Abstract
Disrupted circadian rhythmicity is associated with ethanol (EtOH) abuse, yet little is known about how EtOH affects the mammalian circadian clock of the suprachiasmatic nucleus (SCN). Clock timing is regulated by photic and nonphotic inputs to the SCN involving glutamate release from the retinohypothalamic tract and serotonin (5-HT) from the midbrain raphe, respectively. Our recent in vitro studies in the SCN slice revealed that EtOH blocks photic phase-resetting action of glutamate and enhances the nonphotic phase-resetting action of the 5-HT1A,7 agonist, 8-OH-DPAT. To explore the basis of these effects in the whole animal, we used microdialysis to characterize the pharmacokinetics of intraperitoneal injection of EtOH in the hamster SCN extracellular fluid compartment and then studied the effects of such EtOH treatment on photic and serotonergic phase resetting of the circadian locomotor activity rhythm. Peak EtOH levels (∼50 mM) from a 2 g/kg injection occurred within 20–40 min with a half-life of ∼3 h. EtOH treatment dose-dependently attenuated photic phase advances but had no effect on phase delays and, contrary to in vitro findings, markedly attenuated 8-OH-DPAT-induced phase advances. In a complementary experiment using reverse microdialysis to deliver a timed SCN perfusion of EtOH during a phase-advancing light pulse, the phase advances were blocked, similar to systemic EtOH treatment. These results are evidence that acute EtOH significantly affects photic and nonphotic phase-resetting responses critical to circadian clock regulation. Notably, EtOH inhibition of photic signaling is manifest through direct action in the SCN. Such actions could underlie the disruption of circadian rhythmicity associated with alcohol abuse.
Keywords: suprachiasmatic nucleus, glutamate, serotonin, microdialysis, alcohol
ethanol (EtOH) is highly disruptive to mammalian circadian physiological and behavioral rhythms, including those for melatonin (43, 75), glucocorticoids (2, 70), thyroid-stimulating hormone (20), body temperature (5, 21, 22, 94), and sleep (11, 27, 42, 43, 72). Also, disrupted circadian timing of hypothalamic-pituitary-adrenal axis function is associated with increased EtOH preference in experimental animals, and in humans, may be a risk factor for developing alcoholism and relapsing after abstinence (16, 61, 98). In a related manner, circadian-based sleep problems are implicated in the development of alcoholism and in abstinent alcoholics' propensity to relapse (73, 74).
In mammals, the master circadian clock responsible for generating and maintaining physiological and behavioral rhythms is located in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus (41, 56, 80). SCN clock-driven rhythms are entrained to the daily light-dark (LD) cycle via glutamate release from the retinohypothalamic tract in the SCN (34, 36, 54, 62, 79). Glutamatergic activation of the SCN N-methyl-d-aspartate (NMDA) receptor is essential for light-induced phase shifting to occur (1, 15, 23, 49, 50). In a recent report, we showed that EtOH can block such glutamate-induced phase shifts in the SCN in vitro (68). This finding fits with reports that EtOH inhibits glutamatergic signaling, both presynaptically and postsynaptically in other brain areas (46, 55, 57, 60, 84, 96) and that the NR2B subunit of the NMDA receptor complex involved in SCN photic phase resetting (57) is EtOH sensitive (3, 4, 58, 86). It is notable that EtOH's actions on the NMDA receptor are considered to be the basis for intoxication, dependence, tolerance, addiction, and neuronal damage during withdrawal (13, 14, 29, 33, 40, 88, 90, 91, 92), and thus disrupted photic signaling in the clock may also be related to EtOH effects on the NMDA receptor.
In addition to photic entrainment, the SCN receives a variety of nonphotic (behavior-induced) inputs that are important to circadian rhythm regulation. One such mediator of nonphotic input is serotonin (5-HT), which is supplied by a projection from the median raphe nucleus (49, 57). Serotonergic input also modulates photic signaling in the SCN (32, 47, 63, 64, 71, 82, 97) by inhibiting glutamatergic transmission (9, 12, 85, 87). Relevant to EtOH's disruptive effect on 5-HT-related circadian functions are observations that chronic EtOH increases the release of, and may decrease the reuptake of, central 5-HT (14, 76) and downregulates certain subtypes of 5-HT receptors (45, 59). Moreover, acute EtOH treatment potentiates serotonergic phase advances of the SCN in vitro (68). It is therefore apparent that EtOH effects on critical serotonergic and glutamatergic functions in the SCN could underlie the disordered circadian rhythmicity associated with chronic EtOH use.
To gain a better understanding of the actions of EtOH in the SCN clock, the present experiments were undertaken to characterize the pharmacokinetics of acute systemic EtOH administration in the SCN and to explore the in vivo effects of systemic and intra-SCN EtOH on photic and serotonergic phase-resetting processes in the Syrian hamster. Such information is important for determining the neural substrates of EtOH disruptive effects on circadian timing and whether the SCN represents a direct target for these actions.
MATERIALS AND METHODS
Animals.
Adult, male Syrian hamsters Mesocricetus auratus raised from breeders purchased from Harlan Sprague-Dawley (Madison, IL) were used in this study. Animals were maintained in a temperature-controlled vivarium (23°C) under a 14:10 LD photoperiod with light intensity of 270 lux with food (Prolab 3000; PMI Feeds, St. Louis, MO) and water provided ad libitum. The experiments were approved by the Kent State Institutional Animal Care and Use Committee and were conducted using the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
SCN pharmacokinetics of acute systemic EtOH administration.
In vivo microdialysis was used to characterize the time course of EtOH in the SCN of freely behaving hamsters following intraperitoneal EtOH injection. Procedures for microdialysis of the SCN have been described previously (24). Concentrically designed microdialysis probes were constructed from a 26-gauge stainless-steel outer cannula into which was inserted 32-gauge fused silica tubing. Hemicellulose dialysis membrane tubing (12 kDa mol wt cutoff; 230 μm outer diameter) was secured to the outer cannula with epoxy glue. The active dialysis length was 1.0 mm. Animals were anesthetized with pentobarbital sodium (Nembutal; 50 mg/kg), and Marcaine (0.25% bupivicaine, 0.10 ml) was injected in the scalp area before surgery. Animals received the antibiotic Combi-Pen-48 (15,000 units in 0.05 ml) subcutaneously after surgery. The microdialysis probe was targeted stereotaxically to the SCN 2 days before experimentation, and the implantation site was confirmed histologically after experimentation. The 2-day recovery period was used, since this is considered optimal for tissue recovery (blood-brain barrier reestablishment) and probe function. Sampling was undertaken 1 h before, and extending 7 h after, the intraperitoneal injection (2.0 g/kg) at a flow rate of 1.0 μl/min with a sampling interval of 20 min. EtOH in the microdialysate samples was measured using validated gas chromatography (GC) procedures (HP-5890A gas chromatograph; internal standard = secondary butanol, initial temperature = 40°C, purge time = 1 min, final temperature = 225°C, final time = 5 min). The standard curve for EtOH analysis was linear over the concentration range for SCN microdialysis measurements. The inter- and intra-assay coefficients of variability were 9.2 and 8.6%, respectively. To obtain an in vitro estimate of efficiency of EtOH delivery via our probes, EtOH was measured in dialysate samples collected from probes submerged in known EtOH standards maintained at 35, 37, or 39°C to determine if fluctuations in body temperature within this range would significantly affect probe efficiency. Mean probe efficiency was estimated to be ∼12% and did not vary significantly between the tested temperatures (t = 0; p = 1). Results from this experiment were used to determine the optimal timing of EtOH injection before administration of the light pulses and to estimate an effective EtOH dosage in subsequent experiments.
Effects of acute systemic EtOH administration on photic phase resetting.
