Abstract
This study tested the hypothesis that inducing hyperinsulinemia and hyperglycemia in dogs, by infusing glucose chronically intravenously, would increase tubular sodium reabsorption and cause hypertension. Glucose was infused for 6 days (14 mg·kg−1·min−1 iv) in five uninephrectomized (UNX) dogs. Mean arterial pressure (MAP) and renal blood flow (RBF) were measured 18 h/day using DSI pressure units and Transonic flow probes, respectively. Urinary sodium excretion (UNaV) decreased significantly on day 1 and remained decreased over the 6 days, coupled with a significant, sustained increase in RBF, averaging ∼20% above control on day 6. Glomerular filtration rate and plasma renin activity (PRA) also increased. However, although MAP tended to increase, this was not statistically significant. Therefore, the glucose infusion was repeated in six dogs with 70% surgical reduction in kidney mass (RKM) and high salt intake. Blood glucose and plasma insulin increased similar to the UNX dogs, and there was significant sodium retention, but MAP still did not increase. Interestingly, the increases in PRA and RBF were prevented in the RKM dogs. The decrease in UNaV, increased RBF, and slightly elevated MAP show that glucose infusion in dogs caused a sustained increase in tubular sodium reabsorption by a mechanism independent of pressure natriuresis. The accompanying increase in PRA, together with the failure of either RBF or PRA to increase in the RKM dogs, suggests the site of tubular reabsorption was before the macula densa. However, the volume retention and peripheral edema suggest that systemic vasodilation offsets any potential renal actions to increase MAP in this experimental model in dogs.
Keywords: blood pressure, metabolic syndrome, sodium excretion
hypertension is a major complication of metabolic syndrome and obesity, and its etiology has been linked closely to sodium retention (28, 34, 39). Early studies that showed decreased urinary sodium chloride excretion during acute insulin infusions (9, 11, 26) led to the hypothesis that hyperinsulinemia could contribute to the hypertension in metabolic syndrome (28, 34, 39). However, although there is good evidence that the renin-angiotensin-aldosterone and sympathetic nervous systems contribute to sodium retention and hypertension in metabolic syndrome (8, 10, 21, 27, 33), there remains no direct evidence outside of experimental models in rats that the hyperinsulinemia or impaired glucose homeostasis in metabolic syndrome have a direct, sustained sodium-retaining effect.
An established experimental model of metabolic syndrome is created by feeding rats a diet high in the simple sugars glucose, fructose, or sucrose (12, 20, 22, 23, 30, 36), which causes modest increases in plasma insulin, glucose, and blood pressure. We used 7-day intravenous infusions of insulin plus glucose (4, 5, 25) or glucose alone (2, 7) in rats and also measured increased insulin, glucose, and mean arterial pressure (MAP), but in addition measured sodium retention that was linked to a transient renal vasodilation followed by sustained renal vasoconstriction. These studies in rats supported a role for hyperglycemia and/or hyperinsulinemia to cause sodium retention and hypertension.
However, a major roadblock in translating those results to mechanisms for human disease is that they have not been replicated in humans or nonrodent animal models (32). Chronic sugar feeding does not cause hypertension in humans (41) or dogs (32), and, although dietary-induced obesity in dogs replicates the insulin resistance, hyperinsulinemia, sodium retention, hypertension, and other aspects of metabolic syndrome in humans, chronic insulin plus glucose infusion in dogs actually decreased blood pressure and blood glucose concentration (6, 14, 17). Thus, although the dogs in those insulin infusion studies retained sodium, it is likely that decreased blood pressure played a major role in causing the sodium retention due to reduced pressure natriuresis.
It is important, therefore, that we recently reported a significant, sustained decrease in urinary sodium excretion during chronic intravenous glucose infusion in dogs, which caused modest increases in glucose and insulin concentrations but no decrease in blood pressure (3). Although a statistically significant hypertensive effect was not measured unless we also blocked cyclooxygenase-2 chronically (3), those results were the first evidence in dogs for a direct, chronic sodium-retaining response to the induction of hyperglycemia and hyperinsulinemia. To determine whether that chronic action is the result of renal vasoconstriction or tubular reabsorption, we developed a method for 24 h/day renal blood flow (RBF) measurement in dogs to obtain the most precise assessment of continuous renal function possible in this experimental model. In addition, we repeated the experiment in dogs with reduced kidney mass (RKM) and high salt intake in an attempt to augment the sodium-retaining response to glucose infusion and enable a hypertensive response.
METHODS
Experiments were conducted in conditioned male mongrel dogs weighing between 20 and 25 kg, and the experimental protocols were approved by the Institutional Animal Care and Use Committee of the Medical College of Georgia. In five dogs, under isoflurane anesthesia with aseptic technique, a flow probe (4PSB; Transonic, Ithaca, NY) was placed on the left renal artery via a flank incision and retroperitoneal approach, and the cable was tunneled subcutaneously to the scapular region and exteriorized. The right kidney was removed via a right flank incision. The catheter from a Data Sciences (St. Paul, MN) TA11PA-D70 blood pressure unit was implanted in the right femoral artery, and a standard fluid-filled Tygon catheter was implanted in the right femoral vein. Tygon catheters also were placed in the left femoral artery and vein. The DSI transducer/transmitter body was placed subcutaneously in the right flank, and the Tygon catheters were tunneled subcutaneously to the scapular region and exteriorized, then filled with 1,000 U/ml heparin solution and closed. Dogs were fitted with a canvas jacket that had a pocket to house the catheters and flow probe cable and were under the direct care of the attending veterinarian until recovery was complete and they were transferred to their runs.
RKM dogs.
To augment the sodium-retaining response to glucose infusion in dogs (3) and enhance any potential hypertensive action, the glucose infusion was repeated in six dogs with surgical RKM and high-salt intake. Under isoflurane anesthesia with aseptic technique, the poles of the left kidney were removed, one at a time, via retroperitoneal flank incision using a scalpel and heat cautery followed by application of thrombin-soaked Gelfoam (Pharmacia, Kalamazoo, MI). After both poles were removed, the flow probe was implanted as above. Three weeks were allowed for recovery to ensure complete healing and recovery of left kidney function. During the second surgical procedure 3 wk later, the right kidney was removed via retroperitoneal flank incision to yield an ∼70% total RKM, and the DSI transmitter and Tygon catheters were implanted as described above.
