* | 42.4±3.1* | 43.5±2.4* | 42.7±2.5* | 43.5±3.2 | 44.3±2.3* | 45.1±2.6* | 47.9±3.1† | 48.1±3.0† | 444.6±9.9* |
Values are means ± SE; n = 6. NA, nucleus ambiguus; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazole propionate; NMDA, N-methyl-d-aspartate; GluR, glutamate receptor; NMDAR, NMDA receptor; RA, room air; CIH, chronic intermittent hypoxia.
P < 0.05,
P < 0.01 compared with RA.
Table 2.
AMPA- and NMDA-immunoreactive neurons in external formation of NA
| −800 μm |
−400 μm | 0 μm | +400 μm | +800 μm | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | ||
| AMPA GluR1 | |||||||||||
| RA | 21.0±2.6 | 20.5±2.8 | 20.8±2.7 | 20.6±2.5 | 18.2±2.9 | 19.1±2.5 | 23.3±2.8 | 23.8±2.8 | 24.9±3.2 | 23.9±2.7 | 216.3±15.1 |
| CIH | 15.6±1.7* | 14.9±2.0* | 15.2±1.6* | 15.7±1.9 | 13.9±2.1 | 14.2±1.6* | 18.2±2.3 | 17.9±1.9* | 18.3±2.0* | 19.4±2.2 | 163.4±11.7* |
| AMPA GluR2/3 | |||||||||||
| RA | 22.1±3.0 | 22.3±3.1 | 20.9±3.2 | 20.6±3.0 | 19.9±3.3 | 19.2±3.0 | 25.3±3.2 | 24.9±3.6 | 26.1±2.9 | 26.3±3.1 | 227.7±14.3 |
| CIH | 14.7±1.8* | 15.0±1.7* | 13.8±2.2* | 14.1±2.0* | 13.8±2.1* | 14.6±1.9 | 17.9±1.9* | 18.5±2.3* | 18.5±2.1* | 19.2±2.2* | 160.0±10.2* |
| NMDAR1 | |||||||||||
| RA | 22.4±2.8 | 21.7±2.9 | 23.6±2.6 | 24.1±3.2 | 23.5±2.8 | 22.2±2.5 | 27.8±3.2 | 28.1±3.1 | 29.1±3.5 | 30.1±3.4 | 252.7±11.4 |
| CIH | 16.2±2.1* | 18.0±1.9 | 16.3±2.4* | 15.9±2.0* | 17.1±2.1* | 17.4±2.2 | 20.2±1.8* | 20.6±2.1* | 22.2±2.3* | 22.3±2.5* | 186.3±9.8* |
Values are means ± SE; n = 6.
P < 0.05 compared with RA.
DISCUSSION
We have shown for the first time that injections of both AMPA and NMDA into the NA induce fast, large HR and MAP responses. This supports the hypothesis that both AMPA and NMDA receptors in NA motoneurons mediate cardiovascular responses. In addition, we demonstrated that CIH significantly reduces the HR responses to AMPA and NMDA injections into the NA. In conjunction with such attenuated responses, we found that CIH induced a significant reduction in AMPA (GluR1, GluR2/3) and NMDA (NMDAR1) positively labeled motoneurons. Therefore, we postulate that this attenuation of glutamate receptor-immunoreactive neurons in NA may, in turn, contribute to the reduced baroreflex control of HR (25, 33).
Heart rate is mediated by AMPA and NMDA receptors in NA.
Several studies have previously indicated that excitatory glutamatergic transmission in the NA cardiac motoneurons underlies HR control as follows: 1) Direct microinjection of l-glutamate into the NA region evoked negative chronotropic responses (9, 37). 2) Extensive lesions of the NA with the excitatory neurotoxin domoic acid almost completely abolished the baroreflex control of HR and largely reduced the HR responses to l-glutamate injections into the NA (9). 3) Bradycardia in response to phenylephrine-induced hypertension was reduced by microinjection of kynurenic acid, a glutamate receptor antagonist, into the NA (26). 4) Electrical stimulation of the NTS evoked the synaptic events in NA cardiac motoneurons, which were mediated through both NMDA and non-NMDA receptor-dependent mechanisms (40). 5) NA cardiac motoneurons projecting to cardiac ganglia, which were prelabeled by tracer injection into the pericardiac sac, expressed AMPA subunits GluR1, GluR2, and GluR3 and NMDA subunit NR1 (11). In the present study, we microinjected AMPA and NMDA into the NA and evoked large, comparable bradycardic responses. This finding directly demonstrates that both AMPA and NMDA receptors appear to be involved in HR control. Since we injected AMPA and NMDA within the same site of the NA, we postulate that AMPA and NMDA receptors may colocalize in the same cardiac motoneurons in the NA. The differential roles of AMPA and NMDA in cardiac control deserve further study.
