Abstract
The contribution of CD4 T cells and other CD4+ cells to lung inflammation and airway remodeling remains unclear during bouts of chronic exposure to airborne allergen. Previously, murine models have shown that CD4 T cells are required for initiation of acute inflammation and the remodeling process. However, it is unknown whether CD4 T cells or other CD4+ cells continue to be required for remodeling during ongoing allergen challenges after the development of acute eosinophilic lung inflammation. To test this, mice were sensitized and challenged with ovalbumin (OVA). After acute airway inflammation was established, a CD4 depleting antibody was administered for 4 wk during a period of chronic exposure to intranasal OVA, resulting in effective depletion of CD4+ cells from all organs, including the lung, lung-draining lymph nodes, and spleen. In these mice, levels of peribronchial inflammation, bronchoalveolar (BAL) eosinophils, and lung CD11c+, CD8+, and Siglec-F+CD11c- cells were significantly reduced. However, mucus metaplasia, peribronchial subepithelial fibrosis, and smooth muscle mass were not affected. Additionally, depletion of CD4+ cells before the last week of chronic allergen challenges also led to significant reductions in BAL eosinophils, peribronchial inflammation, and lung CD11c+, CD8+, and Siglec-F+CD11c- cells. These results show that CD4 T cells, and other CD4+ cells including subsets of dendritic cells, iNKT cells, and LTi cells, play a role in ongoing eosinophilic lung inflammation during periods of chronic allergen challenge, but are not required for progressive airway remodeling that develops after initial acute inflammation.
Keywords: CD4 T cells, asthma, Th2
allergic asthma is characterized by a Th2 inflammatory response to inhaled allergens, orchestrated by CD4 T cells producing a characteristic cytokine profile responsible for eosinophilic lung inflammation (7). In asthmatics, numbers of CD4 T cells and Th2 cytokines are elevated in bronchoalveolar lavage (BAL) specimens after allergen challenge, suggesting that Th2 responses drive chronic allergic lung inflammation (34). Despite this, therapies targeting either CD4+ cells or Th2 cytokines, including IL-4 and IL-5, have not been as successful as anticipated (3, 11, 24). Although this may be due to many factors such as patient population, dose, duration, and outcome measures, it is also possible that our understanding of how CD4 T cells or other CD4+ cells contribute to chronic asthma pathogenesis, including airway remodeling, is inadequate.
Progressive decline in lung function in asthma is thought to be due to airway remodeling, characterized by structural changes including mucus metaplasia, subepithelial fibrosis, smooth muscle hypertrophy/hyperplasia, and angiogenesis (5). The relationship between inflammation and remodeling remains unclear, although some studies suggest that the two may be independent. For example, bronchial biopsies from asthmatics have shown similar levels of subepithelial fibrosis after anti-inflammatory therapy with corticosteroids (4, 8, 19). Aside from remodeling, the degree of CD4 T cell involvement in chronic allergic inflammation is unclear because other mechanisms that could support chronic inflammation may exist that are CD4 independent, including Th2 cytokine production by mast cells and eosinophils, as well as release of chemokines such as eotaxin from airway epithelium.
Murine models of acute Th2-driven airway inflammation have shown that CD4+ cells are required for eosinophilic lung inflammation and airway hyperresponsiveness, and adoptively transferred Th2 CD4 T cells can induce these features (15, 16). In addition, models using repetitive antigen challenge to induce airway remodeling have shown that if CD4+ cells are depleted at the time of the initial acute airway challenge, there is reduced inflammation and remodeling (12, 23). Because iNKT cells (up to 90%), lymphoid tissue inducer cells (LTi) (up to 50%), and a subset of dendritic cells (up to 50%) can express CD4, these data might also indicate roles for these cells as well as conventional CD4 T cells for promoting the inflammatory process (1, 22, 30). Furthermore, adoptively transferred CD4 T cells from ovalbumin (OVA)-sensitized rats have been reported to induce increases in airway smooth muscle mass when recipients were repetitively challenged with OVA via the airways (33). Thus, CD4 T cells, and perhaps other CD4+ cells, appear to be required for both initiation of airway inflammation and induction of remodeling. Conversely, CD4 depletion 3 wk after the termination of chronic antigen challenge was found, not surprisingly, to have no effect on features of airway remodeling (28). However, to our knowledge, there are no studies addressing the contribution of CD4+ cells to continued airway inflammation and the progressive remodeling that occurs during ongoing chronic allergen challenge, but after the establishment of acute airway inflammation. Using a CD4 depletion strategy at two different time points during such a chronic allergen challenge, we investigated the effect on airway inflammation and remodeling. Interestingly, we found that although mice depleted of CD4+ cells did display significantly reduced signs of lung inflammation, they still underwent the process of lung remodeling.
MATERIALS AND METHODS
Mouse model of OVA-induced airway inflammation and remodeling.
