Abstract
Exposure to chronic hypoxia (CH; 3–28 days at 380 Torr) induces adaptation in mammalian carotid body such that following CH an acute hypoxic challenge elicits an abnormally large increase in carotid sinus nerve impulse activity. The current study examines the hypothesis that CH initiates an immune response in the carotid body and that chemoreceptor hyperexcitability is dependent on the expression and action of inflammatory cytokines. CH resulted in a robust invasion of ED1+ macrophages, which peaked on day 3 of exposure. Gene expression of proinflammatory cytokines, IL-1β, TNFα, and the chemokine, monocyte chemoattractant protein-1, was increased >2-fold after 1 day of hypoxia followed by a >2-fold increase in IL-6 on day 3. After 28 days of CH, IL-6 remained elevated >5-fold, whereas expression of other cytokines recovered to normal levels. Cytokine expression was not restricted to immune cells. Studies of cultured type I cells harvested following 1 day of in vivo hypoxia showed elevated transcript levels of inflammatory cytokines. In situ hybridization studies confirmed expression of IL-6 in type I cells and also showed that CH induces IL-6 expression in supporting type II cells. Concurrent treatment of CH rats with anti-inflammatory drugs (ibuprofen or dexamethasone) blocked immune cell invasion and severely reduced CH-induced cytokine expression in carotid body. Drug treatment also blocked the development of chemoreceptor hypersensitivity in CH animals. Our findings indicate that chemoreceptor adaptation involves novel neuroimmune mechanisms, which may alter the functional phenotypes of type I cells and chemoafferent neurons.
Keywords: dexamethasone, interleukin-1β, interleukin-6, tumor necrosis factor-α
the mammalian carotid body consists of integrated units of chemoreceptor, neural, glial, and vascular cells surrounded by connective tissue, which collectively constitute a highly adaptive chemosensory organ. Stimulus transduction occurs in paracrine type I (chemoreceptor) cells, which release multiple neuroactive agents in response to hypoxia, hypercapnia, and acidosis. Primary afferent neurons in the petrosal ganglia project axons through the carotid sinus nerve (CSN) to form synaptic terminals on type I cells, whereas glial-like type II cells envelop the type I cells and terminal axon enlargements, forming lobules that are embedded in connective tissue penetrated by a microvascular network of highly permeable sinusoidal capillaries. Fibroblasts, resident macrophages, and a small number of mast cells are also present in the tissue together with postganglionic parasympathetic and sympathetic axons (24).
Prolonged and continuous exposure of mammals to a low Po2 environment (i.e., chronic hypoxia; CH) elicits adaptation in the carotid body involving remarkable morphological and physiological adjustments. These include altered gene expression and increased chemosensitivity in the initial 1–3 days of exposure to hypobaric hypoxia (3, 4, 9). In the rat, exposure to hypobaric hypoxia (380 Torr) for 9–14 days approximately doubles stimulus-evoked CSN activity in response to a standardized acute hypoxic challenge. Moreover, the resting nerve activity in these preparations is substantially elevated (3). Given their exquisite sensitivity to oxygen, it has long been assumed that type I cells initiate and regulate adaptive processes via the autocrine and paracrine action of their secretory products. Indeed, numerous studies have documented altered expression of multiple excitatory as well as inhibitory agents in the chemosensory tissue following CH (2, 27, 41).
Given the proven critical role of type I cells in the chemosensory process, it is not surprising that virtually no attention has been given to the possibility that increased excitability in CH is the consequence of changes initiated by the actions of other cells in the carotid body parenchyma. Interestingly, multiple recent studies have demonstrated that chronic pain in humans and neuropathic and inflammatory pain in animal models involve immune cell-induced hyperexcitability of primary sensory neurons (42). It is now well-documented that invading macrophages and neutrophils as well as resident mast cells and dendritic cells induce remodeling and hypersensitivity of primary nociceptor neurons (42). In addition, mechanoreceptor neurons that do not normally signal pain initiate the production of “pain neurotransmitters” subsequent to the action of inflammatory mediators (25). Even cells that are not commonly associated with immune function may participate in the alteration of peripheral nerve function. For example, fibroblasts become a source of chemoattractant chemokine molecules [e.g., macrophage inflammatory protein-2 and monocyte chemoattractant protein-1 (MCP-1)] that recruit circulating immune cells (primarily neutrophils and macrophages); this is followed by the production and secretion of proinflammatory cytokines (42). Numerous studies of chronic inflammatory and neuropathic pain consistently demonstrate upregulation of three cytokines, IL-1β, IL-6, and TNFα, in addition to the chemokine, MCP-1 (42).
The growing body of evidence that supports a connection between immune cells, inflammatory cytokines, and the development of hyperexcitability in chronic pain appears to provide a model for the development of altered carotid body structure and increased chemosensitivity in CH. The present study uses real-time quantitative PCR (qPCR) and immunofluorescence techniques to demonstrate that CH induces an inflammatory condition in rat carotid body. Furthermore, amplification of RNA from dissociated cell preparations and in situ hybridization histochemistry have been employed to explore whether cytokine production occurs in type I cells in addition to invading immune cells. Finally, common anti-inflammatory drugs ibuprofen and dexamethasone have been used to test the hypothesis that inflammation is a conditional requirement for the development of CH-induced chemosensory adaptation.
METHODS
Animals and exposure to CH.
Eighty-seven rats exposed in a hypobaric chamber were housed in standard rodent cages with food and water. Pressures were reduced from ambient barometric pressure (BP) at the University of Utah (i.e., BP ∼630 Torr; 1,500 m) until a selected pressure equivalent to ∼2,560 m (565 Torr), ∼3,350 m (515 Torr), or ∼5,500 m (380 Torr) was reached and maintained for a selected period (up to 28 days). The chamber was opened every 1 or 2 days to replenish food and water and change litter. Control, normal animals (32 rats), were maintained outside the chamber in ambient conditions. Animal protocols were approved by the University of Utah Institutional Animal Care and Use Committee.
qPCR.