Swiveled and telemetric connections to chronic infusion and recording apparatus.
Following 1–2 wk of recovery, dogs were placed in individual metabolic cages and fitted with a padded lucite harness that was connected via flexible stainless steel tubing (∼ in. outer diameter; Harvard Apparatus, Boston, MA) to a strain relief swivel (Instech, Plymouth Meeting, PA) mounted on the top center of each cage. The strain relief swivel was customized to enable mounting of an Airflyte (Bayonne, NJ) electrical swivel and a 20-gauge hydraulic swivel from Instech. The Airflyte swivel has a pass-through hole through its center to enable routing of the catheters through the swivel. This unique arrangement enables the two swivels to be connected in series and turn synchronously, thus enabling continuous intravenous infusion and electrical connectivity while the dogs have completely unrestricted, 360-degree freedom of movement in the cage 24 h/day. This serial swivel arrangement is similar to our method used for chronic RBF measurement in rats (1). The lucite harnesses, canvas jackets, and all the connectors between the harness, tethering tubing, strain relief swivel, and the Airflyte and Instech swivels were constructed in our laboratory or the Medical College of Georgia machine shop.
Infusion tubing and the flowmeter cable were routed through the steel tethering tubing and connected, respectively, to one of the femoral vein catheters and to the renal flow probe cable at the harness. The other end of the flowmeter cable was cut in two ∼6 in. from the plug. Each cut end was soldered to the appropriate side of the Airflyte electrical swivel, and the plug was inserted in a Transonic 400-series flowmeter on top of the cage. The flowmeter was connected to a DSI data matrix using a CV-11 analog converter. Two RMC-1 DSI receivers were mounted on each cage to collect the transmitter signal, and the DSI A.R.T. software was used to collect the pressure and flow signals from each dog for 10 s each minute at 100 Hz for 18 h/day (1400–0800). The infusion line was connected, via the Instech swivel, to two Instech P720 peristaltic pumps on top of each cage.
Experimental procedure.
Sodium intake was maintained constant (i.e., clamped) at an average of 80 ± 2 meq/day in the uninephrectomized (UNX) dogs and 306 ± 5 meq/day in RKM dogs by feeding a low-sodium diet (Hills H/D) coupled with a continuous intravenous infusion of ∼475 and 1,950 ml of 0.9% saline/day, respectively, throughout the study. In addition, all dogs received ∼975 ml of sterile water/day via continuous intravenous infusion using the second peristaltic pump. The lines from the saline and water pumps were joined at a stopcock, and all solutions were pumped through disposable filters (0.22 mm, Cathivex; Millipore) in each dog's intravenous infusion line. Drinking water was available ad libitum, and antibiotics were administered daily for the duration of the study.
Approximately 2 wk were allowed for the dogs to acclimate to the metabolic cages and be trained to lie quietly for blood sampling. After sodium balance and stable hemodynamics were verified, control measurements were recorded for 3 days. Glucose (∼14 mg·kg−1·min−1) then was infused in all dogs for 6 days by replacing the ∼975 ml sterile water infusion with an equal volume of 50% dextrose solution. After 6 days, the sterile water infusion was restored for a postexperimental control (recovery) period. Blood samples were drawn for hormone and related measurements during the control period, on glucose infusion days 2 and 5, and during the recovery period.
Analytical procedures.
Glomerular filtration rate (GFR) was determined from the total plasma clearance of 125I-labeled iothalamate (Glofil; QOL Medical, Kirkland, WA) (18). Plasma and urine sodium and potassium concentrations were determined with ion sensitive electrodes (Synchron El-ise; Beckman Coulter, Fullerton, CA), plasma protein concentration was measured by refractometry, blood glucose was measured with an Accu-Check meter (Roche, Indianapolis, IN), plasma osmolarity was measured with a microosmometer from Advanced Instruments (Norwood, MA), urinary prostaglandins were measured using enzyme immunoassay (EIA) kits from Cayman Chemicals (Ann Arbor, MI), plasma insulin was measured using an EIA kit from ALPCO Diagnostics (Salem, NH), and plasma renin activity (PRA) was measured by radioimmunoassay (DiaSorin, Stillwater, MN).
Data from RKM and UNX dogs were analyzed with a repeated-measures analysis of variance, using Dunnett's test for comparison of experimental and recovery values with control values. For the large comparisons of daily data (e.g., RBF), an average value for the control period and the R5 value for the recovery period were used in Dunnett's test to compare the recovery period and each experimental day with the control period. The control for time in this experimental design is the recovery period. The ANOVA and Dunnett's test must show that the recovery period is not different from control to assert that any changes measured during the experimental period were independent of time. Statistical significance was considered to be P < 0.05. All data are expressed as means ± SE.
