Abstract
Inappropriate vasopressin (AVP) release causes dilutional hyponatremia in many pathophysiological states such as cirrhosis. The central molecular mechanisms that mediate inappropriate AVP release are unknown. We tested the hypothesis that changes in the expression or trafficking of TRPV4 in the central nervous system may contribute to inappropriate AVP release in the bile duct ligation (BDL) model of cirrhosis in the rat. Four weeks after surgery, BDL rats demonstrated significantly increased plasma vasopressin and plasma renin activity (PRA), hypervolemia, and decreased plasma osmolality. These effects were blocked by providing BDL rats with 2% saline to drink for 15 days. TRPV4 protein expression was significantly increased in brain punches from BDL rats containing the supraoptic nucleus (SON) of the hypothalamus (100% ± 11 to 157% ± 4.8), and this effect was blocked in BDL rats given saline. Immunohistochemistry demonstrated a significant increase in TRPV4-positive cells and the percentage of AVP neurons that also were TRPV4-positive in the SON of BDL rats. In the hypothalamus of BDL rats, TRPV4 lipid raft association increased compared with sham (from 100% ± 2.1 to 326.1% ± 16). This effect was significantly attenuated in BDL rats given 2% saline to drink (174% ± 11). In the brain stem, TRPV4 lipid raft association was reduced by BDL and inversely related to plasma AVP and PRA. We speculate that changes in TRPV4 expression and compartmentalization within lipid rafts could contribute to a feed-forward mechanism related to AVP release in cirrhosis.
Keywords: renin-angiotensin, lipid rafts, supraoptic nucleus, hypothalamus
inappropriate release of vasopressin (AVP) has been shown to contribute to the pathophysiology of several disease states such as hepatic cirrhosis (24, 38, 75) congestive heart failure (13, 24, 39, 41, 63, 67, 69), nephrogenic syndrome (21, 24, 27), and preeclampsia (92). During cirrhosis, inappropriate AVP release results in water retention and dilutional hyponatremia, which has a negative impact on morbidity and mortality (1, 8–10, 24, 38, 59, 63, 69, 75, 78, 89). Treatment with a specific V2-AVP receptor antagonist has been shown to produce solute-free water excretion in cirrhotic patients and animal models of cirrhosis (19, 71, 76, 86). While it is clear that inappropriate AVP release is an important contributing factor to the pathophysiology of cirrhosis, the central pathways and molecular mechanisms responsible are not known.
Traditionally, increased AVP secretion, as well as sympatho-excitation, associated with cirrhosis has been attributed to systemic hypotension and hyperdynamic circulatory syndrome (30–32, 40, 79). Hyperdynamic circulatory syndrome also is reported to lead to activation of the renin-angiotensin system during hepatic cirrhosis, which would be consistent with the traditional view that nonosmotic factors drive vasopressin release associated with this disease state (65, 66, 96). Increased circulating angiotensin could contribute to increased AVP release by acting on central nervous system (CNS) circumventricular organs (57, 58, 64, 83, 91). Recent clinical studies have shown that reducing plasma renin activity (PRA) by giving saline to preascitic cirrhotic patients (93) or blocking angiotensin receptors with losartan (26, 85, 96) prevented or reversed hyponatremia, suggesting a role for the renin-angiotensin system in inappropriate AVP release.
Chronic bile duct ligation (BDL) is a commonly used model of hepatic cirrhosis that is associated with elevated circulating vasopressin, cytokines, and activation of the renin-angiotensin system (11, 25, 55, 61). In addition, BDL rats have increased sodium intake and a trend for increased water intake (45). Previous studies using Fos immunohistochemistry have shown that BDL and portal vein stenosis are associated with chronic activation of medullary and hypothalamic brain regions involved in cardiovascular regulation and vasopressin release (79). Our working hypothesis is that cellular adaptations could be required for sustained activation of these regions. These adaptations could include the conditional expression and partitioning of ion channels and their modulators within microdomains of the plasma membrane of vasopressin neurosecretory cells. TRPV4 is a member of the transient receptor potential (TRP) family of cation channels (68, 72, 88, 90) and has been implicated in osmoregulation (47–50). Of particular interest, it has been reported that TRPV4 knockout mice show impaired AVP release following hypertonic stress (51). TRPV4 is also speculated to sense or respond to systemic osmolality, because it is expressed in several blood-brain barrier-free nuclei in the central nervous system responsible for sensing systemic osmolality (51, 81). At least some TRP channels localize to lipid rafts (6, 54). Gating of TRPV4 appears to require the action of cytoplasmic tyrosine kinases of the Src family (20, 95), which also localize to and are active in lipid rafts (87). Thus, the trafficking of these proteins could have important implications in their function, and long-term changes in the excitability of neural pathways that control sympathetic outflow and AVP release. Lipid rafts and their resident proteins may also play a role in the generation of increased sympathetic tone and inappropriate AVP release associated with cirrhosis.
The purpose of the present study was to evaluate the pattern of expression of TRPV4 in magnocellular vasopressinergic neurons of the hypothalamus and determine whether its expression could be altered in BDL rats with liver cirrhosis. We also speculate whether the association of TRPV4-lipid rafts was globally altered in brain regions that contribute to autonomic and body fluid homeostasis following the BDL model of hepatic cirrhosis associated with syndrome of inappropriate AVP secretion and BDL animals receiving saline to drink to normalize PRA.
MATERIAL AND METHODS
Animals.
Experiments were conducted on adult male Sprague-Dawley rats that weighed 250–350 g (Charles River, Durham, NC). Rats were individually housed and maintained in a temperature-controlled environment on a 12:12-h light-dark cycle. Experimental protocols were approved by the Institutional Animal Care and Use Committee in accordance with the guidelines of the Public Health Service, the American Physiological Society, and the Society for Neuroscience. All rats had ad libitum access to food throughout the experiments unless specified. For some experiments, average daily fluid intake was determined by weighing each rat's fluid bottle every morning.
BDL model for liver cirrhosis.
Each rat was anesthetized with pentobarbital sodium (50 mg/kg ip; SP1; VEDCO, St. Joseph, MO). The abdomen was shaved and cleaned. A midline abdominal incision was performed, and the common bile duct was cut between two ligatures. Liver/body weight ratio was determined for verification of cirrhosis development. Visual inspection of ascetic fluid in the peritoneal cavity was performed daily after surgery. Any rat showing morbidity or ascites of greater than 10% of the body weight was euthanized (Inactin 150 mg/kg ip). Shams rats received the same surgical procedure except the bile duct was neither ligated or cut. Rats were used for experiments 4 wk after surgery. A group of BDL animals was given 2% (wt/vol) NaCl to drink for 15 days beginning 2 wk after surgery.
Micropunch dissection.
Each rat was anesthetized with Inactin (Sigma, St. Louis, MO; 100 mg/kg ip) and decapitated. The brain was removed from the skull and placed in a commercially available brain matrix (Stoelting, Wood Dale, IL). The matrix was used to cut the brain into 1-mm coronal slabs with razor blades. Punches from supraoptic nucleus (SON), paraventricular nucleus (PVN), subfornical organ (SFO), organum vasculosum of the lamina terminalis (OVLT), rostral ventrolateral medulla (RVLM), and nucleus of the solitary tract (NTS) were then collected from the slabs using 1-ml syringes equipped with blunt 23-gauge needles. The punch samples were placed in microcentrifuge tubes and frozen with liquid nitrogen. Punches were sonicated in 50 μl of modified RIPA buffer supplemented with protease and phosphatase inhibitors followed by 30 min incubation on ice. The total homogenates were then centrifuged at 14,000 rpm, 30 min at 4°C, and total protein concentration was determined by the Bradford method (5). Five or ten micrograms of total lysate was resolved by SDS-PAGE, and visualized using Western blot analysis. Whole blood was collected from each rat for analysis of osmolality, plasma AVP, and PRA.
Detergent-free lipid raft preparation and sucrose density analysis.
Each rat was anesthetized with Inactin (Sigma; 100 mg/kg ip) prior to decapitation, and blood was collected from each rat for later analysis. The brain was removed from the skull, and total hypothalamus and brain stem were collected and immediately subjected to fractionation followed by sucrose density analysis modified from Refs. 34 and 35). Each 0.1 g of total hypothalamus and brain stem was homogenized in 1 ml of hypotonic homogenization buffer using a Potter-Elvehjem homogenizer, centrifuged at 1,000 g for 5 min, which removes nuclei and unlysed cells from the homogenate. The resulting supernatant was centrifuged at 15,000 g for 30 min, separating cytosolic proteins from mitochondria, Golgi fragment, and plasma membrane. The pellet was then resuspended in the homogenization buffer containing 500 mM Na2CO3 (80) and subjected to sucrose flotation-gradient fractionation under a 5%/35%/45% discontinuous gradient spun at 175,000 g for 18 h in a preparative ultracentrifuge model XL100K (Beckman Instruments, Palo Alto, CA) using a Swing rotor (SW 55Ti; Beckman Instruments). From this gradient, 500 μl fractions were taken from the top of each layer. Each resulting fraction from sucrose gradient was diluted 2× in hypotonic homogenization buffer and spun 15,000 g for 30 min as described (34, 35). The pellet from sucrose density analysis was resuspended in 50 μl of 1 × Laemmli buffer [62.5 mM Tris·HCl, pH 6.8, 2% (wt/vol) SDS, 160 mM DTT, 0.001% bromophenol blue, 6 M urea]. Between 10–20 μl of each sample (34, 35) were resolved by SDS-PAGE, and visualized using Western blot analysis.
Western blot analysis.