Hamsters under LD were individually caged, and their general circadian locomotor activity rhythms were monitored over a 2-wk period before experimentation using infrared motion detectors interfaced with a computerized data acquisition system (Clocklab; Coulbourn Instruments, Whitehall, PA). Under constant darkness (DD), the onset of activity, designated as circadian time 12, was used as the phase reference point for the beginning of the subjective night. Activity onset was defined as the as the first 6 min that are 1) coincident with an intensity of activity that exceeded 10% of the maximum rate for the day; 2) preceded by a period of at least 4 h of inactivity; and 3) followed by a period of at least 30 min of sustained activity. Phase shifts were calculated as the difference between the projected times of activity onset on the day after stimulation as determined by 1) back extrapolation of the least-squares line through activity onsets on days 3–7 after treatment and 2) extrapolation of the least-squares line calculated from activity onset data collected during the 5 days before treatment.
On the day of the experiment, animals received an intraperitoneal injection of one of three doses of EtOH (0.5, 1.5, and 2.0 g/kg; diluted to 20% vol/vol in physiological saline) or saline vehicle preceeding a 30-min phase-advancing light pulse (270 lux) at Zeitgeber time (ZT) 18.5 or a 30-min phase-delaying light pulse at ZT 14 (where ZT 12 is designated as the onset of the dark phase). Injections were undertaken in the dark using infrared goggles. Immediately after the light pulse, animals were released in DD for 2 wk to assess phase advances and delays using an Aschoff Type II procedure (19). The ‘no light pulse’ control animals received an intraperitoneal injection of saline or EtOH (2.0 g/kg) and did not receive a light pulse.
Effects of intra-SCN EtOH administration on photic phase resetting.
In this experiment, EtOH was administered directly in the SCN via reverse-microdialysis perfusion. Hamsters under LD were individually caged, and their general activity rhythms were monitored over a 2-wk period before experimentation. Before experimentation, each animal received a microdialysis probe implant targeted to the lateral margin of the SCN. On the day of experimentation, the microdialysis probes were perfused with artificial cerebrospinal fluid (ACSF) alone or ACSF containing EtOH (500 mM; based on in vitro measurements of probe efficiency, this provided a theoretical outside tissue concentration of ∼50 mM) from a syringe pump. The concentration of EtOH used was based on the pharmacokinetic data from systemic injections of EtOH in experiment 1. Continuous perfusion of the probes commenced 30 min before, and extended throughout and for 30 min after, a 30-min phase-advancing light pulse (270 lux) administered at ZT 18.5. Immediately after the light pulse, the animals were released into DD for 2 wk to assess phase-shifting responses using the Aschoff Type II procedure. The “no light pulse” controls received microdialysis perfusion of ACSF alone or containing EtOH, but remained in DD. In a separate group of animals, blood alcohol content (BAC) was measured after the 1.5-h perfusion of EtOH (500 mM). Animals were killed, and trunk blood was collected in a 1.5-ml microfuge tube containing heparin USP (1,000 U/ml) and centrifuged to isolate serum. BAC was measured using the Analox AM-1 Alcohol Analyzer (Lunenburg, MA). Location of the microdialysis probes was determined histologically at the end of the experiment from fixed frozen sections mounted and stained with cresyl violet.
Effects of acute systemic EtOH administration on nonphotic (serotonergic) phase resetting.
Hamsters under LD were individually caged, and their general circadian locomotor activity rhythms were monitored over a 2-wk period before experimentation. On the day of the experiment, animals received an intraperitoneal injection of EtOH (2.0 g/kg) or saline vehicle preceding intraperitoneal injection of the serotonin 5-HT1a,7 agonist ( ± )8-OH-DPAT (5.0 mg/kg) at ZT 6 (the time at which the phase-advance portion of the 8-OH-DPAT phase-response curve is maximal). Immediately after injection, animals were released into DD for 2 wk to assess phase advances using the Aschoff Type II procedure. Vehicle controls received intraperitoneal injection of dimethyl sulfoxide instead of 8-OH-DPAT.
Statistical analyses.
Differences in behavioral phase shifts were assessed by ANOVA and subsequent Student-Newman-Keul's post hoc mean comparison test. The level of significance was set at P < 0.05.
RESULTS
Assessment of EtOH pharmacokinetics in the SCN.
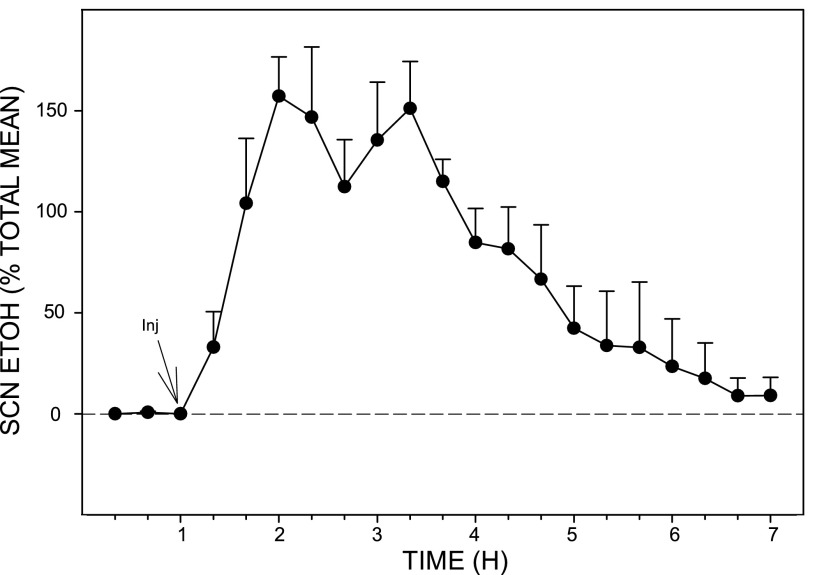
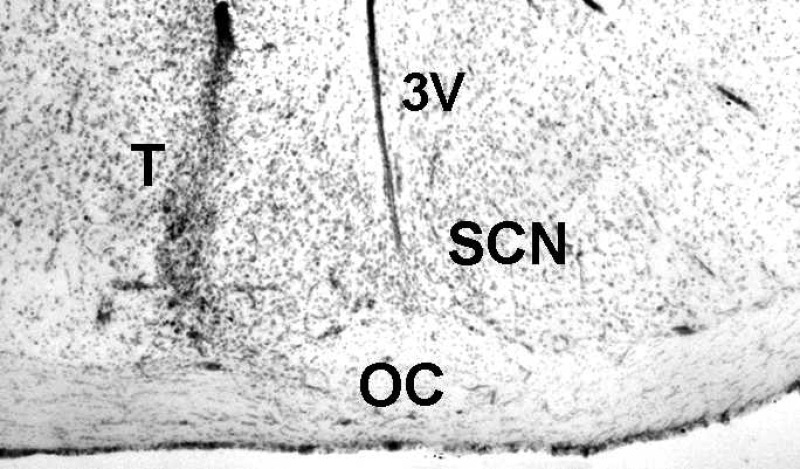
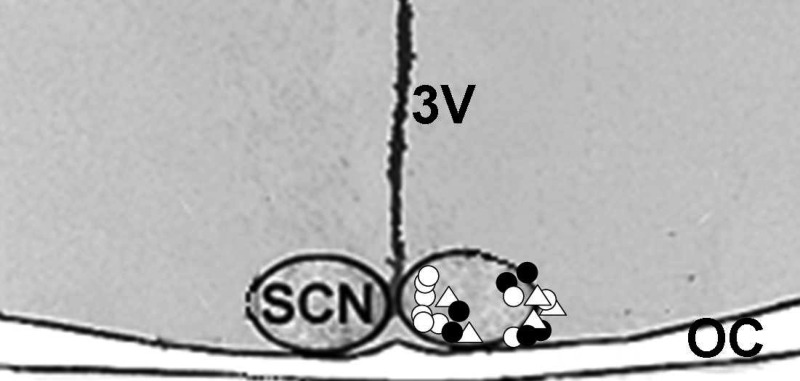
The pharmacokinetic profile of EtOH in the SCN extracellular fluid compartment following intraperitoneal injection of EtOH (2.0 g/kg) as assessed in freely behaving hamsters by microdialysis-GC analysis is presented in Fig. 1. Peak EtOH levels in the SCN occurred within 20–40 min postinjection, and the half-life of the absorbed EtOH (t1/2 elimination) was ∼3 h. Based on the in vitro calculation of microdialysis probe efficiency for EtOH (12%), the peak concentration of EtOH in the SCN was ∼50 mM. Probe location along the lateral margin of the SCN was evaluated histologically as shown in the photomicrograph of Fig. 2, and the locations of probe tips for each animal used in this and subsequent experiments are shown diagrammatically in Fig. 3.
