Abstract
Exposure to clinically relevant doses of glucocorticoids during fetal life increases blood pressure in adult male and female sheep. The purpose of this study was to evaluate the effects of prenatal exposure to betamethasone at 80–81 days of gestation on renal function in ewes and rams at 1.5 yr of age. In prenatal betamethasone-exposed males, compared with the vehicle-exposed animals, basal glomerular filtration rate (GFR) (1.93 ± 0.08 vs. 2.27 ± 0.10 ml·min−1·kg body wt−1) and the ability to excrete an acute Na+ load (37.1 ± 4.4 vs. 53.7 ± 9.7%) were reduced. (P < 0.03 and P = 0.03, respectively). In contrast, prenatal betamethasone exposure had no effect on basal GFR, Na+ excretion, or the percentage of the Na+ load excreted during the experiment in females. Systemic infusions of ANG-(1–7) at 9 ng·min−1·kg−1 for 2 h had minimal effects on basal GFR, renal plasma flow, and Na+ excretion in males but increased Na+ excretion in females. However, the percentage of Na+ load excreted during ANG-(1–7) infusion did not change in prenatal betamethasone-exposed females (113.1 ± 14.2 vs. 98.1 ± 12.2%) compared with the significant increase in vehicle females (139.2 ± 22.3 vs. 92.2 ± 7.5%) (P = 0.01). The data indicate that antenatal betamethasone exposure produces gender-specific alternations in renal function and thus suggest that different mechanisms underlie the antenatal steroid-induced elevations in blood pressure in male and female offspring.
Keywords: prenatal steroid exposure, sodium load, glomerular filtration rate, sodium excretion, angiotensin-(1–7)
since antenatal steroid treatment became the standard of care for enhancing fetal lung maturation in pregnancies threatened by premature labor between 24 and 34 wk of gestation, the use of corticosteroid therapy in the United States has increased from <15% of eligible pregnancies in 1990 to >75% in 1995 (1, 3). However, clinical epidemiological studies show an association between antenatal glucocorticoid administration and altered vascular function (7, 46), suggesting that exposure to excess glucocorticoids in the prenatal period may have untoward consequences in adult offspring.
We and others have shown using sheep and rat experimental models that prenatal steroid exposure results in elevated blood pressure in adulthood (8, 10, 14, 36). Although there are likely multiple targets influencing the effect of steroids on blood pressure, several recent studies have suggested that altered kidney development induced by antenatal glucocorticoids may contribute to the elevations in arterial blood pressure (35, 36, 53). It is well known that the kidneys play a major role in the long-term regulation of arterial pressure and that change in renal function may lead to alterations in Na+ and water balance and blood pressure (19). In a rat model, Ortiz et al. (35, 36) observed that prenatal exposure to dexamethasone on days 15 and 16 of gestation induced 30 and 20% reductions in glomerular number in offspring compared with controls when assessed at 60–70 days and 6–9 mo of age, respectively. This reduction is possibly a consequence of dexamethasone-induced inhibition of ureteric branching morphogenesis (47). Using unbiased stereologic techniques, Wintour and colleagues (53) found that prenatal glucocorticoid treatment very early in sheep gestation (27 days) led to reduced glomerular number in offspring at 7 yr of age and increased blood pressure. A recent report by Dagan and coworkers (8) provides further insight into a possible underlying cause of the hypertension associated with prenatal exposure to glucocorticoids. Specifically, these researchers demonstrated that offspring from prenatal dexamethasone-treated rats exhibited increased Na+/H+ exchanger activity and higher rates of fluid absorption in proximal convoluted tubules compared with controls at 7–8 wk of age (8).
We have previously demonstrated that clinically relevant doses of glucocorticoids given at ∼0.6 of gestation (the nephrogenic period in the sheep fetus) led to decreased nephron number and altered renal ANG II receptor (AT1) expression. Recently, our studies showed that prenatal betamethasone exposure induces an increase in AT1 receptors and decrease in AT2 receptors in nuclear and plasma membranes in the cortex of adult sheep, which could facilitate the development of arterial hypertension (20). These changes are associated with increased blood pressure in adulthood (15, 32). Together these and the aforementioned findings suggest that prenatal exposure to excess glucocorticoid has a significant impact on renal structural and physiological development and may increase the capacity of the kidney to reabsorb Na+ by promoting renal cortical Na+/H+ exchanger activity. However, to date, there is limited information available regarding the consequences of any programming effects induced by glucocorticoids on renal function in adult life.
ANG-(1–7), an active renin-angiotensin system heptapeptide, has been suggested to function as a physiological antagonist of ANG II at the renal level in stimulating water and Na+ excretion (6, 42). These effects of ANG-(1–7) are thought to be mediated through an AT1-like receptor (18) or by the Mas receptor (5). Recent studies indicated that metabolism of ANG-(1–7) yields products that have high affinity for kidney ANG IV receptor (AT4) and could also potentially contribute to various aspects of renal function, including Na+ excretion (21–23). The ability of ANG-(1–7) to modulate the action of ANG II suggests that it plays an important regulatory role in the kidney, and we have evidence that the ratio of ANG-(1–7) to ANG II is decreased in the kidneys of rams exposed to betamethasone before birth (48). Thus the altered renal function in adulthood caused/programmed by prenatal steroid exposure may be related to changes in renal responsiveness to ANG-(1–7). Therefore, in the present studies, we wished to 1) determine if prenatal exposure to a clinically relevant glucocorticoid dose of glucocorticoid during the period of active nephrogenesis would have an impact on reducing renal function in adulthood; 2) and on any steroid-induced changes in renal function involved/induced by altered responses to ANG-(1–7); and 3) we also determined if the effects of prenatal steroid treatment demonstrated gender specificity.
METHODS
Animals.
Mixed-breed, time-dated pregnant sheep obtained from local suppliers were maintained in open pasture with free access to food and water during pregnancy and lactation. Sheep were randomly assigned to receive either two 0.17 mg/kg intramuscular injections of a 1:1 mixture of betamethasone acetate and betamethasone phosphate (Celestone Soluspan; Schering, Kenilworth, NJ) or vehicle alone, which contained 3.4 mg of monobasic sodium phosphate, 7.1 mg of dibasic sodium phosphate, 0.1 mg of sodium ethylenediaminetetraacetic acid, and 0.2 mg of benzalkonium chlorine/ml given 24 h apart at days 80 and 81 of gestation (term is ∼145 days in our flock). Offspring were weaned at 3 mo of age after spontaneous delivery at term and transferred to the laboratory at 1.5 yr of age for study. All procedures were approved by the institutional Animal Care and Use Committee.