Samples from the lipid raft fractions or micropunch total lysates were loaded onto 10% acrylamide SDS gel, eletrophoresed in Tris-glycine buffer under denaturing conditions and transferred to nitrocellulose membrane (Bio-Rad, Hercules, CA) in Tris-glycine buffer with 10–20% methanol. Membranes were blocked for 1 h at room temperature with 5% (wt/vol) nonfat milk in Tris-buffered saline 0.05% (vol/vol) Tween 20 (TBS-Tween; 50 mM Tris base, 200 mM NaCl, 0.05% Tween 20). Membranes were then incubated overnight at 4°C with primary antibody rabbit anti-TRPV4 (Alomone Labs, Jerusalem, Israel). Blots were rinsed 3 times 10 min each with TBS 0.05% Tween 20 and then incubated at room temperature for 1 h in a horseradish peroxidase conjugated secondary antibody against the primary antibody host species (1:5,000; Sigma). The proteins were detected by enhanced chemiluminescence (ECL reagents; Amersham, Piscataway, NJ) exposed to radiographic film (Hyperfilm ECL; Amersham). Densitometry of the immunoreactive bands of interest was performed by acquiring digital images of radiographic film (Hyperfilm ECL; Amersham) using the software Scion Image for Windows. For the lipid raft marker or GAPDH analysis (micropunches), all blots were blocked again at room temperature and incubated with mouse anti-flotillin (BD Biosciences, Franklin Lakes, NJ) or mouse anti-GAPDH (BD Biosciences).
Immunohistochemistry.
Four weeks after BDL or sham surgery, each rat was anesthetized with Inactin (Sigma; 100 mg/kg ip) and perfused transcardially with 0.1 M PBS (30–50 ml) followed by 4% paraformaldehyde in 0.1 M PBS (300–500 ml) as previously described (28, 36). Each brain was removed from the skull and placed in a fixative for 2–3 h before being placed in 30% sucrose in PBS for 2–3 days at 4°C. The brains were cut into three sets of serial 40-μm coronal sections using a crytostat. Sections containing the hypothalamus were processed for vasopressin (1:10,000; guinea pig anti-vasopressin from Peninsula Labs, San Carlos, CA) and TRPV4 (1:1,000 rabbit anti TRPV4 from Alomone Labs) immunofluorescence. Sections were incubated with both primary antibodies for 48 h at 4°C. After rinsing, the sections were incubated with Cy3-labeled anti-guinea pig and Cy2-labeled anti-rabbit secondary antibodies for 5 h. Sections were mounted on coated slides and cover slipped with Permount (Fisher Scientific, Pittsburgh, PA). Digital images of Cy2 and Cy3 immunofluorescence from the SON and PVN were captured using an Olympus BX41 microscope equipped for epifluorescence and an Olympus DP70 digital camera. The numbers of TRVP4-positive and vasopressin-positive cells were counted in the SON and PVN from at least four sections from each rat as previously described (28, 36). Digital images of TRPV4 and AVP immunofluorescence from each section were merged for counting the numbers of cells from each section that were stained for both TRPV4 and AVP. The counts from each section were averaged for each rat for statistical analysis.
Plasma measurements.
Trunk blood was collected into two centrifuge tubes containing EDTA (Sigma) and centrifuged for 5 min (at 10,000 g) at 4°C. The plasma was removed from each sample, placed into screw-top vials, and stored at −80°C until it was sent to the University of Iowa RIA core to be assayed for PRA and plasma AVP (28, 36). A separate sample of whole blood from each rat was collected into a 1.5-ml microcentrifuge tube that did not contain EDTA. Two heparin-containing capillary tubes were filled with blood from this sample for measuring hematocrit. The rest of the sample was centrifuged and a 200-μl sample of serum was removed for measuring osmolality using a vapor pressure osmometer (Wescor, Logan, UT).
Statistics.
Data are presented as group means ± one SE. Data were analyzed by one-way ANOVA with Student Newman-Keuls t-test for post hoc analysis of significant main effects (SigmaStat, v. 2.03; Systat Software, Point Richmond, CA). Significance was set at P < 0.05.
RESULTS
Plasma measurements.
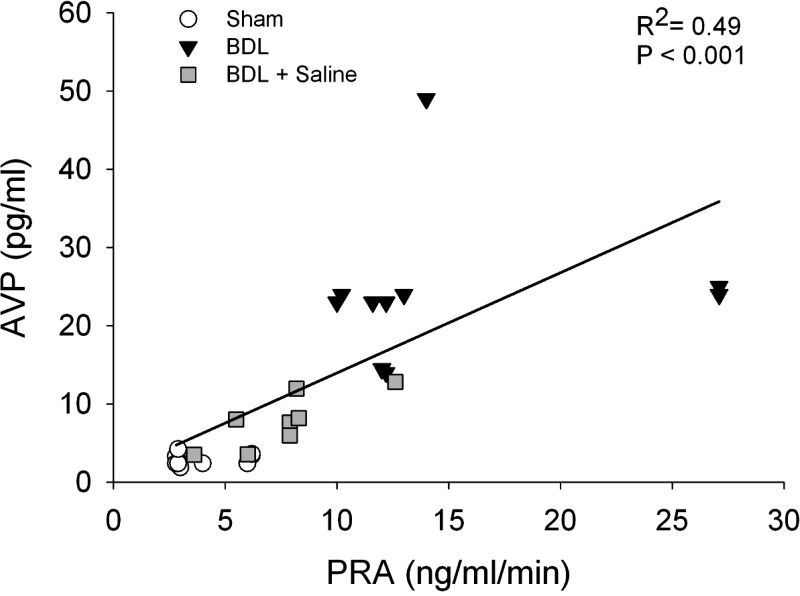
Plasma samples from sham, BDL rats, and BDL rats given saline to drink were taken to evaluate measurements in plasma osmolality, hematocrit, AVP, and PRA (Table 1). In BDL rats, plasma AVP, and PRA were significantly increased compared with sham, while their plasma osmolality and hematocrit were significantly decreased (Table 1). BDL rats given saline to drink for 2 wk, after 2 wk bile duct ligation had average plasma osmolality and hematocrits that were not significantly different from sham controls. Their plasma AVP and PRA were significantly decreased compared with BDL animals but not different from sham rats. Both groups of BDL rats had significantly increased liver weight to body weight ratios (sham 0.03 ± 0.001; BDL 0.07 ± 0.001; BDL + saline 0.06 ± 0.001; both P < 0.01). In the present study, a significant correlation was found between plasma AVP levels and PRA among groups (Fig. 1). High PRA in BDL rats were associated with high plasma AVP in the same group. However, when the PRA was corrected in BDL rats given saline to drink, the plasma AVP levels were distributed to values between BDL and sham (Fig. 1).
Table 1.
Plasma AVP, osmolality, hematocrit, and PRA in Sham, BDL rats, and BDL rats given saline (BDL+Sal)
| Sham (10) | BDL (12) | BDL+Sal (8) | |
|---|---|---|---|
| Osmolality, mOsm/kg | 293±1.39 | 281±2.11* | 291±2.381a |
| Hematocrit, % | 45±0.24 | 40±0.69* | 47±1.13a |
| AVP, pg/ml | 2.95±0.23 | 24.20±2.49* | 7.71±1.21a |
| PRA, ng·ml−1·h−1 | 3.97±0.48 | 14.26±1.76* | 7.5±0.93a |
AVP, arginine vasopressin; PRA, plasma renin activity.
Significantly different from Sham.
Significantly different from BDL.
Fig. 1.
Linear regression analysis of plasma renin activity (PRA) and vasopressin (AVP) from sham-ligated rats (Sham), bile duct ligation rats (BDL), and BDL rats given 2% saline to drink (BDL+saline).
Fluid intake.
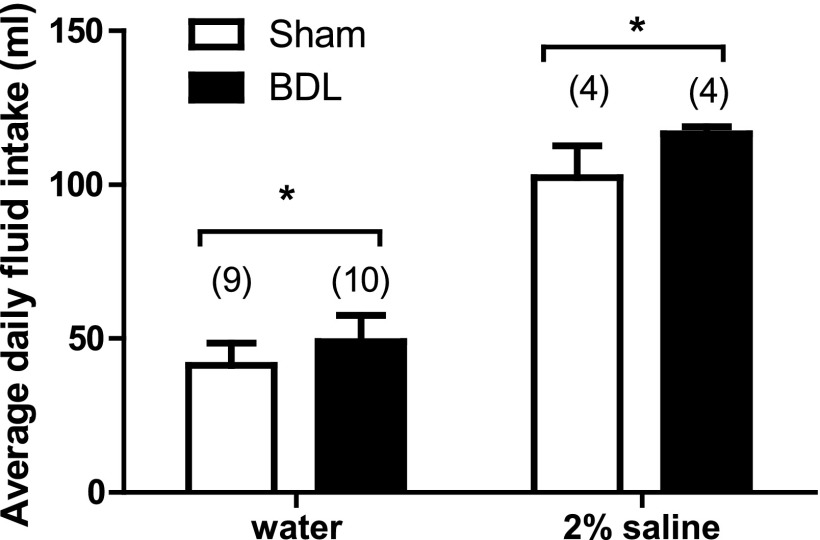
A significant increase in daily average baseline water consumption in BDL rats was observed compared with shams (Fig. 2). In rats given only 2% NaCl to drink, BDL rats also drank significantly more than shams (Fig. 2).
Fig. 2.
Average daily fluid intake from Sham and BDL rats given water or 2% saline to drink for 2 wk. Values are means ± SE, *Statistically significant, P < 0.05. Parentheses indicate number of animals.
Western blot analysis.