Fig. 1.
Pharmacokinetics of ethanol (EtOH) in the suprachiasmatic nucleus (SCN) (n = 5 hamsters) following ip injection (Inj) of EtOH (2.0 g/kg). Peak levels (estimated at ∼50 mM) occurred within 20–40 min of injection, and the half-life (t1/2) for clearance was 3 h. Bars represent means ± SE.
Fig. 2.
Coronal section through the hypothalamus showing the probe tract (T) of a microdialysis probe targeted to the lateral margin of the SCN. 3V, third ventricle; OC, optic chiasm.
Fig. 3.
Histologically verified probe tip locations relative to the SCN for each of the microdialysis experiments. ▵, Pharmacokinetics of EtOH after ip injection; •, reverse dialysis of EtOH; ○, reverse dialysis of vehicle.
Acute systemic EtOH administration attenuates photic phase resetting in vivo.
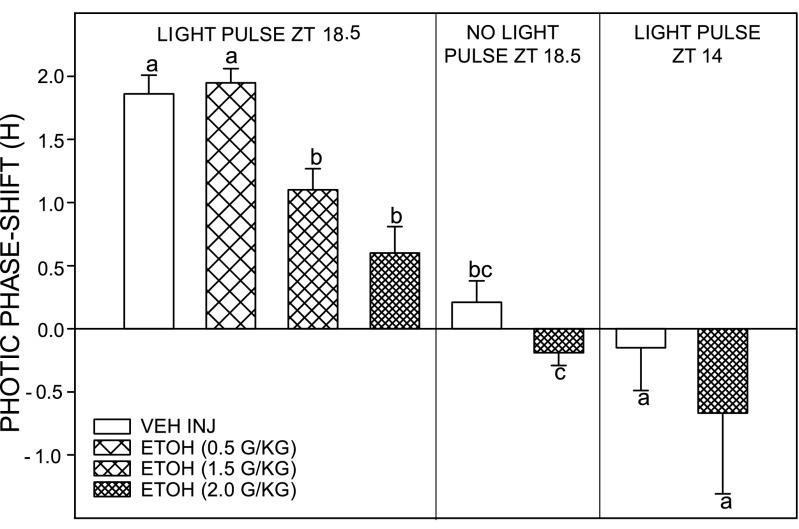
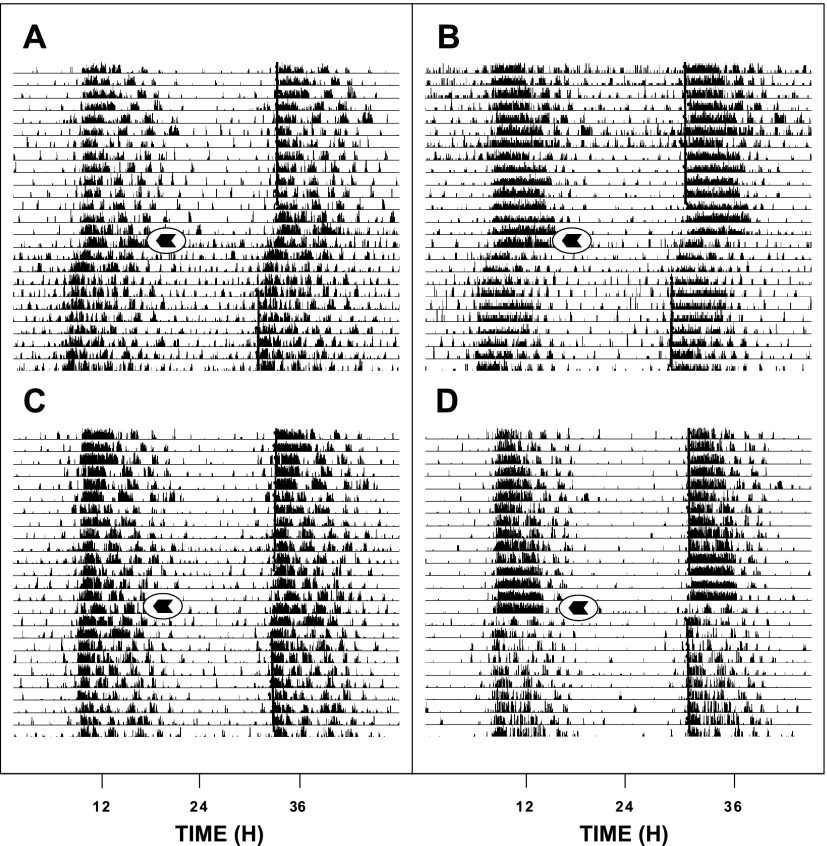
Photic phase-advance shifts at ZT 18.5 were significantly attenuated by intraperitoneal injection of EtOH in a dose-dependent manner [F(3,28) = 14.6; P = 0.0001]. Controls receiving intraperitoneal saline injection had phase advances averaging 1.90 ± 0.20 h (n = 11). Animals receiving intraperitoneal injection of EtOH at a dose range of 0.5 (n = 11), 1.5 (n = 5), and 2.0 (n = 5) g/kg showed diminishing phase advances averaging 2.00 ± 0.10, 1.10 ± 0.20, and 0.60 ± 0.20 h, respectively (Fig. 4). The two higher dosages significantly inhibited photic phase resetting (both P < 0.05 vs. saline controls). EtOH (2.0 g/kg) did not have a phase-resetting effect in the absence of a light pulse at ZT 18.5. No effect of EtOH (2.0 g/kg) was seen on photic phase delays. Controls receiving intraperitoneal saline injection showed phase delays of −0.15 ± 0.34 h (n = 5). Animals receiving intraperitoneal injection of EtOH showed phase delays of −0.67 ± 0.64 h [n = 5; F(1,9) = 1.6; P < 0.24]. Representative actograms for vehicle- and EtOH-treated (2.0 g/kg) animals receiving light pulses at ZT 18.5 are presented in Fig. 5.
Fig. 4.
EtOH dose dependently attenuates photic phase-advance shifts to a light pulse delivered late in the dark phase [Zeitgeber time (ZT) 18.5; left] but has no effect on phase-delay responses to a light pulse delivered at ZT 14 (right). The highest dose of EtOH (2.0 g/kg) had no phase-resetting effect of its own at ZT 18.5 in the absence of a light pulse (middle). For each time point, bars with different letters are significantly different (P < 0.05). Bars represent means ± SE.
Fig. 5.
Representative double-plotted actograms of general locomotor activity showing EtOH attenuation of photic phase-advance responses to light pulses delivered at ZT 18.5. A and B: vehicle-injection. C and D: EtOH (2.0 g/kg) injection. Arrows denote the time of injection.
The SCN is a direct target for EtOH inhibition of photic phase resetting.