Consideration of experimental approaches.
Whether AMPA and NMDA injections into the NA region also activated other neurons in the baroreflex loop that regulate the heart was a concern because the NTS neurons project to the caudal ventrolateral medulla (CVLM) (12). The interneurons in the CVLM are located immediately ventral to the NA and could possibly be activated by AMPA and NMDA injections. These neurons contain AMPA and NMDA receptors, receive NTS glutamatergic inputs, and project to and inhibit sympathetic cardiac neurons in the rostral ventrolateral medulla (RVLM) through a GABAergic inhibitory mechanism. Thus injections of AMPA and NMDA could decrease MAP and HR through this sympathetic pathway as well (39). Previously, Marchenko and Sapru (35) demonstrated that microinjection of l-glutamate into CVLM of rats induces large MAP responses but small HR responses, whereas microinjection of l-glutamate into NA induces large HR responses but small MAP responses. This is consistent with our previous finding that the HR response to l-glutamate injection after application of the β1-blocker atenolol was only mildly attenuated (>80% original maximal HR response) (9) and subsequent injection of the muscarinic receptor blocker methylatropine almost completely abolished HR responses. Therefore, the reduced HR responses to AMPA and NMDA injection into the NA may be primarily due to the NA, with a lesser extent of contribution of the interneurons in the CVLM, whereas the MAP responses may be largely due to the CVLM in normal control rats. However, whether such a relationship, i.e., the parasympathetic system, is primarily responsible for changes in HR and the sympathetic system is primarily responsible for changes in AP is still preserved after CIH is an interesting issue. Nevertheless, since CIH significantly reduced the maximal HR responses to AMPA and NMDA injections as well as AMPA- and NMDA-immunoreactive neurons in NA but did not affect the MAP responses to either drug in our present study, we suggest that CIH-induced HR bradycardia in response to AMPA and NMDA injections is more likely due to the parasympathetic limb. The result that CIH did not significantly alter the CVLM mediation of MAP does not necessarily mean that CIH does not affect the CVLM interneurons. Such a negative result for the sympathetic pathway is probably due to a drug injection centered in the NA rather than the CVLM as shown in Fig. 1. Further studies are needed to investigate whether the interneurons in the sympathetic pathway after CIH are altered by focused injection of AMPA and NMDA into the CVLM as shown in Ref. 35.
Since the NA does not have clear-cut anatomic boundaries, the counts of AMPA and NMDA motoneurons could be relatively inaccurate. To obtain a reliable count, we first injected TMR-D into the nodose ganglia to retrogradely label NA motoneurons. Then we made a map for the location and boundaries of the NA region at each section level by superimposing the TMR-D-labeled NA motoneurons in six animals for each side. In addition, we only counted neurons that fulfilled the criterion of >20-μm maximum diameter, such that this approach allowed us to count NA motoneurons within the defined boundaries and also permitted us to avoid counting of glial cells and local neurons, which are much smaller than the NA motoneurons. In our experiment with retrograde labeling of NA motoneurons with tracer injection of TMR into the vagus, NA motoneurons were larger than 20 μm. This is consistent with a recent report in which NA motoneurons were found to be larger than 20 μm (31). In the brain, interneurons are usually smaller than 15 μm (48). Therefore, we believe that the NA neurons that we counted are most likely motoneurons. The criterion used for quantifying motoneurons in this study is similar to that used by Hottinger et al. (28) and Xu et al. (53).