RESULTS
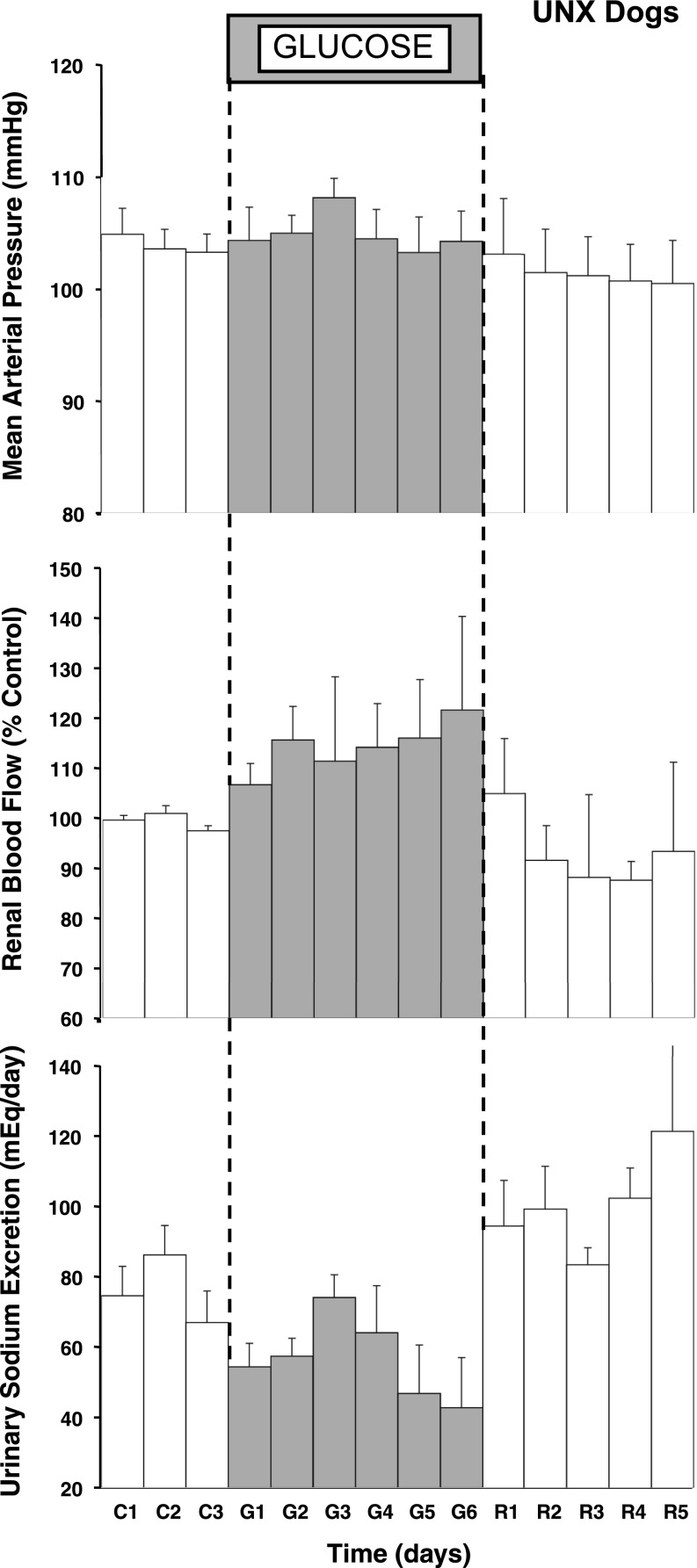
Table 1 shows that glucose infusion caused modest but significant hyperglycemia and an approximate two- to fourfold increase in plasma insulin concentration. Figure 1 shows that there was no significant effect of glucose infusion on MAP in the UNX dogs. This is consistent with our previous report in normal, two-kidney dogs (3), and it is important that there was not a decrease in MAP as reported in dogs infused with insulin plus glucose (3, 6, 14). In fact, there was a tendency for MAP to drift upward, also consistent with our previous findings (3). Heart rate averaged 73 ± 2 beats/min during the control period in the UNX dogs, increased to an average of 89 ± 7 beats/min during glucose infusion, and returned to control levels during recovery. RBF averaged 123 ± 17 ml/min at baseline and increased immediately following glucose infusion, and the increase was sustained, averaging ∼20% above control by day 6 of glucose infusion (Fig. 1). GFR increased significantly by day 5 (Table 1). Urinary sodium excretion decreased immediately, tended to recover, but was below control levels on days 5 and 6, and this sodium excretory response to glucose infusion was remarkably similar to the pattern reported previously in two-kidney dogs (3). Sodium balance increased over the 6 days by an average of 102 ± 13 meq, and the changes in extracellular fluid volume (ECFV) and plasma protein concentration corroborate the volume retention (Table 1).
Table 1.
Plasma concentrations, GFR, ECFV, and hematocrit during each study period
| Variable | Group | Experimental Period |
|||
|---|---|---|---|---|---|
| Control | Glucose D2 | Glucose D5 | Recovery | ||
| Plasma insulin, μU/ml | UNX dogs | 28±4 | 45±12* | 41±13 | 24±6 |
| RKM dogs | 13±4 | 50±12* | 47±13* | 17±6 | |
| Blood glucose, mmol/l | UNX dogs | 6.5±0.2 | 7.3±0.4* | 6.9±0.1 | 7.1±0.2 |
| RKM dogs | 7.2±0.3 | 7.3±0.1 | 8.8±0.7* | 7.2±0.1* | |
| GFR, ml/min | UNX dogs | 55±5 | 60±5 | 64±4* | 50±3 |
| RKM dogs | 45±4 | 50±5 | 51±6 | 44±5 | |
| ECFV, ml | UNX dogs | 7,396±558 | 7,499±780* | 8,286±401* | 8,420±260* |
| RKM dogs | 6,813±489 | 8,459±488* | 8,144±511* | 7,774±530 | |
| Plasma protein, g/dl | UNX dogs | 6.7±0.2 | 6.3±0.2 | 5.9±0.1* | 6.6±0.2 |
| RKM dogs | 6.5±0.2 | 6.1±0.2 | 6.0±0.3* | 6.9±0.3 | |
| Hematocrit, % | UNX dogs | 36±3 | 33±2 | 29±2* | 28±1* |
| RKM dogs | 35±2 | 33±2 | 33±2 | 32±2 | |
Values are means ± SE. UNX, uninephrectomized; RKM, reduced kidney mass; GFR, glomerular filtration rate; ECFV, extracellular fluid volume; D2, day 2; D5, day 5.
P < 0.05, within-group vs. control period.
Fig. 1.
Measurements of mean arterial pressure, renal blood flow, and daily urinary sodium excretion for 18 h/day during the control (C), glucose (G), and recovery (R) periods in uninephretomized (UNX) dogs. *P < 0.05 vs. average control.