Eight- to ten-week-old C57/BL6 mice (Jackson Laboratories) were given intraperitoneal (IP) injections on days 0 and 12 with 50 μg of OVA (Sigma) adsorbed to 0.5 mg of Alum (Pierce). Intranasal challenges of 20 μg of OVA in 20 μl of PBS were given on days 24, 26, and 28. Some mice were killed on day 29 to confirm induction of acute lung inflammation. Further intranasal challenges using the same OVA dose were then performed two times per week for 4 wk to allow progressive airway remodeling (Fig. 1A). Control groups of mice received IP injections of alum without antigen and intranasal challenges with PBS without antigen. Mice were killed 2 days after the last OVA challenge, and BAL fluid, lungs, lung-draining lymph nodes, and spleens were obtained. BAL was performed by intratracheal insertion of catheter and lavaging with 0.8–0.9 ml of 2% filtered BSA (Sigma). The right hilum was tied off and lung was isolated for cellular analysis by FACS. The left lung was instilled with 0.4 ml of 4% paraformaldehyde (PFA) and placed in PFA overnight for histology. The La Jolla Institute for Allergy and Immunology IACUC approved all animal studies.
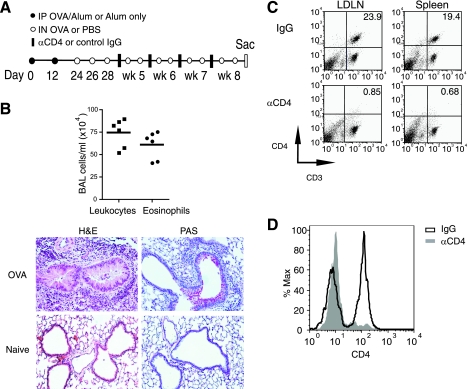
Fig. 1.
Chronic asthma experimental protocol and CD4 cell depletion strategy. A: outline of protocol for chronic airway inflammation and remodeling. Mice were immunized intraperitoneally (IP) on days 0 and 12 with ovalbumin (OVA) (filled circles). Intranasal (IN) challenges were administered on days 24, 26, and 28 and then repeated twice per week for 4 wk (open circles). Anti-CD4 (GK 1.5) or control IgG was given on days 30, 35, 43, and 50 (filled bars). B: bronchoalveolar lavage (BAL) leukocytes and eosinophils (top); H&E and periodic acid-Schiff (PAS) (bottom) on day 29 before CD4 depletion. C: CD4 depletion from spleen and lung-draining lymph nodes (LDLN). Mice were killed 2 days after undergoing the depletion protocol in A, and spleen, LDLN, and lung cells were analyzed for CD3 and CD4. D: CD4 depletion from lung. Lung cells, gated on CD3-positive cells, were analyzed for CD4 expression. Results in B are from 6 mice and 1 representative example of 8 mice/group in C and D.
CD4 cell depletion.
CD4 depleting antibody (GK 1.5) was purified from inhouse hybridoma. GK 1.5 (750 μg) or control rat IgG (Millipore) was administered IP on days 30, 35, 43, and 50 for one experimental protocol (Fig. 1A). In another protocol, 750 μg of GK 1.5 or control rat IgG were given IP once, 3 days before the last two intranasal challenges (see Fig. 3A). In all experiments, verification of CD4 depletion by FACS was performed in lungs, lung-draining lymph nodes, and spleen. For CD4 depletion before the last two intranasal challenges, peripheral blood was taken via capillary tube eye bleed and processed for FACS analysis.
Airway inflammation analysis.
BAL cells were obtained by centrifugation and resuspended in 250 μl of Hanks' balanced salt solution (Gibco). Total cell counts were performed using a hemacytometer (Hausser-Scientific). Differential cell counts of cytospins were performed after Hema 3 (Fisher) staining, a modified Wright-Giemsa stain, by counting 200 leukocytes at ×400. Paraformaldehyde-fixed lung sections were stained with hematoxylin and eosin (H&E). Slides were blinded, peribronchial regions, six to eight per mouse, were evaluated at ×200, and inflammatory infiltrates around airways were graded for severity (0, normal; 1, <3 cell diameter thick; 2, 3–10 cells thick; 3, >10 cells thick) and extent (0, normal; 1, <10% of sample; 2, 10–25%; 3, >25%). Scores were calculated by multiplying severity by extent (max 9).
Airway remodeling analysis.
Paraformaldehyde-fixed lung sections were stained with Masson's Trichrome, periodic acid-Schiff (PAS), and anti-α-smooth muscle antibody (Sigma). Mucus metaplasia was determined by counting the number of PAS+ bronchial epithelial cells. The area of peribronchial fibrosis on trichrome-stained sections was evaluated using an image analysis system (Image-Pro Plus; Media Cybernetics, Silver Spring, MD) (9). Smooth muscle area was evaluated in the same manner. All slides were blinded until completion. Results are expressed as the area of staining per micrometer length of basement membrane of bronchioles. At least six bronchi were counted in each slide.
