Abstract
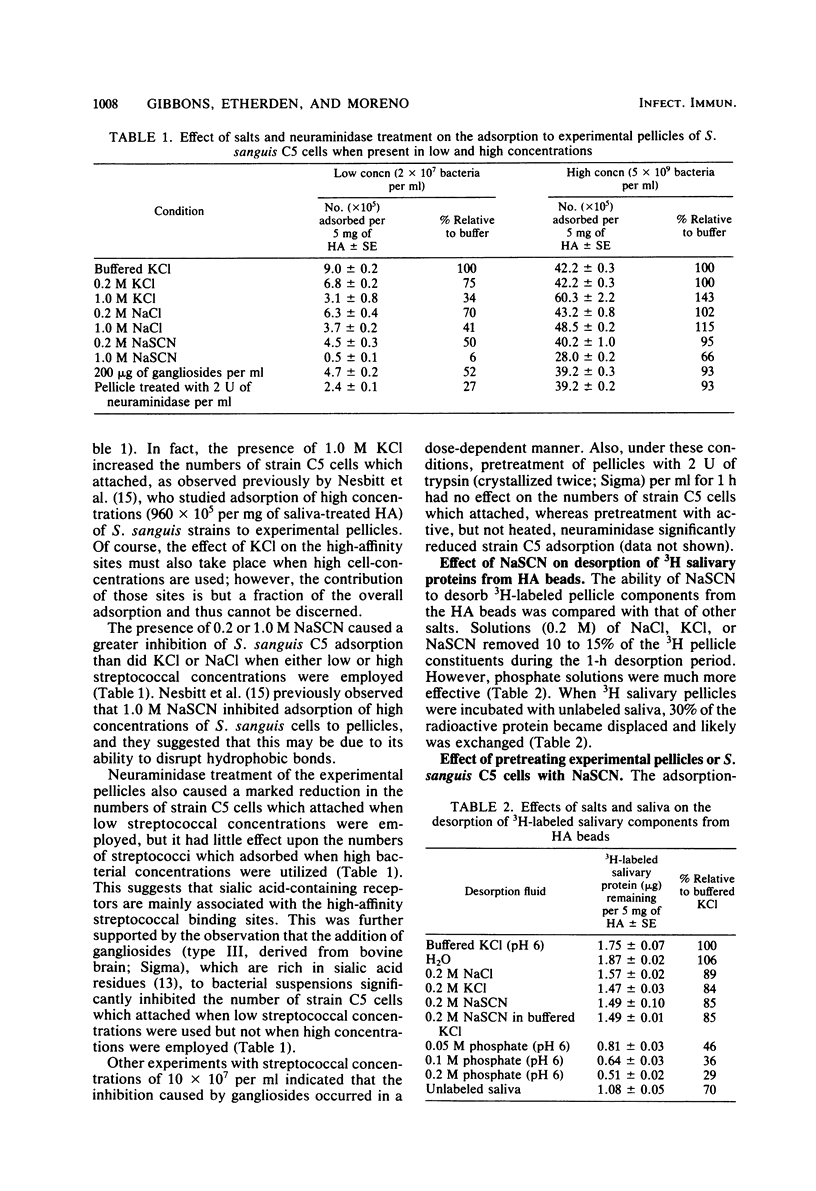
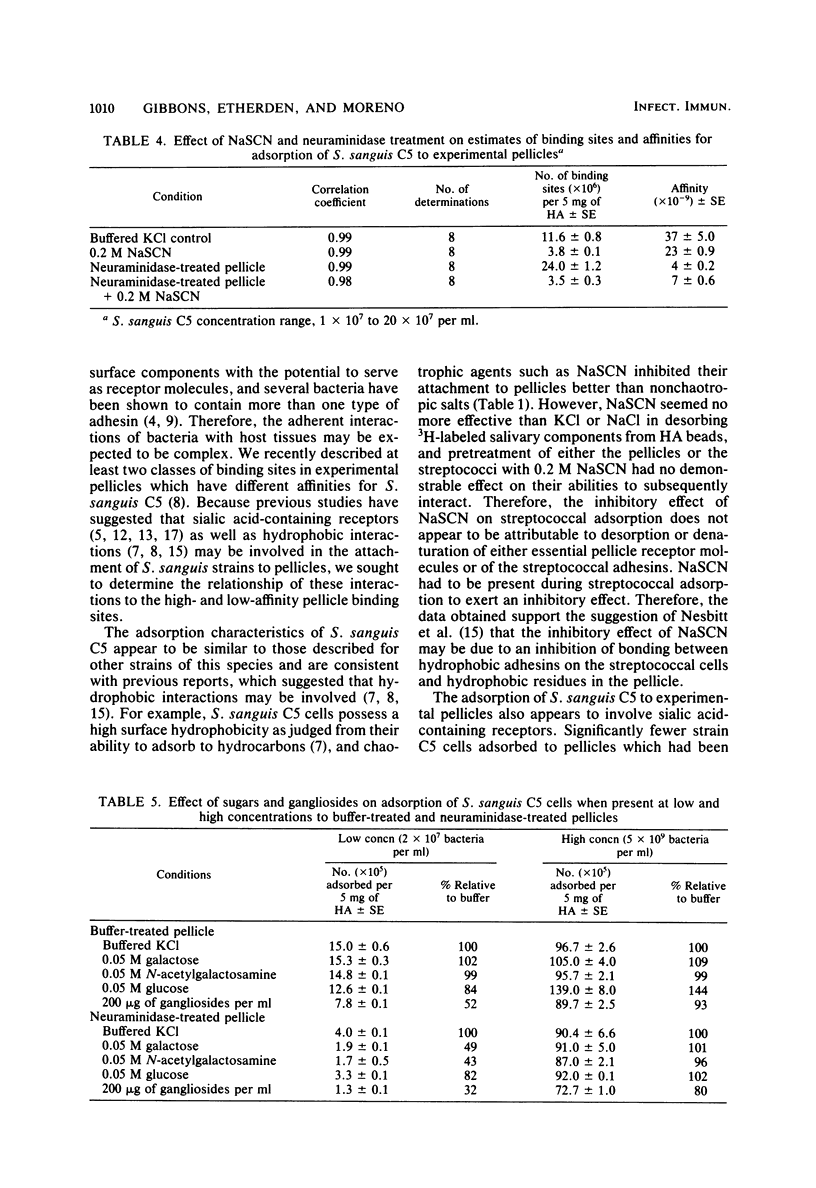
The relationship of neuraminidase-sensitive receptors and putative hydrophobic interactions to high- and low-affinity binding sites in experimental salivary pellicles for Streptococcus sanguis C5 was investigated. NaSCN, an inhibitor of hydrophobic interactions, reduced the number of cells which adsorbed to pellicles to a greater extent than NaCl or KCl when both low and high streptococcal concentrations were used in assays. However, NaSCN was not more effective than NaCl or KCl in desorbing 3H-labeled salivary pellicle components from hydroxyapatite, and NaSCN pretreatment of either strain C5 cells or the salivary pellicles did not destroy or remove either the streptococcal adhesions or the pellicle receptors. Neuraminidase treatment of pellicles or the presence of sialic acid-containing gangliosides only inhibited S. sanguis adsorption when low streptococcal concentrations were used. At these concentrations, S. sanguis adsorbs primarily to high-affinity pellicle binding sites. Adsorption isotherms indicated that neuraminidase-sensitive interactions were mainly responsible for the high affinity of these binding sites, whereas putative hydrophobic interactions inhibitable by NaSCN were mainly associated with the numbers of binding sites available. Sugar inhibition studies suggested that the two classes of binding sites previously demonstrated in untreated salivary pellicles for S. sanguis C5 are not the result of a partial conversion of high-affinity sites to low-affinity sites due to removal of sialic acid residues.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Etherden I. Comparative hydrophobicities of oral bacteria and their adherence to salivary pellicles. Infect Immun. 1983 Sep;41(3):1190–1196. doi: 10.1128/iai.41.3.1190-1196.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Etherden I. Enzymatic modification of bacterial receptors on saliva-treated hydroxyapatite surfaces. Infect Immun. 1982 Apr;36(1):52–58. doi: 10.1128/iai.36.1.52-58.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Etherden I., Skobe Z. Association of fimbriae with the hydrophobicity of Streptococcus sanguis FC-1 and adherence to salivary pellicles. Infect Immun. 1983 Jul;41(1):414–417. doi: 10.1128/iai.41.1.414-417.