Surgery.
At 1.5 yr of age, vehicle- or betamethasone-exposed sheep were brought into the air-conditioned, light-controlled laboratory, where temperature was maintained at 20 ± 2°C. Sheep were fed a standard commercial diet containing 0.75% NaCl and had ad libitum access to water. Before surgery, the animals were fasted for 24 h. General anesthesia was induced (isofluorane in oxygen) and polyvinyl catheters were inserted in the femoral arteries and veins and jugular vein. A Foley catheter was placed directly in the bladder via a suprapubic incision. The catheters were filled with heparinized saline, closed with metal plugs, tunneled subcutaneously, exteriorized to the flank of the sheep, and placed in a plastic ziplock bag. Postoperative ampicillin (1 g) was administered for 3 days, and sheep were allowed to recover for at least 5 days before experimentation.
Preparation of ANG-(1–7).
ANG-(1–7) was obtained from Bachem BioScience (King of Prussia, PA). The peptide was dissolved in distilled water and stored in 0.5-ml volumes (1 mg/ml) at −80°C until required. On the day of study, ANG-(1–7) stock was diluted in sterile isotonic saline to the required concentration.
Experimental protocol.
Sheep were housed in portable metabolic carts during the experiments and were studied on two separate days [day 1 control study, day 2 ANG-(1–7) study] with a 2- to 3-day washout period in between. All studies commenced between 0800 and 0900. During the first 2 h, urine was collected hourly. Intravenous infusions of inulin and p-aminohippuric acid (PAH) were then begun (details follow) and continued for 3 h. One hour into the inulin/PAH infusion, an infusion of hypertonic saline (0.0275 meq·kg−1·min−1) was started at a rate of 0.55 ml/min for 60 min. Following cessation of the saline infusion, the experiment continued for a further hour. Blood and urine samples were collected at hourly intervals during the experiment for the measurement of inulin, PAH, electrolytes, and creatinine. For the ANG-(1–7) study arm, the peptide was infused at the same time as PAH/inulin at a dose of 10 pmol·kg−1·min−1 for 2 h.
All blood and urine samples were centrifuged immediately after collection, separated into aliquots, and stored at −80°C for later analysis. Urine volumes were noted.
Renal function measurements.
Renal function was evaluated by assessing inulin and PAH steady-state output clearance as previously described (34, 44). Briefly, loading doses of 850 mg of inulin and 225 mg of PAH were given as bolus injections followed by constant intravenous infusions of 10 and 11 mg/min, respectively. Clearances of each were calculated by determining plasma concentrations of the indicators after the establishment of steady state during the experiments. Under these conditions, the rate of renal elimination equals the rate of infusion when the plasma level reaches a steady state. Plasma concentrations of inulin and PAH were measured using standard methods (9, 28).
Plasma and urine electrolytes, creatinine, and urinary protein measurements.
Plasma and urinary concentrations of electrolytes were determined using a Medical Easy Lyte instrument (Bedford, MA). Electrolyte excretion was calculated by multiplying the urine volume by the urine electrolyte concentration. The percentage of the Na+ load excreted was calculated by dividing the quantity of Na+ excreted from the start of the hypertonic saline infusion until the end of the experiment by the total amount of Na+ given in the infusion and expressing that quotient as a percentage.
Urinary creatinine concentrations were measured using a commercially available kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer's directions. Under the conditions of the assay, the dynamic range of the kit was 0–15 mg/dl.
Urinary protein concentrations were measured using the Bradford technique.
Blood pressure measurements.
Arterial blood pressure was measured continuously during some experiments using a Cobe transducer connected to a DigiMed analyzer that digitized the signal that was then recorded by computer. Pressure was sampled at 100 Hz and averaged over 1-min intervals. Because of equipment malfunction, blood pressure recordings were not obtained from all animals.
Statistical analysis.
Data are presented as means ± SE. Comparisons between the betamethasone-exposed and vehicle groups were made by ANOVA and Tukey's post hoc test. A P value of <0.05 was considered statistically significant.
RESULTS
Animal characteristics.
Body weights at the birth and at the time of the experiments (1.5 yr of age) were not different in the vehicle and steroid groups (Table 1). Singletons comprised 34% of the vehicle-treated groups and 50% of the betamethasone-treated groups.
Table 1.
Animal characteristics
| Body Wt, kg |
S, T | Singleton Body Wt, kg | Twins Body Wt, kg | ||||
|---|---|---|---|---|---|---|---|
| Birth | 1.5 yr | Birth | 1.5 yr | Birth | 1.5 yr | ||
| Males | |||||||
| Vehicle | 4.8±1.0 | 63.76±6.9 | 6, 6 | 5.0±0.8 | 66.57±5.4 | 4.6±1.1 | 60.95±8.1 |
| β | 4.2±0.7 | 61.55±7.6 | 4, 7 | 4.7±0.6 | 59.40±3.3 | 3.9±0.6 | 63.70±9.3 |
| Females | |||||||
| Vehicle | 5.0±1.0 | 56.54±9.8 | 9, 2 | 5.2±0.8 | 59.23±6.4 | 4.0±0.4 | 53.85±2.3 |
| β | 4.6±1.3 | 59.01±9.9 | 7, 4 | 5.0±1.4 | 61.21±10.1 | 4.0±0.4 | 56.80±7.3 |
Values are means ± SE. β, prenatal betamethasone exposed. S, T: Singeleton, Twins.
Hemodynamics.
Blood pressure was examined in four vehicle-treated males, four betamethasone males, three vehicle-treated females, and three betamethasone females. The blood pressures were 90.2 ± 2.6, 93.6 ± 2.8, 94.8 ± 4.0, and 95.0 ± 2.7 mmHg, respectively, in these groups. There were no significant differences between groups. Effective renal plasma flow (ERPF) was similar in betamethasone- and vehicle-treated males (958 ± 50 vs. 841 ± 45 ml/min) and females (779 ± 29 vs. 892 ± 42 ml/min) in basal conditions. During the period of acute Na+ loading and recovery, no significant changes were observed in either male or female sheep. Similarly, ANG-(1–7) infusion did not cause significant ERPF changes in either gender, with values of 1,074 ± 56 vs. 845 ± 38 ml/min in male groups and 766 ± 80 vs. 814 ± 109 ml/min in female groups. Furthermore, there were no group, gender, or time effects when ERPF was normalized by body weight in the control and ANG-(1–7) infusion studies (data not shown).
Glomerular filtration rate.