It should be pointed out that NA motoneurons project their axons to many other organs in the upper alimentary tract in addition to the heart (2). Furthermore, the NA region is overlapped with other motoneuron groups, such as the ventral respiratory group (VRG) and the rostroventrolateral nucleus (RVL). Thus not all motoneurons counted in the present study were specifically involved in cardiovascular control (2, 14, 15, 27). Injecting cardiac ganglia with a tracer and then counting retrogradely labeled NA neurons might provide additional verification of our findings (11). However, injection of tracers into the pericardial sac may lead to a large variation of the number of labeled NA neurons from animal to animal and also label noncardiac neurons in the NA (24). Unlike those in cats and dogs, numerous cardiac ganglia are not in the “fat pads” in rats and mice and they are distributed in spatially separated ganglionic plexuses on the dorsal surface of atria (1, 6, 7). In our preliminary experiments (unpublished), we found that injections of retrograde tracers into pericardial sac led to a random labeling of cardiac ganglia in spatially separated ganglionated plexuses, which led to a large variation of the number of labeled NA motoneurons. Even though appropriate volumes of tracer injected in the pericardial sac have been shown to selectively label cardiac vagal neurons, unfortunately this approach cannot ensure that all cardiac vagal neurons are labeled. Additional volumes that would be necessary to ensure complete labeling of all cardiac vagal neurons would likely increase the risk of leakage and nonspecific labeling (24). Thus we did not use this retrograde labeling strategy in the present study because variations in the number of labeled NA motoneurons would mask the genuine difference between RA and CIH rats.
Alternatively, we counted the glutamatergic receptor-immunoreactive neurons in the external formation (Table 2). According to Bieger and Hopkins (2), the external formation is cardiac related. Our data indicate that CIH reduces the number of AMPA and NMDA receptor-immunoreactive neurons in the external formation (Table 2). In addition, since the HR responses to AMPA and NMDA injection are reduced, the loss of these motoneurons in NA should include NA cardiac motoneurons.
Reduced number of AMPA- and NMDA-immunoreactive neurons in NA.
Another concern was whether reductions in the number of AMPA- and NMDA-immunoreactive neurons might be due to cellular losses or reduced expression of AMPA and NMDA receptors on each NA neuron. As reported, CIH leads to cortical and hippocampal neuronal cell losses that are associated with significant neurobehavioral deficits in rats and mice (18–21, 23, 32, 41, 42, 44, 45). CIH-induced apoptosis in the brain is associated with oxidative stress (47, 52). Recently, we demonstrated (54) that CIH indeed induces a significant cellular loss in the NA region as well as in VRG and RVL regions, as estimated by Nissl staining. This suggests that CIH may induce neural degeneration in cardiorespiratory motoneurons and the reduced number of AMPA- and NMDA-immunoreactive neurons could be due to cellular losses in the NA as well as other cell groups. However, we have not yet tested other functional changes due to the loss of these cardiopulmonary motoneurons. The signal transduction pathway for CIH-induced apoptosis in NA is not clear and should be elucidated in the future.
According to the literature (16, 37, 38), the caudal, intermediate, and rostral portions of the NA project to different cardiac ganglia and control different aspects of cardiac functions (negative chronotropic, negative dromotropic, and negative inotropic responses). Since the number of AMPA- and NMDA-immunoreactive neurons is reduced throughout the entire NA (Table 1) and HR responses to AMPA and NMDA injections to the NA caudal to the obex are reduced after CIH, we postulate that CIH also decreases NA regulation of atrioventricular conduction and myocardial contractility. Injections of AMPA and NMDA into different locations of the NA are needed to test this speculation.
Perspectives and Significance
Baroreflex impairment has been used as a risk factor for cardiac failure (13, 29). Previously, we demonstrated that CIH significantly attenuates baroreflex control of HR. Baroreflex circuitry includes baroreceptor afferent, NTS interneurons, vagal motoneurons, CVLM interneurons, RVLM presympathetic motoneurons, sympathetic and parasympathetic efferents, sympathetic ganglia, and parasympathetic cardiac ganglia. Changes of any of these components may lead to baroreflex impairment. Recently, Gu et al. (25) demonstrated that impairment of baroreflex control of HR cannot be explained by baroreceptor afferents and vagal efferents because baroreceptor afferent function and vagal efferent control of HR are enhanced after CIH. Instead, a deficit in central autonomic components, possibly via cellular losses in the NA, could contribute to the reduced baroreflex control of HR (25, 54). In the present study, we further demonstrated that CIH reduced the number of AMPA- and NMDA-immunoreactive neurons in NA. Since AMPA and NMDA are primary receptors that are involved in excitatory transmission within the NA, we suggest that the loss of AMPA- and NMDA-immunoreactive neurons contributed to the reduced HR responses to the glutamatergic receptor agonist injections and attenuated central mediation of reflex HR bradycardia after CIH. Since NA also mediates respiratory modulation of HR (50), we speculate that a loss of glutaminergic neurons may result in a reduction of respiratory modulation of HR as well. This study will provide a focus for the future investigation of cellular and molecular mechanisms underlying CIH-induced impairment of baroreflex control of HR, whereas studies on CIH-induced changes of other neural components mediating cardiovascular and respiratory functions are also needed.
