Abstract
When previously sedentary men and women follow exercise training programs with ad libitum feeding, men lose body fat, but women do not. The purpose of this study was to evaluate whether this observation could be related to sex differences in the way energy-regulating hormones and appetite perception respond to exercise. Eighteen (9 men, 9 women) overweight/obese individuals completed four bouts of exercise with energy added to the baseline diet to maintain energy balance (BAL), and four bouts without energy added to induce energy deficit (DEF). Concentrations of acylated ghrelin, insulin, and leptin, as well as appetite ratings were measured in response to a meal after a no-exercise baseline and both exercise conditions. In men, acylated ghrelin area under the curve (AUC) was not different between conditions. In women, acylated ghrelin AUC was higher after DEF (+32%) and BAL (+25%), and the change from baseline was higher than men (P < 0.05). In men, insulin AUC was reduced (−17%) after DEF (P < 0.05), but not BAL. In women, insulin AUC was lower (P < 0.05) after DEF (−28%) and BAL (−15%). Leptin concentrations were not different across conditions in either sex. In men, but not in women, appetite was inhibited after BAL relative to DEF. The results indicate that, in women, exercise altered energy-regulating hormones in a direction expected to stimulate energy intake, regardless of energy status. In men, the response to exercise was abolished when energy balance was maintained. The data are consistent with the paradigm that mechanisms to maintain body fat are more effective in women.
Keywords: acylated ghrelin, leptin, insulin, physical activity, body fat, food intake
evidence from the national weight control registry shows the critical importance of regular aerobic exercise in maintaining lost body weight and body fat (25). On the basis of a strong body of data, the Institute of Medicine indicated that preventing body fat gain over time probably requires 60 min of physical activity per day (4). When previously sedentary individuals begin exercise training programs, however, fat loss is neither inevitable nor consistent across the sexes (11, 36). In general, men lose body fat when they undertake structured exercise training programs with ad libitum eating (9, 22, 36). In contrast, women do not lose body fat in identical protocols (9, 22, 36). For example, Donnelly et al. (11) reported that supervised aerobic exercise 5 days/wk for 16 mo lowered body fat and body weight in men who ate ad libitum. In contrast, there were no changes in body fat and body mass in women. These data are corroborated by similar studies showing sex differences in body fat loss or fat oxidation (19, 20, 36). Taken together, these data suggest that during exercise training, men do not sufficiently increase energy intake to balance their new higher energy expenditure. In contrast, women more precisely match intake with expenditure and therefore maintain body weight and body fat.
Sex differences in body fat loss in response to aerobic exercise may result, at least partially, from changes in key circulating hormones (e.g., acylated ghrelin, insulin, leptin, thyroid hormone, etc.) that mediate energy balance. Higher concentrations of acylated ghrelin and lower concentrations of leptin and insulin stimulate appetite, energy intake, and promote energy conservation (12, 47, 48). The one prior study in which sex differences were evaluated showed that women, but not men, had lower fasting leptin and insulin concentrations (indicating greater stimulus to eat) after 12 wk of exercise training (21). These data provide initial evidence that energy-regulating hormones may respond more robustly to aerobic exercise in a direction expected to stimulate appetite in women compared with men. A more robust change in the hormonal response and appetite would be expected to increase energy intake and minimize body fat loss. To date, a systematic comparison of how the initiation of exercise training impacts key energy-regulating hormones and appetite in men and women has not been published.
A challenge to studying the effects of exercise on hormones that regulate energy intake and expenditure is to separate the independent effects of exercise from the potentially confounding influence of energy deficit. If energy intake is not raised to cover the energy costs of exercise, the resulting energy deficit induces potent hormonal/metabolic effects that overlap with the effects of exercise. For example, energy deficit raises circulating concentrations of acylated ghrelin and lowers insulin and leptin, all of which stimulate appetite and energy intake (15, 36, 38). To tease apart the independent effects of exercise from energy imbalance, subjects must be studied in both energy-deficient (energy intake not raised to match expenditure) and energy-balanced (energy intake raised to match the new higher energy expenditure) conditions.
Because regular exercise training consistently results in greater fat loss in men than in women, the purpose of this study was to evaluate key hormone and appetite responses to exercise that may play a role in mediating this important sex difference. To accomplish this objective, previously sedentary men and women exercised on four consecutive days with energy added to the baseline diet to maintain energy balance, and on another 4 days without energy added to the baseline diet to induce an energy deficit. We hypothesized that 1) the hormonal response to exercise alone (energy balance maintained) would be potentiated by energy deficit, and 2) compared with men, women would have more robust hormonal and appetite responses in a direction expected to stimulate appetite and energy intake and suppress energy expenditure in both energy conditions.
METHODS
Overview.
The effects of exercise on energy-regulating hormones and on appetite were assessed in adult humans. A counter-balanced, cross-over study design, in which men and women served as their own controls, was used (Fig. 1). Concentrations of hormones related to the regulation of energy intake and energy expenditure and perception of hunger and satiety were measured in three conditions: 1) after a no-exercise day with subjects in energy balance, 2) after 4 days of daily exercise with energy added back to the baseline diet to match the higher expenditure and maintain energy balance (BAL), and 3) after 4 days of daily exercise without energy added to the baseline diet, so that subjects were in energy deficit (DEF).
Fig. 1.
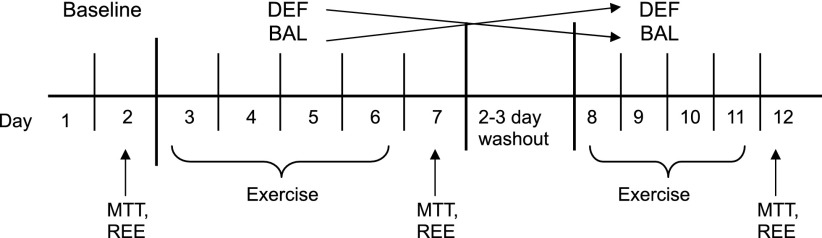
Overview of the study. MTT, meal tolerance test; REE, resting energy expenditure; DEF, exercise in energy deficit; BAL, exercise in energy balance.
Subjects.
Eighteen (9 men and 9 women), previously sedentary (<1 h/wk of aerobic exercise), overweight, or obese individuals between the ages of 19 and 57 were recruited from the surrounding area (Amherst, MA) by flyers and advertisements (Table 1). All volunteers were in good health, disease-free, and weight stable for the previous 6 mo (±2 kg) as determined from a health-history questionnaire. Exclusion criteria included using tobacco products, following a very low or very high-carbohydrate diet (<30% or >70% carbohydrate, respectively), or habitually using antioxidant supplements (e.g., Vitamin C, Vitamin E, etc.) or anti-inflammatory medications (e.g., aspirin, etc.), lipid-lowering (e.g., statin drugs) or diabetes (e.g., metformin) medications. Men and women were excluded if they had had a body mass index (BMI) <25 kg/m2 or body fat <20% for men or <25% for women. Seven of the nine women were on a triphasic birth control regimen that was maintained during the study. Women were tested in the early follicular phase (1 to 4 days after menstruation) of the menstrual cycle. The study was approved by the Institutional Review Board at the University of Massachusetts, Amherst, and written informed consent was obtained from all subjects.
Table 1.
Subject characteristics
| Variable | Men | Women | Exact P Value |
|---|---|---|---|
| Subject | n = 9 | n = 9 | |
| Age, yr | 26.8 (11.8) | 23.3 (8) | 0.07 |
| Body mass, kg | 81.5 (9.5) | 72.9 (10.0) | 0.08 |
| Height, cm | 176.6 (7.2) | 160.9 (6.1) | <0.01 |
| BMI, kg/m2 | 25.7 (2.3) | 28.0 (3.5) | 0.14 |
| Body fat, % | 27.0 (4.9) | 41.2 (4.6) | <0.01 |
| Fat mass, kg | 20.2 (4.3) | 28.1 (6.5) | <0.01 |
| Lean mass, kg | 55.0 (7.6) | 39.4 (3.9) | <0.01 |
| Estimated V̇o2peak, ml·kg−1·min−1 | 44.9 (4.8) | 34.9 (5.2) | <0.01 |
Values are means (SD).