Glomerular filtration rate (GFR) adjusted by body weight in male sheep is shown in Fig. 1. Basal, adjusted GFR was reduced in betamethasone compared with vehicle males (P < 0.03) There was a significant effect of Na+ load on adjusted GFR in males following hypertonic saline infusion (F = 12.5, P = 0.0001), with values increasing in both groups. There was no effect of ANG-(1–7) infusion on adjusted GFR following Na+ loading in male sheep.
Fig. 1.
Glomerular filtration rate (GFR) normalized for body weight in prenatally exposed male sheep to vehicle (○, n = 11) or betamethasone (▪, n = 12) during saline (A) or ANG-(1–7) (B) infusion (9 ng·min−1·kg body wt−1), an acute Na+ load, and 60 min post-Na+ load period. *P < 0.03 vs. vehicle at basal condition. There is an overall effect of Na+ load on GFR (F = 12.5, P = 0.0001).
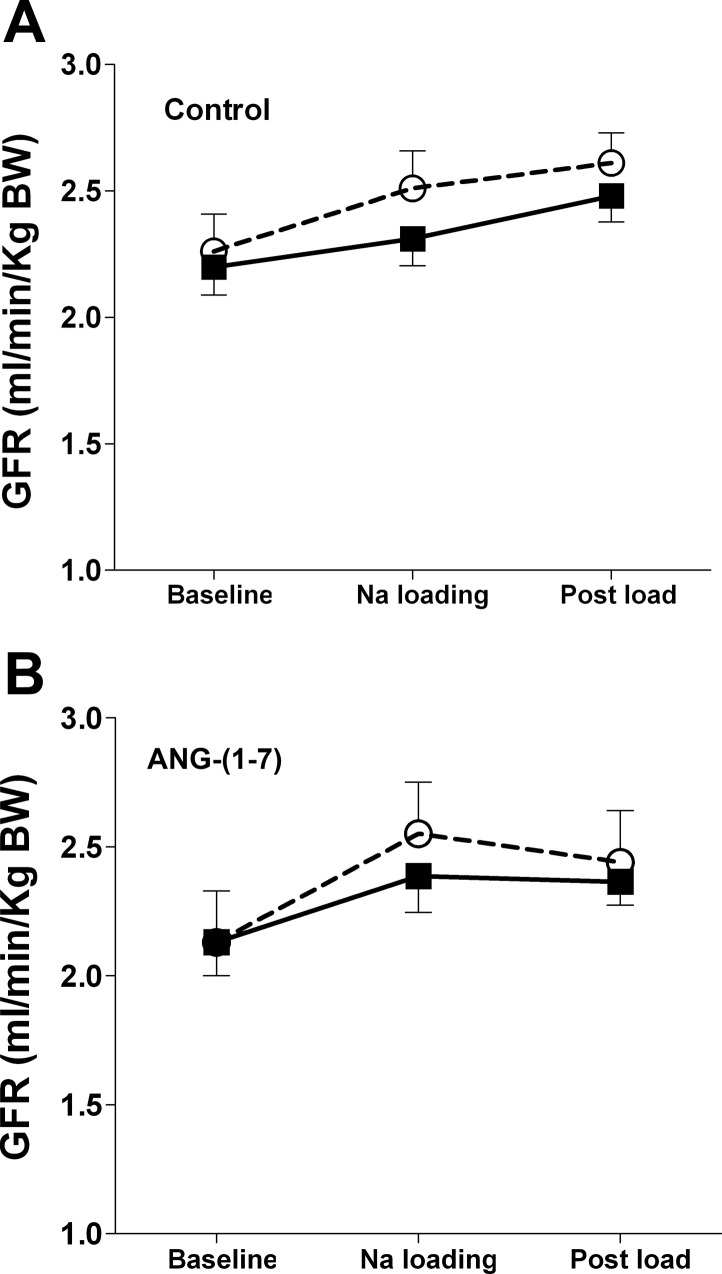
In females, there was no significant difference in basal levels of adjusted GFR between vehicle and betamethasone sheep (Fig. 2), and ANG-(1–7) did not change GFR. However, Na+ loading significantly increased overall GFR (F = 7.5, P = 0.004).
Fig. 2.
GFR normalized for body weight in prenatally exposed female sheep to vehicle (○, n = 10) or Betamethasone (▪, n = 11) during saline (A) or ANG (1–7) (B) infusion (9 ng·min−1·kg body wt−1), an acute Na+ load, and 60 min post-Na+ load period. There is an overall effect of Na+ load on GFR (F = 7.5, P < 0.01).
There were no effects of gender on adjusted GFR in vehicle or betamethasone animals.
Urine volume and Na+ and K+ concentrations in urine and plasma.
Results pertaining to plasma and urine electrolyte concentrations in male and female sheep are presented in Tables 2 and 3, respectively. There was no overall effect of treatment on plasma Na+ or K+ concentrations or on urine volume or Na+ or K+ levels.
Table 2.
Urinary volume, urinary and plasma Na+ and K+ concentrations in male sheep before and after Na+ load and Na+ load with ANG-(1-7) infusion
| Baseline |
Na+ Load | Post-Na+ Load (1 h) | ||||
|---|---|---|---|---|---|---|
| Vehicle | β | Vehicle | β | Vehicle | β | |
| VUrine, ml/h | ||||||
| Control | 93.9±17.6 | 114.3±16.9 | 111.5±11.9 | 101.7±15.4 | 125.9±12.6 | 90.3±13.0 |
| ANG-(1-7) | 139.0±14.7* | 137.3±14.4* | 145.8±26.0 | 142.2±22.0 | 148.6±27.0 | 180.7±33.0* |
| [Na+]Urine, mmol/l | ||||||
| Control | 151.7±17.2 | 148.0±20.0 | 169.4±13.5 | 138.8±17.7 | 173.6±20.1 | 182.2±16.9 |
| ANG-(1-7) | 172.3±22.7 | 130.7±17.6 | 170.1±14.5 | 114.9±14.8 | 178.2±14.7 | 167.9±27.0 |
| [K+]Urine, mmol/l | ||||||
| Control | 151.6±14.7 | 164.6±24.8 | 159.1±11.9 | 163.5±21.0 | 139.2±15.5 | 160.4±13.4 |
| ANG-(1-7) | 154.9±21.0 | 197.5±32.6 | 170.2±22.5 | 192.1±32.0 | 160.1±24.0 | 157.4±30.0 |
| [Na+]Plasma, mmol/l | ||||||
| Control | 147.0±1.0 | 146.6±0.5† | 148.5±1.4† | 147.3±0.6† | 147.7±1.0 | 147.4±0.8† |
| ANG-(1-7) | 144.3±1.0 | 145.1±0.7 | 146.0±0.8 | 147.1±0.9 | 145.6±1.2 | 145.6±0.7 |
| [K+]Plasma, mmol/l | ||||||
| Control | 4.33±0.11 | 4.25±0.10 | 4.19±0.11‡ | 4.16±0.10‡ | 4.14±0.06‡ | 4.22±0.08 |
| ANG-(1-7) | 4.28±0.07 | 4.26±0.10 | 4.24±0.07 | 4.00±0.09‡ | 4.17±0.05‡ | 4.06±0.09‡ |
Values are means ± SE; n = 7-12 sheep. ANG-(1-7), angiotensin 1-7 infusion (9 ng·min−1·kg body wt−1). VUrine, urine volume; [Na+]Urine, urine Na+ concentration; [K+]Urine, urine K+ concentration; [Na+]Plasma, plasma Na+ concentration; [K+]Plasma, plasma K+ concentration.