Carotid bodies were harvested from rats anesthetized with a mixture of ketamine (10 mg/100 g) and xylazine (0.9 mg/100 g). Carotid artery bifurcations were located, excised, and placed in a lucite chamber containing 100% O2-equilibrated modified Tyrode solution at 0–4°C (in mM: 112 NaCl; 4.7 KCl; 2.2 CaCl2; 1.1 MgCl2; 42 sodium glutamate; 5 HEPES buffer; 5.6 glucose; pH 7.4). Each carotid body was carefully dissected from the artery and cleaned of surrounding connective tissue. Tissues were then immediately frozen on Al-foil on dry ice. In accord with the kit instructions (RNAqueous-Micro; Ambion, Austin, TX), total RNA was extracted from homogenized tissue samples pooled from groups of 5 rats for each experiment. Following removal of contaminating DNA (DNase treatment), first-strand complementary DNA was synthesized from 1 μg of total RNA (quantified with a NanoDrop ND-1000 Spectrophotometer) using RETROscript (Ambion). Aliquots of cDNA corresponding to 2 ng of total RNA were introduced into a SYBRgreen reaction mix (25 μl; Qiagen) containing “upstream” and “downstream” primers for selected cytokines (MCP-1, TNFα, IL-1β, and IL-6). All primer pairs were “blasted” against known rat gene sequences. qPCR was conducted in an MJ Research PTC-200 equipped with a Chromo4 detector. From each pooled group of cDNA, 3–5 PCR reactions were initiated at 95°C for 15 min followed by 40 cycles consisting of 30 s at 94°C, 30 s at 58°C, and 30 s at 72°C, with the final cycle extended to 5 min at 72°C. Product purity was evaluated by determination of the melting curve, after which samples were stabilized at 4°C. Sample comparisons were based on the relative standard curve method (29), and data are normalized to 18S rRNA expression. In preliminary studies using cDNA aliquots equivalent to equal amounts of total RNA, we found that 18S rRNA varies <10% in CH vs. normal samples with P > 0.24. Amplifications of samples not treated with RETROscript were performed to exclude possible contamination with genomic DNA.
Amplified RNA.
Carotid bodies were dissected free of surrounding connective tissue and transferred to Ham's F-12 medium (Ca2+- and Mg2+-free) containing 0.2% collagenase and 0.2% trypsin. Each organ was cut into 6–12 pieces and incubated for 40 min in a CO2 incubator (5% CO2-95% air) at 36.5°C. Tissue fragments were rinsed (2 × 10 min, room temperature) in F-12 medium (Ca2+- and Mg2+-free), transferred to poly-l-lysine-coated glass coverslips, and triturated in a small volume of medium plus 10% fetal calf serum and 5 μg/ml insulin. Dissociated single type I cells collected using a patch-clamp pipette were immersed in buffer provided in a PicoPure RNA isolation kit (Microgenomics/Arcturus), and total RNA was isolated according to kit directions. The RNA was further purified with RNeasy MinElute Cleanup Kit (Qiagen). mRNA was amplified according to directions in the MessageBOOSTER cDNA Synthesis Kit (Epicentre Biotechnologies).
Immunocytochemistry.
Anesthetized rats were perfused intracardially with ice-cold 4% paraformaldehyde in 0.1 M PBS. Carotid bodies were removed, cleaned of surrounding connective tissue, immersed in the same fixative for 1 h, rinsed in 20% sucrose/PBS for 2 h, and stored at 4°C in 30% sucrose/PBS for 1 h. Cryostat sections (6 μm) were thaw-mounted onto gelatin-subbed slides. Sections were treated for 20 min with 5% goat serum in PBS plus 0.1% Triton X-100 and then incubated at 4°C overnight in primary antibodies for tyrosine hydroxylase (TH) and the macrophage marker, ED1, or the universal leukocyte marker, CD45, and diluted [1:2,000 for anti-TH (Chemicon); 1:100 for anti-ED1 and anti-CD45 (Serotec)] in PBS containing 2% goat serum and 0.1% Triton X-100. Sections were then rinsed in PBS at room temperature, incubated for 1 h with selected secondary antibodies (diluted 1:200 to 1:400) conjugated with fluorescein or rhodamine in 2% goat serum plus 0.1% Triton X-100, and then rinsed in PBS for 20 min. In all experiments, normal vs. experimental tissue samples and frozen sections were processed simultaneously, and all incubation and reaction conditions were identical. In selected sections, the primary antibody was omitted to assess nonspecific staining of the secondary fluorescent antibodies. Specimens are viewed in a Zeiss Model M30 laser scanning confocal microscope.
In situ hybridization histochemistry.
Tissues harvested from rats were quick-frozen in optimum cutting temperature compound (OCT), sectioned (5–8 μm), mounted on gelatin-subbed glass slides, fixed with 4% paraformaldehyde, dehydrated in an ascending series of ethanols, and stored at room temperature. Sections were rehydrated in a descending series of ethanols, incubated in proteinase K (10 μg/ml; 37°C, 9 min), washed in PBS, treated with triethanolamine (100 μM, 2 min), and acetylated in 0.25% acetic anhydride (10 min). Following a wash in PBS, sections were dehydrated. A 463-base riboprobe for IL-6 was constructed from specified PCR products along with ligated restriction sites and inserted into a pBluescript II KS+ (Stratagene). Restriction enzyme digests were linearized followed by in vitro transcription incorporating the digoxigenin label (DIG RNA Labeling Kit; Roche Diagnostics). The probe was purified on a Micro Bio-Spin P-30 column (Bio-Rad). The hybridization solution consisted of 50% formamide, 600 mM NaCl, 10 mM Tris·HCl (pH 8.0), 1 mM EDTA, 1× Denhardt's solution, 0.25% SDS, 10% dextran sulfate, and 200 μg/ml yeast tRNA. Solution was heated to 85°C for 10 min; incubation at 85°C continued for 3 min following addition of riboprobe. Sections were incubated overnight in a chamber humidified with 50% formamide at 55°C. Slides were washed 2× in SSC (3 M NaCl, 0.3 M sodium citrate, pH 7.0) containing 50% formamide at 60°C for 30 min followed by washes in 2× SSC (20 min, 60°C) and 0.2 SSC (2× 20 min, 60°C). Blocking proceeded in 10% normal sheep serum for 1 h at room temperature followed by treatment with alkaline phosphatase conjugated anti-digoxigenin antibody (1:1,000) overnight at 4°C. After washing, sections were incubated overnight in nitro blue tetrazolium salt (NBT; 0.33 mg/ml) and 5-bromo-4-chloro-3-indolyl phosphate toluidine salt (BCIP; 0.16 mg/ml) in a buffer containing 0.1 M NaCl, 0.1 M Tris, and 5 mM MgCl2 (pH 9.5) (14).