Preliminary tests.
Subjects completed a Physical Activity Readiness Questionnaire, a record of recent physical activity, and health history questionnaire prior to starting the study. Body composition was assessed by dual-energy X-ray absorptiometry (Lunar, Madison, WI) scan.
Peak oxygen consumption (V̇o2peak) was estimated using the Treadwalk test (34). Briefly, subjects warmed up on a treadmill (Life Fitness 9100HR, Schiller Park, IL) for 5 min at a comfortable walking pace they could maintain for 15–20 min. After the warm-up period, subjects then walked at the same pace until one mile was completed. Heart rate (Polar Electro A1, Woodbury, NY) was averaged during the last 2 min of the 1-mile test, and time to complete the 1-mile test was recorded. V̇o2peak was calculated using the equations developed by Pober et al. (34), as previously described. The estimated V̇o2peak was used to set the appropriate workload during the exercise training (see Experimental protocol).
Daily physical activity was assessed during the intervention using an Actigraph monitor (Model GT1M; Actigraph, LLC, Fort Walton Beach, FL) using the Freedson kilocalorie equation (14). The Actigraph was used during the exercise training conditions to monitor the activity of all subjects outside the laboratory. To help ensure accuracy, subjects were instructed to always wear the Actigraph on the right hip.
Energy intake and energy expenditure.
We have previously described a similar protocol to accurately estimate energy intake and expenditure (3, 17, 18). Prior to the intervention, all subjects completed a diet survey to provide an initial estimate of energy intake. Resting energy expenditure (REE) was measured in the morning after an overnight fast using open-circuit indirect calorimetry to estimate energy requirements. After 20 min of relaxation, subjects sat comfortably in a reclining chair for 15–20 min, while expired air was connected to an online metabolic system (Parvomedics Truemax 2400, Consentius Technologies, Sandy, UT). The measured REE was then multiplied by an activity factor from 1.3–1.4 based on habitual physical activity (as determined from the questionnaires) to estimate total daily energy expenditure for each subject. When energy requirements are estimated from REE using the appropriate activity factor, there is a strong correlation (r = 0.73) with energy requirements calculated during 28 days of controlled feeding (24). To ensure that all subjects consumed the appropriate number of kilocalories, all meals were provided throughout the study. The diet was composed of 50–60% carbohydrate, 25–30% fat, and 13–20% protein and consisted of whole and frozen foods (e.g., cereal, bagel, fruits, vegetables, pasta, etc.). Subjects were asked to consume meals at a specific time of the day (breakfast, lunch, dinner, etc.). Subjects were instructed to consume all food provided and to return the used containers at the end of each day, including any uneaten food. All returned food was weighed and subtracted from the daily energy intake. Subjects were instructed to refrain from alcohol and caffeine throughout the intervention. Subjects signed a contract each day, indicating their continued adherence to the protocol.
Experimental protocol.
Baseline testing (Fig. 1) was done after a 24-h period in which subjects performed no structured exercise (i.e., no physical activity beyond daily living) and meals were provided as described above. On the morning of day 2, after a 10- to 12-h overnight fast, subjects reported to the Energy Metabolism Laboratory. They rested quietly for 15–20 min, completed an appetite questionnaire in the energy intake and energy expenditure section, and REE was measured as described. A catheter was inserted into a superficial forearm vein, and a fasting blood sample was taken, followed by a meal tolerance test (MTT). The meal consisted of a bagel with butter and a glass of orange juice. The energy content of the meal was 505 kcal and was composed of 65% carbohydrate (82 g), 24% fat (14 g), and 10% protein (12 g). Subjects had <15 min to complete the meal. Blood samples were collected at 30, 60, 90, and 120 min. All subjects then completed two exercise conditions: 1) daily exercise on four consecutive days with exercise energy replaced to maintain energy balance (BAL), and 2) daily exercise on four consecutive days with exercise energy not replaced, causing an energy deficit (DEF). To control for the effects of the order, in which conditions were presented, eight subjects (4 M, 4 W) were assigned to the BAL condition first, and 10 (5 M, 5 W) to the DEF condition first. A washout period of 2 or 3 days separated the two exercise conditions. During the washout period, subjects were allowed to return to their normal daily activities but were asked not to exercise. After the washout period, the subjects returned to complete the second half of the study in the opposite condition (e.g., DEF → BAL or BAL → DEF).
All exercise bouts were performed in the Energy Metabolism Laboratory under controlled environmental conditions. Each exercise bout was conducted on a treadmill (Life Fitness 9100, Schiller Park, IL) at a moderate intensity (50–65% of estimated V̇o2peak) until 30% of total daily energy expenditure was expended. V̇o2 was measured periodically to maintain the appropriate workload using open-circuit spirometry (Parvomedics Truemax 2400, Consentius Technologies, Sandy, UT). Subjects were allowed short breaks (1–5 min) during exercise as needed. After each exercise bout, subjects were given food for the day and returned the following day for the next exercise bout. On the 5th day of each condition (Fig. 1: days 7 and 12) after a 10- to 12-h fast, the MTT, appetite questionnaire, and REE were administered exactly as described for the baseline condition. Subjects completed a daily questionnaire to rate appetite (i.e., desire to eat, hunger, fullness, how much food can you eat) as previously described (42).
Hormone assays.
Venous blood samples were collected in sterile syringes and transferred to precooled Vacutainers containing EDTA for analysis of acylated ghrelin, total ghrelin, insulin, and leptin. Plasma acylated ghrelin concentrations are unstable in untreated plasma because of the octanoly group at the serine-3 residue. To retard the breakdown of acylated ghrelin, EDTA Vacutainers were pretreated with aprotinin to a final concentration of 500 KIU and 50 μl of 1 N HCL were added per 1 ml to acidify the plasma. Samples used for analysis of total thyroxine (T4), total triiodothyronine (T3), free T4 (fT4), and free T3 (fT3) were transferred to serum Vacutainers and allowed to clot for 10–15 min. Samples used for analysis of plasma glucose concentrations were transferred to a Vacutainer containing sodium fluoride (to inhibit glycolysis) and potassium oxalate. All samples were centrifuged at (3,000 g) for 15 min, and plasma or serum was aliquotted into polystyrene tubes and stored at −80°C until analysis. Quantitative assessment of plasma concentrations of acylated ghrelin, total ghrelin, insulin, and leptin were determined by competitive binding radioimmunoassay (Millipore, St. Charles, MO). Concentrations of T4 and T3, and fT4 and fT3 were assessed by enzyme-linked immunosorbent assay (Diagnostic Systems Laboratory, Webster, TX).
Acylated ghrelin, total ghrelin, acylated ghrelin to total ghrelin ratio (AG/TG), and insulin concentrations were used to calculate area under the curve (AUC) during the MTT using the trapezoidal method.
Statistical analyses.
Raw data are presented as mean (SD). A mixed-model three-factor ANOVA with repeated measures was used to determine (sex × condition × time) interactions for acylated ghrelin, total ghrelin, AG/TG ratio, and insulin in response to the MTT, and the appetite questionnaire. A two-factor repeated-measures ANOVA (sex × condition) was used for REE, AUC, and fasting concentrations for all hormones. If sex differences did not exist for hormone AUC and appetite questionnaire ratings, a one-factor ANOVA was used for men and women independently. Concentrations of acylated ghrelin, total ghrelin, AG/TG, and insulin were not normally distributed, and so the values were log transformed before statistical analysis. Correlations were done between resting energy expenditure, exercise expenditure, body composition, appetite questionnaire ratings and acylated ghrelin, total ghrelin, insulin, leptin, T4, fT4, T3, and fT3 concentrations. By convention, significant differences were defined as α ≤ 0.05. When there were significant differences in the main effects of sex, condition, and/or time, a Fisher least significant difference test was used for post hoc analysis.
RESULTS
Exercise training.