F = 7.8, P = 0.017, control vs. ANG-(1-7).
F = 6.0, P = 0.005, baseline vs. later times in both groups.
F = 4.1, P = 0.024, baseline vs. later times in both groups.
Table 3.
Urinary volume, urinary and plasma Na+ and K+ concentrations in female sheep before and after Na+ load and Na+ load with ANG-(1-7) infusion
| Baseline |
Na Load | Post-Na+ Load (1 h) | ||||
|---|---|---|---|---|---|---|
| Vehicle | Β | Vehicle | β | Vehicle | β | |
| VUrine, ml/h | ||||||
| Control | 99.7±9.2 | 77.6±9.9 | 137.6±23.4† | 97.4±11.0† | 150.9±21.8† | 116.0±17.0† |
| ANG-(1-7) | 142.6±22.9* | 164.7±29.1* | 191.6±34.0*† | 244.6±29.5*† | 171.0±23.9† | 206.7±25.5*† |
| [Na+]Urine, mmol/l | ||||||
| Control | 182.6±24.0 | 180.1±31.8 | 190.3±16.7 | 236.4±27.2 | 257.0±23.3‡ | 272.3±24.2‡ |
| ANG-(1-7) | 187.6±31.3 | 176.1±18.9 | 192.7±30.9 | 193.2±20.9 | 213.2±25.6 | 193.4±19.8 |
| [K+]Urine, mmol/l | ||||||
| Control | 181.1±24.0 | 156.0±27.5 | 192.3±30.8 | 149.0±28.9 | 178.6±30.3 | 143.8±30.2 |
| ANG-(1-7) | 159.8±22.6 | 151.7±22.9 | 154.4±25.6 | 150.3±25.0 | 120.1±18.1 | 155.0±19.3 |
| [Na+]Plasma, mmol/l | ||||||
| Control | 151.4±3.7 | 149.2±1.8 | 153.2±3.4 | 146.1±4.2 | 148.1±3.7 | 149.1±1.9 |
| ANG-(1-7) | 151.9±3.0 | 149.0±4.2 | 151.5±2.1 | 155.8±5.0 | 148.7±2.9 | 152.3±2.5 |
| [K+]Plasma, mmol/l | ||||||
| Control | 4.03±0.08 | 4.12±0.14 | 4.08±0.13 | 3.95±0.14 | 4.22±0.14 | 4.14±0.07 |
| ANG-(1-7) | 4.13±0.07 | 4.29±0.09 | 4.15±0.10 | 4.33±0.11 | 4.03±0.15 | 4.26±0.18 |
Values are means ± SE; n = 6-9 sheep.
F = 11.0, P = 0.005, control vs. ANG-(1-7).
F = 5.0, P = 0.014, baseline versus later times, both groups.
F =7.5, P = 0.003, baseline vs. later times, both groups.
In males, urine volume did not change significantly with time in response to Na+ loading or during the recovery period in either group. Infusion of ANG-(1–7) increased urine output in both groups (F = 7.8, P = 0.017). Urinary Na+ and K+ concentrations did not change during any of the experiments in males. There were significant increases in plasma Na+ concentrations (F = 6.0, P = 0.005) and decreases in plasma K+ concentrations (F = 4.1, P = 0.024) with time in response to Na+ load in both male groups. However, there were no significant changes in plasma Na+ and K+ levels associated with infusion of ANG-(1–7).
In females, urine output increased significantly during Na+ loading and the recovery period in both groups (F = 5.0, P = 0.014). ANG-(1–7) infusion led to a significant increase in urine volume in females (F = 11.0, P = 0.005). Urinary Na+ levels increased during Na+ loading and the recovery period in both groups (F = 7.5, P = 0.003); however, there was no change associated with ANG-(1–7) infusion. Urinary K+ concentrations did not change during any of the experiments in female sheep. Likewise, plasma Na+ and K+ levels did not change in response to Na+ loading or ANG-(1–7) infusion in either group.
There were no between-gender differences in urine volume or electrolyte concentrations nor plasma electrolyte concentrations in vehicle-treated sheep. However, in the betamethasone animals, urinary Na+ concentrations were significantly lower in males compared with females (F = 4.8, P = 0.049). There were no gender differences in urine flow, urine K+ levels, or plasma electrolyte concentrations in betamethasone-exposed sheep.
Normalized Na+ excretion.
Na+ excretion normalized for body weight in males is shown in Fig. 3. The betamethasone males exhibited significantly lower overall urinary excretion of Na+ compared with vehicle males (F = 4.3, P = 0.05). Neither Na+ loading nor ANG-(1–7) infusion changed Na+ excretion.
Fig. 3.
Urinary Na+ excretion in prenatally exposed male sheep to vehicle (○, n = 11) or betamethasone (▪, n = 12) during saline (A) or (B) ANG (1–7) (B) infusion (9 ng·min−1·kg body wt−1), an acute Na+ load, and 60 min post-Na+ load period. There is no significant effect of Na+ load or ANG-(1–7). There is an overall effect of betamethasone on Na+ excretion (F = 4.3, P < 0.05).
In contrast, betamethasone exposure did not reduce Na+ excretion in females (Fig. 4), and Na+ excretion significantly increased with the acute Na+ loading (F = 6.7, P = 0.004). In addition, infusion of ANG-(1–7) resulted in overall enhanced Na+ excretion compared with the control study (F = 8.5, P = 0.01).
Fig. 4.