Flow cytometry.
Lungs were digested in 2 mg/ml collagenase (Roche) for 30 min, and lung, spleen, and lymph node cells were purified using a 70-μm cell strainer (BD Falcon). Red blood cell lysis was used for spleen and peripheral blood (Sigma). Cells were blocked for 10 min and then stained for 30 min with various combinations of PerCP-conjugated anti-CD4, CD8, PE-conjugated Siglec-F, FITC-conjugated CD11c, and anti-CD3 (BD Biosciences). After being washed, cells were fixed with 2% PFA and analyzed with a FACSCalibur flow cytometer (BD Biosciences). Further analyses were performed with Flow Jo software (Tree Star).
Statistical analysis.
Statistical analysis was performed using GraphPad Prism Software (San Diego, CA). The Mann-Whitney test was used where indicated.
RESULTS
CD4 depletion in the lung and lung-draining lymph nodes.
Mice were first sensitized and then challenged on days 24–28 with intranasal OVA on three alternating days and killed 1 day after the last challenge. The hallmark of the OVA-induced allergic airway model is eosinophilic lung inflammation, and we observed high numbers of BAL leukocytes containing 75–80% eosinophils (Fig. 1B). Marked peribronchial cellular infiltration and epithelial mucus production were present as well. Thus, before CD4 depletion, we confirmed the presence of acute eosinophilic lung inflammation and mucus production. Similar to previous analyses, at this time, we found little/no evidence of airway subepithelial fibrosis or smooth muscle changes (data not shown).
Anti-CD4 antibody was initially given 2 days after the last acute intranasal challenge (day 30) and then once a week during the remaining weeks of chronic antigen challenge (Fig. 1A). After the 8-wk protocol, lung, lung-draining lymph node, and spleen were processed to confirm efficient depletion of CD4+ cells. Lung-draining lymph node and splenic cell populations in control mice had 19–24% CD3+CD4+ cells and less than 2% CD3-CD4+ cells (Fig. 1C). The group receiving anti-CD4 had a near complete absence of CD4 cells with less than 2% of the total CD4+ cells remaining. In the lung, the percent of CD3+ cells was reduced by one-third after anti-CD4 administration (data not shown), with an approximate 98% depletion of CD4+ lung cells (Fig. 1D). Thus, anti-CD4 treatment given after the establishment of acute airway inflammation was effective in depleting the majority of CD4+ cells from the animals.
CD4 depletion during chronic airway antigen challenge reduces lung inflammation.
Mice undergoing the 8-wk experimental protocol depicted in Fig. 1A were killed 2 days after the last allergen challenge. Mice that received the CD4-depleting antibody for 4 wk had on average 30–40% reduced numbers of total BAL leukocytes (P = 0.0059) and a similar reduction in peribronchial inflammation as determined by histological scoring (P = 0.0025) (Fig. 2, A and B). BAL eosinophils were further reduced by ∼50% (Fig. 2A), and the total numbers of lung cells determined after collagenase treatment of lung tissue were 70% lower than in control mice (Fig. 2B).
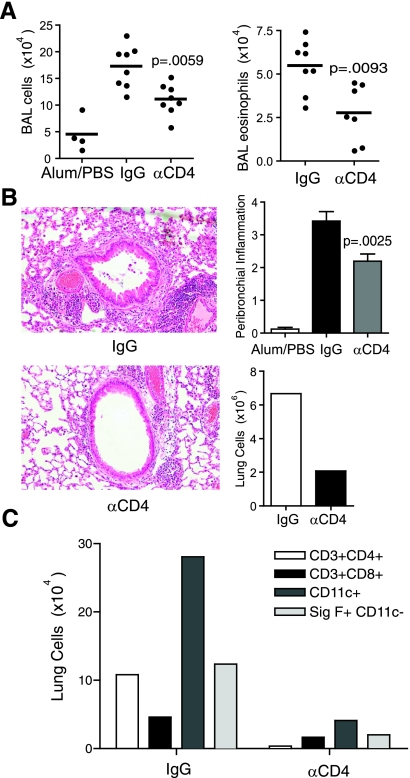
Fig. 2.
CD4 depletion reduces lung inflammation during chronic allergen challenge. A: mice undergoing the protocol in Fig. 1A were killed 2 days after the last intranasal antigen challenge. BAL leukocytes (left) and eosinophils (right) were counted. B: H&E-stained lung sections (left) were scored for levels of peribronchial inflammation (top right), and total lung cells were counted (bottom right). C: pooled lung cells were evaluated by FACS for CD3+CD4+, CD3+CD8+, CD11c+, and Siglec-F+CD11c- cells. Results are from 8 mice per OVA group and 4 mice in the Alum/PBS control group. Pooled lung cells are from 4 mice/group. The Mann-Whitney test was used for P values.