Energy status (DEF or BAL) had no effect on exercise heart rate, V̇o2, or energy expenditure (Table 2). The relative exercise intensity (percent of maximal V̇o2) during exercise was similar between men and women for both conditions (Table 2). By design, each exercise bout comprised 30% of total daily energy expenditure (TDEE). Because the mean TDEE was higher in men than in women, the absolute energy expenditure for each exercise bout was also higher in both conditions (P < 0.05), but the fraction of TDEE was similar across sexes.
Table 2.
Exercise training characteristics
| Measured in | Men |
Women | |||
|---|---|---|---|---|---|
| DEF | BAL | DEF | BAL | ||
| Exercise Expenditure | kcal | 746 (98) | 751 (100) | 600 (66) | 603 (64) |
| kcal/min | 9.4 (1.8) | 9.4 (2.0) | 6.9 (0.8) | 7.0 (0.8) | |
| % of TDEE | 30% | 30% | 30% | 30% | |
| V̇o2 | ml·kg−1·min−1 | 24.0 (3.8) | 24.2 (4.2) | 19.7 (2.5) | 20.0 (2.4) |
| l/min | 1.93 (0.36) | 1.95 (0.42) | 1.41 (0.16) | 1.44 (0.16) | |
| % V̇o2peak | 53.7 (7.1) | 53.5 (6.8) | 56.9 (6.0) | 57.9 (6.2) | |
| Heart rate | bpm | 144 (16) | 144 (15) | 150 (16) | 154 (17) |
| Duration | min | 83 (8) | 83 (8) | 89 (5) | 88 (5) |
| Speed | mph/grade | 3.7/5% | 3.7/5% | 3.5/3% | 3.5/3% |
Values are means (SD). DEF, energy deficit; BAL, energy balance; TDEE, total daily energy expenditure.
Energy intake, energy expenditure, and energy status.
As designed, men and women were in energy balance during BAL and energy deficit during DEF with clear differences between the two conditions (Table 3). In both sexes, energy intake was similar between baseline and DEF and was 30% higher during BAL to meet the 30% rise in energy expenditure (Table 3). The macronutrient composition of the diet as a percentage of total energy intake was consistent in all conditions because the absolute amounts of dietary carbohydrate, fat, and protein were all raised proportionally in BAL (Table 3).
Table 3.
Energy status
| Men |
Women | |||||
|---|---|---|---|---|---|---|
| Baseline | DEF | BAL | Baseline | DEF | BAL | |
| Energy intake, kcal | 2574 (376) | 2540 (301) | 3269 (405) | 2018 (277) | 1960 (237) | 2603 (271) |
| Cho (g) | 363 (45), 57% | 358 (48), 56% | 463 (61), 56% | 297 (44), 58% | 288 (40), 58% | 382 (45), 58% |
| Fat (g) | 85 (10), 30% | 85 (10), 30% | 109 (14), 30% | 61 (9), 28% | 61 (8), 28% | 83 (9), 29% |
| Pro (g) | 85 (13), 13% | 89 (13), 14% | 114 (16), 14% | 74 (10), 15% | 71 (10), 14% | 87 (11), 13% |
| Energy expenditure, kcal | 2538 (317) | 3278 (391) | 3275 (394) | 1986 (238) | 2606 (275) | 2606 (275) |
| Energy balance, kcal | 36 (39) | −738 (135) | −6 (117) | 42 (132) | −646 (115) | −3 (94) |
Values are means (SD). Cho, carbohydrate; Pro, protein.
In men, body mass was slightly but significantly lower (P < 0.05) after DEF (80.7 ± 9.5 kg) and BAL (81.0 ± 9.4) compared with baseline (81.5 ± 9.4). In women, body mass declined slightly from baseline (72.9 ± 10.0 kg) in DEF (72.3 ± 10.1 kg, P < 0.05) but not in BAL (72.6 ± 10.0). Resting energy expenditure did not change across conditions in men (baseline: 1,908 ± 300, DEF: 1,934 ± 220, BAL: 1,949 ± 337 kcal/day) or women (baseline: 1,547 ± 120, DEF: 1,524 ± 162, BAL: 1,583 ± 185 kcal/day). Similarly, there were no differences across conditions in daily counts generated by the Actigraph accelerometer in men (DEF: 28,572 ± 26,951, BAL: 29,438 ± 26,853 counts/24 h) or in women (DEF: 13,394 ± 10,114, BAL: 15,195 ± 11,092 counts/24 h). These latter data indicate that physical activity outside of the structured exercise in the laboratory was not different across condition in either men or women.
Hormones related to energy intake.
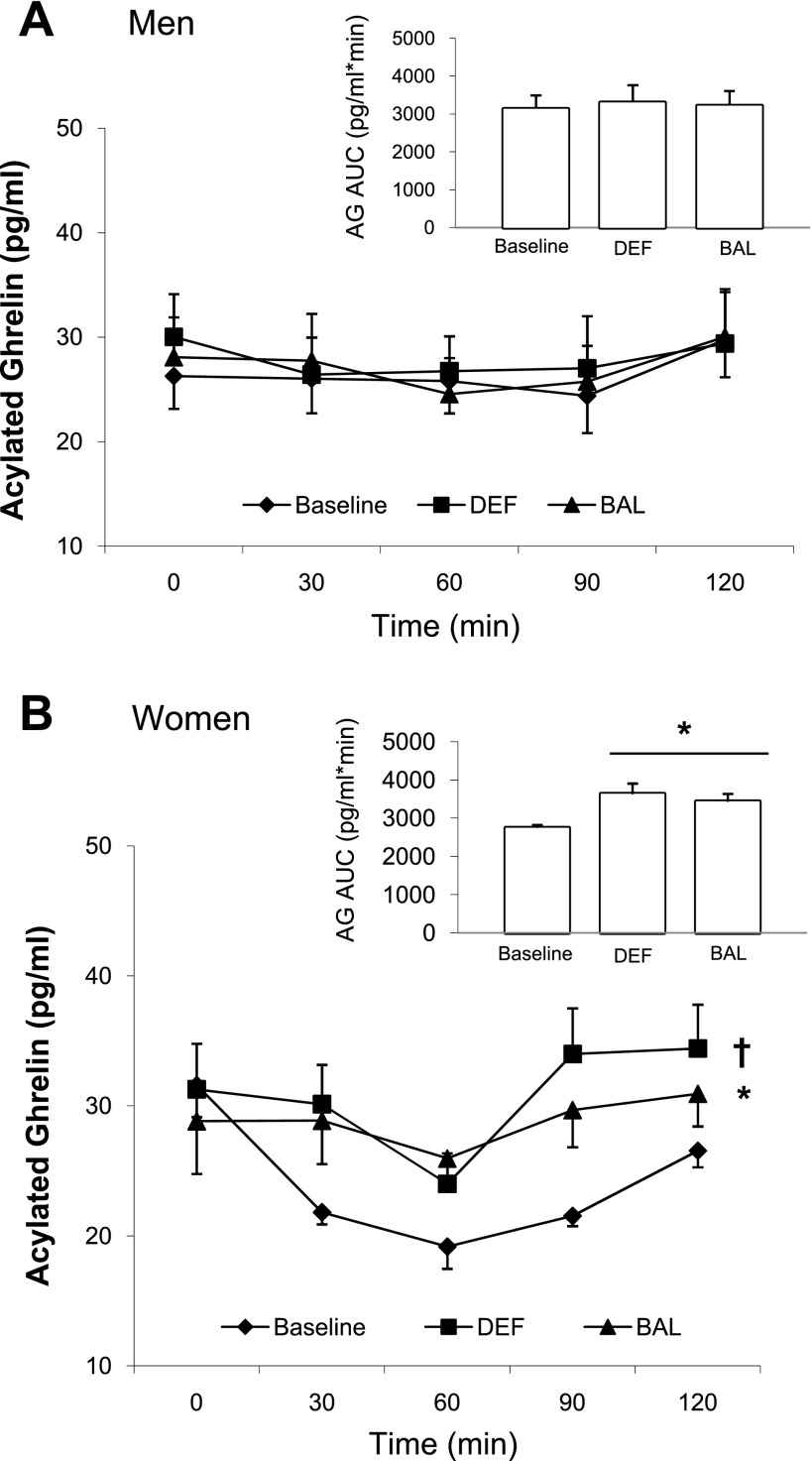
Acylated ghrelin in the fasted state (Table 4) did not differ across condition either in men or in women. The acylated ghrelin response to the MTT also did not vary by condition in men (Fig. 2A). In women, however, the acylated ghrelin response to the MTT (Fig. 2B) was significantly higher after both DEF and BAL compared with baseline with DEF > BAL. Expressed as AUC, acylated ghrelin was elevated from baseline by 32% after DEF and by 25% after BAL (Fig. 2B), with no difference between DEF and BAL. There was a significant main effect of sex (P < 0.05) when acylated ghrelin was calculated as a change from baseline, indicating that the change was significantly greater after DEF (P = 0.042) and BAL (P = 0.05) in women than in men (data not shown).