GRANTS
This work was supported by National Institutes of Health Grants AG-021020 and HL-796369 and the Institutional Fund of the University of Central Florida to Z. J. Cheng.
Acknowledgments
We thank Dr. Mark Chapleau for valuable discussion and comments on the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ai J, Epstein PN, Gozal D, Yang B, Wurster R, Cheng ZJ. Morphology and topography of nucleus ambiguus projections to cardiac ganglia in rats and mice. Neuroscience 149: 845–860, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Bieger D, Hopkins DA. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. J Comp Neurol 262: 546–562, 1987. [DOI] [PubMed] [Google Scholar]
- 3.Bonsignore G, Mancia G. Autonomic cardiac regulation in obstructive sleep apnea syndrome: evidence from spontaneous baroreflex analysis during sleep. J Hypertens 12: 1621–1626, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Bonsignore G, Di Rienzo M. Baroreflex control of heart rate during sleep in severe obstructive sleep apnoea: effects of acute CPAP. Eur Respir J 27: 128–135, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Bradley TD, Tkacova R, Hall MJ, Ando S, Floras JS. Augmented sympathetic neural response to simulated obstructive apnea in human heart failure. Clin Sci (Lond) 104: 231–238, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Cheng Z, Powley TL, Schwaber JS, Doyle FJ. Projections of the dorsal motor nucleus of the vagus to cardiac ganglia of rat atria: an anterograde tracing study. J Comp Neurol 410: 320–341, 1999. [PubMed] [Google Scholar]
- 7.Cheng Z, Powley TL. Nucleus ambiguus projections to cardiac ganglia of rat atria: an anterograde tracing study. J Comp Neurol 424: 588–606, 2000. [PubMed] [Google Scholar]
- 8.Cheng Z, Guo SZ, Lipton AJ, Gozal D. Domoic acid lesions in nucleus of the solitary tract: time-dependent recovery of hypoxic ventilatory response and peripheral afferent axonal plasticity. J Neurosci 22: 3215–3226, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng Z, Zhang H, Yu J, Wurster R, Gozal D. Attenuation of baroreflex sensitivity following domoic acid lesion of the nucleus ambiguus of rats. J Appl Physiol 96: 1137–1145, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Cheng Z, Zhang H, Guo SZ, Wurster R, Gozal D. Differential control over vagal efferent postganglionic neurons in rat intrinsic cardiac ganglia by neurons in the nucleus ambiguus and the dorsal motor nucleus of the vagus: anatomical evidence. Am J Physiol Regul Integr Comp Physiol 286: R625–R633, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Corbett EK, Saha S, Deuchars J, McWilliam PN, Batten TF. Ionotropic glutamate receptor subunit immunoreactivity of vagal preganglionic neurones projecting to the rat heart. Auton Neurosci 105: 105–117, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Dampney RA, Polson JW, Potts PD, Hirooka Y, Horiuchi J. Functional organization of brain pathways subserving the baroreceptor reflex: studies in conscious animals using immediate early gene expression. Cell Mol Neurobiol 23: 597–616, 2003. [DOI] [PubMed] [Google Scholar]
- 13.De Jong MJ, Randall DC. Heart rate variability analysis in the assessment of autonomic function in heart failure. J Cardiovasc Nurs 20: 186–195, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Ellenberger HH, Feldman JL. Subnuclear organization of the lateral tegmental field of the rat. I. Nucleus ambiguus and ventral respiratory group. J Comp Neurol 294: 202–211, 1990. [DOI] [PubMed] [Google Scholar]
- 15.Ellenberger HH, Feldman JL, Zhan WZ. Subnuclear organization of the lateral tegmental field of the rat. II. Catecholamine neurons and ventral respiratory group. J Comp Neurol 294: 212–222, 1990. [DOI] [PubMed] [Google Scholar]