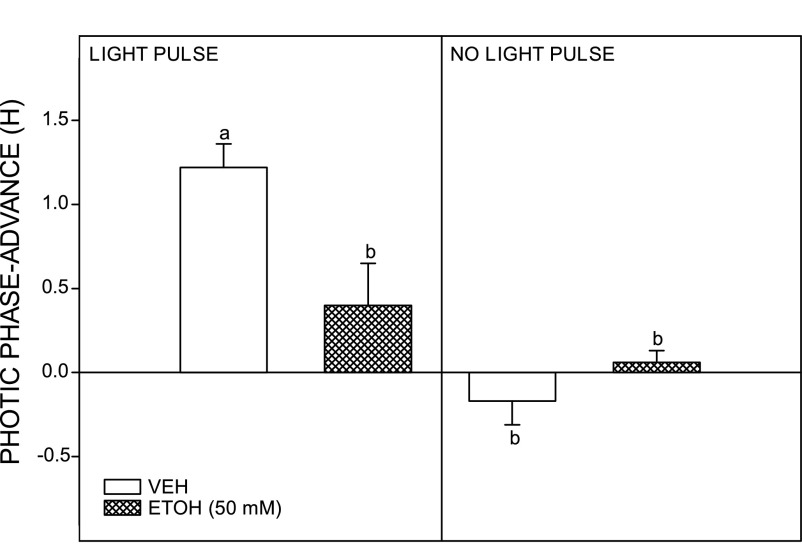
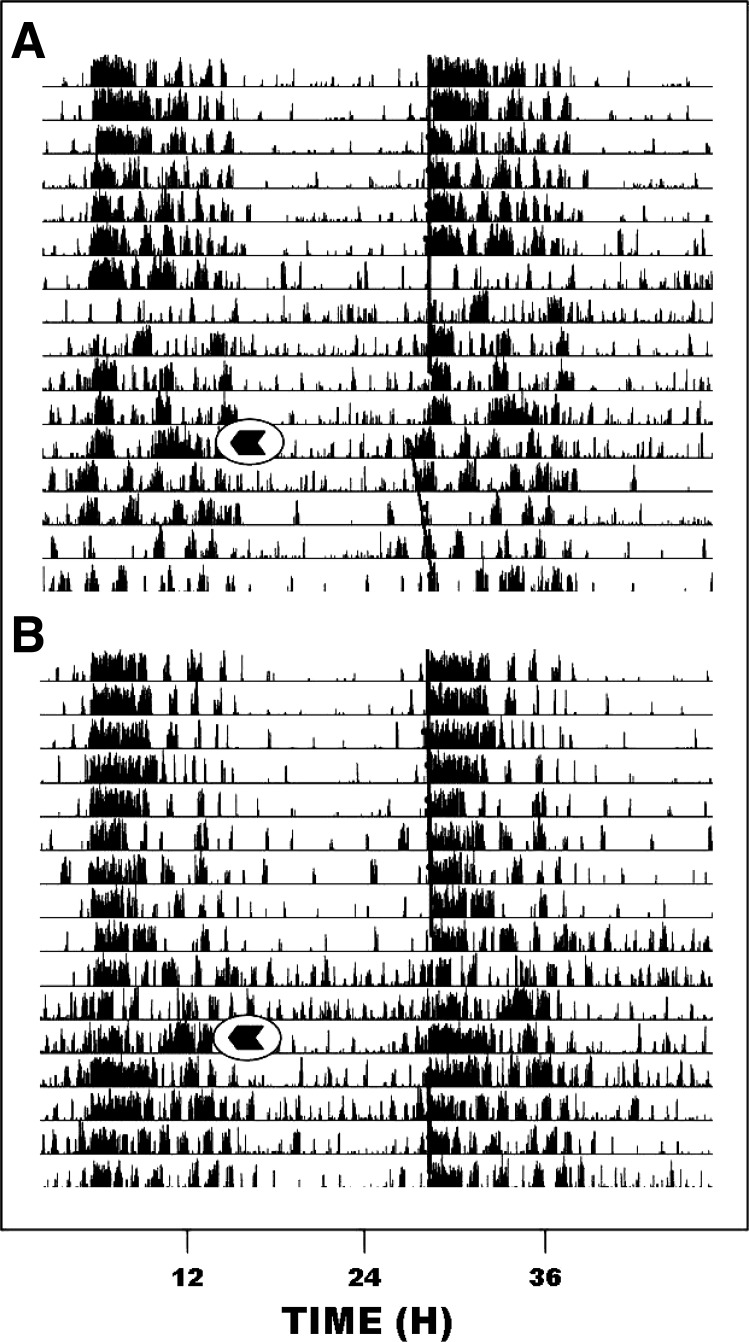
Reverse microdialysis perfusion targeting the SCN region with EtOH (50 mM estimated tissue concentration) significantly attenuated photic phase-advance shifts at ZT 18.5. Animals receiving SCN ACSF perfusion had phase advances averaging 1.22 ± 0.14 h (n = 6; Fig. 6), whereas animals receiving SCN EtOH perfusion showed significantly smaller phase advances averaging 0.40 ± 0.25 h [n = 4; F(1,8) = 9.70; P < 0.015 vs. saline]. Neither ACSF nor EtOH SCN perfusion had a phase-resetting effect in the absence of a light pulse [0.06 ± 0.07 h (n = 3) and −0.17 ± 0.14 h (n = 2), respectively]. Representative actograms for vehicle- and EtOH-perfused animals are shown in Fig. 7. Reverse microdialysis perfusion of EtOH in the SCN did not produce a detectable BAC (n = 2), confirming that the EtOH from the SCN perfusion does not enter in the systemic circulation.
Fig. 6.
Inhibition of photic phase-advance responses to a light pulse delivered late in the dark phase (ZT 18.5) by direct reverse-microdialysis perfusion of EtOH to the SCN. The EtOH perfusion had no phase-resetting effect at ZT 18.5 in the absence of a light pulse. Bars with different letters are significantly different (P < 0.05). Bars represent means ± SE.
Fig. 7.
Representative double-plotted actograms of general locomotor activity showing direct reverse-microdialysis perfusion of EtOH to the SCN attenuates photic phase-advance responses to light pulses delivered at ZT 18.5. A: microdialysis perfusion of vehicle [artificial cerebrospinal fluid (ACSF)]. B: reverse-microdialysis perfusion of EtOH (50 mM). Arrows denote the onset of the 1.5-h perfusion.
Acute systemic EtOH administration inhibits serotonergic phase resetting in vivo.
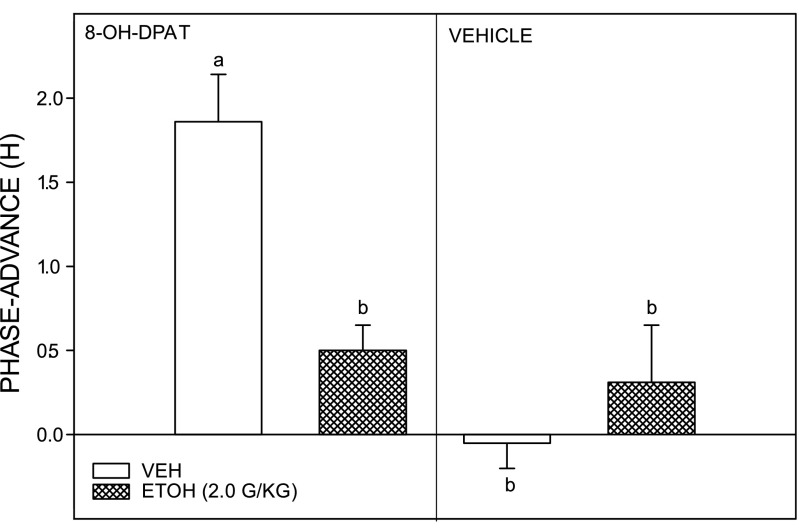
Nonphotic phase-advance shifts caused by intraperitoneal injection of 8-OH-DPAT at ZT 6 were significantly inhibited by EtOH treatment (2.0 g/kg ip). Control animals receiving intraperitoneal saline injection instead of EtOH had 8-OH-DPAT-induced phase advances averaging 1.86 ± 0.28 h (n = 6). In contrast, animals receiving EtOH had diminished 8-OH-DPAT-induced phase-advance shifts averaging 0.50 ± 0.15 h [F(3,20) = 13.95; P < 0.0001 vs. saline; Fig. 8]. Other controls receiving EtOH or saline without 8-OH-DPAT did not have significant phase-resetting responses (0.31 ± 0.34 and −0.05 ± 0.12 h, respectively).
Fig. 8.