Table 4.
Fasting hormone concentrations in men and in women
| Men |
Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | DEF | BAL | Exact P | Baseline | DEF | BAL | Exact P | |
| Acylated ghrelin, pg/ml | 26.3 (9.3) | 30.0 (12.3) | 28.1 (11.5) | 0.58 | 31.6 (7.4) | 31.3 (10.6) | 28.8 (12.2) | 0.69 |
| Δ from baseline | 3.8 | 1.8 | −0.3 | −2.8 | ||||
| 95% CI | (−12.9, 5.3) | (−7.3, 10.9) | (−8.7, 9.3) | (−11.7, 6.2) | ||||
| Insulin, pM | 73.7 (55.0) | 66.1 (53.6) | 68.9 (42.8) | 0.66 | 83.1 (39.6) | 56.2 (28.7)* | 57.9 (20.8)* | <0.01 |
| Δ from baseline | −7.6 | −4.8 | −26.9 | −25.2 | ||||
| 95% CI | (−2.3, 4.8) | (−4.4, 2.8) | (2.1, 6.9) | (−7.4, −1.0) | ||||
| Leptin, ng/ml | 5.5 (3.8) | 4.0 (2.6) | 5.1 (3.6) | 0.06 | 24.0 (8.6)† | 20.9 (10.7)† | 21.8 (7.6)† | 0.10 |
| Δ from baseline | −1.5 | −0.4 | −3.2 | −2.2 | ||||
| 95% CI | (−0.01, 2.9) | (−1.5, 0.7) | (−1.0, 7.3) | (−5.2, 1.1) | ||||
| T4, μg/dl | 5.8 (2.5) | 6.0 (2.2) | 5.3 (2.1) | 0.73 | 7.1 (2.1) | 6.8 (2.1) | 6.4 (2.0) | 0.81 |
| Δ from baseline | 0.2 | −0.5 | −0.3 | −0.6 | ||||
| 95% CI | (−2.0, 1.6) | (−2.3, 1.4) | (−2.3, 2.8) | (−3.2, 1.9) | ||||
| T3, ng/dl | 82.3 (27.1) | 82.1 (19.7) | 84.2 (21.7) | 0.95 | 104.9 (43.2) | 96.5 (25.1) | 98.8 (36.9) | 0.67 |
| Δ from baseline | −0.1 | 1.9 | −8.8 | −6.1 | ||||
| 95% CI | (−15.6, 19.5) | (−17.4, 17.7) | (−15.6, 32.4) | (−29.2, 17.1) | ||||
| fT4, ng/dl | 0.73 (0.16) | 0.71 (0.19) | 0.76 (0.19) | 0.46 | 0.75 (0.21) | 0.74 (0.20) | 0.65 (0.14) | 0.18 |
| Δ from baseline | −0.01 | −0.03 | −0.01 | −0.11 | ||||
| 95% CI | (−0.1, 0.1) | (−0.1, 0.1) | (−0.1, 0.2) | (−0.3, 0.1) | ||||
| fT3, pg/ml | 3.08 (0.83) | 2.88 (0.59) | 3.19 (0.44) | 0.52 | 3.01 (0.48) | 2.97 (0.39) | 2.75 (0.75) | 0.44 |
| Δ from baseline | −0.20 | 0.10 | −0.04 | −0.26 | ||||
| 95% CI | (−4.9, 0.9) | (−5.9, 0.8) | (−0.5, 0.6) | (−0.8, 0.3) | ||||
Significantly different than baseline.
Significantly different than men. Values are means (SD).
Fig. 2.
Plasma acylated ghrelin response during the 2-h MTT and acylated ghrelin area under the curve (AUC) (inset) in men (A) and in women (B). *Significantly different than baseline (P < 0.05). †Significantly different than baseline and BAL (P < 0.05).
In men, total ghrelin in the fasting state was higher after BAL relative to baseline (data not shown). In women, total ghrelin in the fasting state did not differ between conditions (data not shown). In both men and women, total ghrelin concentrations and total ghrelin AUC were significantly higher (P < 0.05) after DEF and BAL compared with baseline (data not shown), and there were no main effects of sex in either total ghrelin response to the MTT or total ghrelin AUC.
To assess the relative proportions of “biologically active” to total ghrelin, we calculated the AG/TG ratio. In men, AG/TG response to the MTT and the AUC was not different between conditions (data not shown). In women, however, the AG/TG response to the MTT was elevated from baseline after DEF (P < 0.05) but not BAL, with no difference between DEF and BAL. Also, the AG/TG AUC was 21% higher (P < 0.05) after DEF and tended to be higher after BAL (+11%; P = 0.08) compared with baseline (data not shown). There was a significant main effect of sex (P = 0.04), indicating that the AUC change from baseline was significantly greater in women than in men (data not shown).
To try and account for the impacts of the clear sex differences in body composition and cardiorespiratory fitness on the acylated or total ghrelin responses, we repeated the statistical analyses using body fat, BMI, and V̇o2peak as covariates. Using any of those characteristics as covariates did not alter the outcomes described above. Taken together, the data indicate that women had a more robust acylated ghrelin response after DEF and BAL, in the direction expected to stimulate appetite and energy intake.