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Moreno E. C., Etherden I. Concentration-dependent multiple binding sites on saliva-treated hydroxyapatite for Streptococcus sanguis. Infect Immun. 1983 Jan;39(1):280–289. doi: 10.1128/iai.39.1.280-289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen S. D., Henrichsen J. Twitching motility and possession of polar fimbriae in spreading Streptococcus sanguis isolates from the human throat. Acta Pathol Microbiol Scand B. 1975 Apr;83(2):133–140. doi: 10.1111/j.1699-0463.1975.tb00083.x. [DOI] [PubMed] [Google Scholar]
- Jentoft N., Dearborn D. G. Labeling of proteins by reductive methylation using sodium cyanoborohydride. J Biol Chem. 1979 Jun 10;254(11):4359–4365. [PubMed] [Google Scholar]
- Levine M. J., Herzberg M. C., Levine M. S., Ellison S. A., Stinson M. W., Li H. C., van Dyke T. Specificity of salivary-bacterial interactions: role of terminal sialic acid residues in the interaction of salivary glycoproteins with Streptococcus sanguis and Streptococcus mutans. Infect Immun. 1978 Jan;19(1):107–115. doi: 10.1128/iai.19.1.107-115.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride B. C., Gisslow M. T. Role of sialic acid in saliva-induced aggregation of Streptococcus sanguis. Infect Immun. 1977 Oct;18(1):35–40. doi: 10.1128/iai.18.1.35-40.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. A., Levine M. J., Tabak L. A., Reddy M. S. Specificity of salivary-bacterial interactions: II. Evidence for a lectin on Streptococcus sanguis with specificity for a NeuAc alpha 2, 3Ga1 beta 1, 3Ga1NAc sequence. Biochem Biophys Res Commun. 1982 May 31;106(2):390–396. doi: 10.1016/0006-291x(82)91122-6. [DOI] [PubMed] [Google Scholar]
- Nesbitt W. E., Doyle R. J., Taylor K. G. Hydrophobic interactions and the adherence of Streptococcus sanguis to hydroxylapatite. Infect Immun. 1982 Nov;38(2):637–644. doi: 10.1128/iai.38.2.637-644.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt W. E., Doyle R. J., Taylor K. G., Staat R. H., Arnold R. R. Positive coooperativity in the binding of Streptococcus sanguis to hydroxylapatite. Infect Immun. 1982 Jan;35(1):157–165. doi: 10.1128/iai.35.1.157-165.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson M. W., Levine M. J., Cavese J. M., Prakobphol A., Murray P. A., Tabak L. A., Reddy M. S. Adherence of Streptococcus sanguis to salivary mucin bound to glass. J Dent Res. 1982 Dec;61(12):1390–1393. doi: 10.1177/00220345820610120101. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]