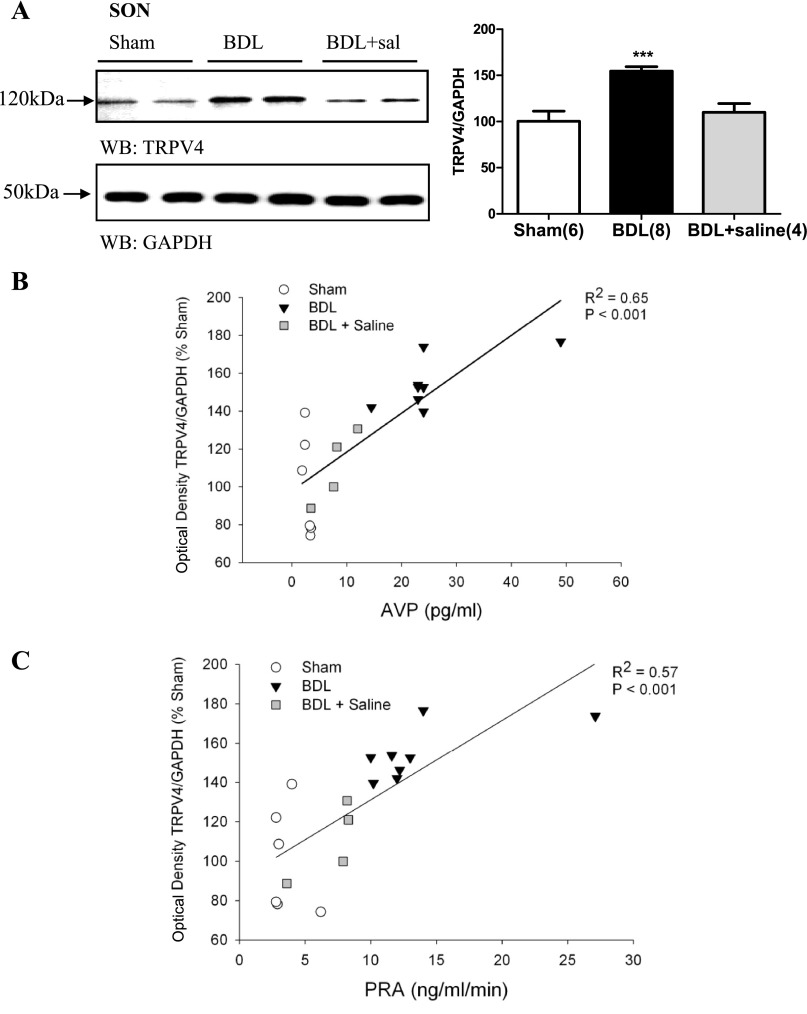
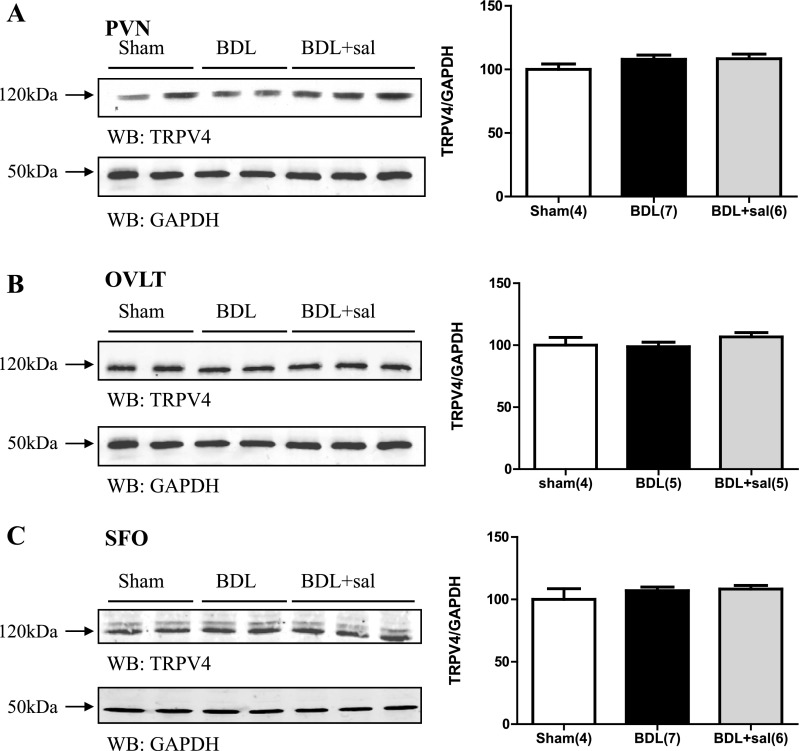
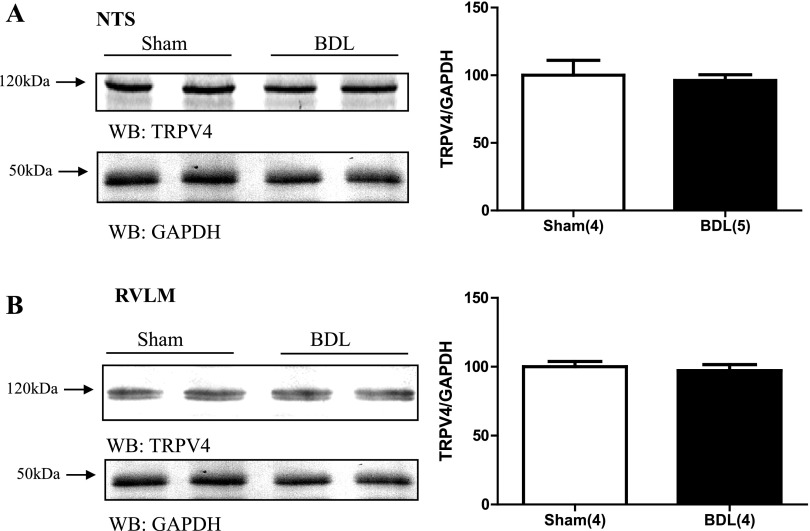
In BDL rats, TRPV4 protein expression was significantly increased in punches containing the SON (Fig. 3A). This effect was blocked in BDL rats given 2% saline to drink for 15 days (Fig. 3A). BDL rats given saline to drink had average optical densities for TRPV4 that were not significantly different from sham (Fig. 3A). The optical density values corresponding to TRPV4 protein abundance from SON punches was significantly correlated with plasma AVP and PRA (Fig. 3, B and C, respectively). We failed to observe similar changes in punches samples from the PVN (Fig. 4A) or the two circumventricular organs OVLT and SFO (Fig. 4, B and C, respectively) where the abundance of TRPV4 protein was not significantly different. BDL was not associated with significant changes in TRPV4 protein expression in either the NTS or RVLM (Fig. 5, A and B).
Fig. 3.
A: digital images of representative Western blot analysis for TRPV4 from brain punches taken from SON of sham-ligated rats, BDL rats, and BDL rats given 2% saline to drink (BDL+sal). Summary of densitometric analysis of TRPV4 immunoreactivity normalized with GAPDH from sham, BDL, and BDL+ saline. Values are expressed as means ± SE. ***Statistically significant, P < 0.001. Parentheses indicate number of animals. B: linear regression analysis of AVP and transient receptor potential vanilloid 4 (TRPV4) immunoreactivity in the SON of sham, BDL, and BDL+ saline rats. C: linear regression analysis of PRA and TRPV4 immunoreactivity in the SON of sham, BDL, and BDL+ saline rats.
Fig. 4.
Digital images of representative Western blot analysis for TRPV4 from brain punches taken from PVN (A), OVLT (B), and SFO (C) from sham, BDL rats, and BDL rats given 2% saline to drink (BDL+sal). Graph represents summary data of densitometric analysis of TRPV4 immunoreactivity in sham, BDL, and BDL+ saline from these regions. Values are expressed as means ± SE. Numbers in parentheses represent number of animals.
Fig. 5.
Digital images of representative Western blot analysis for TRPV4 from brain punches taken from NTS (A) and RVLM (B) from sham, BDL rats. Graph illustates densitometric analysis of TRPV4 immunoreactivity in sham and BDL. Values are expressed as means ± SE. Numbers in parentheses represent number of animals.
Immunohistochemistry.