EtOH (2.0 g/kg) inhibition of nonphotic phase-advance responses to the serotonin 5-HT1a,7 agonist (±)8-OH-DPAT (5.0 mg/kg) administered during the middle of the light phase (ZT 6; left). EtOH had no phase-resetting effect when administered before vehicle (dimethyl sulfoxide) injection at ZT 6 (right). Bars with different letters are significantly different (P < 0.05). Bars represent the means ± SE.
DISCUSSION
Alcohol abuse is associated with marked disturbances in the daily patterns of multiple circadian clock-regulated functions, including overt behavioral rhythms (e.g., the sleep-wake cycle; see Refs. 10, 11, and 44) and internal physiological and endocrine rhythms (28, 76). The circadian timing of these clock-generated rhythms is regulated by photic and nonphotic entrainment pathways to the SCN, and it is thus reasonable to speculate that the adverse chronobiological effects of EtOH could involve some disruption of these pathways. Here we confirm this idea by showing that acute EtOH markedly attenuates the phase-resetting responses of the circadian clock to photic and nonphotic (serotonergic) signals. These inhibitory effects are induced in response to systemic (ip) administration of EtOH and for photic signaling, as well as also by direct reverse-microdialysis administration of EtOH to the SCN. This latter result strongly suggests that the SCN clock is a direct target for EtOH's disruption of photic phase resetting in vivo, which is consistent with our previous finding that acute EtOH blocks the phase-resetting effects of light-related (glutamate-induced) signaling in the isolated mouse SCN slice in vitro (68). Collectively, these results represent the first lines of evidence that acute EtOH can directly affect phase-resetting mechanisms of the circadian clock that regulate its physiological timekeeping activities.
Literature dealing with the circadian effects of EtOH in adult rodents is relatively sparse and is limited primarily to chronic drinking models. In particular, issues regarding site(s) and mechanism(s) of action have not been addressed. Progress in this area has also been hampered by inconsistencies in chronic EtOH drinking effects on various circadian endpoints within and between species. In hamsters, for example, chronic drinking is reported to have minor effects on circadian rhythm stability and period characteristics and does not impede the rate of reentrainment to a shifted LD cycle (51). On the other hand, chronic EtOH has been shown reduce light pulse-induced phase advances (but not phase delays) and inhibit phase advance shifts to triazolam (81). In rats, chronic EtOH alters the free-running period under DD but apparently has no effect on photic phase resetting (77, 78). Although species differences in EtOH response are likely a major factor contributing to this variability, it is probable that methodological differences in the amount and duration of EtOH consumed could also be problematic. Another important consideration relating to chronic drinking is the development of compensatory responses and/or tolerance that could impact the effects of EtOH on circadian regulation. This is relevant to photic (glutamate-mediated) phase-resetting studies (i.e., light pulse phase shifting and reentrainment to LD shifts), since glutamate signaling pathways show compensation to chronic EtOH (30, 38, 39). Moreover, EtOH compensation could also factor into nonphotic phase-resetting responses because the activity of serotonergic pathways is significantly altered by chronic EtOH (45, 59). In this regard, the present experiments based on acute EtOH administration eliminate the potential confounds associated with compensation and thus may reveal a more defined picture of rapid neurophysiological actions of EtOH in the circadian system.
An interesting observation in the present study is that, while acute EtOH strongly inhibits photic phase-advance shifts during the late night, it does not affect photic phase-delay shifts during the early night. This result is largely consistent with a previous study in chronically drinking hamsters where EtOH inhibited photic advances but not delays (81). The basis of this differential effect of EtOH on phase resetting is not clear but could reflect a distinct inhibitory action directed at a component of the intracellular photic clock-resetting pathway devoted to phase-advance shifting. Conceptually, such an action could be registered at a signaling step downstream from NMDA receptor activation and the subsequent generation of nitric oxide (NO) that are initial steps leading to photic phase advances and phase delays (23). Arguing against this, however, is the finding in the mouse SCN slice that EtOH may act upstream in this pathway to inhibit glutamate induction of NO (R. A. Prosser and H. M. Lee, unpublished observations). However, it must be noted that EtOH blocks glutamate-induced phase advances and phase delays in the mouse SCN in vitro, suggesting that there may be a species difference in the mode of EtOH inhibitory action in the SCN photic signaling pathway between hamsters and mice. Other possibilities are that there are EtOH actions outside the SCN that counter the inhibition or that there may be compensatory mechanisms in vivo that help with delays more than advances.
With respect to the effects of EtOH administered directly to the SCN via reverse-microdialysis perfusion, it is noteworthy that the estimated tissue concentration of EtOH outside the probe (∼50 mM) attenuated photic phase advances to the same degree as when EtOH was administered intraperitoneally at 2.0 g/kg. These results are also consistent with those in the mouse SCN slice, where EtOH at a similar concentration range (20–50 mM) blocked glutamate-induced phase advances (68). Importantly, no differences in behavior (e.g., loss of balance, sedation, locomotor stimulation) other than the attenuation of photic phase advances was noted during or after the period of EtOH perfusion, and no detectable BAC was produced by this procedure. These observations, together with the in vitro evidence, strongly support the idea that EtOH effects on photic phase resetting are manifest within the SCN clock. In this regard, however, it is possible that EtOH inhibition of photic and nonphotic entrainment may be secondary to its hypothermic effects. This is unlikely, however, since EtOH does not induce hypothermia at room temperature at which the present experiments were undertaken (17, 31, 37, 94).
In addition to its effects on photic circadian clock resetting, EtOH also modulates the activity of neurotransmitters involved in mediating nonphotic signaling in the clock. Among these is 5-HT, which is strongly implicated in mediating behavioral phase resetting (reviewed in Ref. 52) and modulating photic input to the SCN (63, 71, 82, 97). Although the role of 5-HT in nonphotic shifting responses remains speculative, potent phase-resetting actions of 5-HT agonists are well documented. In particular, the 5-HT1A,7 agonist 8-OH-DPAT, used widely in circadian studies, has a phase-response curve (PRC) resembling behavioral PRCs and produces in vivo phase shifts of similar magnitude as nonphotic stimuli (∼1.5 h) when administered systemically (8, 89), or in the SCN (25), and large shifts (∼3 h) when administered to the SCN in vitro (69, 83). It is notable that acute EtOH affects central serotonergic activity by increasing the release and decreasing the reuptake of 5-HT (14, 76). In addition, chronic EtOH treatment downregulates 5-HT receptors (45, 59). The present finding that acute EtOH markedly diminished the phase-advancing action of 8-OH-DPAT (by 72%) appears inconsistent with these observations in that EtOH would be expected to increase serotonergic phase resetting. However, evidence of very rapid (∼5 min) agonist-induced downregulation of 5-HT receptors has been seen in vitro (6), and pretreatments with 5-HT agonists have been shown to block 8-OH-DPAT-induced phase shifts in the SCN in vitro (67). Whereas no information on the dynamics of 5-HT1A or 5-HT7 receptor downregulation exists, it is conceivable that rapid internalization of these receptors may have occurred in the SCN or other circadian-related areas in response to an EtOH-induced increase in the availability of 5-HT in the synapse, causing the inhibition of serotonergic phase resetting seen here. In contrast with the present results, however, is our recent observation in the mouse SCN brain slice preparation that acute EtOH administration dose dependently enhances daytime 8-OH-DPAT-induced phase advances to a maximal ∼30% increase (68). It was hypothesized that this enhancing effect of EtOH is manifest through its inhibition of glutamate (photic) signaling in the SCN. This idea is based on the concept that, since glutamate agonists inhibit nonphotic phase resetting (7, 65), suppression of glutamate response would lead to enhanced serotonergic shifting. The reason for the differential 8-OH-DPAT shifting response to acute EtOH between the in vivo and in vitro studies is unknown but could relate to species difference or more likely a strong shift-inhibiting action of EtOH on 5-HT pathways registered outside of the SCN in the in vivo experiment, which would not be seen in the deafferented SCN slice.