Urinary Na+ excretion in prenatally exposed female sheep to vehicle (○, n = 10) or betamethasone (▪, n = 11) during saline (A) or ANG-(1–7) (B) infusion (9 ng·min−1·kg body wt−1), an acute Na+ load, and 60 min post-Na+ load period. There is no significant effect of betamethasone exposure. There are overall effects of Na+ load (F = 6.7, P = 0.004) and ANG-(1–7) on Na+ excretion (F = 8.5, P = 0.01).
In the vehicle-exposed animals, Na+ excretion was significantly higher in females compared with males during the control (F = 13.3, P = 0.002) and ANG-(1–7) infusion experiments (F = 10.1, P = 0.006). Significantly higher rates of Na+ excretion were also evident in betamethasone females compared with males in both the control (F = 23.0, P = 0.0002) and ANG-(1–7) infusion studies (F = 18.3, P = 0.0007).
Percentage of total Na+ load excreted.
Figure 5 shows the percentage of the total Na+ load excreted during and after hypertonic saline infusion. Betamethasone males excreted a significantly smaller percentage of the acute Na+ load than did vehicle-treated males (F = 5.6, P = 0.03). Infusion of ANG-(1–7) did not alter the percentage of the administered Na+ that was excreted (Fig. 5A).
Fig. 5.
Ability to excrete Na+ in response to an acute Na+ load as percentage of the Na+ load in prenatally exposed sheep to vehicle (○, n = 11) or betamethasone (▪, n = 12) during saline (control) or ANG-(1–7) infusion (9 ng·min−1·kg body wt−1) followed by 60 min post-Na+ load period. There was an overall effect of betamethasone in males (*F = 5.6, P = 0.03). There was an effect of ANG-(1–7) in the females (#F = 8.4, P = 0.01) that was confined to the saline-treated animals.
Contrary to the situation in the males, there was no effect of betamethasone on the percentage of the Na+ load excreted in females. Both vehicle and betamethasone females eliminated over 90% of the Na+ load by the end of the control experiment. ANG-(1–7) infusion markedly increased the percentage of the Na+ load excreted in vehicle females only (F = 8.4, P = 0.01; Fig. 5B). Females excreted a higher percentage of the total Na+ load than did the males for both treatment groups (vehicle: F = 9.5, P = 0.007; betamethasone: F = 25.1, P = 0.001).
Urinary protein-to-creatinine ratio.
The ratio of urinary protein to urinary creatinine concentration was determined in a single urine sample obtained from morning urine collections, and the ratio was expressed as milligram of protein per milligram of creatinine. There were no significant differences between the vehicle and betamethasone groups for males [1.47 ± 0.23 (n = 9) vs. 1.90 ± 0.35 (n = 11)] or females [0.55 ± 0.17 (n = 5) vs. 0.87 ± 0.25 (n = 5)]. However, the ratio was significantly higher in males compared with females in both vehicle (P = 0.04) and betamethasone animals (P = 0.05).
DISCUSSION
Evidence from a growing number of studies demonstrates that fetal exposure to excess glucocorticoids leads to a reduction in nephron number and the development of hypertension in adult life (35, 36, 53). What is not clear is whether these changes are associated with altered kidney function. Therefore, the present studies were aimed at determining the effects of prenatal betamethasone exposure on renal function. We found that prenatal exposure to clinically relevant doses of glucocorticoids at the time of nephrogenesis leads to impaired Na+ excretion in adult male sheep. In addition, we have shown that adult female offspring are protected from such impairment. This suggests that prenatal glucocorticoid exposure has gender-specific effects on renal function and raises the possibility that the mechanism by which antenatal steroid treatment increases blood pressure differs in male and female offspring.
We found that baseline GFR adjusted for body weight was significantly decreased in male but not female adult sheep prenatally exposed to clinically relevant doses of betamethasone. Findings from a number of studies have demonstrated lower nephron endowment, high blood pressure, and altered ANG receptor expression in glucocorticoid-exposed models of fetal programming (11, 33, 54, 55). To date, few studies have assessed whether these changes result in impaired or altered renal function, especially in adult offspring. In rats, GFR was decreased by 40% in the offspring of dams that received long-term dexamethasone treatment throughout pregnancy (4). In contrast, Ortiz et al. (36) reported that GFR was not altered in anesthetized 60-day-old male offspring from dams treated with dexamethasone on two consecutive days during pregnancy. Similarly, Martins et al. (30) reported that the combination of prenatal dexamethasone and high protein diet after birth did not alter GFR in anesthetized male and female rats studied at 70 days of age. However, the impact of anesthesia on renal hemodynamics may have obscured differences in GFR caused by antenatal steroid treatment (25, 50).
The fact that we found measurable changes in GFR in adult male offspring following prenatal betamethasone exposure suggests the existence of a functional deficit that could contribute to long-term increases in blood pressure. Interestingly, a similar modest reduction in GFR has been reported in a group of young adults who were exposed to a single course of betamethasone in utero. These subjects were all born at <32 wk of gestation, and the reduction in GFR was found in both young men and women (17). This suggests that steroid exposure alone is sufficient to reduce GFR in male offspring while an additional insult (prematurity) may be required to alter GFR in females. A “two-hit” hypothesis has been proposed to explain the development of hypertension in response to prenatal programming (52).
The mechanism by which prenatal betamethasone exposure results in altered GFR in male offspring remains unclear. In a previous study, we found that glomerular number was decreased by 25.5% (in the absence of any change in kidney weight) after exposure to betamethasone at 0.6 of gestation (16, 32). This finding, in combination with our current observation that GFR was decreased by 14.6% in males, suggests that single-nephron filtration may be increased and that there is compensatory growth in nephron structures in these animals. If true, such changes may have important implications for the progression of renal hypertension (56, 57).
Our finding that prenatal betamethasone exposure did not significantly affect basal GFR in adult female sheep is consistent with reports by others regarding rodent models of maternal protein restriction-induced hypertension (58) and hypertension induced by postnatal AT1 receptor antagonist treatment (29, 39, 40). Our data also agree with the findings that treatment of pregnant ewes very early in gestation with low dose of dexamethasone does not change the whole kidney GFR in adult female offspring (12). The lack of alterations in whole kidney GFR and renal blood flow in females suggests an increase in single nephron hemodynamics when taking into consideration the similarity in the reduced nephron number in males and females by antenatal dexamethasone exposure (15, 32).