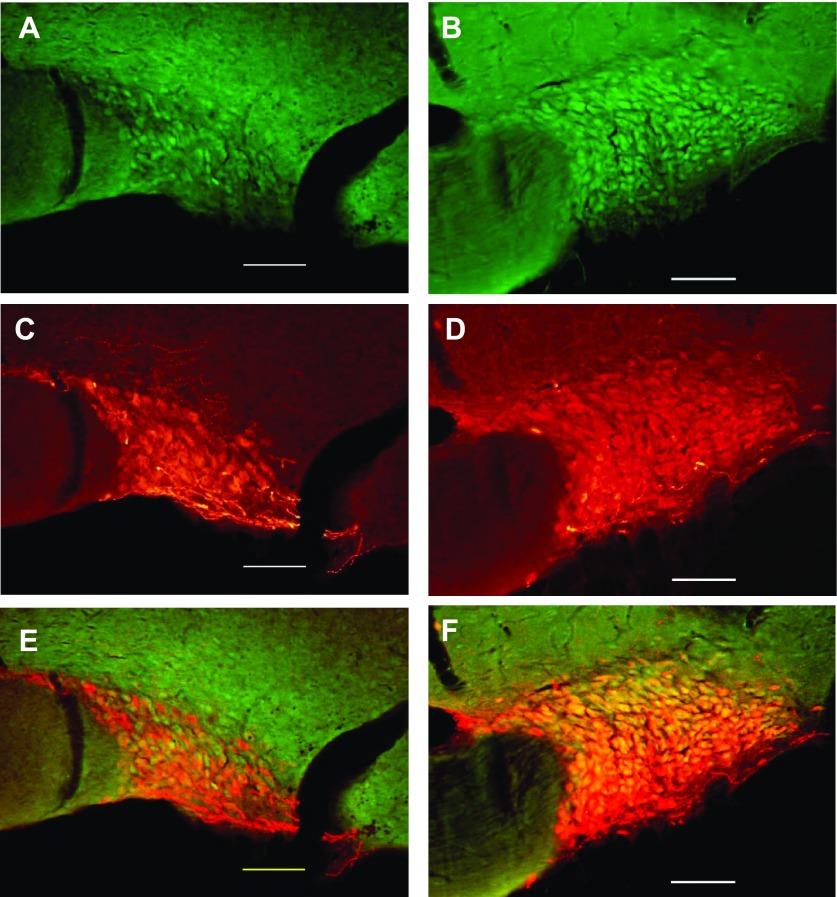
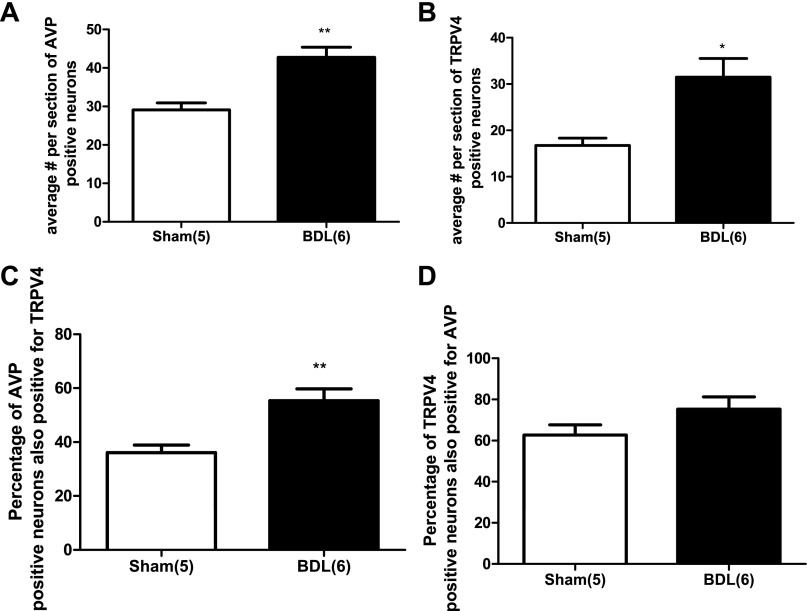
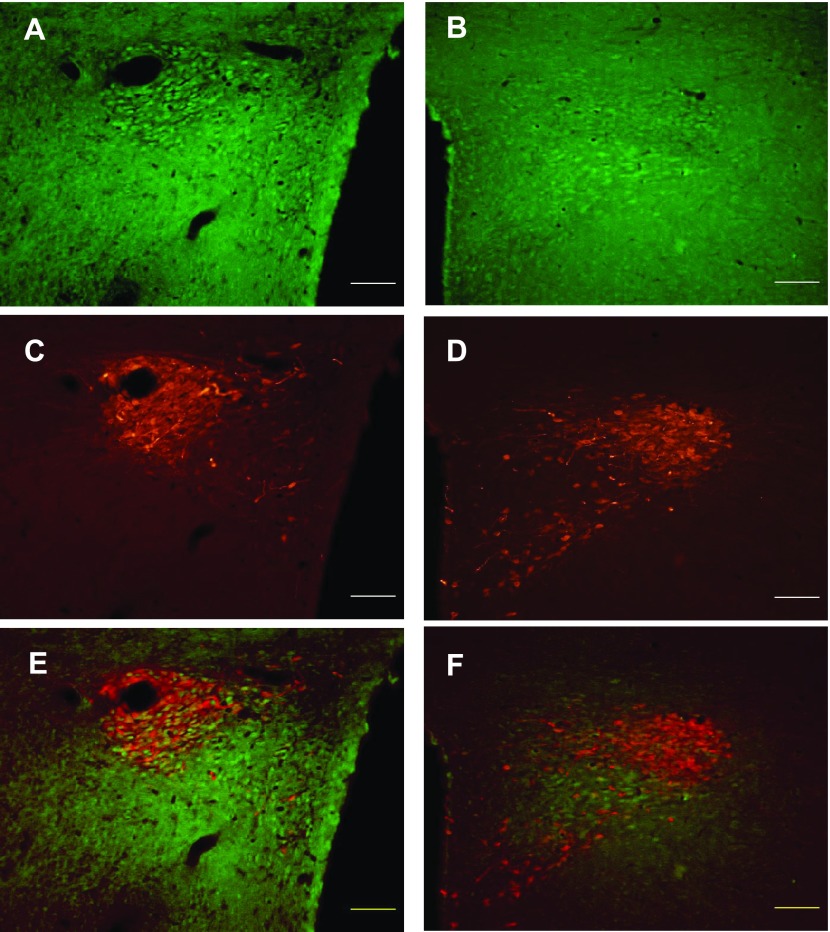
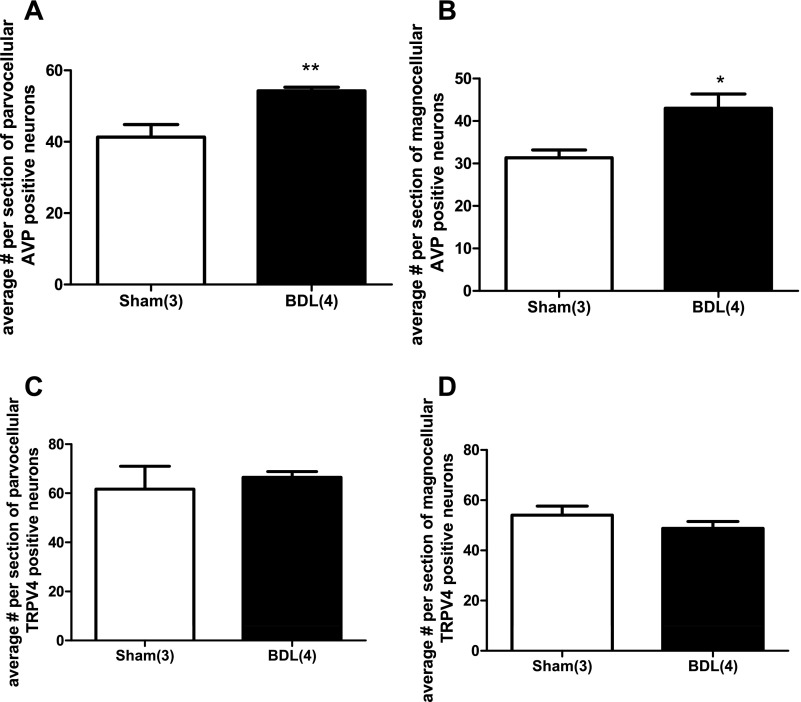
We investigated, by double-labeling immunohistochemistry, whether TRPV4 staining was present in AVP neurons of the SON and PVN in sham and BDL animals. Fig. 6 shows TRPV4 staining within the SON in sham (Fig. 6A) and BDL rats (Fig. 6B). The AVP staining in the same section is shown in Fig. 6C (sham) and Fig. 6D (BDL). Figure 6, E and F shows the merged images for Sham and BDL, respectively. As can be seen in the images, TRPV4 was colocalized with AVP in the SON of both groups. Quantitative analysis indicated that the average number of AVP-positive neurons were higher in BDL rats compared with sham (Fig. 7A). The average numbers of TRPV4-positive cells per section was also significantly increased in BDL rats compared with sham (Fig. 7B), and the percentage of AVP-positive neurons that were also positive for TRPV4 significantly increased from 36% ± 3 to 55.3% ± 4.3 (P = 0.0062) (Fig. 7C). However, the percentage of TRPV4 neurons also expressing AVP was not significantly affected after BDL, suggesting that TRPV4 may have increased in nonvasopressinergic cells [sham (n = 5) 62.7 ± 4.8; BDL (n = 6) 75.2 ± 5.9; P = 0.14]. TRPV4-positive cells were found in both the magnocellular and parvocellular portions of the PVN in sham and BDL rats, and in both regions of the PVN, TRPV4 was colocalized with AVP (Fig. 8). As observed in the SON, the average numbers of AVP-positive neurons in magnocellular PVN were significantly increased in BDL rats compared with sham (Fig. 9A). In contrast, BDL did not significantly affect the average number of TRPV4-positive cells per section in magnocellular PVN (Fig. 9B). The average percentage of AVP-positive magnocellular neurons also positive for TRPV4 was significantly decreased [sham (n = 3) 75.5 ± 10.15; BDL (n = 4) 26.7 ± 6.9; P = 0.0091]. This could be due to the increased number of AVP-positive cells in the PVN but no change in TRPV4 staining associated with BDL. BDL produced similar effects in parvocellular PVN (Fig. 9, C and D). The percentage of parvocellular AVP-positive neurons also positive for TRPV4 was not statistically different [sham (n = 3) 47.5 ± 6.6; BDL (n = 4) 31.8 ± 6.8; P = 0.16].
Fig. 6.
Digital images of representative examples of immunohistochemistry for TRPV4 (green: A and B) and AVP (red: C and D) staining in the SON of sham (A and C) and BDL rats (B and D). Merged images (E and F) illustrate colocalization of TRPV4 and AVP in the SON of sham and BDL rats. Scale bar in each image is 100 μm.
Fig. 7.
Quantitative analysis of changes in AVP and TRPV4 staining in the SON from sham-ligated and BDL rats. *Significantly different from sham, P < 0.01. **Significantly different from sham, P < 0.001. A: average number per section of AVP-positive neurons. B: average number per section of TRPV4-positive neurons. C: percentage of AVP-positive neurons also positive for TRPV4. D: percentage of TRPV4-positive neurons also positive for AVP. Numbers in parentheses represent number of animals.
Fig. 8.
Digital images of representative examples of immunohistochemistry for TRPV4 (green: A and B) and AVP (red: C and D) staining in the PVN of sham (A and C) and BDL rats (B and D). Merged images (E) and (F) illustrate colocalization of TRPV4 and AVP in the SON of sham and BDL rats. Scale bar in each image is 100 μm.
Fig. 9.
Quantitative analysis of changes in AVP and TRPV4 staining in the PVN from sham-ligated and BDL rats. A: average number per section of parvocellular AVP-positive neurons. B: average number per section of magnocellular AVP-positive neurons. C: average number per section of parvocellular TRPV4-positive neurons. D: average number per section of parvocellular TRPV4-positive neurons. *Significantly different from sham, P < 0.01. **Significantly different from sham, P < 0.001. Numbers within parentheses represent number of animals.
Detergent-free lipid raft preparation.