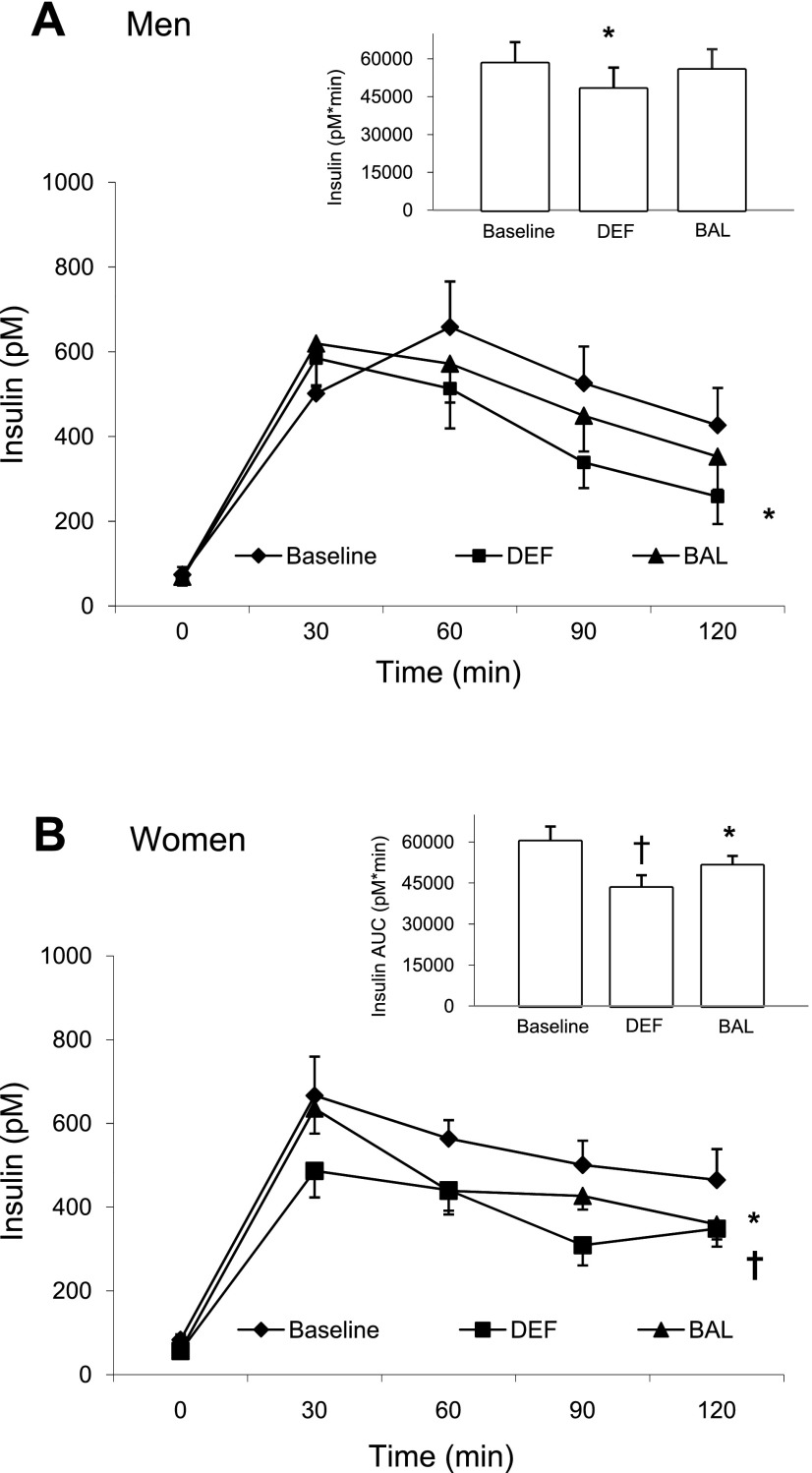
In men, fasting insulin concentrations were not different between conditions but, in women, they were significantly lower after DEF and BAL compared with baseline (Table 4). The insulin response to the MTT and the insulin AUC (−17%) were lower after DEF compared with either baseline or BAL in men (Fig. 3A). In women, the insulin response to the MTT was significantly lower after DEF and BAL compared with baseline, and DEF was significantly lower than BAL (Fig. 3B). Insulin AUC was 28% lower after DEF and 15% lower after BAL, compared with baseline, with a significant difference between DEF and BAL (Fig. 3B). Although the pattern of insulin responses appeared to be different, there was no main effect of sex for insulin AUC. Using body fat, BMI, or V̇o2peak as covariates again had no impact on the outcomes observed. The data indicate that in men, insulin concentrations after the MTT were lower than baseline in DEF but restored to baseline values in BAL. In women, however, restoration of energy balance did not fully reverse the effect of DEF to lower both fasting insulin and the insulin response to the MTT.
Fig. 3.
Plasma insulin response during the 2-h MTT and insulin AUC (inset) in men (A) and in women (B). *Significantly different than baseline (P < 0.05). †Significantly different than baseline and BAL (P < 0.05).
Fasting leptin concentrations were significantly higher in women than in men but were not different across conditions. In both sexes, fasting glucose concentrations, the glucose response to the MTT, and glucose AUC was not different (P > 0.05) across conditions (data not shown).
Hormones related to energy expenditure.
In men, fasting total T4, fT4, total T3, and fT3 concentrations were not different across conditions (Table 4). There was no statistical difference across conditions in women either, although mean concentrations of all four thyroid-related hormones tended to be lower after both DEF and BAL, relative to baseline (Table 4). There was no main effect of sex for total T4, total T3, fT4, and fT3 concentrations.
Subjective appetite response.
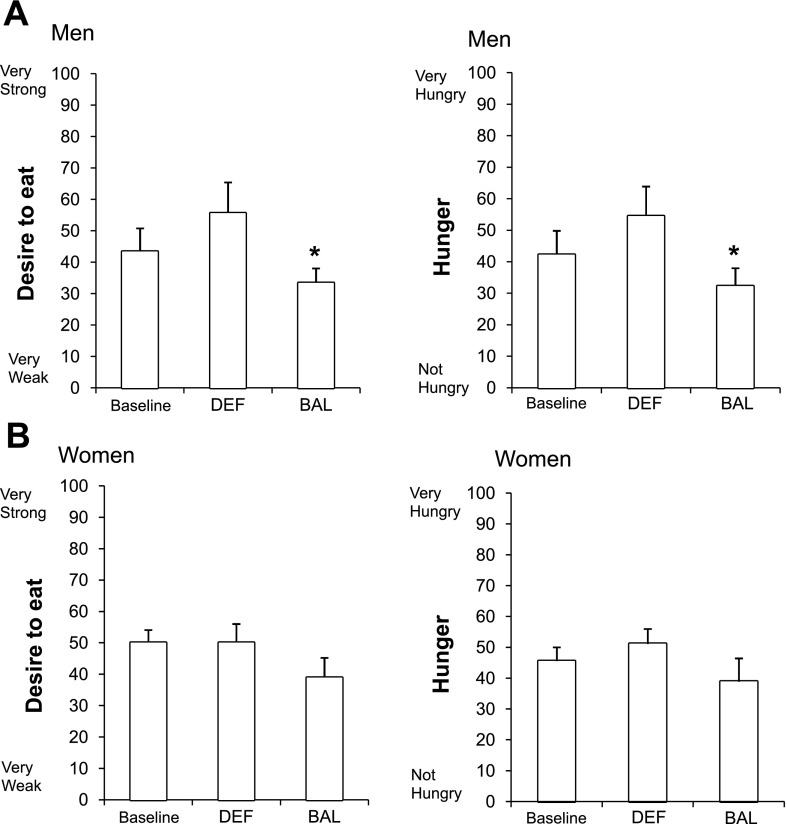
Compared with DEF, men had a significantly lower “desire to eat” (Fig. 4A), lower “hunger” (Fig. 4A), and lower “how much food you can eat” (data not shown) ratings during BAL. There was no difference across conditions in “fullness” (data not shown). In stark contrast, women reported no difference in “desire to eat” (Fig. 4B), “hunger” (Fig. 4B), “fullness,” and “how much food you can eat” (data not shown) in any of the three conditions. Despite these clear differences in the pattern of response observed across conditions, there was no significant main effect of sex or a sex × condition interaction (P > 0.05).
Fig. 4.
Desire to eat and hunger ratings in men (A) and in women (B). *Significantly different than DEF (P < 0.05).
Correlations.
Body fat was strongly related to fasting leptin concentrations (P < 0.05; r2 = 0.87) in both sexes. There were no significant correlations between body fat and any other hormone.
In men, lower insulin concentrations were related to lower “desire to eat,” “fullness,” and to greater “hunger” after DEF (P < 0.05; data not shown). In addition, lower leptin was related to lower “desire to eat”, “fullness,” and to more “hunger” after DEF (P < 0.05; data not shown). Other correlations were weak and not significant (P > 0.05).
In women, there were no significant correlations between any hormone and any of the appetite ratings (P > 0.05). These data indicate that a decrease in the anorexigenic hormones insulin and leptin is accompanied by a decrease in appetite in men but not in women.
DISCUSSION
This study demonstrated a clear sex difference in the way exercise, with or without energy deficit, affected energy-regulating hormones and appetite ratings. In the current study, we showed that compared with men, women responded to the initiation of exercise training with higher acylated ghrelin and lower insulin, both of which would be expected to stimulate energy intake. This pattern was observed, though attenuated, even when energy intake was increased to restore energy balance. In men, however, the acylated ghrelin and insulin responses to the initiation of exercise training were less pronounced than in women. When energy intake was increased to restore energy balance, the effects of exercise were notably absent. There were also sex differences in the appetite response to exercise. In men, adding dietary energy to restore energy balance reduced appetite. In women however, the addition of dietary energy to cover costs of exercise had no effect on appetite. These results support our hypothesis that women, compared with men, have a more robust hormonal response in a direction expected to stimulate appetite and (possibly) energy intake. However, in contrast with our hypotheses, exercise with or without energy deficit, had no effect on resting energy expenditure, leptin, or thyroid hormones.