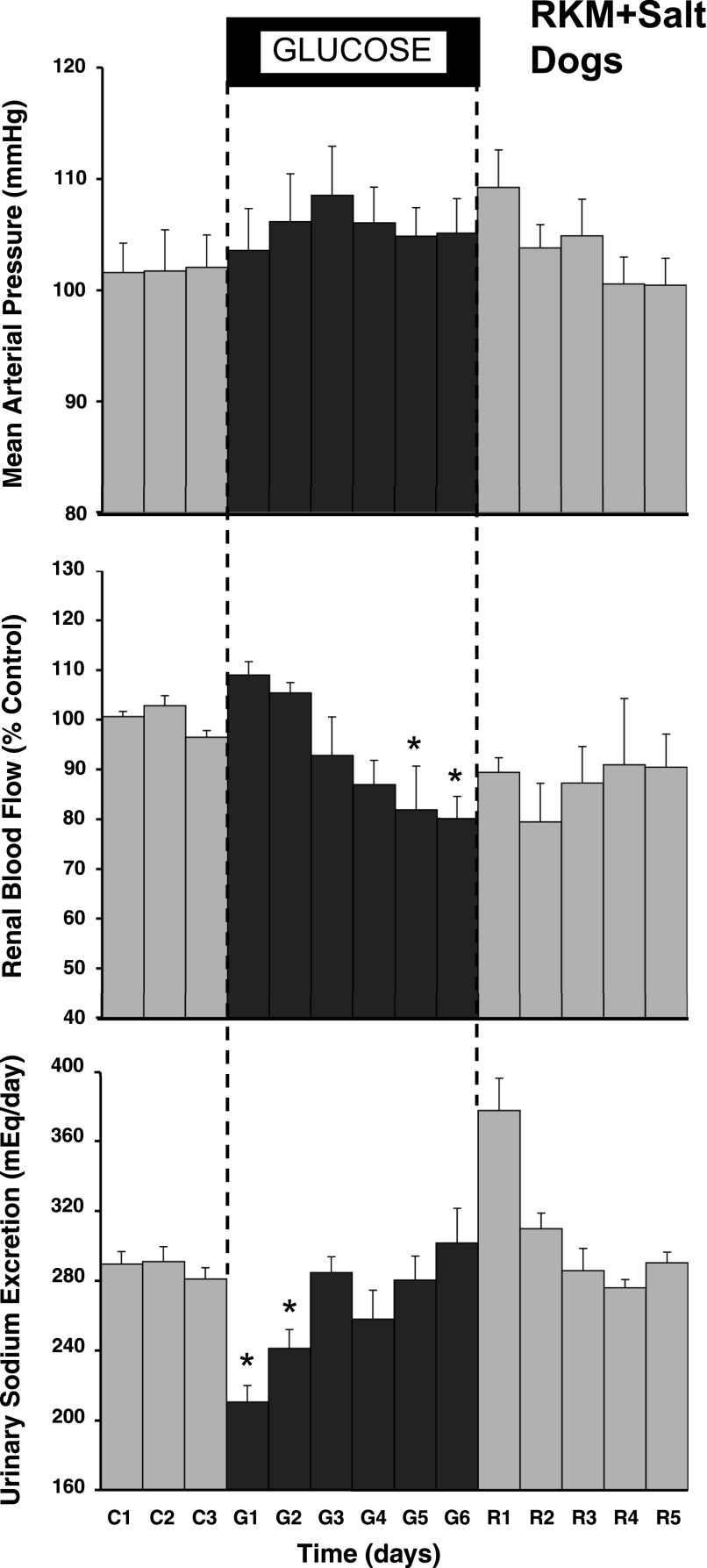
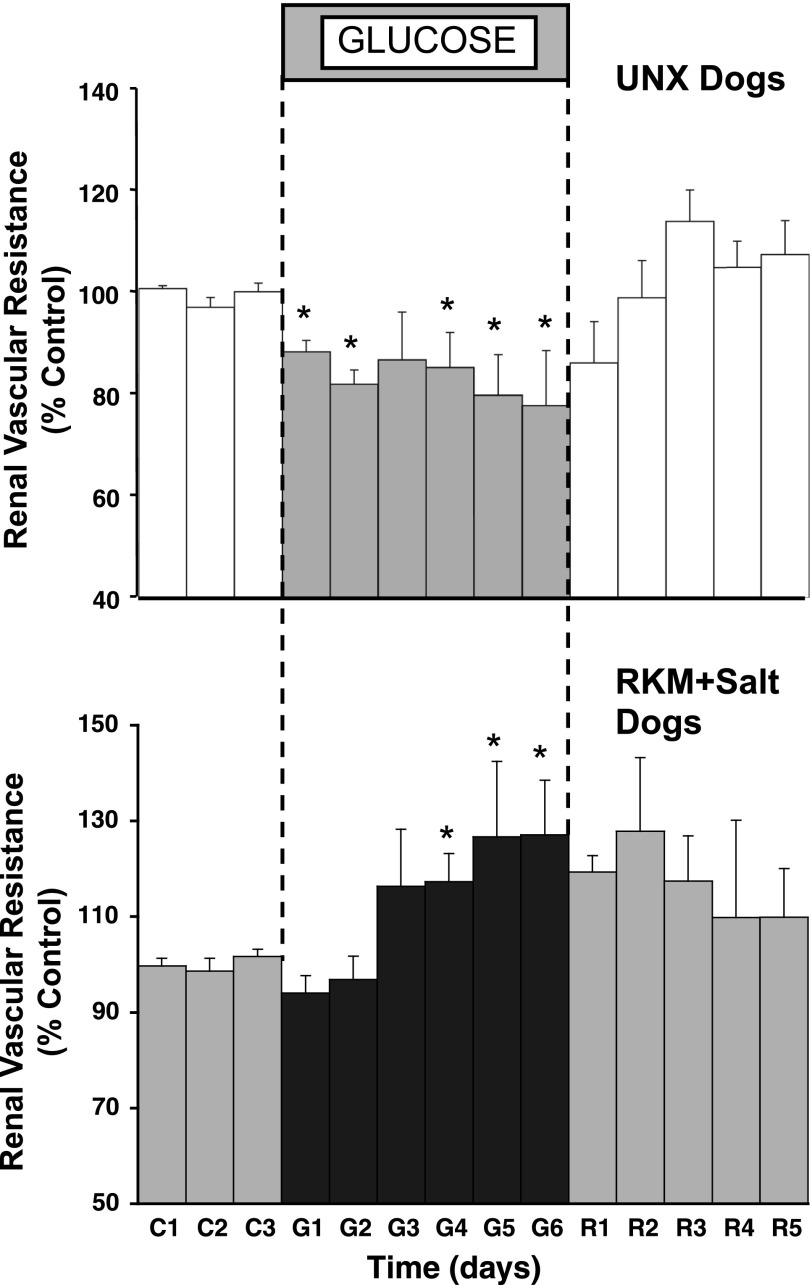
In the RKM dogs, there was a similar, immediate reduction in urinary sodium excretion (Fig. 2), and sodium balance increased by 148 ± 38 meq by day 6 of glucose infusion. RBF, which averaged 84 ± 18 ml/min during baseline, began to increase over the first 2 days of glucose infusion, similar to the response in the UNX dogs, but the effect waned and actually was decreased significantly by the end of the period. MAP tended to increase during glucose infusion, similar to the response in the UNX dogs, but this was not statistically significant. Figure 3, however, shows that there was a significant increase in renal vascular resistance in the RKM dogs during glucose infusion compared with the renal vasodilator response in UNX dogs. Plasma protein concentration and ECFV in the RKM dogs changed similar to the response in UNX dogs (Table 1), and there was clear evidence of pitting edema on examination in both groups. The heart rate response also was similar, averaging 78 ± 2 beats/min in the RKM dogs at baseline, increasing to an average of 86 ± 3 beats/min during the 6-day glucose period, and returned to control levels during recovery.