Another potential target for EtOH nonphotic effects is the γ-aminobutyric acid (GABA)-A/benzodiazepine receptor. GABA-A receptor activity is affected by EtOH (53), and central components of the circadian system express GABA and the GABA-A receptor subtype (26). Moreover, treatment of hamsters with the benzodiazapine triazolam produces phase-advance and phase-delay shifts thought to be mediated by the GABA-A receptor (93). It is notable in this regard that these phase-resetting actions of triazolam are inhibited by chronic EtOH, an effect that could be based on the well-documented desensitizing effects of chronic EtOH both on the GABA-A receptor and benzodiazepine action (30, 48). We also have preliminary data that treatment of the mouse SCN slice with a GABA-A antagonist (RO15–4513) blocks EtOH's inhibition of photic (glutamate) phase-delay shifts and enhancement of 8-OH-DPAT phase-advance shifts (66), strengthening the argument for a role of the GABA-A receptor in mediating EtOH actions on photic and nonphotic phase-resetting pathways in the SCN.
The profile of EtOH in the SCN region following intraperitoneal injection of EtOH was assessed to characterize the central pharmacokinetics of EtOH in the Syrian hamster to offer a comparison with other rodents and to confirm in our light pulse experiment that the timing of the light pulse overlaps completely with sufficiently raised EtOH levels in the SCN. This was confirmed, since the 30-min light pulse was started ∼20 min after EtOH injection, which coincided with near peak levels of SCN EtOH occurring 20–40 min postinjection, and lasting over an additional 40-min period. The estimated peak concentration of EtOH in the SCN extracellular fluid compartment was ∼50 mM, which is within the effective dose range for blocking glutamate-induced phase-advance shifts in the mouse SCN slice (68). This pharmacokinetic profile of EtOH in the hamster SCN is similar to that seen in the rat nucleus accumbens (18, 95) and striatum (35) following intraperitoneal injection of the same dosage of EtOH as used here (2.0 g/kg), with peak levels (∼60 mM) occurring within 15–30 min postinjection. The t1/2 for clearance estimated from graphs from these papers ranged from ∼1.5 to 3.0 h, which is similar to the 3-h period in the present study.
Perspectives and Significance
The present study is the first to demonstrate that acute EtOH dose dependently blocks in vivo light-induced phase-advance shifts. Such an effect, in theory, could severely impair photic entraining input to the circadian clock. We also provide evidence that serotonergic signaling in circadian pathways for nonphotic phase regulation is impaired by EtOH. This dual blockade of photic and nonphotic entrainment mechanisms could underlie the highly disruptive effects of alcohol abuse on rhythms such as the sleep-wake cycle and those of the hypothalamic-pituitary-adrenal axis associated with developing alcoholism. Notably, this study also provides a framework for future investigations by confirming that the SCN clock is a direct target for EtOH's effects in the circadian timing system.
GRANTS
This work was supported by National Institute on Alcohol and Alcohol-Related Disorders Grant AA-015948 to R. A. Prosser and J. D. Glass.
Acknowledgments
We thank Dr. Mahinda Gangoda for assistance in measuring EtOH with GC.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abe H, Rusak B, Robertson HA. Photic induction of Fos protein in the suprachiasmatic nucleus is inhibited by the NMDA receptor antagonist MK-801. Neurosci Lett 127: 9–12, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Adinoff B, Risher-Flowers D, Dee Jong J, Ravitz B, Bone GHA, Nutt DJ, Roehrich L, Martin PR, Linnoila M. Disturbances of hypothalamic-pituitary-adrenal axis functioning during ethanol withdrawal in six men. Am J Psychiat 148: 1023–1025, 1991. [DOI] [PubMed] [Google Scholar]
- 3.Allgaier C Ethanol sensitivity of NMDA receptors. Neurochem Int 41: 377–382, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Alvestad RM, Grosshans DR, Coultrap SJ, Nakazawa T, Yamamoto T, Browning MD. Tyrosine dephosphorylation and ethanol inhibition of N-methyl-d-aspartate receptor function. J Biol Chem 278: 11020–11025, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Baird TJ, Briscoe RJ, Vallett M, Vanecek SA, Holloway FA, Gauvin DV. Phase-response curve for ethanol: alterations in circadian rhythms of temperature and activity in rats. Pharmacol Biochem Behav 61: 303–315, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Berry SA, Shah MC, Khan N, Roth BL. Rapid agonist-induced internalization of the 5-hydroxytryptamine2A receptor occurs via the endosome pathway in vitro. Mol Pharmacol 50: 306–313, 1996. [PubMed] [Google Scholar]
- 7.Biello SM, Golombek DA, Harrington ME. Neuropeptide Y and glutamate block each other's phase shifts in the suprachiasmatic nucleus in vitro. Neuroscience 77: 1049–1057, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Bobrzynska KJ, Godfrey MH, Mrosovsky N. Serotonergic stimulation and nonphotic phase-shifting in hamsters. Physiol Behav 59: 221–230, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Bradbury MJ, Dement WC, Edgar DM. Serotonin-containing fibers in the suprachiasmatic hypothalamus attenuate light-induced phase delays in mice. Brain Res 768: 125–134, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Brower KJ Alcohol's effects on sleep in alcoholics. Alcohol Res Health 25: 101–109, 2001. [PMC free article] [PubMed] [Google Scholar]
- 11.Brower KJ, Aldrich MS, Robinson EAR, Zucker RA, Greden JF. Insomnia, self-medication, and relapse to alcoholism. Am J Psychiatry 158: 399–404, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Challet E, Pevet P. Interactions between photic and nonphotic stimuli synchronize the master circadian clock in mammals. Front Biosci 8: 246–257, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Chandler LJ Ethanol and brain plasticity: receptors and molecular networks of the postsynaptic density as targets of ethanol. Pharmacol Ther 99: 311–326, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Chastain G Alcohol, neurotransmitter systems, behavior. J Gen Psychol 133: 329–335, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Colwell CS, Foster RG, Menaker M. NMDA receptor antagonists block the effects of light on circadian behavior in the mouse. Brain Res 554: 105–110, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Costa A, Bono G, Martignoni E, Merlo P, Sances G, Nappi G. An assessment of hypothalamo-pituitary-adrenal axis functioning in non-depressed, early abstinent alcoholics. Psychoneuroendocrinology 21: 263–275, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Crawshaw L, O'Connor C, Wollmuth L. Ethanol and the neurobiology of temperature regulation. In: Alcohol and Neurobiology: Brain Development and Hormone Regulation, edited by Watson R. Boca Raton, FL: CRC, 1992.