A novel finding in the current investigation is the clear sex difference in how energy-regulating hormones responded to exercise. In women, concentrations of acylated ghrelin were higher (indicating more stimulus to eat) after exercise, regardless of energy status (deficit or balance). In contrast, we observed no change to acylated ghrelin in men. When scaled to total ghrelin concentrations, the AG/TG ratio was higher after the energy deficit condition (and tended to be higher after energy balance condition) in women, whereas there was no change in men. Acylated ghrelin, and not total or desacyl ghrelin, is widely recognized as the compound that stimulates energy intake because acylation is necessary to bind to the ghrelin receptor within the arcuate nucleus of the hypothalamus, hind brain, and other tissues (8, 16). Acylated ghrelin concentrations have been measured in a few prior studies, and none have directly compared the responses in men and women. In the one published study assessing the acylated ghrelin response during and after exercise, Broom et al. (5) reported that a single bout of high-intensity exercise reduced the acylated ghrelin AUC in lean, active men (energy balance was maintained for a short period of time). Sex differences in total ghrelin concentrations were evaluated in only one other study related to short-term energy imbalance (15). Gayle et al. (15) reported that female rats had higher total ghrelin concentrations and increased food consumption after a 12-h fast compared with male rats. Others have found that exercise-induced weight loss increases total ghrelin concentrations in women (13, 28, 29), whereas men have no change in total ghrelin concentrations after exercise-induced weight loss (37). Our findings are generally consistent with, and extend the results of, those previous studies to show that regular exercise, regardless of energy status, changes acylated ghrelin concentrations in a direction expected to stimulate energy intake in women but not in men.
By design, we controlled energy intake and expenditure in the current study and so are unable to determine whether higher postmeal acylated ghrelin concentrations in women increase actual food intake. In other research studies, infusing acylated ghrelin into the peripheral circulation stimulated food intake (49). In addition, fasting, which is associated with higher ghrelin concentrations, resulted in hyperphagia in animals (1, 45). But to our knowledge, no studies have been published showing that variations in postmeal acylated ghrelin concentrations increases subsequent food intake.
Further supporting the sex difference in how exercise impacts energy-regulating hormones, circulating insulin concentrations also changed more dramatically in women. In women, fasting insulin and insulin AUC were lower (indicating more stimulus to eat) after the initiation of exercise training, regardless of energy status. In men, insulin AUC was lower after the energy deficit condition, but, unlike in women, insulin AUC was restored to pre-exercise values when energy intake was increased to maintain energy balance. These results are concordant with the one previous study evaluating sex differences in the insulin response to exercise (21). Hickey et al. (21) found that 12 wk of regular exercise, without a change in body fat or body mass, lowered fasting insulin concentrations by 19% in women. Exercise training had no effect on fasting insulin concentrations in men. Our results in men are consistent with other previous well-controlled exercise studies (3, 39, 40). In general, these studies showed that an energy deficit lowers insulin concentrations, and replacing the exercise energy expenditure to restore energy balance prevents this decline (3, 39, 40). These data, combined with our finding that acylated ghrelin was higher after exercise in women, suggest that in women exercise alone (energy balance maintained) alters the hormonal response in a direction expected to stimulate energy intake, and an energy deficit potentiates this response. In men, however, exercise alone has no impact on energy-regulating hormones, and the effect of energy deficit is less pronounced than it is in women.
A lower circulating concentration of insulin, similar to leptin, is thought to stimulate energy intake because it is associated with an increase in neurotransmitters (e.g., neuropeptide Y, etc.) within the arcuate nucleus of the hypothalamus that are known to stimulate food intake (33). The hypothesis is not supported by data, however. Recent results from Clegg and colleagues (6) showed that the central nervous systems of female rats were more sensitive than those of male rats to the effects of leptin, but not to insulin. To date, there are no published results showing that lower postmeal insulin concentrations stimulate subsequent ad libitum food intake. The lack of prior data limits our ability to infer that women would have increased actual energy intake in response to our exercise intervention.
In the current investigation, leptin and thyroid hormone (in particular T3) concentrations did not change in either sex in response to exercise. These results are in agreement with one (27) but not the majority of prior studies (23, 30, 35). Loucks and colleagues (23, 30) showed that, relative to a no-exercise control, 24-h leptin and thyroid hormone concentrations were lower after 4 days of exercise-induced energy deficit in women. Because leptin and thyroid hormone concentrations have a diurnal variation (30, 31), we may have missed a change in these hormones by assessing a single fasting sample. In addition, the total amount of exercise energy expenditure may influence concentrations of leptin and thyroid hormone, and ultimately resting energy expenditure (23, 30, 35). Hilton and Loucks (23) showed that 4 days of exercise (total exercise expenditure ∼5,520 kcal), without a concurrent increase in dietary energy, lowered leptin and thyroid hormone concentrations. Similarly, Poehlman et al. (35) showed that thyroid hormone concentrations and resting energy expenditure were lower after 22 days of exercise-induced energy deficit (total exercise expenditure ∼22,000 kcal). In the current study, the 4-day exercise energy expenditure was ∼3,000 kcal in men and 2,400 kcal in women. Therefore, our exercise protocol may have been of insufficient duration and/or intensity to alter leptin and thyroid hormone concentrations or resting energy expenditure. Finally, it is possible that the sample and effect sizes were inadequate to the necessary statistical power to detect changes in leptin and thyroid hormone concentrations.
Similar to the effects of acylated ghrelin and insulin concentrations, we found that the initiation of exercise training affected subjective appetite ratings differently in men and women. In men, appetite ratings (e.g., “desire to eat,” “hunger,” and “how much food can you eat”) were lower when energy intake matched energy expenditure, as opposed to when they were in energy deficit. In contrast, appetite responses did not decline when energy was added to replace exercise energy expenditure in women. “Desire to eat,” “hunger,” “fullness,” and “how much food can you eat” ratings were not different regardless of energy state. These data are consistent with a recent study, in men, showing that appetite was reduced after a single bout of exercise when energy balance was maintained (5). However, our results are in disagreement with several previous studies that report regular exercise (1–9 bouts), with or without a concurrent energy deficit, does not affect appetite ratings in men or in women (41, 42). A major distinction from our study is that in those experiments, energy intake was evaluated by self-report and appetite responses to exercise were assessed in subjects that ate ad libitum (41, 42). Using self-reported intake from food diaries typically underreports energy intake (26). To minimize the potentially confounding impact of self-reported data, we evaluated appetite responses to a systematic manipulation of energy intake and energy expenditure. Because we took great care to control energy balance (e.g., all food was provided, exercise was done under controlled conditions in the laboratory, etc.), we are confident that the reduced appetite response we observed in men when energy balance was restored is a true response to the intervention.
As mentioned previously, because we controlled energy intake and expenditure in the current investigation (both independent variables), we are unable to determine whether there are sex differences in actual food intake after exercise. In general, we saw no change in appetite ratings after several days of exercise in women, which is in line with previous studies (41, 43). Stubbs et al. (43) found that raising energy expenditure with exercise for 7 days (expenditure = 453 to 812 kcal/day) did not alter subjective appetite ratings in women. In that study, women increased ad libitum food intake to partially balance the new higher energy expenditure, even though appetite was not higher. In contrast, men did not change their food intake when energy expenditure was raised by the addition of exercise. To more fully understand the impacts of exercise and/or energy balance on the hormonal regulation of appetite and food intake, it will be important to assess all three as dependent variables in the same study.
Our data indicate that in men, the appetite response to 4 days of exercise is inhibited when energy intake is raised to meet the new higher energy expenditure. In women, increasing energy intake to maintain energy balance did not inhibit appetite. However, in women, given our relatively small sample size, we may not have been sufficiently powered to detect a significant difference in appetite.