Pooled lung homogenates were processed for flow cytometry and analyzed for CD8, CD11c, and Siglec-F positive cells. Siglec-F is a sialic acid-recognizing lectin present on the surface of mouse eosinophils and is upregulated during allergic lung inflammation (40). Other cells, including pulmonary macrophages, may also express Siglec-F but are successfully excluded by costaining with CD11c, with the CD11c-negative population of Siglec-F-positive lung cells identifying eosinophils (35). Lung Siglec-F-positive, CD11c-negative cells were reduced by more than 75% following anti-CD4 administration (Fig. 2C).
In mice receiving anti-CD4, total lung CD8+(CD3+) T cells were moderately reduced suggesting a subpopulation of CD8 T cells present in the chronically inflamed lung is dependent on CD4+ cells. Interestingly, 80% fewer total lung CD11c+ cells were found, which would partially result from direct depletion of CD4+ dendritic cells, but also indicated that a majority of infiltrating dendritic cells were dependent on the presence of CD4+ T cells or other CD4+ cells. Overall, these results show that CD4+ cells play a substantial role in contributing to the inflammatory infiltrate that is found with chronic allergen challenge, and they are required after the establishment of initial acute inflammation. However, CD4+ cells are not absolutely necessary as significant eosinophilia was found even when they were absent.
CD4 depletion after several weeks of chronic airway challenge reduces eosinophilic inflammation.
Given the requirement for CD4+ cells to maintain strong lung inflammation during 4 wk of allergen challenge (Fig. 2), further experiments were performed to evaluate if this reflected only an early role, or if continued involvement of CD4 cells was required at later times when mice had already been exposed to chronic allergen for several weeks. Anti-CD4 or control antibody was therefore administered for 1 wk, 3 days before the last two OVA challenges on wk 8, as shown in Fig. 3A. Analysis of peripheral blood, taken 2 days after anti-CD4 was given, revealed successful depletion of CD4+ cells before the remaining intranasal challenges (Fig. 3B). Lung cell suspensions made at the time of death also confirmed CD4 depletion (Fig. 3C).
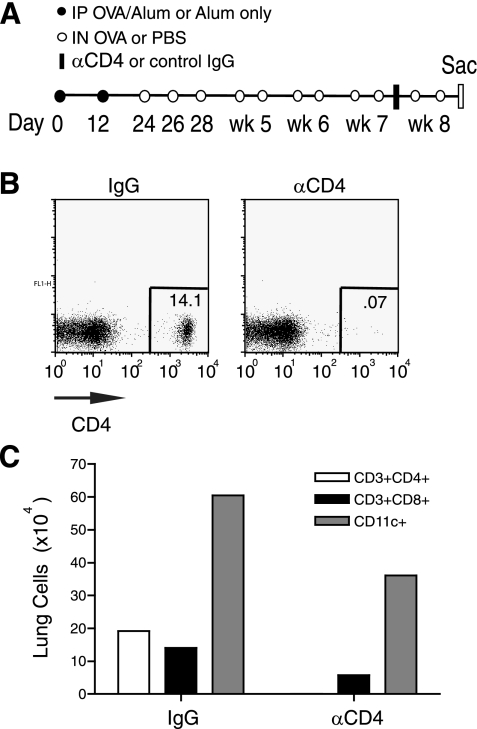
Fig. 3.
Strategy to deplete CD4+ cells several weeks after the initiation of chronic allergen challenge. A: protocol for CD4 depletion during the final week of chronic intranasal challenges. Mice were immunized IP on days 0 and 12 with OVA (filled circles). Intranasal challenges were administered on days 24, 26, and 28 and then repeated twice per week for 4 wk (open circles). Anti-CD4 (GK 1.5) or control IgG was given 3 days before the second to last intranasal challenge (filled bar). B: peripheral blood CD4 depletion. Peripheral blood was taken 1 day before the second to last intranasal challenge and analyzed by FACS for CD4+ cells. C: lungs were processed and analyzed by FACS for CD3+CD4+, CD3+CD8+, or CD11c+ cells. Results in B are 1 representative of 4 mice/group, and pooled lungs from 4 mice/group were analyzed in C; 1 of 2 independent experiments.