Electrophysiological recording of CSN activity.
As has been described previously (3), the carotid bifurcations were excised from rats under ketamine-xylazine anesthesia and placed in a lucite chamber containing 100% O2-equilibrated modified Tyrode solution at 0–4°C. Each carotid body along with its attached nerve was carefully dissected from the artery and cleaned of surrounding connective tissue. Preparations were then placed in a conventional flow chamber where the carotid body was continuously superfused (up to 4 h) with modified Tyrode solution maintained at 37°C and equilibrated with a selected gas mixture. The CSN was drawn up into the tip (∼100-μm inner diameter) of a glass suction electrode for monopolar recording of chemoreceptor activity. Basal neural activity was established in superfusates maintained at Po2 = 450 Torr. The Po2 was lowered to 120 Torr in superfusates equilibrated with air to provide a moderately hypoxic stimulus. Neural activity was led to an alternating current (AC)-coupled preamplifier, filtered, and transferred to a window discriminator and a frequency-to-voltage converter. Signals were processed by an analog-to-digital converter for display of frequency histograms on a PC monitor. Data were expressed as impulses per second and analyzed using Student's t-test and ANOVA.
RESULTS
CH-induced immune cell invasion and cytokine gene expression in carotid body.
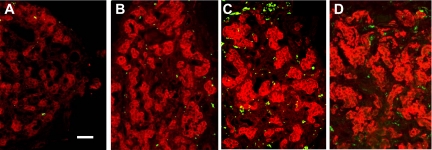
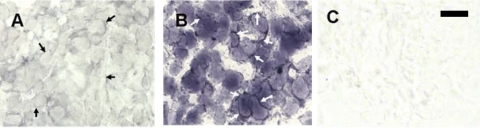
Immunofluorescence assessments indicate that increased numbers of invasive ED1+ macrophages (20) are present in the carotid body following hypoxia. In Fig. 1, type I cells immunostained for TH are red, and macrophages expressing the ED1 antigen are green. Macrophages are rare in normal carotid body, but their incidence increased noticeably after 1 day of CH. Moreover, 3 days of CH elicited a marked elevation in the incidence of ED1+ cells in perivascular and connective tissue, consistent with extravasation and differentiation of monocytes, the circulating precursors of macrophages. In addition, TH immunofluorescence is elevated following 1 and 3 days of hypoxia, in accord with previous reports of CH-induced upregulation of this catecholaminergic enzyme (6). After 7 days of CH, TH levels remain high in type I cells, but the incidence of macrophages, although above normal, has markedly receded.
Fig. 1.
Immunofluorescence in rat carotid body of type I cell marker tyrosine hydroxylase (TH; red) and immune cell antigen ED1 (green). A: normal. B, C, and D: 1, 3, and 7 days of chronic hypoxia (CH), respectively. Scale bar = 50 μm.
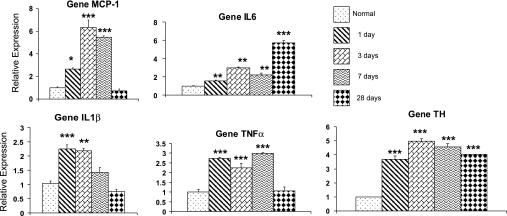
The data in Fig. 2 show the effects of 1, 3, 7, and 28 days of CH (380 Torr) on the expression of the chemokine, MCP-1, and principal inflammatory cytokines, IL-6, IL-1β, and TNFα. All values are expressed relative to levels measured in carotid bodies from control animals maintained at the University of Utah (1,500 m, ∼630 Torr). A particularly prominent finding is the early elevation in carotid body after only 1 day of hypoxia, of transcript for the inflammatory mediators. These indicators of inflammation are also significantly increased on day 3, and three of the four cytokines remain elevated after 7 days of CH. However, following a 28-day exposure, only IL-6 remains at high levels; expression of MCP-1, IL-1β, and TNFα is similar to normal in these long-exposure animals. Data in Fig. 2 also show a similar analysis of the effects of CH on expression of TH in the carotid body. These findings confirm a previous study that showed that TH expression increases approximately three- to fourfold following 24–48 h of hypoxia (6), and, in addition, they demonstrate that, unlike the inflammatory cytokines, TH levels remain elevated for up to 4 wk of continuous exposure.
Fig. 2.
Time course of CH-induced inflammatory cytokine and TH gene expression in rat carotid body. Quantitative PCR (qPCR) data are normalized to 18S RNA and expressed relative to mRNA levels in normal tissue. MCP-1, monocyte chemoattractant protein-1. *, **, And *** indicate P < 0.05, 0.01, and 0.001 vs. normal, respectively. CH: exposure at 380 Torr for time indicated.
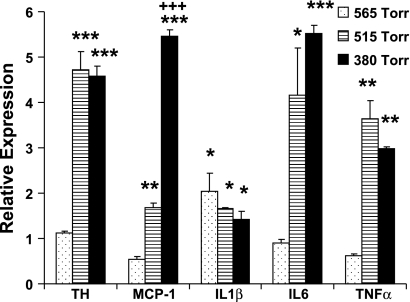
Figure 3 compares the effects of less severe hypoxia with that evoked by 380 Torr on cytokine and TH expression in carotid body. Exposure for 7 days at 565 Torr (equivalent to ∼2,560 m or ∼8,400 ft) did not evoke increases in the expression of TH and the inflammatory cytokines IL-6 and TNFα as well as the chemokine, MCP-1. It is noteworthy that 565 Torr is only slightly lower than the lowest pressure (575 Torr) allowed by the United States Federal Aviation Administration in commercial airliner cabins (8). Thus it may not be surprising that this modest stimulus evoked a significant increase in IL-1β but not in other cytokines or TH. CH at 515 Torr (∼3,350 m, 11,000 ft) evoked significant increases in expression of all inflammatory cytokines in addition to TH. Quantitatively, these changes were similar to expression at 380 Torr with the exception that levels of the chemokine, MCP-1, were significantly lower at 515 vs. 380 Torr, and they were even lower at 565 Torr, indicating a graded effect of hypoxia. Expression of IL-1β was similar at all levels of hypoxia following 7 days of CH. Data presented in Fig. 2 show the level of IL-1β peaked at 3 days and recovered to near normal on CH day 7. Thus more robust changes in IL-1β may be observed following shorter exposures to 515 Torr.