- 18.Crippens D, White ML, George MA, Jaworski JN, Brunner LJ, Lancaster FE, Gonzales RA. Gender differences in blood levels, but not brain levels, of ethanol in rats. Alcohol Clin Exp Res 23: 414–420, 1999. [PubMed] [Google Scholar]
- 19.Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: Pacemaker as a clock. J Comp Physiol 106: 291–331, 1976. [Google Scholar]
- 20.Danel T, Touitou Y. Alcohol decreases the nocturnal peak of TSH in healthy volunteers. Psychopharmacology 170: 213–214, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Danel T, Libersa C, Touitou Y. The effect of alcohol consumption on the circadian control of human core body temperature is time dependent. Am J Physiol Regul Integr Comp Physiol 281: R52–R55, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Devaney M, Graham D, Greeley J. Circadian variation of the acute and delayed response to alcohol: investigation of core body temperature variations in humans. Pharmacol Biochem Behav 75: 881–887, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Ding JM, Chen D, Weber ET, Faiman LE, Rea MA, Gillette MU. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science 266: 1713–1717, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Dudley TE, DiNardo LA, Glass JD. Endogenous regulation of serotonin release in the hamster suprachiasmatic nucleus. J Neurosci 18: 5045–5052, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehlen JC, Grossman GH, Glass JD. In vivo resetting of the hamster circadian clock by 5-HT7 receptors in the suprachiasmatic nucleus. J Neurosci 21: 5351–5357, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehlen JC, Paul KN. A role for extrasynaptic GABAA receptors in the circadian system (Abstract). Soc Neurosci Abst 833: 11, 2007. [Google Scholar]
- 27.Ehler CL, Slawecki CJ. Effects of chronic ethanol exposure on sleep in rats. Alcohol 20: 173–179, 2000. [DOI] [PubMed] [Google Scholar]
- 28.el-Guebaly N Alcohol, alcoholism and biological rhythms. Alcohol Clin Exp Res 11:139–143, 1987. [DOI] [PubMed] [Google Scholar]
- 29.Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol 56: 385–431, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Faingold CL, N'Gouemo P, Riaz A. Ethanol and neurotransmitter interactions- from molecular to integrative effects. Prog Neurobiol 55: 509–535, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Gibbs FP Temperature dependence of the hamster circadian pacemaker. Am J Physiol Regul Integr Comp Physiol 244: R607–R610, 1983. [DOI] [PubMed] [Google Scholar]
- 32.Glass JD, Selim M, Rea MA. Modulation of light-induced c-fos expression in the suprachiasmatic nuclei by 5-HT receptor agonists. Brain Res 462: 235–242, 1994. [DOI] [PubMed] [Google Scholar]
- 33.Heinz A, Schafer M, Higley JD, Krystal JH, Goldman D. Neurobiological correlates of the disposition and maintenance of alcoholism. Pharmacopsychiatry 36: S255–S258, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Hendrikson AE, Wagoner N, Cowan WM. An autoradiographic and electron microscope study of retinohypothalamic connections. Z Zellforsch Mikrosk Anat 135: 1–26, 1972. [DOI] [PubMed] [Google Scholar]
- 35.Job MO, Ramachandra V, Anders S, Low MJ, Gonzales RA. Reduced basal and ethanol stimulation of striatal extracellular dopamine concentrations in dopamine D2 receptor knockout mice. Alcohol Clin Exp Res 27: 1573–1582, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Johnson RF, Morin LP, Moore RY. Retinohypothalamic projections in the hamster and rat demonstrated using cholera toxin. Brain Res 462: 301–312, 1988. [DOI] [PubMed] [Google Scholar]
- 37.Kalant H, Le A. Effects of ethanol on thermoregulation. In: Thermoregulation: Pathology, Pharmacology and Therapy, edited by Schonbaum E and Lomax P. New York, NY: Pergamon, 1991.
- 38.Kalluri HS, Ticku MK. Effect of ethanol on phosphorylation of the NMDAR2B subunit in mouse cortical neurons. Mol Brain Res 68: 159–168, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Kalluri HS, Metha AK, Ticku MK. Up-regulation of NMDA receptor subunits in adult brain following chronic ethanol treatment. Mol Brain Res 58: 221–224, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Kelley AE Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron 44: 161–179, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Klein DC, Moore RM, Reppert SM. Suprachiasmatic Nucleus: The Mind's Clock. New York, NY: Oxford Univ Press, 1991.
- 42.Kubota R, De A, Brown RA, Simasko SM, Krueger JM. Diurnal effects of acute and chronic administration of ethanol on sleep in rats. Alcohol Clin Exp Res 26: 1153–1161, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Kuhlwein W, Hauger RL, Irwin MR. Abnormal nocturnal melatonin secretion and disordered sleep in abstinent alcoholics. Biol Psych 54: 1437–1443, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Landolt HP, Gillin J. Sleep abnormalities during abstinence in alcohol-dependent patients. Aetiol Man CNS Drugs 15: 413–425, 2001. [DOI] [PubMed] [Google Scholar]
- 45.LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse and dependence: Findings of animal studies. Biol Psychiat 36: 395–421, 1994. [DOI] [PubMed] [Google Scholar]
- 46.Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science 243: 1721–1724, 1989. [DOI] [PubMed] [Google Scholar]
- 47.Meyer-Bernstein EL, Morin LP. Differential serotonergic innervation of the suprachiasmatic nucleus and the intergeniculate leaflet and its role in circadian rhythm modulation. J Neurosci 16: 2097–2111, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mihic SJ, Kalant H, Liu JF, Wu PH. Role of the gamma-aminobuyric acid receptor/chloride channel complex in tolerance to ethanol and cross-tolerance to diazepam and pentobarbital. J Pharm Expt Ther 261: 108–113, 1992. [PubMed] [Google Scholar]
- 49.Mintz EM, Albers HE. Microinjection of NMDA into the SCN region mimicks the phase shifting effect of light in hamsters. Brain Res 758: 245–249, 1997. [DOI] [PubMed] [Google Scholar]
- 50.Mintz EM, Marvel CL, Gillespie CF, Price KM, Albers HE. Activation of NMDA receptors in the suprachiasmatic nucleus produces light-like phase shifts of the circadian clock in vivo. J Neurosci 19: 5124–5130, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mistlberger RE, Nadeau J. Ethanol and circadian rhythms in the Syrian hamster: effects on entrained phase, reentrainment rate, and period. Pharmacol Biochem Behav 43: 159–165, 1992. [DOI] [PubMed] [Google Scholar]
- 52.Mistlberger RE, Antle MC, Glass JD, Miller JD. Behavioral and serotonergic regulation of circadian rhythms. Biol Rhythm Res 31: 240–283, 2000. [Google Scholar]
- 53.Mody I Extrasynaptic GABAA receptors in the crosshairs of hormones and ethanol. Neurochem Int 52: 60–64, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moga MM, Moore RY. Organization of neural inputs to the suprachiasmatic nucleus in the rat. J Comp Neurol 389: 508–534, 1997. [DOI] [PubMed] [Google Scholar]
- 55.Moghaddam B, Bolinao ML. Biphasic effect of ethanol on extracellular accumulation of glutamate in the hippocampus and nucleus accumbens. Neurosci Lett 178: 99–102, 1994. [DOI] [PubMed] [Google Scholar]
- 56.Moore RY Organization and function of a central nervous system circadian oscillator: the suprachiasmatic nucleus. Fed Proc 42: 2783–2789, 1983. [PubMed] [Google Scholar]
- 57.Moriya T, Horikawa K, Akiyama M, Shibata S. Correlative association between N-methyl-d-aspartate receptor-mediated expression of Period genes in the suprachiasmatic nucleus and phase shifts in behavior with photic entrainment of clock in hamsters. Mol Pharmacol 58: 1554–1562, 2000. [DOI] [PubMed] [Google Scholar]
- 58.Morrow AL, Ferrani-Kile K, Davis MI, Shumilla JA, Kumar S, Maldve R, Pandey SC. Ethanol effects on cell signaling mechanisms. Alcohol Clin Exp Res 28: 217–227, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Nevo I, Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochem Int 26: 305–336, 1995. [DOI] [PubMed] [Google Scholar]
- 60.Nie Z, Madamba SG, Siggins GR. Ethanol inhibits glutamatergic neurotransmission in nucleus accumbens neurons by multiple mechanisms. J Pharmacol Exp Ther 271: 1566–73, 1994. [PubMed] [Google Scholar]
- 61.O'Callaghan MJ, Croft AP, Jacquot C, Little HJ. The hypothalamopituitary-adrenal axis and alcohol preference. Brain Res Bull 68: 171–178, 2005. [DOI] [PubMed] [Google Scholar]
- 62.Pickard GE The afferent connection of the suprachiasmatic nucleus of the golden hamster with emphasis on the retinohypothalamic projection. J Comp Neurol 211: 65–83, 1982. [DOI] [PubMed] [Google Scholar]
- 63.Pickard GE, Smith BN, Belenky M, Rea MA, Dudek FE, Sollars PJ. 5HT1B receptor mediated presynaptic inhibition of retinal input to the suprachiasmatic nucleus. J Neurosci 19: 4034–4045, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pickard GE, Weber ET, Scott PA, Riberdy AF, Rea MA. 5HT1B receptor agonists inhibit light-induced phase shifts of behavioral circadian rhythms and expression of the immediate-early gene c-fos in the suprachiasmatic nucleus. J Neurosci 16: 8208–8220, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prosser RA Glutamate blocks serotonergic phase-advances of the mammalian circadian pacemaker through AMPA and NMDA receptors. J Neurosci 21: 7815–7822, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prosser RA, Glass JD, McElroy B, Zakaria A. Potential GABA receptor involvement in ethanol actions on in vitro suprachiasmatic nucleus circadian rhythms (Abstract). Soc Res Biol Rhythms Abst P202, 2008.