By virtue of our study design, we are unable to determine the relative contributions of energy or carbohydrate deficit to the hormonal and appetite changes observed. A main goal of this study was to determine the interactions between energy status and exercise on hormones that regulate energy intake and expenditure. Because we kept the macronutrient composition constant in all 3 conditions (∼55–60% carbohydrate, ∼25–30% fat, ∼15% protein), when energy was restricted, subjects were also in a “carbohydrate deficit” relative to the BAL condition. In men, carbohydrate intake was 4.4 g/kg body mass in DEF vs. 5.7 in BAL, and in women, it was 4.0 g/kg body mass in DEF and 5.3 in BAL. In addition to the differences in carbohydrate intake between conditions, the carbohydrate intake (in absolute terms) was also higher in men because they weighed considerably more than the women. In some studies, ghrelin and insulin concentrations were more responsive to changes in dietary carbohydrate than to changes in energy intake per se (38, 44). Therefore, it is possible that more dietary carbohydrate, not more energy, blunted or negated changes in acylated ghrelin and insulin concentrations in men in both conditions (deficit and balance). Because neither carbohydrate intake nor energy intake were correlated with any hormone we measured, we cannot rule out either of them playing a role in the observed sex differences.
Regular exercise can reduce body fat directly by increasing energy expenditure to generate an overall energy deficit. When energy intake is raised to compensate for exercise energy expenditure, however, body fat is maintained. Previous studies suggest that with the initiation of exercise training, women match the new higher energy expenditure by raising energy intake or have no change in postexercise fat oxidation (19, 20), and therefore do not lose body fat (11, 36). In contrast, men lose body fat because they do not sufficiently raise energy intake to match the higher exercise energy expenditure (11, 36). Potteiger et al. (36) reported that supervised aerobic exercise performed 5 days/wk for 9 mo lowered body fat and body weight in men who ate ad libitum. In stark contrast, there were no changes in body fat and body mass in women. In the current study, the observed sex difference in energy-regulating hormones and appetite may explain, at least partially, the sex differences reported for energy intake and body fat loss in response to exercise training reported in previous studies. The real-world impact of these male-female differences in hormones that regulate energy intake will depend upon if/how these effects manifested over time. The hormones that regulate energy intake respond quickly to alterations in energy status, well prior to measurable changes in body fat (3, 18, 38). If the hormonal pattern of changes that we observed is maintained over time, body fat loss may be accentuated in men compared with women.
In women, better matching of energy intake to demand in response to a metabolic or physiological challenge may be driven by the critical relationship between energy balance and reproductive success. Energy deficiency, with or without exercise, suppresses ovulatory cycles, inhibits gonadotrophin-releasing hormone secretion, reduces pulsatility of luteinizing hormone, and stops copulatory behavior (2, 10, 31, 46). In women, higher acylated ghrelin and lower insulin concentrations in response to elevated energy expenditure may be some of the mechanisms in place to defend energy balance and preserve reproduction function. Surprisingly, few data exist supporting the role for ghrelin and insulin as primary signals in reproductive function (6, 32). Martini et al. (32) observed that desacyl ghrelin, not acylated ghrelin, inhibits luteinizing hormone secretion. Recent data from Clegg and colleagues (6) show that male rats decrease food intake in response to insulin administration. In females, however, insulin had no impact on food intake. It is likely that the relationships between insulin, and possibly ghrelin, on reproductive function are related to their roles as indicators of energy availability rather than as primary reproductive signals (46).
In the current investigation, to control for the menstrual cycle phase, the majority of women were on hormonal contraceptives. It is possible that changing concentrations of progesterone and estrogen across the menstrual cycle phase may have impacted the results of this study. We tried to limit this potential confound by women starting the intervention in the early follicular phase of the menstrual cycle during the pill-free week. In a recent study in normal menstruating women not on hormonal contraceptives, Dafopolous et al. (7) showed no change in acylated ghrelin concentrations across different phases of the menstrual cycle. However, the results of this study may be specific to women on hormonal contraceptives and are not generalizable to women not on these contraceptives.
In the current study, we did not match men and women for body composition or cardiorespiratory fitness. Doing so would minimize the real-world relevance of the results because, in general, those differences in body fat and cardiorespiratory fitness are true sex differences. It is possible that the male-female differences we observed in energy-regulating hormones may be related to lower fitness level and/or higher body fat in women. However, all of the correlations between V̇o2peak or body fat and any hormone we measured were weak and nonsignificant. In addition, when we used V̇o2peak and body fat as covariates, the changes in acylated ghrelin and insulin concentrations persisted. These analyses suggest that the observed sex differences in energy intake regulating hormones are a true response to the intervention and not confounded by differences in subject characteristics. Still, it remains that the less physically fit women might simply have exerted themselves less and, hence, recovered more rapidly.
Another limitation of the present study is that we measured only a few of the hormones that are known to regulate energy intake and energy expenditure. Clearly, other hormones (e.g., cholecystokinin, PYY3–36, and glucagon like peptide-1) and other factors (e.g., behaviors not under direct hormonal control) that we did not measure are also involved in the regulation of energy intake, expenditure, and appetite. The present study was focused on circulating factors that have strong support in the literature as major players in the regulation of energy balance. Clearly, there is much more work that can be done in this area, both at the molecular/cellular (binding of hormones in the arcuate nucleus of the hypothalamus, hindbrain, etc., paracrine effects of peptides that cannot be measured appropriately in the general circulation), and behavioral (role for social cues, environmental context) levels.
Perspectives and Significance
In summary, we observed a clear sex difference in the way exercise alters hormones and appetite related to energy intake. Women had a more robust hormonal response to exercise in a direction expected to stimulate appetite and energy intake, regardless of energy status (deficit or balance). In men however, the hormonal response to exercise with concurrent energy deficit was more subtle than it was in women, and it was completely reversed when energy balance was restored. This sex difference in the regulation of energy balance has implications both for our understanding of basic human biology (e.g., defending body fat for reproductive success in women) and in terms of exercise and dietary recommendations for the lay public. Anecdotally, there is a consensus in the scientific community that women may need both to increase energy expenditure and decrease energy intake to achieve body fat loss. The results of the current study suggest at least one potential biological explanation.
GRANTS
This investigation was supported by a grant from the Baystate/University of Massachusetts Amherst Biomedical Research program and National Institutes of Health Grant NS10873-34.