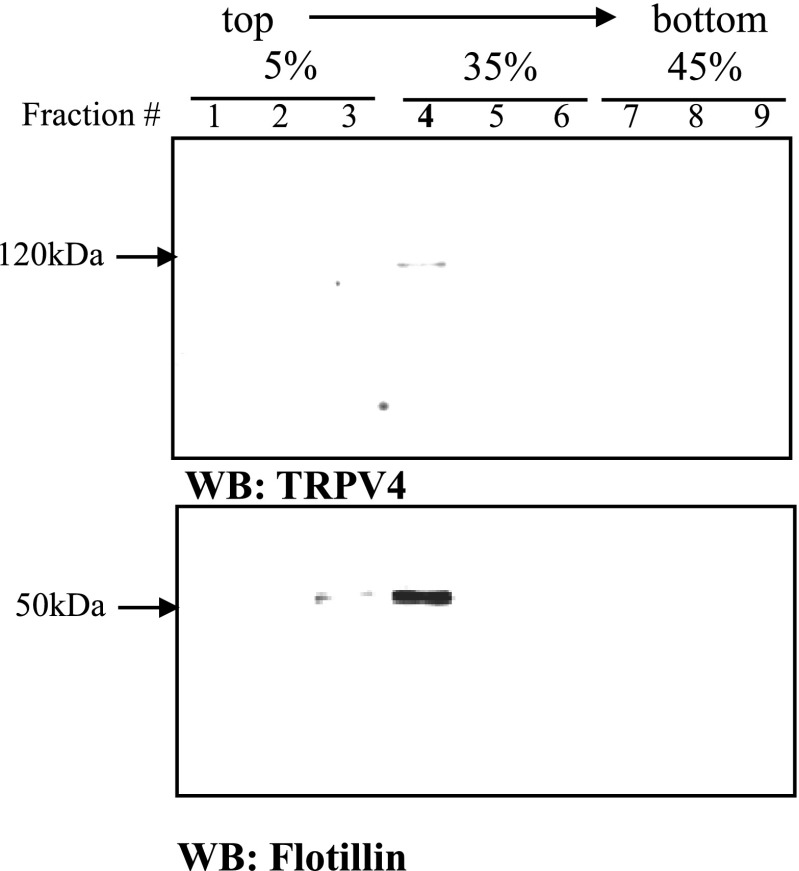
Brain tissues from the hypothalamus containing the OVLT, PVN, and SON and brain stem containing NTS, RVLM, caudal ventrolateral medulla, and area postrema were subjected to a centrifugal fractionation to obtain crude membrane fractions. The crude membrane fractions were combined with sodium carbonate, and the mixture was fractionated in a 5%/35%/45% discontinuous sucrose gradient by ultracentrifugation. From the sucrose density gradient, nine fractions were collected and resolved by Western blot analysis (Fig. 10). The lipid raft neuronal marker flotillin immunoreactivity peaked in fraction number 4 (Fig. 10), which was near the interface of 5%/35% sucrose gradient. Since low density and flotillin expression are characteristics of lipid raft microdomains, these results suggest that this methodology successfully isolated lipid rafts.
Fig. 10.
TRPV4 and flotillin distributions over sucrose density gradients from total hypothalamus. Total hypothalamus of sham animals was homogenized, fractionated, and the crude plasma membrane fractions were homogenized by the Na2CO3 method, and the fractions were resolved by SDS-PAGE and Western blot analysis. The fraction number 4 corresponds to the lipid raft fraction.
TRPV4 lipid raft association is increased in the hypothalamus followed by BDL.
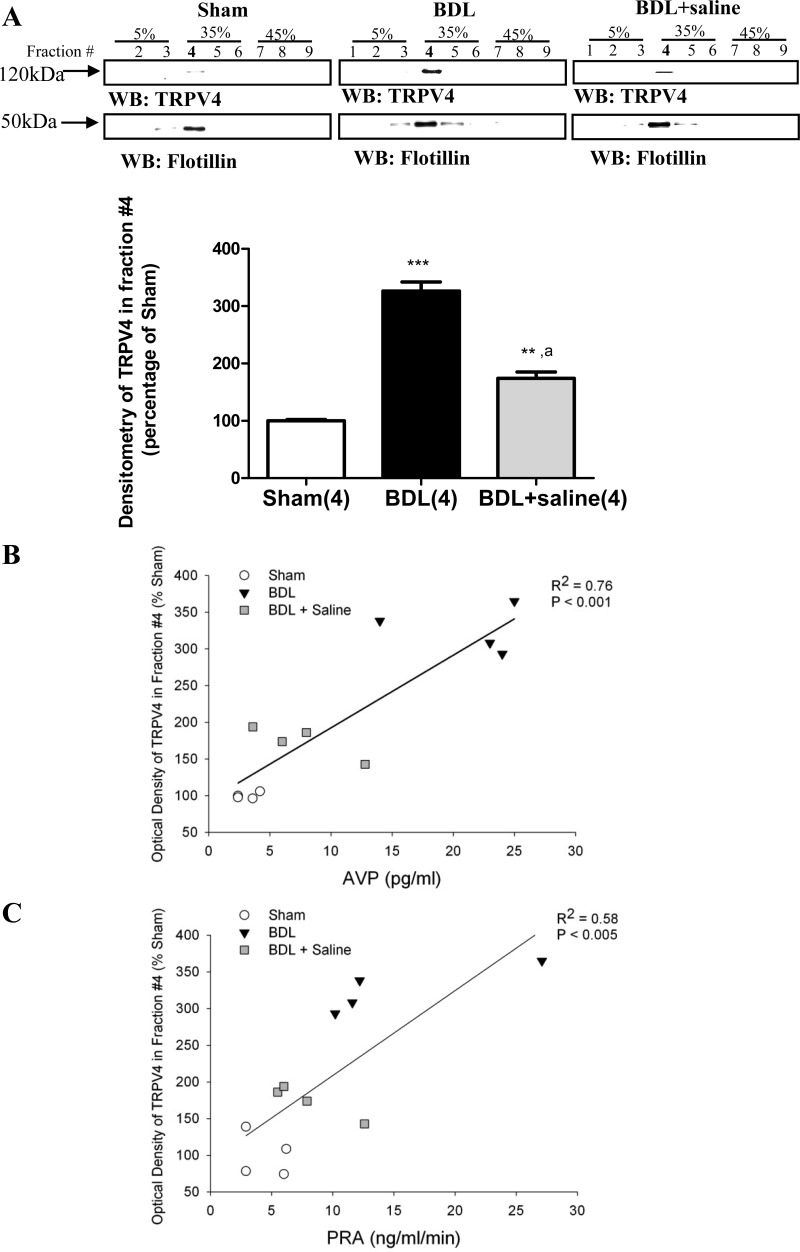
To clarify the role of TRPV4 partitioning in lipid rafts microdomains the nondetergent method of Na2CO3 treatment of crude plasma membrane preparations was utilized to separate lipid raft microdomains, and their associated proteins from other plasma membrane structures. Proteins were resolved via centrifugation through a sucrose gradient containing 250 mM Na2CO3. Following this protocol, flotillin-1, a known lipid raft marker in neuronal cell models (46, 82) was found to cofractionate with TRPV4 in lipid raft fraction (fraction number 4). In the hypothalamus of BDL rats, TRPV4 lipid raft association increased to 326.1% ± 16 compared with the sham animals (100% ± 2.1) (Fig. 11). A significant correlation was found between the abundance of TRPV4 associated with lipid raft and both plasma AVP (Fig. 11B) and PRA (Fig. 11C).
Fig. 11.
A: TRPV4 and flotillin distributions over sucrose density gradients in the hypothalamus of BDL rats and BDL rats given 2% saline to drink (BDL+sal). The fraction number 4 corresponds to the lipid raft fraction. TRPV4 immunoreactivity was quantified by densitometry using the Scion program, and the respective values were normalized by the flotillin densitometry in four independent experiments. Values are expressed as means ± SE, ***Statistically significant different from sham, P < 0.001. **Statistically significant different from sham, P < 0.01. aStatistically significant difference from BDL, P < 0.001. B: linear regression analysis of AVP and TRPV4 immunoreactivity in lipid raft (fraction #4) from total hypothalamus in all three groups. C: linear regression analysis of PRA and TRPV4 immunoreactivity in lipid raft (fraction number 4) of total hypothalamus from sham, BDL, and BDL+ saline.
When BDL animals were given 2% saline to drink a significant decrease in TRPV4 lipid raft association was observed (Fig. 11, A–C), although the association was not fully back to sham levels.
TRPV4 lipid raft association in brain stem was decreased followed by BDL.
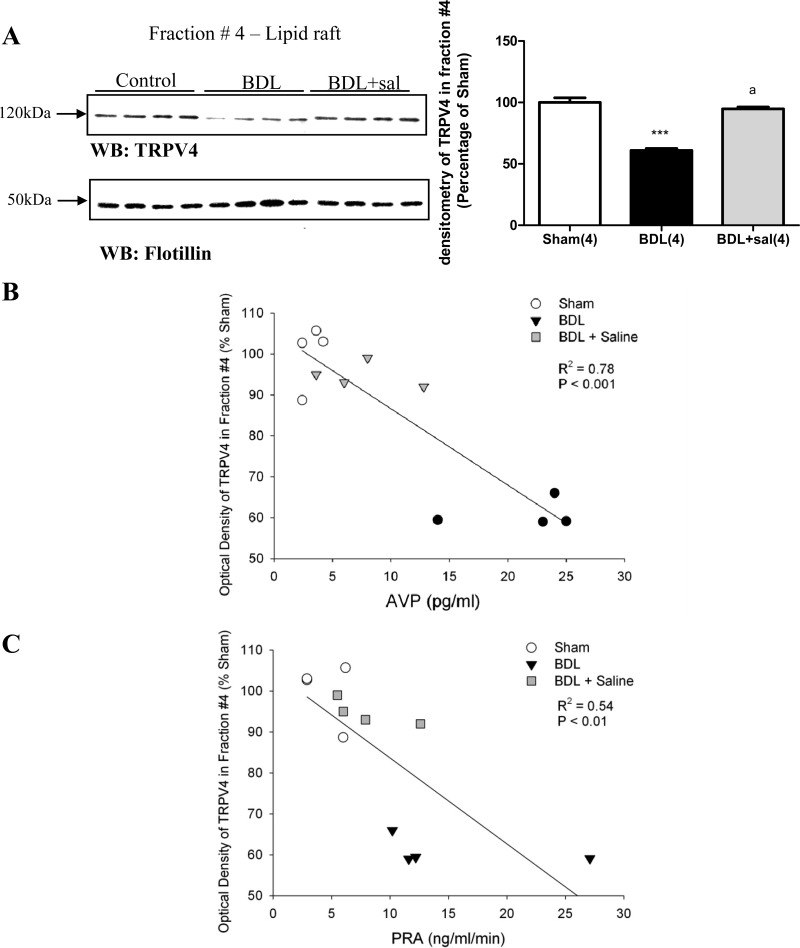
In the brain stem of BDL rats, TRPV lipid raft association decreased compared with the sham animals (Fig. 12). When BDL animals were given 2% saline to drink, TRPV4 lipid raft association was increased compared with BDL rats (Fig. 12). Significant negative correlations were found between the abundance of TRPV4 in lipid rafts and plasma AVP (Fig. 12B) and PRA (Fig. 12C).