- 16.Gatti PJ, Johnson TA, Massari VJ. Can neurons in the nucleus ambiguus selectively regulate cardiac rate and atrio-ventricular conduction? J Auton Nerv Syst 57: 123–127, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Goldbart A, Cheng ZJ, Brittian KR, Gozal D. Intermittent hypoxia induces time-dependent changes in the protein kinase B signaling pathway in the hippocampal CA1 region of the rat. Neurobiol Dis 14: 440–446, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Goldbart A, Row BW, Kheirandish L, Schurr A, Gozal E, Guo SZ, Payne RS, Cheng Z, Brittian KR, Gozal D. Intermittent hypoxic exposure during light phase induces changes in cAMP response element binding protein activity in the rat CA1 hippocampal region: water maze performance correlates. Neuroscience 122: 585–590, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci 21: 2442–2450, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gozal D, Row BW, Kheirandish L, Liu R, Guo SZ, Qiang F, Brittian KR. Increased susceptibility to intermittent hypoxia in aging rats: changes in proteasomal activity, neuronal apoptosis and spatial function. J Neurochem 86: 1545–1552, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Gozal D, Xue YD, Simakajornboon N. Hypoxia induces c-Fos protein expression in NMDA but not AMPA glutamate receptor labeled neurons within the nucleus tractus solitarii of the conscious rat. Neurosci Lett 262: 93–96, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Gozal E, Sachleben LR Jr, Rane MJ, Vega C, Gozal D. Mild sustained and intermittent hypoxia induce apoptosis in PC-12 cells via different mechanisms. Am J Physiol Cell Physiol 288: C535–C542, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Grkovic I, Fernandez K, McAllen RM, Anderson CR. Misidentification of cardiac vagal pre-ganglionic neurons after injections of retrograde tracer into the pericardial space in the rat. Cell Tissue Res 321:335–340, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Gu H, Lin M, Liu J, Gozal D, Scrogin KE, Wurster RD, Chapleau MW, Ma X, Cheng ZJ. Selective impairment of central mediation of baroreflex in anesthetized young adult Fischer 344 rats after chronic intermittent hypoxia. Am J Physiol Heart Circ Physiol 293: H2809–H2818, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Guyenet PG, Flitz TM, Donaldson SR. Role of excitatory amino acids in the vagal and sympathetic baroreflexes. Brain Res 407: 272–284, 1987. [DOI] [PubMed] [Google Scholar]
- 27.Hopkins DA, Bieger D, deVente J, Steinbusch WM. Vagal efferent projections: viscerotopy, neurochemistry and effects of vagotomy. Prog Brain Res 107: 79–96, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Hottinger AF, Azzouz M, Déglon N, Aebischer P, Zurn AD. Complete and long-term rescue of lesioned adult motoneurons by lentiviral-mediated expression of glial cell line-derived neurotrophic factor in the facial nucleus. J Neurosci 20: 5587–5593, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansson M, Gao SA, Friberg P, Annerstedt M, Carlström J, Ivarsson T, Jensen G, Ljungman S, Mathillas O, Nielsen FD, Strömbom U. Baroreflex effectiveness index and baroreflex sensitivity predict all-cause mortality and sudden death in hypertensive patients with chronic renal failure. J Hypertens 25: 163–168, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Lai CJ, Yang CC, Hsu YY, Lin YN, Kuo TB. Enhanced sympathetic outflow and decreased baroreflex sensitivity are associated with intermittent hypoxia-induced systemic hypertension in conscious rats. J Appl Physiol 100: 1974–1882, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Lewinter RD, Scherrer G, Basbaum AI. Dense transient receptor potential cation channel, vanilloid family, type 2 (TRPV2) immunoreactivity defines a subset of motoneurons in the dorsal lateral nucleus of the spinal cord, the nucleus ambiguus and the trigeminal motor nucleus in rat. Neuroscience 151: 164–173, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li RC, Row BW, Kheirandish L, Brittian KR, Gozal E, Guo SZ, Sachleben LR Jr, Gozal D. Nitric oxide synthase and intermittent hypoxia-induced spatial learning deficits in the rat. Neurobiol Dis 17: 44–53, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Lin M, Liu R, Gozal D, Wead WB, Chapleau MW, Wurster R, Cheng ZJ. Chronic intermittent hypoxia impairs baroreflex control of heart rate but enhances heart rate responses to vagal efferent stimulation in anesthetized mice. Am J Physiol Heart Circ Physiol 293: H997–H1006, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Marchenko V, Sapru HN. Cardiovascular responses to chemical stimulation of the lateral tegmental field and adjacent medullary reticular formation in the rat. Brain Res 977: 247–260, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnea. Exp Physiol 92: 27–37, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Massari VJ, Johnson TA, Gillis RA, Gatti PJ. What are the roles of substance P and neurokinin-1 receptors in the control of negative chronotropic or negative dromotropic vagal motoneurons? A physiological and ultrastructural analysis. Brain Res 71: 197–207, 1996. [DOI] [PubMed] [Google Scholar]