Fig. 2.
Measurements of mean arterial pressure, renal blood flow, and daily urinary sodium excretion for 18 h/day during the control, glucose, and recovery periods in reduced kidney mass (RKM) dogs. *P < 0.05 vs. average control.
Fig. 3.
Calculated renal vascular resistance during control, glucose, and recovery periods in UNX dogs (top) and in RKM dogs (bottom). *P < 0.05 vs. average control.
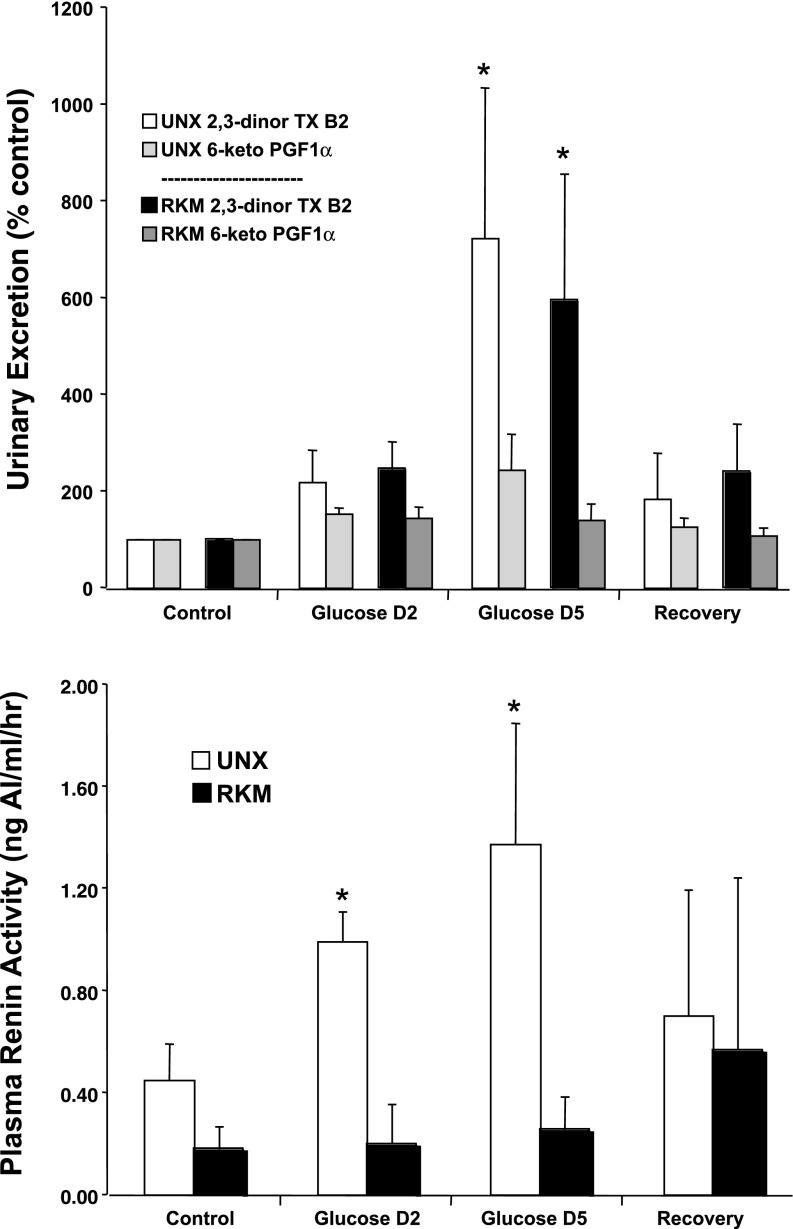
Figure 4 shows the increase in urinary 2,3-dinor thromboxane B2 excretion caused by glucose infusion in both groups. There were no significant changes in excretion of the prostacyclin metabolite 6-keto-PGF1α (Fig. 4) or in PGE metabolites (data not shown). Figure 4 also shows that PRA increased in the UNX dogs, consistent with our previous report (3). Baseline PRA was low in the RKM dogs, consistent with decreased kidney mass and a fourfold greater sodium intake, and it did not increase with glucose infusion.
Fig. 4.
Urinary excretion of thromboxane and prostaglandin metabolites (top) and plasma renin activity (bottom) during the control period, days 2 and 5 of the glucose period, and the recovery period in the UNX and RKM dogs. *P < 0.05 vs. within-group control.
Baseline RBF: Methodological issues.