Fig. 12.
A: TRPV4 and flotillin distributions in sucrose density gradients in the brain stem of sham, BDL rats, and BDL rats given 2% saline to drink (BDL + sal). The fraction number 4 corresponds to the lipid raft fraction. Values are expressed as means ± SE. ***Statistically significant different from sham, P < 0.001. aStatistically significant different from BDL, P < 0.001. B: linear regression analysis of vasopressin (AVP) and TRPV4 immunoreactivity in lipid raft (fraction number 4) of total brain stem from sham, BDL, and BDL+ saline. C: linear regression analysis of PRA and TRPV4 immunoreactivity in lipid raft (fraction number 4) of total brain stem from sham, BDL, and BDL+ saline.
DISCUSSION
Although an association between liver cirrhosis and inappropriate AVP release has long been recognized (24, 38, 59, 75, 89), the molecular mechanisms among areas of the brain that contribute to the pathology is poorly understood. The present study tested the expression of the transient receptor potential TRPV4 within microdomains of the plasma membrane in forebrain and hindbrain regions implicated in body fluid and cardiovascular regulation during the pathology of cirrhosis.
Our results demonstrate that TRPV4 abundance is significantly increased in the SON of BDL rats. The results from our immunohistochemistry experiments showed that TRPV4 is colocalized in AVP neurons. In BDL-induced cirrhosis, the percentage of AVP neurons in the SON expressing TRPV4 was significantly increased, which is consistent with the results from our Western blot studies. However, it should be noted that results suggest that TRPV4 was also increased in nonvasopressinergic cells. Nevertheless, these results suggest that BDL was associated with a significant increase in TRPV4 in vasopressinergic SON neurons. Additional Western blot studies using punch samples collected from other brain regions involved in cardiovascular regulation and body fluid homeostasis, including the PVN indicate that this effect on TRPV4 expression was regionally specific to the SON.
In both the SON and PVN, the average number of vasopressin neurons was significantly increased in BDL rats. Traditionally, magnocellular neurosecretory neurons have been described as having either a vasopressin or oxytocin phenotype based on their anatomical location and their electrophysiological properties (2, 70). However, using single-cell RT-PCR, it has been demonstrated that most magnocellular neurons in the SON contain mRNA for both vasopressin and oxytocin (94). In nearly all of the SON cells, either vasopressin or oxytocin mRNA was expressed in excess of the other supporting the existence of possibly distinct vasopressin and oxytocin phenotypes. However, a third possible phenotype was observed in which vasopressin and oxytocin mRNAs were expressed in nearly equivalent amounts. On the basis of the results of Xi et al. (94), it could be suggested that the increase in vasopressin-positive profiles in the SON and PVN associated with BDL could be the result of activity-dependent changes in gene expression that converted this third, intermediate phenotype of SON neurons into a vasopressin phenotype. Given the limitations of the immunohistochemical approach used in the current study, additional studies will be required to address this issue.
Clinical studies have demonstrated that providing preascitic cirrhosis patients with increased salt lowers their PRA and improves their fluid retention (93). It is well established that synthesis and secretion of renin are inversely related to salt intake (29, 33). In the current study, we observed that giving BDL rats 2% saline to drink for 15 days significantly lowered both their PRA and their plasma AVP levels and improved their plasma volume and osmolality. This experimental manipulation also blocked the increased expression of TRPV4 in the SON of BDL rats, and our data demonstrated a significant correlation between PRA and TRPV4 in BDL rats. These results suggest that the peripheral renin-angiotensin system may contribute to nonosmotic AVP release during cirrhosis. Circulating angiotensin acts on the CNS through two forebrain circumventricular organs, the OVLT and the SFO (57, 58, 64, 83). These circumventricular organs influence AVP release through direct and indirect projections to SON and PVN in the hypothalamus (22, 57, 58, 62). Therefore, it seems reasonable to suggest that the renin-angiotensin system could act on the CNS to contribute to nonosmotic AVP release during cirrhosis. This is supported by recent clinical studies showing that reducing PRA or blocking angiotensin receptors with losartan prevented or reversed hyponatremia associated with cirrhosis (26, 85, 93).
Because TRP channels localize to lipid rafts (6, 54) and Src kinases, which may participate in the gating of TRPV4, are localized in lipid rafts (20, 87, 95), we conducted membrane fractionation studies with Western blot analysis to determine whether changes in membrane trafficking of TRPV4 also occurs during BDL. We observed a significant increase of TRPV4 expression in lipid rafts in the hypothalamus of BDL rats. This effect was blocked by providing BDL rats with 2% saline to drink. These results suggest that during cirrhosis, TRPV4 is preferentially targeted to lipid raft microdomains, increasing their possible interactions with Src kinases in the hypothalamus. Furthermore, providing BDL rats with saline to drink blocked this effect, similar to its effect on increased expression of TRPV4 in the SON. This suggests a possible role for the central effects of the renin-angiotensin system on the expression and trafficking of TRPV4 in BDL rats. Additional studies will be necessary to determine whether BDL is associated with increased localization of TRPV4 in lipid rafts in the SON or in other regions of the hypothalamus, where we failed to find changes in TRPV4 abundance associated with BDL.
Because TRPV4 is a cationic channel, we speculate that their increased expression in lipid rafts could lead to changes in the activation of SON neurons that would be consistent with increased AVP release in cirrhotic rats. Previous studies have shown that the mechanosensitive currents in SON neurons are also modulated by neuropeptides that act through GPCR (4, 12). It could be that increased expression of TRPV4 in lipid rafts could increase the potential interaction between TRPV4 and peptide-sensitive GPCRs (15).
While we did not observe changes in TRPV4 expression in brain punch samples from the medulla, TRPV4 localization in lipid rafts from the brain stem significantly decreased in BDL rats, and this decrease was prevented by providing BDL rats with 2% saline to drink. These results could be due to changes in TRPV4 expression in brain stem regions that were not included in our study using punch samples of regions known to be involved in cardiovascular regulation. Alternatively, these results could indicate that the expression of TRPV4 in the brain stem was not affected by BDL but that the trafficking of TRPV4 to lipid rafts was influenced. This question will need to be addressed in future studies by examining TRPV4 localization in lipid rafts using punch samples from specific regions in the brain stem.
In this study, BDL rats drank significantly more fluid on average than sham-ligated controls. Previous studies have shown that BDL rats have an increased sodium appetite but did not find evidence of increase water intake (23, 44, 45). The differences in these findings could be due to a different strain of animals used in the earlier work (23, 44, 45). In the physiological context of decreasing plasma osmolality, increased water intake associated with cirrhosis would also be inappropriate and could contribute to eventual hyponatremia along with vasopressin release. The increased sodium appetite in this model appears to be related more too systemic hypotension than activation of the renin-angiotensin system (23, 45). Hypotension could also contribute to increased water intake in BDL rats. While the OVLT and SFO are involved in the central control of fluid intake related to changes in plasma osmolality and angiotensin (37, 56, 58), we did not observe changes in TRPV4 expression in either of these regions associated with BDL. The regions and molecular mechanisms responsible for increased water intake in BDL remain to be determined.
Perspectives and Significance
Inappropriate AVP release associated with cirrhosis has traditionally been attributed to a nonosmotic mechanism (24, 38, 74, 75). Our current results and previous studies suggest that the renin-angiotensin system may be one of these contributing mechanisms. This is based on the observation that the BDL rats given 2% NaCl to drink showed significant decreases both plasma AVP and PRA. For the first time, we have demonstrated that activation of the renin-angiotensin system is correlated with increased trafficking of TRPV4 to lipid rafts in the hypothalamus. Lipid rafts and their resident proteins may also play a role in how these channels are gated. As mentioned earlier, some TRP channels are localized in lipid rafts (6, 54), and TRPV4 is likely among them. It has been shown that TRPV1 membrane expression and function in rat nociceptors is dependent on cholesterol (53), which, in turn, is an essential component of lipid rafts (97). In endothelial cells, caveolin-1 expression modulates membrane localization and activity of TRPV4, thus impacting the endothelium-derived hyperpolarizing factor (EDHF)-mediated relaxation (73). Gating of TRPV4 by hypotonicity appears to require the action of cytoplasmic tyrosine kinases of the Src family (20, 95), which also are localized to and are active in lipid rafts (87). The increased localization of TRPV4 to lipid rafts could also affect their modulation by peptides through GPCRs (15) or by MAP kinase pathways that are affected by osmolality (7, 17, 18, 42, 43). It remains to be established whether lipid rafts in conjunction with their protein cargo are instrumental in regulating the activity of TRPV4.
Our data also demonstrate that TRPV4 expression is increased in the AVP neurons in the SON in association with BDL-induced inappropriate vasopressin release. Bourque's laboratory has demonstrated that mechanosensitive ion channels mediate osmosensitivity in the SON, as well as in the OVLT (3, 4, 16). Studies in TRPV1 (77) and TRPV4 (49, 51, 52) knockout mice suggest that channels participate in the osmotic regulation of AVP release and fluid intake, although a recent study failed to support such a role for TRPV1 (84). Several studies suggest that TRPV4 may mediate responses related to increases and decreases in osmotic pressure (14, 47, 48, 50, 52, 60). It could be that increased expression of TRPV4 in AVP neurons in the SON of BDL rats resets their osmosensitivity, allowing them to continue to release AVP in the face of decreasing osmolality and hyponatremia. We speculate that changes in TRPV4 expression and trafficking to different microdomains of the plasma membrane could contribute to the remodeling of electrogenic membrane properties leading to a feed-forward mechanism associated with the pathophysiology of liver cirrhosis. Further electrophysiology analysis should be performed to evaluate how changes in expression and membrane compartmentation impact the vasopressinergic neurons and contribute to inappropriate AVP secretion in disease states, such as liver cirrhosis and congestive heart failure.