Acknowledgments
We would like to thank all of the volunteers for their time and participation in this investigation. We are grateful to Samantha Reusch and Gregory Ead for helping with data collection. We thank Patty Freedson, Rebecca Hasson, Steven Malin, and Kirsten Granados for helping with various aspects of the study.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA, Kasuga M. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 120: 337–345, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Berriman SJ, Wade GN, Blaustein JD. Expression of Fos-like proteins in gonadotropin-releasing hormone neurons of Syrian hamsters: effects of estrous cycles and metabolic fuels. Endocrinology 131: 2222–2228, 1992. [DOI] [PubMed] [Google Scholar]
- 3.Black SE, Mitchell E, Freedson PS, Chipkin SR, Braun B. Improved insulin action following short-term exercise training: role of energy and carbohydrate balance. J Appl Physiol 99: 2285–2293, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Brooks GA, Butte NF, Rand WM, Flatt JP, Caballero B. Chronicle of the Institute of Medicine physical activity recommendation: how a physical activity recommendation came to be among dietary recommendations. Am J Clin Nutr 79: 921S–930S, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Broom DR, Stensel DJ, Bishop NC, Burns SF, Miyashita M. Exercise-induced suppression of acylated ghrelin in humans. J Appl Physiol 102: 2165–2171, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes 55: 978–987, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Dafopoulos K, Sourlas D, Kallitsaris A, Pournaras S, Messinis IE. Blood ghrelin, resistin, and adiponectin concentrations during the normal menstrual cycle. Fertil Steril In press. [DOI] [PubMed]
- 8.Davenport AP, Bonner TI, Foord SM, Harmar AJ, Neubig RR, Pin JP, Spedding M, Kojima M, Kangawa K. International Union of Pharmacology. LVI Ghrelin receptor nomenclature, distribution, and function. Pharmacol Rev 57: 541–546, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Despres JP, Bouchard C, Savard R, Tremblay A, Marcotte M, Theriault G. The effect of a 20-week endurance training program on adipose-tissue morphology and lipolysis in men and women. Metab Clin Exp 33: 235–239, 1984. [DOI] [PubMed] [Google Scholar]
- 10.Dickerman RW, Li HY, Wade GN. Decreased availability of metabolic fuels suppresses estrous behavior in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol 264: R568–R572, 1993. [DOI] [PubMed] [Google Scholar]
- 11.Donnelly JE, Hill JO, Jacobsen DJ, Potteiger J, Sullivan DK, Johnson SL, Heelan K, Hise M, Fennessey PV, Sonko B, Sharp T, Jakicic JM, Blair SN, Tran ZV, Mayo M, Gibson C, Washburn RA. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: the Midwest Exercise Trial. Arch Intern Med 163: 1343–1350, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Flier JS Clinical review 94: What's in a name? In search of leptin's physiologic role. J Clin Endocrinol Metab 83: 1407–1413, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Foster-Schubert KE, McTiernan A, Frayo RS, Schwartz RS, Rajan KB, Yasui Y, STS, Cummings D. Human plasma ghrelin levels increase during a one-year exercise program. J Clin Endocrinol Metab 90: 820–825, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc 30: 777–781, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Gayle DA, Desai M, Casillas E, Beloosesky R, Ross MG. Gender-specific orexigenic and anorexigenic mechanisms in rats. Life Sci 79: 1531. –1536, 2006. [DOI] [PubMed]
- 16.Ghigo E, Broglio F, Arvat E, Maccario M, Papotti M, Muccioli G. Ghrelin: more than a natural GH secretagogue and/or an orexigenic factor. Clin Endocrinol(Oxf) 62: 1–17, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Hagobian TA, Braun B. Interactions between energy surplus and short-term exercise on glucose and insulin responses in healthy people with induced, mild insulin insensitivity. Metabolism 55: 402–408, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Hagobian TA, Sharoff CG, Braun B. Effects of short-term exercise and energy surplus on hormones related to regulation of energy balance. Metabolism 57: 393–398, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Henderson GC, Fattor JA, Horning MA, Faghihnia N, Johnson ML, Luke-Zeitoun M, Brooks GA. Glucoregulation is more precise in women than in men during postexercise recovery. Am J Clin Nutr 87: 1686–1694, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Henderson GC, Fattor JA, Horning MA, Faghihnia N, Johnson ML, Mau TL, Luke-Zeitoun M, Brooks GA. Lipolysis and fatty acid metabolism in men and women during the postexercise recovery period. J Physiol 584: 963–981, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hickey MS, Houmard JA, Considine RV, Tyndall GL, Midgette JB, Gavigan KE, Weidner ML, McCammon MR, Israel RG, Caro JF. Gender-dependent effects of exercise training on serum leptin levels in humans. Am J Physiol Endocrinol Metab 272: E562–E566, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Hill JO, Sparling PB, Shields TW, Heller PA. Effects of exercise and food restriction on body composition and metabolic rate in obese women. Am J Clin Nutr 46: 622–630, 1987. [DOI] [PubMed] [Google Scholar]
- 23.Hilton LK, Loucks AB. Low energy availability, not stress, suppresses diurnal rhythm of leptin in healthy young women. Am J Physiol Endocrinol Metab 278: E43–E49, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Kien CL, Ugrasbul F. Prediction of daily energy expenditure during a feeding trial using measurements of resting energy expenditure, fat-free mass, or Harris-Benedict equations. Am J Clin Nutr 80: 876–880, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klem ML, Wing RR, McGuire MT, Seagle HM, Hill JO. A descriptive study of individuals successful at long-term maintenance of substantial weight loss. Am J Clin Nutr 66: 239–246, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Klesges RC, Eck LH, Ray JW. Who underreports dietary intake in a dietary recall? Evidence from the Second National Health and Nutrition Examination Survey. J Consult Clin Psychol 63: 438–444, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Kyriazis GA, Caplan JD, Lowndes J, Carpenter RL, Dennis KE, Sivo SA, Angelopoulos TJ. Moderate exercise-induced energy expenditure does not alter leptin levels in sedentary obese men. Clin J Sport Med 17: 49–51, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Leidy HJ, Dougherty KA, Frye BR, Duke KM, Williams NI. Twenty-four-hour ghrelin is elevated after calorie restriction and exercise training in non-obese women. Obesity 15: 446–455, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Leidy HJ, Gardner JK, Frye BR, Snook ML, Schuchert MK, Richard EL, Williams NI. Circulating ghrelin is sensitive to changes in body weight during a diet and exercise program in normal-weight young women. J Clin Endocrinol Metab 89: 2659–2664, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Loucks AB, Heath EM. Induction of low-T3 syndrome in exercising women occurs at a threshold of energy availability. Am J Physiol Regul Integr Comp Physiol 266: R817-R823, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Loucks AB, Thuma JR. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J Clin Endocrinol Metab 88: 297–311, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Martini AC, Fernandez-Fernandez R, Tovar S, Navarro VM, Vigo E, Vazquez MJ, Davies JS, Thompson NM, Aguilar E, Pinilla L, Wells T, Dieguez C, Tena-Sempere M. Comparative analysis of the effects of ghrelin and unacylated ghrelin on luteinizing hormone secretion in male rats. Endocrinology 147: 2374–2382, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Morton GJ, Schwartz MW. The NPY/AgRP neuron and energy homeostasis. Int J Obes Relat Metab Disord 25 Suppl 5: S56–S62, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Pober DM, Freedson PS, Kline GM, McInnis KJ, Rippe JM. Development and validation of a one-mile treadmill walk test to predict peak oxygen uptake in healthy adults ages 40 to 79 years. Can J Appl Physiol 27: 575–589, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Poehlman ET, Tremblay A, Nadeau A, Dussault J, Theriault G, Bouchard C. Heredity and changes in hormones and metabolic rates with short-term training. Am J Physiol Endocrinol Metab 250: E711–E717, 1986. [DOI] [PubMed] [Google Scholar]
- 36.Potteiger JA, Jacobsen DJ, Donnelly JE, Hill JO. Glucose and insulin responses following 16 months of exercise training in overweight adults: the Midwest Exercise Trial. Metab Clin Exp 52: 1175–1181, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Ravussin E, Tschop M, Morales S, Bouchard C, Heiman ML. Plasma ghrelin concentration and energy balance: overfeeding and negative energy balance studies in twins. J Clin Endocrinol Metab 86: 4547–4551, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Romon M, Gomila S, Hincker P, Soudan B, Dallongeville J. Influence of weight loss on plasma ghrelin responses to high-fat and high-carbohydrate test meals in obese women. J Clin Endocrinol Metab 91: 1034–1041, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, Janssen I. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med 133: 92–103, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Segal KR, Edano A, Abalos A, Albu J, Blando L, Tomas MB, and Pi-Sunyer FX. Effect of exercise training on insulin sensitivity and glucose metabolism in lean, obese, and diabetic men. J Appl Physiol 71: 2402–2411, 1991. [DOI] [PubMed] [Google Scholar]
- 41.Stubbs RJ, Hughes DA, Johnstone AM, Whybrow S, Horgan GW, King N, Blundell J. Rate and extent of compensatory changes in energy intake and expenditure in response to altered exercise and diet composition in humans. Am J Physiol Regul Integr Comp Physiol 286: R350–R358, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Stubbs RJ, Sepp A, Hughes DA, Johnstone AM, Horgan GW, King N, Blundell J. The effect of graded levels of exercise on energy intake and balance in free-living men, consuming their normal diet. Eur J Clin Nutr 56: 129–140, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Stubbs RJ, Sepp A, Hughes DA, Johnstone AM, King N, Horgan G, Blundell JE. The effect of graded levels of exercise on energy intake and balance in free-living women. Int J Obes Relat Metab Disord 26: 866–869, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Tannous dit El Khoury D., Obeid O, Azar ST, Hwalla N. Variations in postprandial ghrelin status following ingestion of high-carbohydrate, high-fat, and high-protein meals in males. Ann Nutr Metab 50: 260–269, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 407: 908–913, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Wade GN, Jones JE. Neuroendocrinology of nutritional infertility. Am J Physiol Regul Integr Comp Physiol 287: R1277–R1296, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Woods SC, Gotoh K, Clegg DJ. Gender differences in the control of energy homeostasis. Exp Biol Med (Maywood) 228: 1175–1180, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 86: 5992, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 141: 4325–4328, 2000. [DOI] [PubMed] [Google Scholar]