Baseline RBF averaged 123 ± 17 ml/min in the UNX dogs, which is lower than we predicted. In dogs this weight, we have reported that 24 h/day cardiac output averaged 2.3 ± 0.1 l/min (6), so we predicted ∼230 ml/min RBF if each kidney is assumed to receive ∼10% of cardiac output. This is the first study to our knowledge in which RBF has been measured 24 h/day, chronically, in dogs, but we have revised our surgical methods in ongoing studies to address this. In short, by filling the probe window with sterile lubricating jelly, wrapping it in dacron mesh, and covering it in quick-hardening latex, we believe we have prevented infiltration of the probe window by fat after wound closure and recovery, and we now obtain daily RBF measurements in the 200–400 ml/min range, as would be expected with adequate compensation after uninephrectomy. It is important to note that our RBF comparisons were within-group, and the recovery of RBF during the postexperimental period verifies the stability of the probe and the measurements. The baseline value is what we are addressing here.
DISCUSSION
The use of 24 h/day methods for measuring RBF and arterial pressure in this study demonstrated that chronic glucose infusion caused significant sodium retention in dogs by increasing tubular sodium reabsorption. This is because, 1) the tendency for MAP to increase indicates that a decrease in blood pressure did not contribute to the sodium retention via reduced pressure natriuresis, and 2) the only way that significant decreases in urinary sodium excretion can occur in the presence of significant, sustained renal vasodilation and no change in sodium intake is if renal tubular sodium reabsorption is increased. However, despite the sodium retention, there was no significant increase in MAP, even in the RKM dogs that were predisposed to volume-dependent hypertension. This suggests that marked systemic vasodilation counteracted the potential for the sodium retention to cause hypertension in this experimental model.
There has long been interest in the potential for dysregulation of glucose metabolism to cause hypertension. Glomerular injury late in the course of type I and type II diabetes provides a ready mechanism to help explain hypertension after years of diabetes, but hypertension occurs much sooner in patients destined to develop type II diabetes, and GFR actually is elevated during those early stages. The obesity, hyperglycemia, hyperinsulinemia, and hypertension in those patients are part of a constellation of findings collectively called metabolic syndrome, and the increases in plasma glucose and insulin are two factors that have been hypothesized to mediate the early increases in blood pressure (28, 35, 38, 39). This is due in part to acute experiments that support an effect of insulin to stimulate renal tubular sodium reabsorption (9, 26), but direct links between hyperinsulinemia and/or hyperglycemia, sodium retention, and hypertension have not been established convincingly in a chronic experimental model that translates readily to humans. This study and our previous study (3) show that chronic glucose infusion in dogs increases plasma insulin and glucose concentrations similar to the effect of metabolic syndrome in humans.
Chronic fat feeding in dogs has been shown to induce obesity and hypertension and to be consistent with many findings in obese, hypertensive human subjects, including hyperinsulinemia and hyperglycemia (10, 13, 16). The increase in circulating insulin in the glucose-infused dogs in this study is similar to the increase measured after 5 wk of fat feeding in dogs (13). The increase in blood glucose concentration also is similar to the response to induction of obesity in dogs, and it contrasts sharply with the decrease in glucose reported previously during insulin plus glucose infusion in dogs that decreased MAP (6, 14, 17). Thus, the intravenous glucose infusion in dogs produced modest hyperinsulinemia and hyperglycemia comparable to what occurs in patients with metabolic syndrome, and also consistent with the increases in those variables in fat-fed dogs that develop obesity and hypertension. Of course one key response that was missing in the current study was significant hypertension, even when kidney mass was reduced and salt intake was raised in an effort to facilitate volume-mediated hypertension. The increase in ECFV and the observation of peripheral edema suggest that there still was sufficient systemic vasodilation even in the RKM dogs to prevent the sodium retention from causing hypertension, and it is important to note that these levels of plasma insulin and glucose took 5 wk, rather than 3–5 days, to be reached in obese dogs, paralleling the increases in body weight and blood pressure.
Nonetheless, these results shed considerable light on the mechanism for the sodium retention caused by glucose infusion. Chronic, 18 h/day measurement of RBF provided a much more detailed picture of renal function than has been obtainable previously from spot measurements with clearance methods, and the significant, sustained renal vasodilation in the UNX dogs (Figs. 1 and 3), together with the increase in GFR, is strong evidence that the decrease in urinary sodium excretion caused by glucose infusion was the result of increased tubular reabsorption. In the steady state with fixed sodium intake, the only way urinary sodium excretion can be decreased is through renal vasoconstriction or increased tubular reabsorption. Each action has secondary effects on the other, but the RBF measurements in this study rule out renal vasoconstriction as a primary cause for the sodium retention, although effects on the renal medullary circulation per se cannot be discerned in this chronic setting. Ecelbarger and others (37, 40) have provided intriguing new evidence for actions of insulin on renal sodium chloride transporters that could play a role, but because chronic intrarenal infusion of insulin in dogs was shown previously to cause only modest and intermittent decreases in urinary sodium excretion (15), significant effects due to insulin acting alone in the kidney also are unlikely. One possible explanation could be the hyperglycemia acting alone or in concert with the hyperinsulinemia in the kidney, for instance, if the increase in filtered load of glucose was sufficient to cause significant stimulation of proximal tubular glucose-linked sodium transport. In fact, previous acute dog studies (42) have linked a proximal tubular, sodium-reabsorbing effect of glucose to renal vasodilation, increased GFR, and increased renin secretion via tubuloglomerular feedback (TGF), and the significant increases in PRA and RBF in the UNX dogs in this study are consistent with that possibility, or at least with increased reabsorption before the macula densa. However, this experimental model does not enable determination of the relative roles of insulin and glucose in causing the sodium retention, or whether the response was because of direct tubular actions or mediated via systemic mechanisms such as activation of the renal nerves, as the increased heart rate response may implicate.