GRANTS
This research was supported by the National Institutes of Health (Grant R01 HL62579 to J. T. Cunningham).
Acknowledgments
The authors would like to acknowledge the expert technical assistance of Joel T. Little.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anderson RJ, Chung HM, Kluge R, Schrier RW. Hyponatremia: a prospective analysis of its epidemiology and the pathogenetic role of vasopressin. Ann Intern Med 102: 164–168, 1985. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong WE Morphological and electrophysiological classification of hypothalamic supraoptic neurons. Prog Neurobiol 47: 291–339, 1995. [PubMed] [Google Scholar]
- 3.Bourque CW, Ciura S, Trudel E, Stachniak TJ, Sharif-Naeini R. Neurophysiological characterization of mammalian osmosensitive neurones. Exp Physiol 92: 499–505, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourque CW, Voisin DL, Chakfe Y. Stretch-inactivated cation channels: cellular targets for modulation of osmosensitivity in supraoptic neurons. Prog Brain Res 139: 85–94, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Bradford MM A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 6.Brazer SC, Singh BB, Liu X, Swaim W, Ambudkar IS. Caveolin-1 contributes to assembly of store-operated Ca2+ influx channels by regulating plasma membrane localization of TRPC1. J Biol Chem 278: 27208–27215, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burg MB, Kwon ED, Kultz D. Osmotic regulation of gene expression. FASEB J 10: 1598–1606, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Cardenas A, Arroyo V. Hepatorenal syndrome. Ann Hepatol 2: 23–29, 2003. [PubMed] [Google Scholar]
- 9.Cardenas A, Arroyo V. Mechanisms of water and sodium retention in cirrhosis and the pathogenesis of ascites. Best Pract Res Clin Endocrinol Metab 17: 607–622, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Cardenas A, Bataller R, Arroyo V. Mechanisms of ascites formation. Clin Liver Dis 4: 447–465, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Cardenas A, Gines P. Pathogenesis and treatment of fluid and electrolyte imbalance in cirrhosis. Semin Nephrol 21: 308–316, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Chakfe Y, Bourque CW. Excitatory peptides and osmotic pressure modulate mechanosensitive cation channels in concert. Nat Neurosci 3: 572–579, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee K Neurohormonal activation in congestive heart failure and the role of vasopressin. Am J Cardiol 95: 8B–13B, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Liu C, Liu L. Changes in osmolality modulate voltage-gated calcium channels in trigeminal ganglion neurons. Brain Res 1208C: 56–66, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chini B, Parenti M. G-protein coupled receptors in lipid rafts and caveolae: how, when and why do they go there? J Mol Endocrinol 32: 325–338, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Ciura S, Bourque CW. Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality. J Neurosci 26: 9069–9075, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen DM The transient receptor potential vanilloid-responsive 1 and 4 cation channels: role in neuronal osmosensing and renal physiology. Curr Opin Nephrol Hypertens 16: 451–458, 2007. [DOI] [PubMed] [Google Scholar]
- 18.De NE, Alepuz PM, Posas F. Dealing with osmostress through MAP kinase activation. EMBO Rep 3: 735–740, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Decaux G Difference in solute excretion during correction of hyponatremic patients with cirrhosis or syndrome of inappropriate secretion of antidiuretic hormone by oral vasopressin V2 receptor antagonist VPA-985. J Lab Clin Med 138: 18–21, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Essandri-Haber N, Dina OA, Joseph EK, Reichling DB, Levine JD. Interaction of transient receptor potential vanilloid 4, integrin, and SRC tyrosine kinase in mechanical hyperalgesia. J Neurosci 28: 1046–1057, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feldman BJ, Rosenthal SM, Vargas GA, Fenwick RG, Huang EA, Matsuda-Abedini M, Lustig RH, Mathias RS, Portale AA, Miller WL, Gitelman SE. Nephrogenic syndrome of inappropriate antidiuresis. N Engl J Med 352: 1884–1890, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferguson AV, Bains JS. Electrophysiology of the circumventricular organs. Front Neuroendocrinol 17: 440–475, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Fitts DA, Lane JR, Starbuck EM, Li CP. Drinking and blood pressure during sodium depletion or ANG II infusion in chronic cholestatic rats. Am J Physiol Regul Integr Comp Physiol 276: R23–R31, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Gines P, Abraham WT, Schrier RW. Vasopressin in pathophysiological states. Semin Nephrol 14: 384–397, 1994. [PubMed] [Google Scholar]
- 25.Gines P, Berl T, Bernardi M, Bichet DG, Hamon G, Jimenez W, Liard JF, Martin PY, Schrier RW. Hyponatremia in cirrhosis: from pathogenesis to treatment. Hepatology 28: 851–864, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Girgrah N, Liu P, Collier J, Blendis L, Wong F. Haemodynamic, renal sodium handling, and neurohormonal effects of acute administration of low dose losartan, an angiotensin II receptor antagonist, in preascitic cirrhosis. Gut 46: 114–120, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gitelman SE, Feldman BJ, Rosenthal SM. Nephrogenic syndrome of inappropriate antidiuresis: a novel disorder in water balance in pediatric patients. Am J Med 119: S54–S58, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Gottlieb HB, Ji LL, Jones H, Penny ML, Fleming T, Cunningham JT. Differential effects of water and saline intake on water deprivation-induced c-Fos staining in the rat. Am J Physiol Regul Integr Comp Physiol 290: R1251–R1261, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev 70: 1067–1116, 1990. [DOI] [PubMed] [Google Scholar]
- 30.Henriksen JH, Moller S. Hypertension and liver disease. Curr Hypertens Rep 6: 453–461, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Henriksen JH, Moller S. Liver cirrhosis and arterial hypertension. World J Gastroenterol 12: 678–685, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henriksen JH, Moller S, Schifter S, Bendtsen F. Increased arterial compliance in decompensated cirrhosis. J Hepatol 31: 712–718, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Ingert C, Grima M, Coquard C, Barthelmebs M, Imbs JL. Effects of dietary salt changes on renal renin-angiotensin system in rats. Am J Physiol Renal Physiol 283: F995–F1002, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Jeske NA, Glucksman MJ, Roberts JL. EP24.15 is associated with lipid rafts. J Neurosci Res 74: 468–473, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Jeske NA, Glucksman MJ, Roberts JL. Metalloendopeptidase EC3.4.24.15. is constitutively released from the exofacial leaflet of lipid rafts in GT1–7 cells. J Neurochem 90: 819–828, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Ji LL, Gottlieb HB, Penny ML, Fleming T, Toney GM, Cunningham JT. Differential effects of water deprivation and rehydration on Fos and FosB/DeltaFosB staining in the rat brain stem. Exp Neurol 203: 445–456, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Johnson AK The sensory psychobiology of thirst and salt appetite. Med Sci Sports Exer 39: 1388–1400, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Kashani A, Landaverde C, Medici V, Rossaro L. Fluid retention in cirrhosis: pathophysiology and management. QJM 101: 71–85, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Kim JK, Michel JB, Soubrier F, Durr J, Corvol P, Schrier RW. Arginine vasopressin gene expression in chronic cardiac failure in rats. Kidney Int 38: 818–822, 1990. [DOI] [PubMed] [Google Scholar]
- 40.Kirstetter P, Moreau R, Soupison T, Cailmail S, Hartleb M, Lebrec D. Role of sympathetic cardiovascular tone in control of arterial pressure in rats with cirrhosis. Liver 16: 263–266, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Kortas C, Bichet DG, Rouleau JL, Schrier RW. Vasopressin in congestive heart failure. J Cardiovasc Pharmacol 8 Suppl 7: S107–S110, 1986. [DOI] [PubMed] [Google Scholar]
- 42.Kultz D, Burg M. Evolution of osmotic stress signaling via MAP kinase cascades. J Exp Biol 201: 3015–3021, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Kultz D, Burg MB. Intracellular signaling in response to osmotic stress. Contrib Nephrol 123: 94–109, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Lane JR, Purhar KK, Fitts DA. Drinking in sodium-depleted rats with bile duct, vena cava or portal vein obstruction. Physiol Behav 62: 1145–1154, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Lane JR, Starbuck EM, Johnson AK, Fitts DA. Effects of bile duct ligation and captopril on salt appetite and renin-aldosterone axis in rats. Physiol Behav 66: 419–425, 1999. [DOI] [PubMed] [Google Scholar]