Fig. 3.
Effect of exposure to hypoxia at 565, 515, or 380 Torr for 7 days on expression of TH and inflammatory cytokines in rat carotid body. Data are expressed relative to normal nonhypoxic rats. *, **, And *** indicate P < 0.05, 0.01, and 0.001, respectively, vs. normal; +++P < 0.001 vs. 515-Torr group.
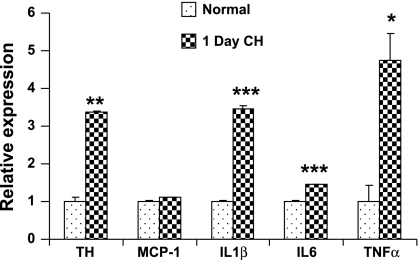
The production of high levels of IL-1β and TNFα on day 1 of hypoxia appears to conflict with the immunofluorescence data showing relatively low levels of ED1+ macrophages at this time. However, because virtually all cells are capable of producing cytokines (26), an important question is whether nonimmune cells participate in the inflammatory response. Figure 4 presents data using the amplified RNA (aRNA) technique. Originally developed by Van Gelder and colleagues (36), aRNA produces large amounts of mRNA from a few cells while maintaining the proportionality between the expression of different genes. We harvested mRNA from dissociated carotid body cells that were identified based on size and morphological characteristics commonly used in our laboratory to select cells for examination of O2-sensitive K+-currents (17, 18). Following amplification, mRNA was converted to cDNA and subjected to qPCR for selected genes. The data show that following 1 day of in vivo hypoxia, expression of TH is elevated >3-fold. In addition, expression of IL-1β, IL-6, and TNFα were significantly increased. The short hypoxic exposure did not alter expression of MCP-1. Complementary results using the in situ hybridization technique (Fig. 5) show that, after 3 days of CH, IL-6 expression is upregulated in slender cellular processes consistent with the morphology of sustentacular type II cells; there also appears to be a lesser but substantial increase in IL-6 mRNA in cell lobules, consistent with type I cells. These findings suggest that type I as well as type II cells participate in the initial phase of cytokine production. A recent report likewise indicated that CH upregulates production of inflammatory cytokines in type I cells (22).
Fig. 4.
Effect of 24-h in vivo hypoxia on expression of TH, the chemokine, MCP-1, and inflammatory cytokines IL-1β, IL-6, and TNFα in rat type I cells. Data are obtained from 15 normal and 15 hypoxic cells using amplified RNA (aRNA)/qPCR technology. See text for details. *, **, And *** indicate P < 0.05, 0.01, and 0.001, respectively, vs. normal.
Fig. 5.
In situ hybridization histochemistry for cytokine IL-6 in normal (A) and 3-day CH (380 Torr; B) carotid body. CH induces increased IL-6 expression in slender cell processes, consistent with the morphology of type II cells (arrows in A vs. B). Upregulation of IL-6 expression is also indicated in type I cells (enveloped by type II cell processes). Incubation in “sense probe” (C) shows background staining. Scale bar = 20 μm.
Effect of anti-inflammatory drugs on CH-induced inflammation and chemoreceptor adaptation.
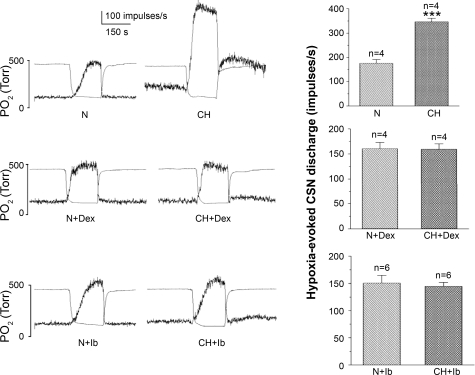
The possibility that inflammation plays a role in CH-induced chemoreceptor adaptation was examined in CH animals concurrently treated with common anti-inflammatory drugs ibuprofen and dexamethasone. Data presented in the top left of Fig. 6 show that CH elicits a marked hypersensitivity as indicated by CSN responses to a standardized acute hypoxic challenge after 8 days of CH at 380 Torr. Summary data (Fig. 6, right) show that CH induces a doubling of the averaged hypoxia-evoked nerve discharge. Separate groups of animals received ibuprofen (4 mg·kg−1·day−1) or dexamethasone (0.1 mg·kg−1·day−1) during exposure to either CH or normoxia for 8–10 days (Fig. 6, middle and bottom). Drug doses were chosen based on their ability to suppress inflammation-induced phenotypic changes in rat primary sensory neurons (38). In normoxic rats, drug treatment did not affect basal or hypoxia-evoked CSN activity. However, typical records of basal and stimulus-evoked nerve activity show that CH did not induce increased chemosensitivity in rats treated with the anti-inflammatory agents. Summary histograms (Fig. 6, right) show the averaged impulses per second evoked over the 150 s of hypoxia from each of 6 or 4 preparations treated with ibuprofen or dexamethasone, respectively, indicating that adaptation does not occur in the CH drug-treated groups.
Fig. 6.
Left: carotid sinus nerve (CSN) activity evoked by a standard hypoxic stimulus (indicated by separate trace of bath Po2) in normal (N) vs. 8- to 10-day CH preparations. CH elicits a robust increase in hypoxic sensitivity and basal resting activity; however, note that hypersensitivity to hypoxic challenge is absent in CH animals concurrently treated with dexamethasone (Dex; 0.1 mg·kg−1·day−1) or ibuprofen (Ib; 4 mg·kg−1·day−1). Right: summary data (averaged evoked impulses per second) from 4 or 6 preparations in each group: drug-free (top) or concurrently treated with either dexamethasone or ibuprofen (middle and bottom). ***P < 0.001 vs. normal.