- 67.Prosser RA, Lee HM, Wehner A. Serotonergic pre-treatments block in vitro serotonergic phase shifts of the mouse suprachiasmatic nucleus circadian clock. Neuroscience 142: 547–555, 2006. [DOI] [PubMed] [Google Scholar]
- 68.Prosser RA, Mangrum CA, Glass JD. Acute ethanol modulates glutamatergic and serotonergic phase shifts of the mouse circadian clock in vitro. Neuroscience 152: 837–848, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prosser RA, Miller JD, Heller HC. A serotonin agonist phase-shifts the circadian clock in the suprachiasmatic nucleus in vitro. Brain Res 534: 336–339, 1990. [DOI] [PubMed] [Google Scholar]
- 70.Rasmussen DD, Boldt BM, Bryant CA, Mitton DR, Larsen SA, Wilkinson CW. Chronic daily ethanol and withdrawal. 1. Long-term changes in the hypothalamo-pituitary-adrenal axis. Alcohol Clin Exp Res 24: 1836–1849, 2000. [PubMed] [Google Scholar]
- 71.Rea MA, Glass JD, Colwell CS. Serotonin modulates photic responses in the hamster suprachiasmatic nuclei. J Neurosci 14: 3635–3642, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roehrs T, Petrucelli N, Roth T. Sleep restriction, ethanol effects and time of day. Human Psychopharm 11: 199–204, 1996. [Google Scholar]
- 73.Roehrs T, Roth T. Sleep, sleepiness, and alcohol use. Alcohol Res Health 25: 101–109, 2001. [PMC free article] [PubMed] [Google Scholar]
- 74.Roehrs T, Roth T. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Med Rev 5: 287–297, 2001. [DOI] [PubMed] [Google Scholar]
- 75.Rojdmark S, Wikner J, Adner N, Andersson DEH, Wetterberg L. Inhibition of melatonin secretion by ethanol in man. Metab Clin Exp 42: 1047–1051, 1993. [DOI] [PubMed] [Google Scholar]
- 76.Rosenwasser AM Alcohol, antidepressants, and circadian rhythms. Alcohol Res Health 25: 126–135, 2001. [PMC free article] [PubMed] [Google Scholar]
- 77.Rosenwasser AM, Fecteau ME, Logan RW. Effects of ethanol intake and ethanol withdrawal on free-running activity rhythms in rats. Physiol Behav 84: 537–542, 2005. [DOI] [PubMed] [Google Scholar]
- 78.Rosenwasser AM, Logan RW, Fecteau ME. Chronic ethanol intake alters circadian period-responses to brief light pulses in rats. Chronobiol Int 22: 227–236, 2005. [DOI] [PubMed] [Google Scholar]
- 79.Rusak B Involvement of the primary optic tracts in mediation of light effects on hamster circadian rhythms. J Comp Physiol 118: 165–172, 1977. [Google Scholar]
- 80.Rusak B, Zucker I. Neural regulation of circadian rhythms. Physiol Rev 59: 449–526, 1979. [DOI] [PubMed] [Google Scholar]
- 81.Seggio JA, Logan RW, Rosenwasser AM. Chronic ethanol intake modulates photic and non-photic circadian phase responses in the Syrian hamster. Pharmacol Biochem Behav 87: 297–305, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Selim M, Glass JD, Hauser UE, Rea MA. Serotonergic inhibtion of light-induced fos protein expression and extracellular glutamate in the suprachiasmatic nuclei. Brain Res 621: 181–188, 1993. [DOI] [PubMed] [Google Scholar]
- 83.Shibata S, Tsuneyoshi A, Hamada T, Tominaga K, Watanabe S. Phase-shifting effect of 8-OH-DPAT, a serotonin1A receptor agonist, on the circadian rhythm of firing rate in the rat suprachiasmatic nuclei in vitro. Brain Res 582: 353–356, 1992. [DOI] [PubMed] [Google Scholar]
- 84.Simson PE, Criswell HE, Breese GR. Inhibition of NMDA-evoked electrophysiological activity by ethanol in selected brain regions: evidence for ethanol-sensitive and ethanol-insensitive NMDA-evoked responses. Brain Res 607: 9–16, 1993. [DOI] [PubMed] [Google Scholar]
- 85.Smith BN, Sollars PJ, Dudek FE, Pickard GE. Serotonergic modulation of retinal input to the mouse suprachiasmatic nucleus mediated by 5-HT1B and 5-HT7 receptors. J Biol Rhythms 16: 25–38, 2001. [DOI] [PubMed] [Google Scholar]
- 86.Snell LD, Nunley KR, Lickteig RL, Browning MD, Tabakoff B, Hoffman PL. Regional and subunit specific changes in NMDA receptor mRNA and immunoreactivity in mouse brain following chronic ethanol ingestion. Mol Brain Res 40: 71–78, 1996. [DOI] [PubMed] [Google Scholar]
- 87.Sollars PJ, Olgilvie MD, Rea MA, Pickard GE. 5-HT1B receptor knockout mice exhibit an enhanced response to constant light. J Biol Rhythms 17: 428–437, 2002. [DOI] [PubMed] [Google Scholar]
- 88.Stephens DN A glutamatergic hypothesis of drug dependence: extrapolations from benzodiazapine receptor ligands. Behav Pharmacol 6: 425–446, 1995. [PubMed] [Google Scholar]
- 89.Tominaga K, Shibata S, Ueki S, Watanabe S. Effects of 5-HT1A receptor agonists on the circadian rhythm of wheel-running activity in hamsters. Europ J Pharm 214: 79–84, 1992. [DOI] [PubMed] [Google Scholar]
- 90.Tsai G Glutamatergic neurotransmission in alcoholism. J Biomed Sci 5: 309–320, 1998. [DOI] [PubMed] [Google Scholar]
- 91.Tsai G, Coyle JT. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annu Rev Med 49: 173–184, 1998. [DOI] [PubMed] [Google Scholar]
- 92.Tsai G, Gastfriend DR, Coyle JT. The glutamatergic basis for human alcoholism. Am J Psychiatry 152: 332–340, 1995. [DOI] [PubMed] [Google Scholar]
- 93.Van Reeth O, Turek FW. Stimulated activity mediates phase shifts in the hamster circadian clock induced by dark pulses or benzodiazepines. Nature 339: 49–50, 1989. [DOI] [PubMed] [Google Scholar]
- 94.Wasielewski JA, Holloway FA. Alcohol's interactions with circadian rhythms. A focus on body temperature. Alcohol Res Health 25: 94–100, 2001. [PMC free article] [PubMed] [Google Scholar]
- 95.Yan QS Extracellular dopamine and serotonin after ethanol monitored with 5-minute microdialysis. Pharmacol Biochem Behav 52: 29–34, 1995. [DOI] [PubMed] [Google Scholar]
- 96.Yang X, Criswell HE, Simson P, Moy S, Breese GR. Evidence for a selective effect of ethanol on N-methyl-d-aspartate responses: ethanol affects a subtype of the ifenprodil-sensitive N-methyl-d-aspartate receptors. J Pharmacol Exp Ther 278: 114–124, 1996. [PubMed] [Google Scholar]
- 97.Ying SW, Rusak B. Effects of serotonergic agonists on firing rates of photically responsive cells in the hamster suprachiasmatic nucleus. Brain Res 651: 37–46, 1994. [DOI] [PubMed] [Google Scholar]
- 98.Zimmermann U, Hundt W, Spring K, Grabner A, Holsboer F. Hypothalamic-pituitary-adrenal system adaptation to detoxification in alcohol-dependent patients is affected by family history of alcoholism. Biol Psychiat 53: 75–84, 2003. [DOI] [PubMed] [Google Scholar]