In the present study, we found that prenatal treatment with a clinically relevant dose of betamethasone had a significant inhibitory effect on Na+ excretion in adult male but not female offspring under control conditions and following a nonpressor Na+ load. Indeed, when expressed as a percentage of the Na+ load, the steroid-exposed males were able to excrete only ∼40% of the Na++ they received during the experimental period. To our knowledge, this is the first report of a gender-specific effect of prenatal steroid exposure on Na+ excretion in adulthood. Sheep in our study were fed a commercial diet containing 7.5 g Na+ and 8 g K+/kg. Na+ intake was ∼11 g/day in females and 15 g/day in males, and basal plasma Na+ and K+ concentrations were in the same range as in other studies (2, 49). The acute hypertonic Na+ load (0.0275 meq·kg−1·min−1) administered in this investigation was the equivalent of ∼20% of normal daily Na+ intake (1.65 meq·kg−1·day−1) and led to a small increase in plasma Na+ concentrations in males but not in females. It is likely that the lack of increase in plasma Na+ in females is because accumulation of Na+ in the plasma may have been too slow to offset the velocity of excretion. Our finding that the NaCl infusion did not alter blood pressure is consistent with other data in the literature showing lack of an effect on blood pressure with a similar infusion in conscious dogs (41). However, the present study indicated that there was an overall increase in GFR in male betamethasone sheep after Na+ loading, whereas renal blood flow did not change. This implies that filtration fraction, an indirect marker of intraglomerular pressure, was slightly increased in these sheep and suggests that an increase in post-, but not in preglomerular, vasoconstriction may have mediated inadequate regulation of tubuloglomerular feedback. Our original hypothesis was that the Na+ load would cause a natriuresis that would be enhanced by infusion ANG-(1–7) (24, 51) and that Na+ excretion would be compromised by antenatal betamethasone. Although impaired Na++ excretion was found after the betamethasone treatment, the lack of a natriuretic response in the males was counter to our hypothesis. However, there is evidence in male human beings and in female dogs that the dietary Na+ intake can modulate responses to acute NaCl infusions (37, 41). Our data suggest that the natriuretic response of male sheep to an acute Na+ load may be more sensitive to the influence of dietary Na+ than the response in females.
We speculate that the reduced ability to excrete a Na+ load in the betamethasone-exposed males may be a consequence of increased tubular Na+ reabsorption. The mechanisms underlying such an effect are yet to be elucidated. Previously, we reported upregulation of Na+/H+ exchanger 3 protein expression in renal proximal tubules from offspring of ewes treated with glucocorticoids during pregnancy (31). In addition, Dagan et al. (8) reported a 50% increase in Na+/H+ exchanger activity in proximal convoluted tubules from rats exposed to prenatal dexamethasone. These findings suggest that abnormal transcellular Na+ reabsorption in proximal tubules may have contributed to the impaired natriuretic response to Na+ loading in this study.
The deficiency in Na+ excretion capability may lead to the development of salt-sensitive hypertension. Consistent with this idea is the observation of Salazar et al. (40) of the existence of a salt responsive hypertension in a rat model of fetal programming in which a reduction in nephron number was associated with AT1 receptor blockade during the nephrogenic period.
There were marked differences in the responses of the females to the experimental manipulations compared with the males. For example, in contrast to what occurred in males, there were significant increases in GFR (in the absence of renal blood flow changes) after Na+ loading in female vehicle- and betamethasone-exposed offspring. This finding agrees with a previous study that showed hypertonic Na+ loading at a dose of 0.03 meq·kg−1·min−1 for 90 min elicited an increase in GFR in normal Na+-replete female dogs (41). We also demonstrated that the vehicle-treated female offspring exhibited significant natriuretic responses and an enhanced ability to excrete Na+ upon Na+ challenge when infused with ANG-(1–7). Such responses were absent in the males. Most importantly, there were no significant effects of betamethasone exposure on basal GFR, basal Na+ excretion rate, and the capacity to excrete the Na+ load in females. These findings suggest that females may be protected from some of the steroid-induced changes in renal function seen in males. However, betamethasone did abolish the natriuretic effect of ANG-(1–7) in the females. ANG-(1–7) enabled the vehicle-treated females to excrete well over 100% of the Na+ load administered while the betamethasone females showed no enhanced response to the hypertonic NaCl infusion. The reason for this is unclear but may reflect effects of prenatal betamethasone exposure on expression of the presumed ANG-(1–7) receptor [recently identified as the mas oncogene (43)]. Last, the urinary protein-to-creatinine ratio, a sensitive marker of proteinuria (27, 38), was significantly higher in males regardless of treatment. These findings underline the importance of consideration of gender when examining programming effects on renal physiology in sheep.
We have recently reported that prenatal betamethasone exposure resulted in hypertensive adult offspring from 6 mo to 3 yr of age in the basal, environment-maintained conditions (13, 45). In the current renal study, we were unable to detect significant blood pressure differences between the vehicle and betamethasone animals. This is likely because of the substantial level of human activity in the laboratory environment during the experiments necessitated by the logistics of the studies. Such activity can influence blood pressure in sheep. The relatively small number of animals in which blood pressure was successfully monitored also reduced our ability to find significant differences between the groups.
In this study, prenatal steroid treatment had no effect on birth weight in male and female groups. This observation is similar to an earlier study in which a single injection of betamethasone, given at 104 days’ gestation, did not change weight at birth (26). This suggests that the steroid effects on renal function were not related to marked changes in fetal growth. It is doubtful that the renal responses to prenatal steroid exposure in the offspring were related to twinning pregnancy, since the proportion of twins was similar in three of the four groups.
In conclusion, we have demonstrated that prenatal exposure to a clinically relevant dose of betamethasone leads to impaired Na+ excretion, especially in response to acute Na+ loading in male offspring. This reduced capability to excrete Na+ following prenatal steroid exposure may play an important role in mediating the development and maintenance of programmed hypertension in male offspring. However, gender differences in the renal effects of prenatal steroid exposure suggest that different mechanisms underlie the antenatal steroid-induced elevations in blood pressure in males and females.
Perspectives and Significance
The use of antenatal glucocorticoid therapy to enhance fetal lung maturation when the possibility of premature delivery exists has reduced the morbidity and mortality associated with preterm birth dramatically; consequently, it has become an accepted standard of care in obstetrics since 1994 (1). Considering that the percentage of preterm births is large and that 75–80% of individuals who are appropriate candidates for such treatment receive it in the United States means that any deleterious effects may be present in a significant number of young people born since the mid-1990s. Our data suggest that, even in the absence of any effects of prematurity, glucocorticoid exposure before birth may have significant effects on renal function. These effects are associated with elevations in blood pressure. Thus it may be of benefit to consider some screening of young adults exposed to antenatal steroid treatment for impairments in renal function and blood pressure regulation. Early knowledge of increased risk in this population could pave the way for interventions to reduce any long-term adverse consequences on health in adulthood.