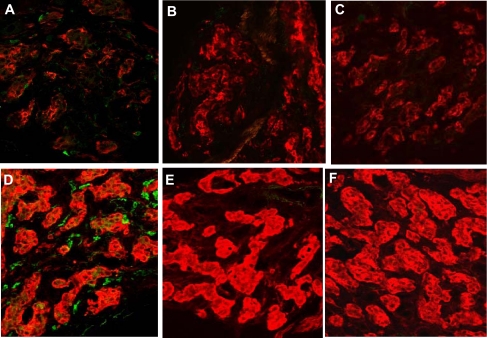
Data in Fig. 7 show immunofluorescence images of carotid body stained for TH (red) and the universal leukocyte antigen, CD45 (green). Figure 7A shows the presence of a few CD45+ cells in normal carotid body. Exposure at 380 Torr for 3 days elicits an enhanced incidence of CD45+ cells (Fig. 7B). Following hypoxia, type I cells show a robust increase in TH immunostaining intensity. In addition, individual CD45+ cells display a remarkable increase in fluorescence, consistent with the previous demonstrations of elevated expression of this antigen in inflamed tissue (31). CH (380 Torr) and concurrent treatment with dexamethasone (0.1 mg·kg−1·day−1; Fig. 7E) or ibuprofen (4 mg·kg−1·day−1; Fig. 7F) virtually eliminated expression of CD45 in carotid body, and, likewise, few CD45+ immune cells were present in normoxic carotid bodies treated with the anti-inflammatory drugs (Fig. 7, B and C).
Fig. 7.
Effect of anti-inflammatory drugs on CH-induced immune cell activity in rat carotid body. Green cells are immunostained for CD45, a universal leukocyte marker; red indicates TH. A: normal carotid body contains a few immune cells that weakly express CD45. Following 3 days of CH (D), the tissue contains numerous cells that express higher levels of CD45. In normoxic carotid bodies from animals treated with dexamethasone (0.1 mg·kg−1·day−1; B) or ibuprofen (4 mg·kg−1·day−1; C) few or no detectable CD45+ immune cells are visible. Likewise, in CH animals treated with dexamethasone (E) or ibuprofen (F), CD45+ cells are virtually absent. Notice that TH fluorescence is increased in animals treated with dexamethasone.
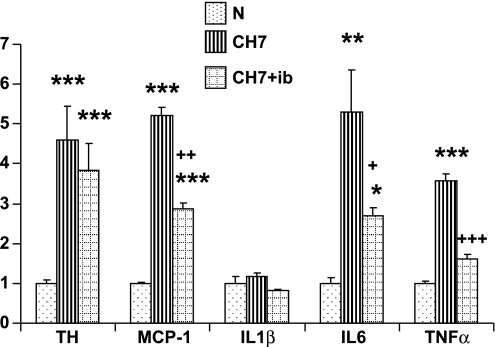
Data from separate real-time PCR experiments shown in Fig. 8 indicate that the anti-inflammatory drugs inhibit expression of proinflammatory cytokines. A 7-day course of ibuprofen (4 mg·kg−1·day−1) concurrent with CH (380 Torr) inhibited >50% of the hypoxia-induced upregulation of the inflammatory genes TNFα and IL-6 in carotid body. Expression of the chemokine, MCP-1, is also substantially less in the presence of ibuprofen. Note that after 7 days at 380 Torr, expression of IL-1β had returned to normal levels (see Fig. 2), and treatment with ibuprofen did not cause any further decrease in the expression of this cytokine. Importantly, CH-induced expression of TH was not significantly hampered in ibuprofen-treated animals, suggesting that increased expression of this gene is not dependent on inflammation. This latter finding is consistent with the immunocytochemical data presented in Fig. 7.
Fig. 8.
Effect of ibuprofen (ib; 4 mg·kg−1·day−1) on 7-day CH-induced gene expression of TH and cytokines in rat carotid body. *, **, And *** indicate P < 0.05, 0.01, and 0.001, respectively, vs. normal; +, ++, and +++, P < 0.05, 0.01, and 0.001, respectively, vs. 7-day CH (CH7).
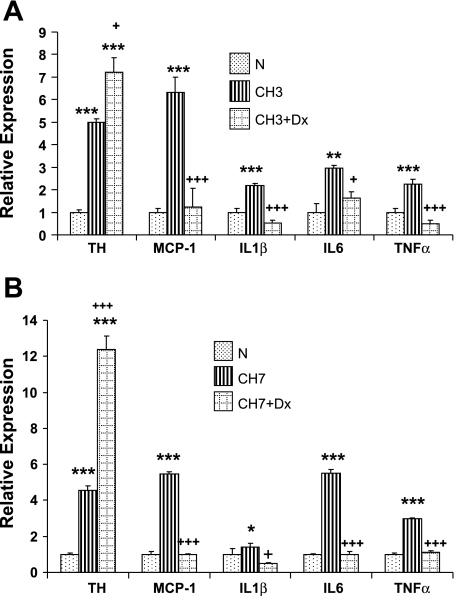
In separate experiments, we examined the effects of dexamethasone on inflammatory gene expression following 3 and 7 days of CH (380 Torr). Data in Fig. 9A demonstrate that the corticosteroid depresses expression of all four inflammatory genes, including IL-1β following 3 days of CH, when both the incidence of immune cells and cytokine expression are at high levels in carotid body. Moreover, TH expression is elevated in the presence of dexamethasone, in accord with previously demonstrated effects of this drug on carotid body (15). TH expression is further enhanced following 7 days of CH in the presence of dexamethasone (Fig. 9B), but expression of the proinflammatory genes remains at levels comparable with normal.
Fig. 9.
Effect of dexamethasone (Dx; 0.1 mg·kg−1·day−1) on CH-induced gene expression of TH and cytokines in rat carotid body. A: 3-day CH (CH3). B: CH7. *, **, And *** indicate P < 0.05, 0.01, and 0.001, respectively, vs. normal; +, and +++, P < 0.05 and 0.001, respectively, vs. CH.