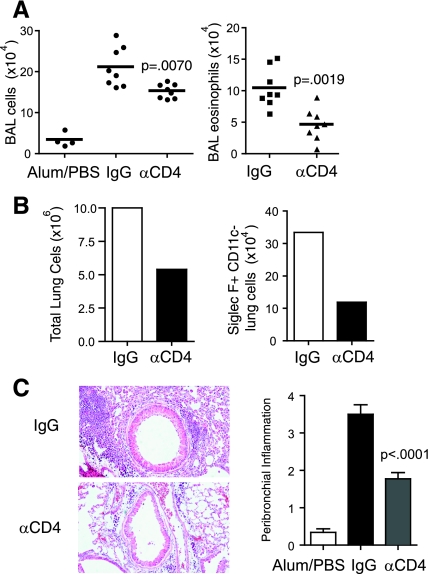
Significantly, mice receiving anti-CD4 showed an ∼50% reduction in total CD11c+ cells and CD8 T cells that accumulated in the lung (Fig. 3C). The number of BAL leukocytes were modestly reduced (P = 0.0070) after 1 wk of CD4 depletion (Fig. 4A). However, these mice had ∼50% fewer BAL eosinophils (P = 0.0019), total lung cells, and peribronchial inflammation as measured by histological scoring (<0.001) (Fig. 4, A–C). Assessment of lung eosinophils by analysis of Siglec-F+CD11c- cells also revealed an ∼60% reduction compared with mice receiving the control antibody. Thus, CD4+ cells continue to play a strong role in controlling the extent of lung inflammation during periods of exposure to airborne antigen, even weeks after the onset of chronic exposure. However, again, they are not absolutely necessary as eosinophilia and influx of some dendritic cells and CD8 T cells can occur in their absence.
Fig. 4.
CD4 depletion several weeks after the initiation of chronic allergen challenge reduces eosinophilic lung inflammation. A: mice undergoing the protocol in Fig. 3A were killed 2 days after the last intranasal antigen challenge. BAL leukocytes (left) and eosinophils (right) were enumerated. B: pooled lung cells were counted (left) and evaluated by FACS for Siglec-F+CD11c- cells. C: H&E-stained lung sections (left) were scored for levels of peribronchial inflammation (right). Results are from 8 mice per OVA group, and 4 mice in the control Alum/PBS group. Pooled lung cells are from 4 mice/group. The Mann-Whitney test was used for P values.
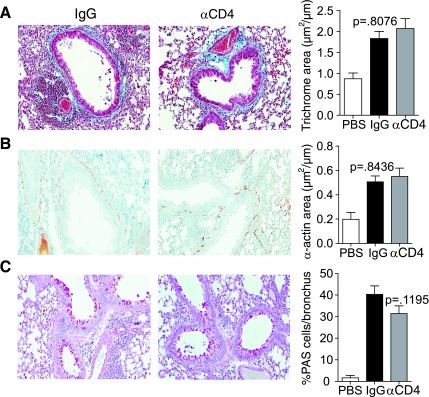
CD4 depletion during chronic allergen challenge does not reduce airway remodeling.
Last, we assessed airway remodeling. Previous studies have shown that remodeling occurs progressively over 4 wk of chronic exposure to antigen following the initial acute lung inflammatory response (6). After repetitive antigen challenge, airway remodeling features were present in control mice that included strong increases in peribronchial subepithelial fibrosis, smooth muscle area, and the number of mucus-producing epithelial cells (Fig. 5). However, most significantly, the extent of fibrosis and smooth muscle mass was not any different between mice receiving the CD4-depleting antibody and the control antibody (P = 0.8076). There was a slight trend toward a reduction in PAS-positive epithelial cells, representing mucus metaplasia, but this did not reach statistical significance (P = 0.1195). Therefore, although CD4+ cells contribute to chronic inflammation, they are not required for progressive airway remodeling that occurs following the acute lung inflammatory response.
Fig. 5.
CD4-depleted mice exhibit normal airway remodeling. Mice undergoing the protocol in Fig. 1A were killed 2 days after the last intranasal challenge, and lung sections were stained for Masson's Trichrome (A), α-smooth muscle actin (B), and PAS (C). A and B: peribronchial regions were analyzed and scored as described in materials and methods. C: percent PAS-positive cells per bronchus were calculated. Results are from 8 mice per OVA group, and 4 mice in the control group that received Alum/PBS but no antigen. Six to eight peribronchial regions per mouse were analyzed and shown as means ± SE (Mann-Whitney).
DISCUSSION
Allergic asthma is characterized by type 2 inflammatory responses in the lung, presumed to be orchestrated by CD4 T cells. Here, we used a chronic Th2-driven airway model of allergic asthma to define the role of CD4+ cells, including CD4 T cells, during the chronic phase of response separable from any action in regulating the acute phase of inflammation. We found that CD4+ cells continue to contribute significantly to eosinophilic lung inflammation after the establishment of acute inflammation, throughout the period of chronic antigen exposure as well as several weeks after the initiation of chronic allergen challenge. Interestingly, the depletion of CD4+ cells in our study was greater than 95% effective, whereas levels of inflammation were ∼50% of control mice. Thus, although CD4+ cells play a substantial role in chronic inflammation, CD4-independent mechanisms that support recruitment and maintenance of inflammatory cells are operable, which might include chemokine and cytokine release from mast cells, eosinophils, and airway epithelium (6, 14, 17, 39). Most significantly, although it is recognized that CD4+ cells are required at an early stage to allow initiation of the airway remodeling process, our data strongly demonstrate that CD4+ cells are not collectively required after an initial acute lung inflammatory response for the structural features of remodeling to develop.