The reason why the sodium retention waned and RBF decreased in the second half of the glucose infusion period in the RKM dogs is not known, because greater sodium retention was predicted. It is interesting that the decrease in sodium excretion that was measured in those dogs occurred without evidence for an increase in PRA, which questions the role of that system in mediating the sodium retention. On the other hand, the progressive rise in PRA in the UNX dogs and “flat” PRA response in the RKM dogs provides a feasible explanation for the continued decrease in sodium excretion in the former and return to balance in the latter, and the different PRA levels at the end of the 6-day period also may explain the rapid natriuresis in the RKM dogs vs. the slower response in the UNX dogs during the recovery period. A role for the renin-angiotensin system in contributing to the sodium retention does not rule out the possibility that renin secretion was a consequence of glucose-driven sodium reabsorption in the proximal tubule. Thus one could hypothesize that, in the RKM dogs with the greatly elevated tubular flow rates and high salt intake, this effect would be minimized, thereby also explaining why there was not a TGF-mediated increase in RBF. The renal vasculature in the RKM dogs also could have been at or near maximum dilation just due to the drastic reduction in nephron mass; however, it is important to note that the RKM, high-salt dogs were added simply as an attempt to “force” glucose infusion to cause hypertension, and the study design is not appropriate to enable accurate assessment of these possibilities.
Nevertheless, the unexpected and strikingly different renal responses in the RKM dogs deserve attention, and another possible explanation for the lack of renal vasodilation, and indeed vasoconstriction, in the RKM dogs is the change in thromboxane. The increases in thromboxane were most evident in the later phase of the infusion at the same time when RBF decreased in the RKM dogs, and the surgically ablated kidney in the RKM dogs may have been more sensitive than the normal kidney in the UNX dogs to the renal vasoconstrictor actions of ANG II and thromboxane (17, 19) However, that effect would not explain why urinary sodium excretion did not remain low. It is interesting that blockade of ANG II and thromboxane each has been shown to prevent fructose hypertension in rats (12, 20, 23) and the hypertension caused by insulin plus glucose infusion in rats (4, 24, 25), and acute hyperglycemia also has been reported to stimulate the renin-angiotensin system in humans (29, 31). The role of those systems in this chronic dog model, including the UNX dogs, remains to be tested, as well as the potential contributions of volume overload via mechanisms such as atrial natriuretic peptide in mediating the escape from sodium retention in the RKM dogs, but it is important to note that a high-salt UNX group would be necessary to draw specific conclusions about differences in sodium balance in the RKM dogs.
In summary, the 18 h/day measurements of RBF and MAP in this study revealed a direct effect of chronic glucose infusion to increase renal tubular sodium reabsorption. Whether systemic responses to glucose infusion, such as the sympathetic nervous or renin-angiotensin systems, thromboxane, and hyperinsulinemia, mediate the sodium-retaining action, or whether direct renal actions of insulin and/or glucose also contribute to the response, is not known, but an indirect effect due to low blood pressure and reduced pressure natriuresis is not supported by these data. These data support the hypothesis that glucose infusion in dogs has a sustained sodium-retaining effect caused by increasing tubular reabsorption. However, the blood pressure impact of these renal actions in insulin-resistant states, independent of the systemic vasodilatory response, remains to be determined.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-56259, HL-75625, and HL-74167.
Acknowledgments
We acknowledge the technical assistance of Vanessa Springfield and Cassandra Henry and the surgical assistance of Cedrick Bouey.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bell TD, DiBona GF, Wang Y, Brands MW. Mechanisms for renal blood flow control early in diabetes as revealed by chronic flow measurement and transfer function analysis. J Am Soc Nephrol 17: 2184–2192, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Brands MW, Fitzgerald SM. Chronic intravenous glucose infusion causes moderate hypertension in rats. Am J Hypertens 13: 99–102, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Brands MW, Hailman AE, Fitzgerald SM. Long-term glucose infusion increases arterial pressure in dogs with cyclooxygenase-2 inhibition. Hypertension 37: 733–738, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Brands MW, Harrison DL, Keen HL, Garnder A, Shek EW, Hall JE. Insulin-induced hypertension in rats depends on an intact renin-angiotensin system. Hypertension 29: 1014–1019, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Brands MW, Lee WF, Keen HL, Alonso-Galicia M, Zappe DH, Hall JE. Cardiac output and renal function during insulin-hypertension in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 271: R276–R281, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Brands MW, Mizelle HL, Gaillard CA, Hildebrandt DA, Hall JE. The hemodynamic response to chronic hyperinsulinemia in conscious dogs. Am J Hypertens 4: 164–168, 1991. [DOI] [PubMed] [Google Scholar]
- 7.Claxton CR, Cameron JA, Fitzgerald SM, Brands MW. Inhibition of nitric oxide synthesis potentiates hypertension during chronic glucose infusion in rats. Hypertension 35: 451–456, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Cooper SA, Whaley-Connell A, Habibi J, Wei Y, Lastra G, Manrique C, Stas S, Sowers JR. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol 293: H2009–H2023, 2007. [DOI] [PubMed] [Google Scholar]
- 9.DeFronzo RA The effect of insulin on renal sodium metabolism. Diabetologia 21: 165–171, 1981. [DOI] [PubMed] [Google Scholar]
- 10.de Paula RB, da Silva AA, Hall JE. Aldosterone antagonism attenuates obesity-induced hypertension and glomerular hyperfiltration. Hypertension 43: 41–47, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Friedberg CE, van Buren M, Bijlsma JA, Koomans HA. Insulin increases sodium reabsorption in diluting segment in humans: evidence for indirect mediation through hypokalemia. Kidney Int 40: 251–256, 1991. [DOI] [PubMed] [Google Scholar]
- 12.Galipeau D, Arikawa E, Sekirov I, McNeill JH. Chronic thromboxane synthase inhibition prevents fructose-induced hypertension. Hypertension 38: 872–876, 2001. [PubMed] [Google Scholar]