- 46.Lang DM, Lommel S, Jung M, Ankerhold R, Petrausch B, Laessing U, Wiechers MF, Plattner H, Stuermer CA. Identification of reggie-1 and reggie-2 as plasmamembrane-associated proteins which cocluster with activated GPI-anchored cell adhesion molecules in non-caveolar micropatches in neurons. J Neurobiol 37: 502–523, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Liedtke W TRPV4 as osmosensor: a transgenic approach. Pflügers Arch 451: 176–180, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Liedtke W TRPV4 plays an evolutionary conserved role in the transduction of osmotic and mechanical stimuli in live animals. J Physiol 567: 53–58, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liedtke W TRPV channels' role in osmotransduction and mechanotransduction. Handb Exp Pharmacol 179: 473–487, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103: 525–535, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4-/- mice. Proc Natl Acad Sci USA 100: 13698–13703, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liedtke W, Tobin DM, Bargmann CI, Friedman JM. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc Natl Acad Sci USA 100 Suppl 2: 14531–14536, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu M, Huang W, Wu D, Priestley JV. TRPV1, but not P2X, requires cholesterol for its function and membrane expression in rat nociceptors. Eur J Neurosci 24: 1–6, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Lockwich TP, Liu X, Singh BB, Jadlowiec J, Weiland S, Ambudkar IS. Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J Biol Chem 275: 11934–11942, 2000. [DOI] [PubMed] [Google Scholar]
- 55.Martin PY, Schrier RW. Pathogenesis of water and sodium retention in cirrhosis. Kidney Int Suppl 59: S43–S49, 1997. [PubMed] [Google Scholar]
- 56.McKinley MJ, Johnson AK. The physiological regulation of thirst and fluid intake. News Physiol Sci 19: 1–6, 2004. [DOI] [PubMed] [Google Scholar]
- 57.McKinley MJ, Mathai ML, McAllen RM, McClear RC, Miselis RR, Pennington GL, Vivas L, Wade JD, Oldfield BJ. Vasopressin secretion: osmotic and hormonal regulation by the lamina terminalis. J Neuroendocrinol 16: 340–347, 2004. [DOI] [PubMed] [Google Scholar]
- 58.McKinley MJ, McAllen RM, Davern P, Giles ME, Penschow J, Sunn N, Uschakov A, Oldfield BJ. The sensory circumventricular organs of the mammalian brain. Adv Anat Embryol Cell Biol 172: III-122, back, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Miller M Hyponatremia and arginine vasopressin dysregulation: mechanisms, clinical consequences, and management. J Am Geriatr Soc 54: 345–353, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Mizuno A, Matsumoto N, Imai M, Suzuki M. Impaired osmotic sensation in mice lacking TRPV4. Am J Physiol Cell Physiol 285: C96–C101, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Natarajan SK, Basivireddy J, Ramachandran A, Thomas S, Ramamoorthy P, Pulimood AB, Jacob M, Balasubramanian KA. Renal damage in experimentally-induced cirrhosis in rats: Role of oxygen free radicals. Hepatology 43: 1248–1256, 2006. [DOI] [PubMed] [Google Scholar]
- 62.Oldfield BJ, Davern PJ, Giles ME, Allen AM, Badoer E, McKinley MJ. Efferent neural projections of angiotensin receptor (AT1) expressing neurones in the hypothalamic paraventricular nucleus of the rat. J Neuroendocrinol 13: 139–146, 2001. [DOI] [PubMed] [Google Scholar]
- 63.Oren RM Hyponatremia in congestive heart failure. Am J Cardiol 95: 2B–7B, 2005. [DOI] [PubMed] [Google Scholar]
- 64.Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: converging signals for neurogenic hypertension. Curr Hypertens Rep 9: 228–235, 2007. [DOI] [PubMed] [Google Scholar]
- 65.Paizis G, Cooper ME, Schembri JM, Tikellis C, Burrell LM, Angus PW. Up-regulation of components of the renin-angiotensin system in the bile duct-ligated rat liver. Gastroenterology 123: 1667–1676, 2002. [DOI] [PubMed] [Google Scholar]
- 66.Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM, Angus PW. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut 54: 1790–1796, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patel GP, Balk RA. Recognition and treatment of hyponatremia in acutely ill hospitalized patients. Clin Ther 29: 211–229, 2007. [DOI] [PubMed] [Google Scholar]
- 68.Plant TD, Strotmann R. TRPV4. Handb Exp Pharmacol 189–205, 2007. [DOI] [PubMed]
- 69.Rai A, Whaley-Connell A, McFarlane S, Sowers JR. Hyponatremia, arginine vasopressin dysregulation, and vasopressin receptor antagonism. Am J Nephrol 26: 579–589, 2006. [DOI] [PubMed] [Google Scholar]
- 70.Renaud LP, Bourque CW. Neurophysiology and neuropharmacology of hypothalamic magnocellular neurons secreting vasopressin and oxytocin. Prog Neurobiol 36: 131–169, 1991. [DOI] [PubMed] [Google Scholar]
- 71.Ros J, Fernandez-Varo G, Munoz-Luque J, Arroyo V, Rodes J, Gunnet JW, Demarest KT, Jimenez W. Sustained aquaretic effect of the V2-AVP receptor antagonist, RWJ-351647, in cirrhotic rats with ascites and water retention. Br J Pharmacol 146: 654–661, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saito S, Shingai R. Evolution of thermoTRP ion channel homologs in vertebrates. Physiol Genomics 27: 219–230, 2006. [DOI] [PubMed] [Google Scholar]
- 73.Saliez J, Bouzin C, Rath G, Ghisdal P, Desjardins F, Rezzani R, Rodella LF, Vriens J, Nilius B, Feron O, Balligand JL, Dessy C. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation 117: 1065–1074, 2008. [DOI] [PubMed] [Google Scholar]
- 74.Schrier RW Water and sodium retention in edematous disorders: role of vasopressin and aldosterone. Am J Med 119: S47–S53, 2006. [DOI] [PubMed] [Google Scholar]
- 75.Schrier RW, Fassett RG, Ohara M, Martin PY. Vasopressin release, water channels, and vasopressin antagonism in cardiac failure, cirrhosis, and pregnancy. Proc Assoc Am Physicians 110: 407–411, 1998. [PubMed] [Google Scholar]
- 76.Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 355: 2099–2112, 2006. [DOI] [PubMed] [Google Scholar]
- 77.Sharif Naeini R, Witty MF, Seguela P, Bourque CW. An N-terminal variant of Trpv1 channel is required for osmosensory transduction. Nat Neurosci 9: 93–98, 2006. [DOI] [PubMed] [Google Scholar]
- 78.Smith DM, McKenna K, Thompson CJ. Hyponatraemia. Clin Endocrinol (Oxf) 52: 667–678, 2000. [DOI] [PubMed] [Google Scholar]
- 79.Song D, Sharkey KA, Breitman DR, Zhang Y, Lee SS. Disordered central cardiovascular regulation in portal hypertensive and cirrhotic rats. Am J Physiol Gastrointest Liver Physiol 280: G420–G430, 2001. [DOI] [PubMed] [Google Scholar]
- 80.Song KS, Li S, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J Biol Chem 271: 9690–9697, 1996. [DOI] [PubMed] [Google Scholar]
- 81.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol 2: 695–702, 2000. [DOI] [PubMed] [Google Scholar]
- 82.Stuermer CA, Lang DM, Kirsch F, Wiechers M, Deininger SO, Plattner H. Glycosylphosphatidyl inositol-anchored proteins and fyn kinase assemble in noncaveolar plasma membrane microdomains defined by reggie-1 and -2. Mol Biol Cell 12: 3031–3045, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sunn N, McKinley MJ, Oldfield BJ. Circulating angiotensin II activates neurones in circumventricular organs of the lamina terminalis that project to the bed nucleus of the stria terminalis. J Neuroendocrinol 15: 725–731, 2003. [DOI] [PubMed] [Google Scholar]
- 84.Taylor AC, McCarthy JJ, Stocker SD. Mice lacking the transient receptor vanilloid potential 1 channel display normal thirst responses and central Fos activation to hypernatremia. Am J Physiol Regul Integr Comp Physiol 294: R1285–R1293, 2008. [DOI] [PubMed] [Google Scholar]
- 85.Therapondos G, Hol L, Benjaminov F, Wong F. The effect of single oral low-dose losartan on posture-related sodium handling in post-TIPS ascites-free cirrhosis. Hepatology 44: 640–649, 2006. [DOI] [PubMed] [Google Scholar]
- 86.Thuluvath PJ, Maheshwari A, Wong F, Yoo HW, Schrier RW, Parikh C, Steare S, Korula J. Oral V2 receptor antagonist (RWJ-351647) in patients with cirrhosis and ascites: a randomized, double-blind, placebo-controlled, single ascending dose study. Aliment Pharmacol Ther 24: 973–982, 2006. [DOI] [PubMed] [Google Scholar]
- 87.Van LF, Leo O. Membrane lipid rafts: new targets for immunoregulation. Curr Mol Med 2: 557–570, 2002. [DOI] [PubMed] [Google Scholar]
- 88.Vennekens R, Owsianik G, Nilius B. Vanilloid transient receptor potential cation channels: an overview. Curr Pharm Des 14: 18–31, 2008. [DOI] [PubMed] [Google Scholar]
- 89.Verbalis JG Disorders of body water homeostasis. Best Pract Res Clin Endocrinol Metab 17: 471–503, 2003. [DOI] [PubMed] [Google Scholar]
- 90.Voets T, Prenen J, Vriens J, Watanabe H, Janssens A, Wissenbach U, Bodding M, Droogmans G, Nilius B. Molecular determinants of permeation through the cation channel TRPV4. J Biol Chem 277: 33704–33710, 2002. [DOI] [PubMed] [Google Scholar]
- 91.Watson AM, Hood SG, May CN. Mechanisms of sympathetic activation in heart failure. Clin Exp Pharmacol Physiol 33: 1269–1274, 2006. [DOI] [PubMed] [Google Scholar]
- 92.Wilson HJ, Shutt LE. Syndrome of inappropriate ADH secretion in a woman with preeclampsia. Int J Obstet Anesth 16: 360–362, 2007. [DOI] [PubMed] [Google Scholar]
- 93.Wong F, Liu P, Blendis L. Sodium homeostasis with chronic sodium loading in preascitic cirrhosis. Gut 49: 847–851, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xi D, Kusano K, Gainer H. Quantitative analysis of oxytocin and vasopressin messenger ribonucleic acids in single magnocellular neurons isolated from supraoptic nucleus of rat hypothalamus. Endocrinology 140: 4677–4682, 1999. [DOI] [PubMed] [Google Scholar]
- 95.Xu H, Zhao H, Tian W, Yoshida K, Roullet JB, Cohen DM. Regulation of a transient receptor potential (TRP) channel by tyrosine phosphorylation. SRC family kinase-dependent tyrosine phosphorylation of TRPV4 on TYR-253 mediates its response to hypotonic stress. J Biol Chem 278: 11520–11527, 2003. [DOI] [PubMed] [Google Scholar]
- 96.Yang YY, Lin HC, Huang YT, Lee TY, Hou MC, Lee FY, Liu RS, Chang FY, Lee SD. Effect of 1-week losartan administration on bile duct-ligated cirrhotic rats with portal hypertension. J Hepatol 36: 600–606, 2002. [DOI] [PubMed] [Google Scholar]
- 97.Zajchowski LD, Robbins SM. Lipid rafts and little caves. Compartmentalized signalling in membrane microdomains. Eur J Biochem 269: 737–752, 2002. [DOI] [PubMed] [Google Scholar]