Despite a significant reduction in lung inflammatory infiltrate, we found that components of airway remodeling (mucus metaplasia, subepithelial fibrosis, and smooth muscle increase) were unchanged after CD4 depletion for 4 wk. Previous findings from studies with protocols that have depleted CD4+ cells at the time of the first acute antigen challenge showed a subsequent reduction in both airway remodeling and inflammation (12, 23). Thus, the timing of CD4 depletion is critical to remodeling outcomes, suggesting that CD4+ cells are required for some type of unknown inductive event, but they are not required for progression or maintenance of remodeling. Adding to the significance of this is the fact that CD4 depletion not only removes CD4+ Th2 cells, but it also eliminates the vast majority of iNKT cells that have been described to contribute to defective lung function in acute models of asthma. Many LTi cells are also CD4 positive, and some studies have hypothesized that they might regulate development of tertiary lymphoid structures in inflamed tissue and possibly the maintenance of populations of memory T cells. CD4 depletion will also eliminate the CD4+ subset of dendritic cells, although less than 2% of lung CD4+ cells were CD3 negative (data not shown), suggesting that these dendritic cells represent a minor fraction of the lung infiltrate. However, we found that CD4 depletion strongly results in a reduction in the total numbers of all CD11c+ dendritic cells that accumulate in the lung. Lung dendritic cells expressing CD11c most likely rely on CD4 T cells for homeostasis and proliferation, and signaling through CD40 and the lymphotoxin β-receptor (LTβR) during the interaction of dendritic cells with CD4 T cells may mediate these processes (37, 41). Thus, the range of cell types affected by CD4 depletion is great, but we conclude that these varying populations are not needed at all, or are not needed in great numbers, for the progressive remodeling that occurs over 4 wk of chronic allergen challenge.
We also observed a significant decrease in CD8 T cell lung populations after administration of anti-CD4 for 1 or 4 wk. In different model systems, CD8 T cell responses are known to require CD4 T cell help and may explain this result (2, 18). Interestingly, CD8 T cells have been shown to play a role in airway inflammation and hyperresponsiveness (32, 38) and hence their reduced numbers in the lungs may have contributed to the reduction in inflammation we observed. On the other hand, the remaining CD8 T cells might be required for the continued eosinophilic inflammation that we observed in the absence of CD4+ cells, and this needs to be investigated in future studies depleting this subset of cells.
One last cell type that needs to be considered is the CD4+ regulatory T cell. CD4+CD25+Foxp3+ Tregs generally represent 5–15% of the total CD4+ population in a mouse, and CD4 depletion will eliminate these cells as well. A study in OVA-sensitized rats revealed that CD25+ αβT cells in the tracheal mucosa begin to increase after the first intranasal challenge of OVA and remained elevated after 10 daily challenges (36). These cells were able to suppress OVA-specific T cell proliferation ex vivo. Other reports have shown that depletion of CD4+CD25+ cells before acute airway allergen challenges exacerbated lung eosinophilia, and adoptive transfer of these cells can also significantly protect against developing lung inflammation (26, 29). Not surprisingly, mice deficient in Foxp3 have increased lung inflammation and smooth muscle mass after repetitive antigen challenge (10). Additionally, a study employing adoptive transfer of OVA-specific CD4+CD25+ cells into mice, after acute lung inflammation was established, revealed that these cells could significantly decrease airway inflammation and remodeling brought about by subsequent repetitive airway challenges (21). Interestingly, when these cells were transferred at a later time point after remodeling was established, there was no reversal of remodeling features or inflammation. However, there is little direct data showing that any endogenous Treg cells continue to be active in a more physiological chronic inflammatory response in the lung, and, in particular, during the time period we targeted here after acute inflammation was established. Thus, although previous studies suggest that these cells have the potential to be suppressive in a chronic response, the effect of depleting CD4+ regulatory T cells is unclear in our model system. Given the reduction in measures of airway inflammation after CD4 depletion, it seems likely that the CD4 effector population is playing a larger or more dominant role than CD4 regulatory cells.
Collectively, these results then support a model whereby lung inflammation and airway remodeling become disconnected at a certain point during a chronic asthmatic response. Consistent with this, a recent report demonstrated that IL-13 had persistent proremodeling effects in the lung long after inflammation had resolved (13). Furthermore, in human asthmatics, airway expression of procollagen I and α-smooth muscle actin were found to increase 7 days after allergen challenge when inflammatory cells had returned to baseline (20). Perhaps, after initiation of the remodeling process, which collectively requires CD4 T cells, other CD4+ cells, and eosinophilic inflammation, a number of growth factors and cytokines, including TGF-β1 and VEGF, produced by CD4-negative cell types such as macrophages or activated epithelial cells, are sufficient for maintenance of remodeling as implied from other reports (25, 31). Although our data support an uncoupling of inflammation and remodeling similar to prior observations (13, 20, 27, 31), the mechanisms that lead to such a divergence have yet to be fully elucidated.