DISCUSSION
Most previous studies of carotid body adaptation have implicitly assumed that the adaptive process is a primary and exclusive function of type I cells. Because of their exquisite sensitivity to O2 and ability to secret multiple neuroactive agents, these cells have been viewed as the master regulators of chemosensitivity in both acute and chronic hypoxia. Our data for the first time suggest that CH-induced increased chemoreceptor excitability is a process that is heavily influenced by a local immune response in the carotid body. The importance of inflammation is strongly supported by the finding that low doses of ibuprofen or dexamethasone prevent increased cytokine production as well as chemosensory adaptation in CH. In addition, the upregulation of cytokines begins within the first 24 h of hypoxia, and the tissue is invaded by numerous macrophages within 72 h. In a previous study, we (3) established that increased CSN sensitivity is detectable after 3 days of CH but not following 24 h, a time course consistent with the development of inflammation. A particularly interesting finding is that, with the exception of IL-6, levels of cytokine expression recede after 7 days of CH and are fully recovered on day 28. A caveat to our measurements is that gene expression is not necessarily quantitatively linked to protein production. It is intriguing that, in a study of the temporal course of cytokine expression following peripheral nerve injury, Winkelstein et al. (45) similarly showed that spinal cord IL-6 expression remained above normal for up to 2 wk, whereas expression of TNFα spiked on day 1 and returned to normal on day 3. Importantly, these authors showed that allodynia and thermal hyperalgesia persisted throughout the course of the study even while expression and protein levels of cytokines had recovered. Thus, in accord with the physiological hypersensitivity developed in nociceptors in response to chronic pain (42), our data indicate that increased responsiveness of arterial chemoreceptors following CH likewise involves unique neuroimmune mechanisms.
Dvorakova et al. (10) first demonstrated the presence of a small population of resident ED1+ macrophages in the normal carotid body. Our immunocytochemical results confirm these findings and show, in addition, that CH elicits a progressive increase in the number of ED1+ cells consistent with extravasation and differentiation of circulating monocytes. Activated macrophages are sources of inflammatory cytokines IL-1β, IL-6, and TNFα. However, our data indicate that cytokine production also occurs in nonimmune cells in the carotid body parenchyma, including O2-sensing type I and neuroglia-like type II cells. Contemporary studies have now firmly established that virtually all cells are capable of producing cytokines; thus these unique signalling molecules are not the exclusive domain of immune cells (26). Peripheral nerve damage or the introduction of inflammatory agents elicit local cytokine production in Schwann cells and fibroblasts (32, 42). Moreover, prolonged depolarization induces IL-6 production in chromaffin-derived pheochromocytoma (PC12) cells (29), which are considered functional analogs of type I cells because they contain high levels of catecholamine and express O2-sensitive K+-channels (7, 21, 47). Our data from type I cells indicate a robust increase in IL-1β and TNFα after only 1 day of hypoxia, a time when the incidence of ED1+ macrophages is low, suggesting that the upregulation of cytokine production in type I cells may not require the presence of immune cells. Collectively, our single-cell PCR data and in situ hybridization findings demonstrate the participation of type I and type II cells in the production of cytokines, thus further implicating these cells as key components of the adaptive mechanism. In accord with cytokine action, recent studies have demonstrated receptors for IL-1, IL-6, and TNFα on type I cells (22, 39, 40). Moreover, the notion that primary sensory chemoafferent neurons may be affected by cytokines is supported by studies that documented expression of specific cytokine receptors on primary sensory dorsal root neurons (34, 44). Moreover, the administration of NSAIDs has been shown to block inflammation-induced phenotypic changes in DRG neurons (38).
Our finding that cytokine expression occurs in type I cells following CH is also in accord with a recent report by Lam et al. (22). However, our respective observations differ on several important points. First, Lam et al. (22) did not report the presence of invading immune cells in their preparations. This difference may be due to the small size of immune cells relative to type I cells, which were immunostained for proinflammatory cytokines. Moreover, our studies used antibodies directed against ED1 and CD45, antigens expressed specifically by leukocytes. Second, Lam et al. (22) reported that breathing 10% O2 for 7 days elicited increases of inflammatory cytokines of only 15–30%. At a similar level of hypoxia, we have documented a more robust increase in expression of IL-6 (∼200%) and TNFα (∼300%) following 7 days of exposure. An explanation for these discrepancies may lie in the choice of internal control genes for normalization of PCR data. Using qPCR assays, we have found that expression of β-actin [the control gene used by Lam et al. (22)] is increased by ∼250% following 3 and 7 days of CH. In contrast, our data indicate that 18S RNA varies by <10% in CH vs. normal samples. A final point is that, with the exception of IL-6, expression of inflammatory cytokines recovers to normal levels following 28 days of CH. Using an assessment of tissue area occupied by positive immunocytochemical staining, Lam et al. (22) estimated that expression of all cytokines remained above normal on day 28 of exposure. However, previous studies demonstrated a high incidence of mitotic activity in type I cells and an increased proportion of tissue occupied by these cells following CH (1, 5, 19). Without quantifying staining intensity in individual type I cells and/or assessment of gene expression in cells or tissues, it is difficult to ascertain whether physiologically meaningful changes in cytokine activity have occurred.
Inhibition of cytokine production in animals concurrently treated with ibuprofen or dexamethasone is consistent with the known anti-inflammatory effects of these agents. Ibuprofen was developed some 50 yr ago; along with aspirin, it was characterized as a nonselective inhibitor of cyclooxygenases 1 and 2 (COX1 and COX2) (28). The anti-inflammatory effects of ibuprofen and similar drugs were therefore attributed to blocking the production of proinflammatory prostanoids. However, studies conducted within the last decade have shown that, in addition to COX inhibition, ibuprofen mediates COX-independent anti-inflammatory effects (35). Perhaps most important is inhibition of nuclear translocation of the transcription factor, NF-κB, which mediates cytokine production, including TNFα, IL-1β, and IL-6 (30). Thus the ibuprofen-mediated block of cytokine expression in CH carotid body suggests the possible involvement of NF-κB in the adaptive process. In addition, cytokine mediated production of high levels of inflammatory prostanoids may also contribute to carotid body adaptation. In fact, numerous studies have demonstrated the involvement of selected prostaglandins in the development of nociceptor hyperexcitability in inflamed skin (37). It is relevant that previous studies of normal rabbit carotid body showed that acute hypoxia elicits release of PGE2, whereas inhibition of PGE2 synthesis with indomethacin augmented type I cell activity as indicated by increased release of catecholamine (12, 13). These findings indicate that PGE2 promotes an inhibitory effect on type I cells in acute hypoxia. However, chronic local treatment with PGE2 has been shown to induce a hypersensitive state in nociceptors (11), suggesting the possibility of divergent short- vs. long-term effects of prostanoids. Moreover, prostanoid effects on type I cells may differ from those on chemoafferent nerve terminals.