- 13.Hall JE, Brands MW, Dixon WN, Smith MJ Jr. Obesity-induced hypertension. Renal function and systemic hemodynamics. Hypertension 22: 292–299, 1993. [DOI] [PubMed] [Google Scholar]
- 14.Hall JE, Brands MW, Kivlighn SD, Mizelle HL, Hildebrandt DA, Gaillard CA. Chronic hyperinsulinemia and blood pressure. Interaction with catecholamines? Hypertension 15: 519–527, 1990. [DOI] [PubMed] [Google Scholar]
- 15.Hall JE, Brands MW, Mizelle HL, Gaillard CA, Hildebrandt DA. Chronic intrarenal hyperinsulinemia does not cause hypertension. Am J Physiol Renal Fluid Electrolyte Physiol 260: F663–F669, 1991. [DOI] [PubMed] [Google Scholar]
- 16.Hall JE, Brands MW, Zappe DH, Dixon WN, Mizelle HL, Reinhart GA, Hildebrandt DA. Hemodynamic and renal responses to chronic hyperinsulinemia in obese, insulin-resistant dogs. Hypertension 25: 994–1002, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Hall JE, Coleman TG, Mizelle HL, Smith MJJ. Chronic hyperinsulinemia and blood pressure regulation. Am J Physiol Renal Fluid Electrolyte Physiol 258: F722–F731, 1990. [DOI] [PubMed] [Google Scholar]
- 18.Hall JE, Guyton AC, Farr BM. A single-injection method for measuring glomerular filtration rate. Am J Physiol Renal Fluid Electrolyte Physiol 232: F72–F76, 1977. [DOI] [PubMed] [Google Scholar]
- 19.Hall JE, Mizelle HL, Brands MW, Hildebrandt DA. Pressure natriuresis and angiotensin II in reduced kidney mass, salt-induced hypertension. Am J Physiol Regul Integr Comp Physiol 262: R61–R71, 1992. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh PS Reversal of fructose-induced hypertension and insulin resistance by chronic losartan treatment is independent of AT2 receptor activation in rats. J Hypertens 23: 2209–2217, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Kassab S, Kato T, Wilkins FC, Chen R, Hall JE, Granger JP. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension 25: 893–897, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Katakam PVG, Ujhelyi MR, Hoenig ME, Miller AW. Endothelial dysfunction precedes hypertension in diet-induced. Am J Physiol Regul Integr Comp Physiol 275: R788–R792, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Katovich MJ, Iyer SN. Effect of chronic losartan potassium treatment on fructose-induced hypertension. Life Sci 55: 134–144, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Keen HL, Brands MW, Smith MJJ, Hall JE. Maintenance of baseline angiotensin II potentiates insulin hypertension in rats. Hypertension 31: 637–642, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Keen HL, Brands MW, Smith MJJ, Shek EW, Hall JE. Inhibition of thromboxane synthesis attenuates insulin-hypertension in rats. Am J Hypertens 10: 1125–1131, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Kirchner KA Insulin increases loop segment chloride reabsorption in the euglycemic rat. Am J Physiol Renal Fluid Electrolyte Physiol 255: F1206–F1213, 1988. [DOI] [PubMed] [Google Scholar]
- 27.Landsberg L Insulin sensitivity in the pathogenesis of hypertension and hypertensive complications. Clin Exp Hypertens 18: 337–346, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Lastra G, Manrique C, Sowers JR. Obesity, cardiometabolic syndrome, and chronic kidney disease: the weight of the evidence. Adv Chronic Kidney Dis 13: 365–373, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Miller JA Impact of hyperglycemia on the renin angiotensin system in early human type 1 diabetes mellitus. J Am Soc Nephrol 10: 1778–1785, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Nishimoto Y, Tomida T, Matsui H, Ito T, Okumura K. Decrease in renal medullary endothelial nitric oxide synthase of fructose-fed, salt-sensitive hypertensive rats. Hypertension 40: 190–194, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Osei SY, Price DA, Fisher ND, Porter L, Laffel LM, Hollenberg NK. Hyperglycemia and angiotensin-mediated control of the renal circulation in healthy humans. Hypertension 33: 559–564, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Pamies-Andreu E, Fiksen-Olsen M, Rizza R, Romero JC. High-fructose feeding elicits insulin resistance without hypertension in normal mongrel dogs. Am J Hypertens 8: 732–738, 1995. [DOI] [PubMed] [Google Scholar]
- 33.Robles RG, Villa E, Santirso R, Martinez J, Ruilope LM, Cuesta C, Sancho JM. Effects of captopril on sympathetic activity, lipid and carbohydrate metabolism in a model of obesity-induced hypertension in dogs. Am J Hypertens 6: 1009–1019, 1993. [DOI] [PubMed] [Google Scholar]
- 34.Sarafidis PA, Bakris GL. The antinatriuretic effect of insulin: an unappreciated mechanism for hypertension associated with insulin resistance? Am J Nephrol 27: 44–54, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Sarafidis PA, Whaley-Connell A, Sowers JR, Bakris GL. Cardiometabolic syndrome and chronic kidney disease: what is the link? J Cardiometab Syndr 1: 58–65, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Shinozaki K, Ayajiki K, Nishio Y, Sugaya T, Kashiwagi A, Okamura T. Evidence for a causal role of the renin-angiotensin system in vascular dysfunction associated with insulin resistance. Hypertension 43: 255–262, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Song J, Hu X, Shi M, Knepper MA, Ecelbarger CA. Effects of dietary fat, NaCl, and fructose on renal sodium and water transporter abundances and systemic blood pressure. Am J Physiol Renal Physiol 287: F1204–F1212, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Sowers JR Insulin resistance and hypertension. Am J Physiol Heart Circ Physiol 286: H1597–H1602, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Strazzullo P, Barbato A, Galletti F, Barba G, Siani A, Iacone R, D'Elia L, Russo O, Versiero M, Farinaro E, Cappuccio FP. Abnormalities of renal sodium handling in the metabolic syndrome. Results of the Olivetti Heart Study. J Hypertens 24: 1633–1639, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Tiwari S, Riazi S, Ecelbarger CA. Insulin's impact on renal sodium transport and blood pressure in health, obesity, and diabetes. Am J Physiol Renal Physiol 293: F974–F984, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Van der Schaaf MR, Koomans HA, Joles JA. Dietary sucrose does not increase twenty-four-hour ambulatory blood pressure in patients with either essential hypertension or polycystic kidney disease. J Hypertens 17: 453–454, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Woods LL, Mizelle HL, Hall JE. Control of renal hemodynamics in hyperglycemia: possible role of tubuloglomerular feedback. Am J Physiol Renal Fluid Electrolyte Physiol 252: F65–F71, 1987. [DOI] [PubMed] [Google Scholar]