In summary, this study demonstrates that depletion of CD4+ cells, after the establishment of acute Th2-driven airway inflammation, has no effect on mucus metaplasia, peribronchial fibrosis, or smooth muscle thickness. However, CD4 cells continue to contribute to eosinophilic inflammation, even at late times after chronic exposure to allergen has occurred. Thus, successful therapies of chronic asthma might need to use combination approaches that target airway remodeling distinct from those that target Th2 cells and inflammation.
GRANTS
This work was supported by National Institutes of Health Grants AI-070535 and CA-91837 (to M. Croft) and AI-007469-14 (to T. A. Doherty).
Acknowledgments
We thank Yanfei Adams and Jae Youn Cho for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Akbari O, Stock P, Meyer EH, Freeman GJ, Sharpe AH, Umetsu DT, DeKruyff RH. ICOS/ICOSL interaction is required for CD4+ invariant NKT cell function and homeostatic survival. J Immunol 180: 5448–5456, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett SR, Carbone FR, Karamalis F, Miller JF, Heath WR. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med 186: 65–70, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borish LC, Nelson HS, Corren J, Bensch G, Busse WW, Whitmore JB, Agosti JM. Efficacy of soluble IL-4 receptor for the treatment of adults with asthma. J Allergy Clin Immunol 107: 963–970, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Boulet LP, Turcotte H, Laviolette M, Naud F, Bernier MC, Martel S, Chakir J. Airway hyperresponsiveness, inflammation, and subepithelial collagen deposition in recently diagnosed versus long-standing mild asthma. Influence of inhaled corticosteroids. Am J Respir Crit Care Med 162: 1308–1313, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Broide DH Immunologic and inflammatory mechanisms that drive asthma progression to remodeling. J Allergy Clin Immunol 121: 560–570; quiz 571–572, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broide DH, Lawrence T, Doherty T, Cho JY, Miller M, McElwain K, McElwain S, Karin M. Allergen-induced peribronchial fibrosis and mucus production mediated by IkappaB kinase beta-dependent genes in airway epithelium. Proc Natl Acad Sci USA 102: 17723–17728, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busse WW, Lemanske RF Jr. Asthma. N Engl J Med 344: 350–362, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Chakir J, Shannon J, Molet S, Fukakusa M, Elias J, Laviolette M, Boulet LP, Hamid Q. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol 111: 1293–1298, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Cho JY, Miller M, Baek KJ, Han JW, Nayar J, Lee SY, McElwain K, McElwain S, Friedman S, Broide DH. Inhibition of airway remodeling in IL-5-deficient mice. J Clin Invest 113: 551–560, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity 29: 114–126, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil's role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med 167: 199–204, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Foster PS, Yang M, Herbert C, Kumar RK. CD4(+) T-lymphocytes regulate airway remodeling and hyper-reactivity in a mouse model of chronic asthma. Lab Invest 82: 455–462, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Fulkerson PC, Fischetti CA, Hassman LM, Nikolaidis NM, Rothenberg ME. Persistent effects induced by IL-13 in the lung. Am J Respir Cell Mol Biol 35: 337–346, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fulkerson PC, Fischetti CA, McBride ML, Hassman LM, Hogan SP, Rothenberg ME. A central regulatory role for eosinophils and the eotaxin/CCR3 axis in chronic experimental allergic airway inflammation. Proc Natl Acad Sci USA 103: 16418–16423, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gavett SH, Chen X, Finkelman F, Wills-Karp M. Depletion of murine CD4+ T lymphocytes prevents antigen-induced airway hyperreactivity and pulmonary eosinophilia. Am J Respir Cell Mol Biol 10: 587–593, 1994. [DOI] [PubMed] [Google Scholar]
- 16.Hogan SP, Koskinen A, Matthaei KI, Young IG, Foster PS. Interleukin-5-producing CD4+ T cells play a pivotal role in aeroallergen-induced eosinophilia, bronchial hyperreactivity, and lung damage in mice. Am J Respir Crit Care Med 157: 210–218, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, Gerard C. A critical role for eosinophils in allergic airways remodeling. Science 305: 1776–1779, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421: 852–856, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Jeffery PK, Godfrey RW, Adelroth E, Nelson F, Rogers A, Johansson SA. Effects of treatment on airway inflammation and thickening of basement membrane reticular collagen in asthma. A quantitative light and electron microscopic study. Am Rev Respir Dis 145: 890–899, 1992. [DOI] [PubMed] [Google Scholar]