Glucocorticoids, including dexamethasone, are known to inhibit the release of IL-1β and TNFα from activated macrophages (16). Given that IL-6 synthesis and release occur as a consequence of the actions of IL-1β and TNFα (16), it is not surprising that expression of this cytokine was decreased in dexamethasone/CH rats. Recent studies also indicate that dexamethasone inhibits gene expression of MCP-1, a chemokine that promotes leukocyte migration (46). Thus our data are also consistent with the known ability of dexamethasone to inhibit the recruitment of monocytes and macrophages into affected tissue and account for the decreased numbers of ED1+ and CD45+ cells in the carotid body of drug-treated animals. In addition to their anti-inflammatory effects, glucocorticoids can suppress pituitary-adrenal function and promote the induction of anabolic enzymes. The extent to which these latter processes may be involved in suppressing CH-induced chemoreceptor adaptation are unknown. However, the use of dexamethasone in humans indicates that non-anti-inflammatory effects require prolonged treatment (multiple weeks to months; Ref. 16), whereas our assessments of CSN activity were completed after 8–10 days.
Although the present findings strongly support a role for inflammation in resetting chemosensory sensitivity, they do not directly identify the targets of inflammatory mediators nor the precise changes in cellular physiology that result in receptor hyperexcitability. Proinflammatory cytokines and downstream inflammatory mediators are known to alter multiple phenotypic properties of primary sensory neurons including expression of ion channels and neurotransmitters (23, 42, 43). Moreover, recent studies of normal rat type I cells have demonstrated that IL-1β inhibits O2-sensitive K+-channels and evokes a transient rise in intracellular Ca2+, presumably via action at specific IL-1 receptors (33, 39). In CH, sustained high levels of specific cytokines may mediate short-term stimulatory effects as well as long-term gene-induced phenotypic changes, which contribute to the adaptive process. These actions may occur in multiple cell types in the chemoafferent pathway including type I cells and primary sensory (chemoafferent) neurons. Collectively, our data suggest that novel neuroimmune interactions are a heretofore unrecognized feature of the regulation of chemoreceptor sensitivity.
GRANTS
This study was supported by National Institutes of Health Grants NS-12636, NS-07938, and HL-086508.
Acknowledgments
We thank Dr. Alan Light, University of Utah Department of Anesthesiology, for valuable discussions.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bee D, Pallot DJ, Barer GR. Division of type I and endothelial cells in the hypoxic rat carotid body. Acta Anat (Basel) 126: 226–229, 1986. [DOI] [PubMed] [Google Scholar]
- 2.Bisgard GE Carotid body mechanisms in acclimatization to hypoxia. Respir Physiol 121: 237–246, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, He L, Dinger B, Stensaas L, Fidone S. Role of endothelin and endothelin A-type receptor in physiological adaptation of the carotid body during chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 282: L1314–L1323, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, He L, Dinger B, Stensaas L, Fidone S. Chronic hypoxia upregulates connexin43 expression in rat carotid body and petrosal ganglion. J Appl Physiol 92: 1480–1486, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, He L, Liu X, Dinger B, Stensaas L, Fidone S. Effect of the endothelin receptor antagonist bosentan on chronic hypoxia-induced morphological and physiological changes in rat carotid body. Am J Physiol Lung Cell Mol Physiol 292: L1257–L1262, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Czyzyk-Krzeska M, Bayliss DA, Lawson EE, Millhorn DE. Regulation of tyrosine hydroxylase gene expression in the rat carotid body by hypoxia. J Neurochem 58: 1538–1546, 1992. [DOI] [PubMed] [Google Scholar]
- 7.Czyzyk-Krzeska MF, Furnari BA, Lawson EE, Millhorn DE. Hypoxia increases rate of transcription and stability of tyrosine hydroxylase mRNA in pheochromocytoma (PC12) cells. J Biol Chem 269: 760–764, 1994. [PubMed] [Google Scholar]
- 8.Dine CJ, Kreider ME. Hypoxia altitude simulation test. Chest 133: 1002–1005, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Dinger B, He L, Chen J, Stensaas L, Fidone S. Mechanisms of morphological and functional plasticity in the chronically hypoxic carotid body. In: Oxygen Sensing: Responses and Adaptation to Hypoxia, edited by Lahiri S, Semenza G, and Prabhakar N. New York: Dekker, 2003, p. 439–465.
- 10.Dvorakova M, Hohler B, Vollerthun R, Fischbach T, Kummer W. Macrophages: a major source of cytochrome b558 in the rat carotid body. Brain Res 852: 349–354, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira SH, Lorenzetti BB, De Campos DI. Induction, blockade and restoration of a persistent hypersensitive state. Pain 42: 365–371, 1990. [DOI] [PubMed] [Google Scholar]
- 12.Gómez-Niño A, Almaraz L, González C. In vitro activation of cyclo-oxygenase in the rabbit carotid body: effect of its blockade on [3H]catecholamine release. J Physiol 476: 257–267, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gómez-Niño A, López-López JR, Almaraz L, González C. Inhibition of [3H]catecholamine release and Ca2+ currents by prostaglandin E2 in rabbit carotid body chemoreceptor cells. J Physiol 476: 269–277, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guitteny AF, Fouque B, Mougin C, Teoule R, Bloch B. Histological detection of messenger RNAs with biotinylated synthetic oligonucleotide probes. J Histochem Cytochem 36: 563–571, 1988. [DOI] [PubMed] [Google Scholar]
- 15.Hanbauer I Long-term regulatory mechanisms for tyrosine hydroxylase in sympathetic ganglia and carotid body. Adv Biochem Psychopharmacol 15: 475–489, 1976. [PubMed] [Google Scholar]
- 16.Haynes RC Adrenocorticotropic hormone; adrenocortical steroids and their synthetic analogs; inhibitors of synthesis and actions of adrenocortical hormones. In: The Pharmacological Basis of Therapeutics, edited by Gilman AG, Rall TW, Nies AS, and Taylor P. New York: Pergamon, 1990, p. 1431–1462.