- 38.Massari VJ, Dickerson LW, Gray AL, Lauenstein JM, Blinder KJ, Newsome JT, Rodak DJ, Fleming TJ, Gatti PJ, Gillis RA. Neural control of left ventricular contractility in the dog heart: synaptic interactions of negative inotropic vagal preganglionic neurons in the nucleus ambiguus with tyrosine hydroxylase immunoreactive terminals. Brain Res 802: 205–220, 1998. [DOI] [PubMed] [Google Scholar]
- 39.McKitrick DJ, Calaresu FR. Reciprocal connection between nucleus ambiguus and caudal ventrolateral medulla. Brain Res 770: 213–220, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Neff RA, Mihalevich M, Mendelowitz D. Stimulation of NTS activates NMDA and non-NMDA receptors in rat cardiac vagal neurons in the nucleus ambiguus. Brain Res 792: 277–282, 1998. [DOI] [PubMed] [Google Scholar]
- 41.Pae EK, Chien P, Harper RM. Intermittent hypoxia damages cerebellar cortex and deep nuclei. Neurosci Lett 375: 123–128, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Payne RS, Goldbart A, Gozal D, Schurr A. Effect of intermittent hypoxia on long-term potentiation in rat hippocampal slices. Brain Res 1029: 195–199, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Prabhakar NR, Dick TE, Nanduri J, Kumar GK. Systemic, cellular and molecular analysis of chemoreflex-mediated sympathoexcitation by chronic intermittent hypoxia. Exp Physiol 92: 39–44, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Row BW, Kheirandish L, Cheng Y, Rowell PP, Gozal D. Impaired spatial working memory and altered choline acetyltransferase (CHAT) immunoreactivity and nicotinic receptor binding in rats exposed to intermittent hypoxia during sleep. Behav Brain Res 177: 308–314, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Row BW, Liu R, Xu W, Kheirandish L, Gozal D. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Respir Crit Care Med 167: 1548–1553, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Sapru HN Glutamate circuits in selected medullo-spinal areas regulating cardiovascular function. Clin Exp Pharmacol Physiol 29: 491–496, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Shan X, Chi L, Ke Y, Luo C, Qian S, Gozal D, Liu R. Manganese superoxide dismutase protects mouse cortical neurons from chronic intermittent hypoxia-mediated oxidative damage. Neurobiol Dis 28: 206–215, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shetty AK, Turner DA. Fetal hippocampal grafts containing CA3 cells restore host hippocampal glutamate decarboxylase-positive interneuron numbers in a rat model of temporal lobe epilepsy. J Neurosci 20: 8788–8801, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J, Irnaten M, Neff RA, Venkatesan P, Evans C, Loewy AD, Mettenleiter TC, Mendelowitz D. Synaptic and neurotransmitter activation of cardiac vagal neurons in the nucleus ambiguus. Ann NY Acad Sci 940: 237–246, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Jones JF, Jeggo RD, de Burgh Daly M, Jordan D, Ramage AG. Effect of pulmonary C-fibre afferent stimulation on cardiac vagal neurones in the nucleus ambiguus in anaesthetized cats. J Physiol 526: 157–165, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitney GM, Ohtake PJ, Simakajornboon N, Xue YD, Gozal D. AMPA glutamate receptors and respiratory control in the developing rat: anatomic and pharmacological aspects. Am J Physiol Regul Integr Comp Physiol 278: R520–R528, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Xu W, Chi L, Row BW, Xu R, Ke Y, Xu B, Luo C, Kheirandish L, Gozal D, Liu R. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience 126: 313–323, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Xu Z, Chen S, Li X, Luo G, Li L, Le W. Neuroprotective effects of (−)-epigallocatechin-3-gallate in a transgenic mouse model of amyotrophic lateral sclerosis. Neurochem Res 31: 1263–1269, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Yan B, Soukhova-O'Hare GK, Li LH, Lin Y, Gozal D, Wead WB, Wurster RD, Cheng ZJ. Attenuation of heart rate control and neural degeneration in nucleus ambiguus (NA) following chronic intermittent hypoxia (CIH) in young adult Fischer 344 rats. Neuroscience 153: 709–720, 2008. [DOI] [PubMed] [Google Scholar]