GRANTS
This work was supported by National Institutes of Health Grants HD-47584, HL-68728, and HD-17644.
Acknowledgments
We acknowledge the help of David Jones and Eric Lesane with the animals.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.American Medical Association. NIH Consensus Development Conference 1995. Effects of corticosteroid for fetal maturation on perinatal outcomes. J Am Med Assoc 273: 413–418, 1995. [Google Scholar]
- 2.Boyd ND, Benediktsson H, Vimy MJ, Hooper DE, Lorscheider FL. Mercury from dental “silver” tooth fillings impairs sheep kidney function. Am J Physiol Regul Integr Comp Physiol 261: R1010–R1014, 1991. [DOI] [PubMed] [Google Scholar]
- 3.Brandon AE, Boyce AC, Lumbers ER, Zimanyi MA, Bertram JF, Gibson KJ. Glomerular hypertrophy in offspring of subtotally nephrectomized ewes. Anat Rec (Hoboken) 291: 318–324, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Celsi G, Kistner A, Aizman R, Eklof AC, Ceccatelli S, de S, Jacobson SH. Prenatal dexamethasone causes oligonephronia, sodium retention, and higher blood pressure in the offspring. Pediatr Res 44: 317–322, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Chappell MC Emerging evidence for a functional angiotensin-converting enzyme 2-angiotensin-(1–7)-MAS receptor axis: more than regulation of blood pressure? Hypertension 50: 596–599, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Chappell MC, Diz DI, Yunis C, Ferrario CM. Differential actions of angiotensin-(1–7) in the kidney. Kidney Int Suppl 68: S3–S6, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Crowley PA Antenatal corticosteroid therapy: a meta-analysis of the randomized trials, 1972 to 1994. Am J Obstet Gynecol 173: 322–335, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Dagan A, Gattineni J, Cook V, Baum M. Prenatal programming of rat proximal tubule Na+/H+ exchanger by dexamethasone. Am J Physiol Regul Integr Comp Physiol 292: R1230–R1235, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson WD, Sackner MA. Simplification of the anthrone method for the deternination of inulin in clearance studies. J Lab Clin Med 62: 351–356, 1963. [PubMed] [Google Scholar]
- 10.Dodic M, Abouantoun T, O'Connor A, Wintour EM, Moritz KM. Programming effects of short prenatal exposure to dexamethasone in sheep. Hypertension 40: 729–734, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Dodic M, May CN, Wintour EM, Coghlan JP. An early prenatal exposure to excess glucocorticoid leads to hypertensive offspring in sheep. Clin Sci 94: 149–155, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Dodic M, Tersteeg M, Jefferies A, Wintour EM, Moritz K. Prolonged low-dose dexamethasone treatment, in early gestation, does not alter blood pressure or renal function in adult sheep. J Endocrinol 179: 275–280, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Figueroa JP, Jie Z. Blood pressure in prenatal betamethasone exposure in adult offspring in sheep (Abstract). Reprod Sci 15: 271A, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Figueroa JP, Acuna G, Rose JC, Massmann GA. Maternal antenatal steroid administration at 0.55 gestation increases arterial blood pressure in young adult sheep offspring (Abstract). J Soc Gynecol Invest 11: 358A, 2004. [Google Scholar]
- 15.Figueroa JP, Rose JC, Massmann GA, Zhang J, Acuna G. Alterations in fetal kidney development and elevations in arterial blood pressure in young adult sheep after clinical doses of antenatal glucocorticoids. Pediatr Res 58: 510–515, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Figueroa JP, Rose JC, Zhang J, Massmann GA. Single maternal betamethasone course at 0.55 of gestation decreases nephron number in late gestation fetal sheep (Abstract). J Soc Gynecol Invest Suppl 10: 292A, 2003. [Google Scholar]
- 17.Finken MJ, Keijzer-Veen MG, Dekker FW, Frolich M, Walther FJ, Romijn JA, van der Heijden BJ, Wit JM. Antenatal glucocorticoid treatment is not associated with long-term metabolic risks in individuals born before 32 weeks of gestation. Arch Dis Child Fetal Neonatal Ed 93: 442–447, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Gironacci MM, Coba MP, Pena C. Angiotensin-(1–7) binds at the type 1 angiotensin II receptors in rat renal cortex. Regul Pept 84: 51–54, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Guyton AC, Coleman TG, Cowley AV Jr, Scheel KW, Manning RD Jr, Norman RA Jr. Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med 52: 584–594, 1972. [DOI] [PubMed] [Google Scholar]
- 20.Gwathmey-Williams T, Shaltout HA, Diz DI, Figueroa JP, Rose JC, Chappell MC. Steroid-induced fetal programming alters angiotensin II receptor subtype expression in the sheep kidney. FASEB J 22: 735–739, 2008. [Google Scholar]
- 21.Handa RK Angiotensin-(1–7) can interact with the rat proximal tubule AT(4) receptor system. Am J Physiol Renal Physiol 277: F75–F83, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Handa RK Metabolism alters the selectivity of angiotensin-(1–7) receptor ligands for angiotensin receptors. J Am Soc Nephrol 11: 1377–1386, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Handa RK, Krebs LT, Harding JW, Handa SE. Angiotensin IV AT4-receptor system in the rat kidney. Am J Physiol Renal Physiol 274: F290–F299, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Heller J, Kramer HJ, Maly J, Cervenka L, Horacek V. Effect of intrarenal infusion of angiotensin-(1–7) in the dog. Kidney Blood Press Res 23: 89–94, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Holstein-Rathlou NH, Christensen P, Leyssac PP. Effects of halothane-nitrous oxide inhalation anesthesia and Inactin on overall renal and tubular function in Sprague-Dawley and Wistar rats. Acta Physiol Scand 114: 193–201, 1982. [DOI] [PubMed] [Google Scholar]
- 26.Huang WL, Beazley LD, Quinlivan JA, Evans SF, Newnham JP, Dunlop SA. Effect of corticosteroids on brain growth in fetal sheep. Obstet Gynecol 94: 213–218, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Hutchison AS, O'Reilly DS, MacCuish AC. Albumin excretion rate, albumin concentration, and albumin/creatinine ratio compared for screening diabetics for slight albuminuria. Clin Chem 34: 2019–2021, 1988. [PubMed] [Google Scholar]
- 28.Jung K, Klotzek S, Schulze BD. Refinements of assays for low concentrations of inulin in serum. Nephron 54: 360–361, 1990. [DOI] [PubMed] [Google Scholar]