- 17.He L, Dinger B, Fidone S. Cellular mechanisms involved in carotid body inhibition produced by atrial natriuretic peptide. Am J Physiol Cell Physiol 278: C845–C852, 2000. [DOI] [PubMed] [Google Scholar]
- 18.He L, Dinger B, Sanders K, Hoidal J, Obeso A, Stensaas L, Fidone S, Gonzalez C. Effect of p47phox gene deletion on ROS production and oxygen sensing in mouse carotid body chemoreceptor cells. Am J Physiol Lung Cell Mol Physiol 289: L916–L924, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Hellstrom S, Pequignot JM. Morphometric studies on intact and sympathectomised carotid bodies of long-term hypoxic rats. A light and electron microscopial study. In: The Peripheral Arterial Chemoreceptors, edited by Pallot DJ. London: Croom Helm, 1982, p. 293–301.
- 20.Hu P, McLachlan EM. Distinct functional types of macrophage in dorsal root ganglia and spinal nerves proximal to sciatic and spinal nerve transections in the rat. Exp Neurol 184: 590–605, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi S, Conforti L, Pun RY, Millhorn DE. Adenosine modulates hypoxia-induced responses in rat PC12 cells via the A2A receptor. J Physiol 508: 95–107, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lam SY, Tipoe GL, Liong EC, Fung ML. Chronic hypoxia upregulates the expression and function of proinflammatory cytokines in the rat carotid body. Histochem Cell Biol 130: 549–559, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci 22: 10662–10670, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald DM Peripheral chemoreceptors: structure-function relationships of the carotid body. In: Regulation of Breathing, Part I, edited by Hornbein TF. New York & Basel: Dekker, 1981, p. 105–319.
- 25.Neumann S, Doubell TP, Leslie T, Woolf CJ. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature 384: 360–364, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Oppenheim JJ, Feldmann M. Introduction to the role of cytokine in innate host defence and adaptive immunity. In: Cytokine Reference, edited by Oppenheim JJ and Feldmann M. New York: Elsevier, 2000, p. 3–20.
- 27.Powell FL The influence of chronic hypoxia upon chemoreception. Respir Physiol Neurobiol 157: 154–161, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rainsford KD Discovery, mechanisms of action and safety of ibuprofen. Int J Clin Pract Suppl 135: 3–8, 2003. [PubMed] [Google Scholar]
- 29.Sallmann S, Juttler E, Prinz S, Petersen N, Knopf U, Weiser T, Schwaninger M. Induction of interleukin-6 by depolarization of neurons. J Neurosci 20: 8637–8642, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheuren N, Bang H, Munster T, Brune K, Pahl A. Modulation of transcription factor NF-kappaB by enantiomers of the nonsteroidal drug ibuprofen. Br J Pharmacol 123: 645–652, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sedgwick JD, Schwender S, Imrich H, Dorries R, Butcher GW, ter M, V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci USA 88: 7438–7442, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shamash S, Reichert F, Rotshenker S. The cytokine network of Wallerian degeneration: tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta. J Neurosci 22: 3052–3060, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shu HF, Wang BR, Wang SR, Yao W, Huang HP, Zhou Z, Wang X, Fan J, Wang T, Ju G. IL-1beta inhibits IK and increases [Ca2+]i in the carotid body glomus cells and increases carotid sinus nerve firings in the rat. Eur J Neurosci 25: 3638–3647, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett 361: 184–187, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Stuhlmeier KM, Li H, Kao JJ. Ibuprofen: new explanation for an old phenomenon. Biochem Pharmacol 57: 313–320, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Van Gelder RN, von Zastrow ME, Yool A, Dement WC, Barchas JD, Eberwine JH. Amplified RNA synthesized from limited quantities of heterogeneous cDNA. Proc Natl Acad Sci USA 87: 1663–1667, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verri WA, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH. Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol Ther 112: 116–138, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci 21: 8026–8033, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Wang BR, Duan XL, Zhang P, Ding YQ, Jia Y, Jiao XY, Ju G. Strong expression of interleukin-1 receptor type I in the rat carotid body. J Histochem Cytochem 50: 1677–1684, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Zhang XJ, Xu Z, Li X, Li GL, Ju G, Wang BR. Morphological evidence for existence of IL-6 receptor alpha in the glomus cells of rat carotid body. Anat Rec A Discov Mol Cell Evol Biol 288: 292–296, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Wang ZY, Bisgard GE. Chronic hypoxia-induced morphological and neurochemical changes in the carotid body. Microsc Res Tech 59: 168–177, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Watkins LR, Maier SF. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol Rev 82: 981–1011, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Waxman SG, Cummins TR, Dib-Hajj SD, Black JA. Voltage-gated sodium channels and the molecular pathogenesis of pain: a review. J Rehabil Res Dev 37: 517–528, 2000. [PubMed] [Google Scholar]
- 44.White FA, Jung H, Miller RJ. Chemokines and the pathophysiology of neuropathic pain. Proc Natl Acad Sci USA 104: 20151–20158, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winkelstein BA, Rutkowski MD, Sweitzer SM, Pahl JL, DeLeo JA. Nerve injury proximal or distal to the DRG induces similar spinal glial activation and selective cytokine expression but differential behavioral responses to pharmacologic treatment. J Comp Neurol 439: 127–139, 2001. [PubMed] [Google Scholar]
- 46.Zhou Y, Ling EA, Dheen ST. Dexamethasone suppresses monocyte chemoattractant protein-1 production via mitogen activated protein kinase phosphatase-1 dependent inhibition of Jun N-terminal kinase and p38 mitogen-activated protein kinase in activated rat microglia. J Neurochem 102: 667–678, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Zhu WH, Conforti L, Czyzyk-Krzeska MF, Millhorn DE. Membrane depolarization in PC-12 cells during hypoxia is regulated by an O2-sensitive K+ current. Am J Physiol Cell Physiol 271: C658–C665, 1996. [DOI] [PubMed] [Google Scholar]