- 20.Kariyawasam HH, Aizen M, Barkans J, Robinson DS, Kay AB. Remodeling and airway hyperresponsiveness but not cellular inflammation persist after allergen challenge in asthma. Am J Respir Crit Care Med 175: 896–904, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Kearley J, Robinson DS, Lloyd CM. CD4+CD25+ regulatory T cells reverse established allergic airway inflammation and prevent airway remodeling. J Allergy Clin Immunol 122: 617–624 e616, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim MY, Rossi S, Withers D, McConnell F, Toellner KM, Gaspal F, Jenkinson E, Anderson G, Lane PJ. Heterogeneity of lymphoid tissue inducer cell populations present in embryonic and adult mouse lymphoid tissues. Immunology 124: 166–174, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komai M, Tanaka H, Masuda T, Nagao K, Ishizaki M, Sawada M, Nagai H. Role of Th2 responses in the development of allergen-induced airway remodelling in a murine model of allergic asthma. Br J Pharmacol 138: 912–920, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kon OM, Sihra BS, Compton CH, Leonard TB, Kay AB, Barnes NC. Randomised, dose-ranging, placebo-controlled study of chimeric antibody to CD4 (keliximab) in chronic severe asthma. Lancet 352: 1109–1113, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, Kang MJ, Cohn L, Kim YK, McDonald DM, Elias JA. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med 10: 1095–1103, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leech MD, Benson RA, De Vries A, Fitch PM, Howie SE. Resolution of Der p1-induced allergic airway inflammation is dependent on CD4+CD25+Foxp3+ regulatory cells. J Immunol 179: 7050–7058, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Leigh R, Ellis R, Wattie J, Southam DS, De Hoogh M, Gauldie J, O'Byrne PM, Inman MD. Dysfunction and remodeling of the mouse airway persist after resolution of acute allergen-induced airway inflammation. Am J Respir Cell Mol Biol 27: 526–535, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Leigh R, Southam DS, Ellis R, Wattie JN, Sehmi R, Wan Y, Inman MD. T-cell-mediated inflammation does not contribute to the maintenance of airway dysfunction in mice. J Appl Physiol 97: 2258–2265, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, Sproles AA, Shah JS, Kohl J, Belkaid Y, Wills-Karp M. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J Exp Med 202: 1549–1561, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLellan AD, Kampgen E. Functions of myeloid and lymphoid dendritic cells. Immunol Lett 72: 101–105, 2000. [DOI] [PubMed] [Google Scholar]
- 31.McMillan SJ, Xanthou G, Lloyd CM. Manipulation of allergen-induced airway remodeling by treatment with anti-TGF-beta antibody: effect on the Smad signaling pathway. J Immunol 174: 5774–5780, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Miyahara N, Swanson BJ, Takeda K, Taube C, Miyahara S, Kodama T, Dakhama A, Ott VL, Gelfand EW. Effector CD8+ T cells mediate inflammation and airway hyper-responsiveness. Nat Med 10: 865–869, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Ramos-Barbon D, Presley JF, Hamid QA, Fixman ED, Martin JG. Antigen-specific CD4+ T cells drive airway smooth muscle remodeling in experimental asthma. J Clin Invest 115: 1580–1589, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med 326: 298–304, 1992. [DOI] [PubMed] [Google Scholar]
- 35.Stevens WW, Kim TS, Pujanauski LM, Hao X, Braciale TJ. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J Immunol Methods 327: 63–74, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strickland DH, Stumbles PA, Zosky GR, Subrata LS, Thomas JA, Turner DJ, Sly PD, Holt PG. Reversal of airway hyperresponsiveness by induction of airway mucosal CD4+CD25+ regulatory T cells. J Exp Med 203: 2649–2660, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Summers-DeLuca LE, McCarthy DD, Cosovic B, Ward LA, Lo CC, Scheu S, Pfeffer K, Gommerman JL. Expression of lymphotoxin-alphabeta on antigen-specific T cells is required for DC function. J Exp Med 204: 1071–1081, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wells JW, Cowled CJ, Giorgini A, Kemeny DM, Noble A. Regulation of allergic airway inflammation by class I-restricted allergen presentation and CD8 T-cell infiltration. J Allergy Clin Immunol 119: 226–234, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Yu M, Tsai M, Tam SY, Jones C, Zehnder J, Galli SJ. Mast cells can promote the development of multiple features of chronic asthma in mice. J Clin Invest 116: 1633–1641, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang M, Angata T, Cho JY, Miller M, Broide DH, Varki A. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood 109: 4280–4287, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang R, Lifson JD, Chougnet C. Failure of HIV-exposed CD4+ T cells to activate dendritic cells is reversed by restoration of CD40/CD154 interactions. Blood 107: 1989–1995, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]