- 29.Loria A, Reverte V, Salazar F, Saez F, Llinas MT, Salazar FJ. Changes in renal hemodynamics and excretory function induced by a reduction of ANG II effects during renal development. Am J Physiol Regul Integr Comp Physiol 293: R695–R700, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Martins JP, Monteiro JC, Paixao AD. Renal function in adult rats subjected to prenatal dexamethasone. Clin Exp Pharmacol Physiol 30: 32–37, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Massmann GA, Zhang J, Rose JC, Figueroa JP. Antenatal steroid administration at 0.55 of gestation alters sodium transporter expression in adult offspring in sheep (Abstract). J Soc Gynecol Investig 12: 44A, 2005. [Google Scholar]
- 32.Massmann GA, Zhang J, Rose JC, Figueroa JP. Acute and long-term effects of clinical doses of antenatal glucocorticoids in the developing fetal sheep kidney. J Soc Gynecol Investig 13: 174–180, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Moritz KM, Johnson K, Douglas-Denton R, Wintour EM, Dodic M. Maternal glucocorticoid treatment programs alterations in the renin-angiotensin system of the ovine fetal kidney. Endocrinology 143: 4455–4463, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Orlando R, Floreani M, Padrini R, Palatini P. Determination of inulin clearance by bolus intravenous injection in healthy subjects and ascitic patients: equivalence of systemic and renal clearances as glomerular filtration markers. Br J Clin Pharmacol 46: 605–609, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ortiz LA, Quan A, Weinberg A, Baum M. Effect of prenatal dexamethasone on rat renal development. Kidney Int 59: 1663–1669, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ortiz LA, Quan A, Zarzar F, Weinberg A, Baum M. Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension 41: 328–334, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasmussen MS, Simonsen JA, Sandgaard NC, Hoilund-Carlsen PF, Bie P. Mechanisms of acute natriuresis in normal humans on low sodium diet. J Physiol Lond 546: 591–603, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruggenenti P, Gaspari F, Perna A, Remuzzi G. Cross sectional longitudinal study of spot morning urine protein:creatinine ratio, 24 hour urine protein excretion rate, glomerular filtration rate, and end stage renal failure in chronic renal disease in patients without diabetes. Br Med J 316: 504–509, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saez F, Castells MT, Zuasti A, Salazar F, Reverte V, Loria A, Salazar FJ. Sex differences in the renal changes elicited by angiotensin II blockade during the nephrogenic period. Hypertension 49: 1429–1435, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Salazar F, Reverte V, Saez F, Loria A, Llinas MT, Salazar FJ. Age- and sodium-sensitive hypertension and sex-dependent renal changes in rats with a reduced nephron number. Hypertension 51: 1184–1189, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Sandgaard NC, Andersen JL, Bie P. Hormonal regulation of renal sodium and water excretion during normotensive sodium loading in conscious dogs. Am J Physiol Regul Integr Comp Physiol 278: R11–R18, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Santos RA, Campagnole-Santos MJ, Andrade SP. Angiotensin-(1–7): an update. Regul Pept 91: 45–62, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Santos RA, Simoes Silva AC, Maric C, Silva DM, Machado RP, de B, I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA 100: 8258–8263, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schnurr E, Lahme W, Kuppers H. Measurement of renal clearance of inulin and PAH in the steady state without urine collection. Clin Nephrol 13: 26–29, 1980. [PubMed] [Google Scholar]
- 45.Shaltout HA, Figueroa JP, Rose JC, Chappell MC, Averill DB, Diz DI. Deleterious cardiovascular effects of antenatal betamethasone exposure in young and adult sheep (Abstract). FASEB J 22: 1129.16, 2008. [Google Scholar]
- 46.Sinclair JC Meta-analysis of randomized controlled trials of antenatal corticosteroid for the prevention of respiratory distress syndrome: discussion. Am J Obstet Gynecol 173: 335–344, 1995. [DOI] [PubMed] [Google Scholar]
- 47.Singh RR, Moritz KM, Bertram JF, Cullen-McEwen LA. Effects of dexamethasone exposure on rat metanephric development: in vitro and in vivo studies. Am J Physiol Renal Physiol 293: F548–F554, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Smith J, Nakahara S, Westwood B, Figueroa J, Chappell M, Rose JC. Prenatal betamethasone exposure alters renin angiotensin system enzyme expression in adult male sheep (Abstract). Reprod Sci 15: 274A, 2008.18421022 [Google Scholar]
- 49.Sun Q, Dimopoulos G, Nguyen DN, Tu Z, Nagy N, Hoang AD, Rogiers P, De BD, Vincent JL. Low-dose vasopressin in the treatment of septic shock in sheep. Am J Respir Crit Care Med 168: 481–486, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Tucker BJ, Peterson OW, Ziegler MG, Blantz RC. Analysis of adrenergic effects of the anesthetics Inactin and alpha-chloralose. Am J Physiol Renal Fluid Electrolyte Physiol 243: F253–F259, 1982. [DOI] [PubMed] [Google Scholar]
- 51.Vallon V, Heyne N, Richter K, Khosla MC, Fechter K. [7-d-ALA]-angiotensin 1–7 blocks renal actions of angiotensin 1–7 in the anesthetized rat. J Cardiovasc Pharmacol 32: 164–167, 1998. [DOI] [PubMed] [Google Scholar]
- 52.Vehaskari VM Developmental origins of adult hypertension: new insights into the role of the kidney. Pediatr Nephrol 22: 490–495, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Wintour EM, Moritz K, Johnson K, Ricardo S, Samuel CS, Dodic M. Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J Physiol Lond 549: 929–935, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wintour EM, Moritz KM, Johnson K, Ricardo S, Samuel CS, Dodic M. Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J Physiol 549: 929–935, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woods LL, Weeks DA. Prenatal programming of adult blood pressure: role of maternal corticosteroids. Am J Physiol Regul Integr Comp Physiol 289: R955–R962, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Woods LL Maternal glucocorticoids and prenatal programming of hypertension. Am J Physiol Regul Integr Comp Physiol 291: R1069–R1075, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Woods LL, Rasch R. Perinatal ANG II programs adult blood pressure, glomerular number, and renal function in rats. Am J Physiol Regul Integr Comp Physiol 275: R1593–R1599, 1998. [DOI] [PubMed] [Google Scholar]
- 58.Woods LL, Weeks DA, Rasch R. Programming of adult blood pressure by maternal protein restriction: role of nephrogenesis. Kidney Int 65: 1339–1348, 2004. [DOI] [PubMed] [Google Scholar]