Abstract
The innate immune functions of human airways include mucociliary clearance and antimicrobial peptide activity. Both functions may be affected by changes in epithelial ion transport. Interleukin-17A (IL-17A), which has a receptor at the basolateral membrane of airway epithelia, is a T cell cytokine that has been shown to increase mucus secretion and antimicrobial peptide production by human bronchial epithelial (HBE) cells. Furthermore, IL-17A levels are increased in sputum from patients during pulmonary exacerbations of cystic fibrosis. Therefore, we investigated the effects of IL-17A on basal, amiloride-sensitive, and forskolin-stimulated ion transport in mature, well-differentiated HBE cells. Exposure of HBE monolayers to IL-17A for 48 h induced a novel forskolin-stimulated bicarbonate secretion in addition to forskolin-stimulated chloride secretion and resulted in alkalinization of liquid on the mucosal surface of polarized cells. IL-17A-induced bicarbonate secretion was cystic fibrosis transmembrane conductance regulator (CFTR)-dependent, mucosal chloride-dependent, partially Na+-dependent, and sensitive to serosal, but not mucosal, stilbene inhibition. These data suggest that IL-17A modulates epithelial bicarbonate secretion and implicate a mechanism by which airway surface liquid pH changes may be abnormal in cystic fibrosis.
Keywords: cystic fibrosis, airway surface liquid, pH, cystic fibrosis transmembrane conductance regulator, anion exchange
the innate defense of the lung against inhaled pathogens and toxins incorporates both physical and immunological mechanisms. A primary physical innate defense is mucociliary clearance, the trapping of inhaled particles in the airway surface liquid, and propulsion of trapped particles toward the oropharynx by a combination of ciliary beating and cough clearance. Immunological defense mechanisms of the airway epithelium in response to both innate and adaptive immune recognition include the production and secretion of antimicrobial peptides and antimicrobial chemicals such as hydrogen peroxide. Both mucociliary clearance and antimicrobial activity are regulated in part by epithelial ion transport, which may be altered through the impact of cytokines on human bronchial epithelial (HBE) cells (3, 8–10).
Interleukin (IL)-17A is a homodimeric, disulfide-linked cytokine produced predominantly by CD4-positive T cells of the Th17 lineage (14, 25), although it may be produced by other T cell subsets (21). It is a member of a unique cytokine family comprising six members, IL-17A through IL-17F. IL-17A binds preferentially to the IL-17RA, which is expressed in the basolateral membranes of bronchial epithelial cells (23). In the lung, IL-17A plays a critical role in the pulmonary inflammatory response to extracellular, gram-negative organisms such as Klebsiella pneumonia and Pseudomonas aeruginosa (5, 13). In vitro studies have demonstrated that binding of IL-17A to its receptor on HBE cells results in the production of proinflammatory cytokines and chemokines (23), increased mucin production (2), and increased production of antimicrobial peptides (17). These data suggest that IL-17A is a potent mediator of epithelial host defense in the airways. Furthermore, IL-17A levels are elevated in sputum from patients during pulmonary exacerbations of CF and subsequently decrease with intravenous antibiotic therapy (23), suggesting that IL-17A may have a role in the pathophysiology of pulmonary exacerbations of CF.
Because mucociliary clearance, antimicrobial peptide activity, and antimicrobial systems such as reactive oxygen species production are host defense mechanisms that depend on ion transport, we investigated the effects of IL-17A on the basal, amiloride-sensitive, and forskolin-stimulated ion transport properties of mature, well-differentiated HBE cells. Understanding how IL-17A affects epithelial ion transport will provide insights into the physiology of the airways during periods of inflammation and may lead to novel therapies for diseases of chronic airways inflammation such as cystic fibrosis (CF).
MATERIALS AND METHODS
Cell culture.
HBE cells, purchased from Lonza (Walkersville, MD), were cultured according to Gray and colleagues (11). Primary human CF airway cells were obtained from excess pathological tissue remaining after lung transplantation of ΔF508 homozygous CF patients, as previously described (24) under a protocol approved by the University of Pittsburgh Institutional Review Board. P2 HBEs were seeded in plastic T-75 flasks (Costar) and grown in bronchial epithelial cell growth medium (BEGM) (Biowhittaker) supplemented with bovine pituitary extract (52 μg/ml), hydrocortisone (0.5 μg/ml), human recombinant epidermal growth factor (0.5 ng/ml), epinephrine (0.5 μg/ml), transferrin (10 μg/ml), insulin (5 μg/ml), retinoic acid (0.1 μg/ml), triiodothyronine (6.5 μg/ml), gentamicin (50 μg/ml), and amphotericin-B (50 ng/ml). Media were changed every 48 h until cells were 90% confluent. Cells were then passaged and seeded on Transwell permeable inserts (Costar) in differentiation media containing 50% DMEM in BEGM with the same supplements as above but without triiodothyronine and with a final retinoic acid concentration of 50 nM (all-trans retinoic acid; Sigma). Cells were seeded at a density of 3 × 104 per 0.33 cm2. HBEs were maintained submerged for the first 7 days in culture after which time they were exposed to an apical air interface for the remainder of the culture period. Medium was refreshed two to three times each week. At all stages of culture, cells were maintained at 37°C in 5% CO2 in an air incubator. Under these conditions, HBEs formed a well-differentiated mucociliary phenotype with the classical ion transport phenotype associated with this tissue. A total of five tissue donors were used to complete these studies.
Solutions.
The normal bath solution contained (in mM): 120 NaCl, 25 NaHCO3, 3.3 KH2PO4, 0.8 K2HPO4, 1.2 MgCl2, 1.2 CaCl2, and 10 glucose. The pH of this solution was 7.3–7.4 when gassed with a mixture of 95% O2-5% CO2 at 37°C. For Cl−-free solutions, Cl− was replaced with equimolar gluconate. For Na+-free solutions, Na+ was replaced with equimolar N-methyl-d-glucamine. HCO3−-free solutions contained (in mM): 145 NaCl, 3.3 KH2PO4, 0.8 K2HPO4, 1.2 MgCl2, 1.2 CaCl2, 10 HEPES, and 10 glucose; pH 7.4. This solution was bubbled with air rather than O2/CO2. Cl− and HCO3−-free solutions contained (in mM): 145 sodium gluconate, 3.3 KH2PO4, 0.8 K2HPO4, 1.2 magnesium gluconate, 1.2 calcium gluconate, 10 HEPES, and 10 glucose, pH 7.4, and were bubbled with air.
Two different solutions were used for calibration of pH with fluorescence emissions. For calibration of SNARF-free acid (Invitrogen, Carlsbad, CA) the solution contained (in mM): 120 NaCl, 25 sodium gluconate, 5 KCl, 1.2 MgCl2, 1.2 CaCl2, and 20 HEPES at five different pH (6.8, 7.2, 7.6, 8.0, and 8.4). For calibration of SNARF-1 dextran (Invitrogen) and intracellular pH, solutions contained (in mM): 120 KCl, 20 NaCl, 1 CaCl2, 1 MgCl2, 20 HEPES, plus 10 μM nigericin, 10 μM valinomycin, and 10 μM forskolin. pH was adjusted to 6.5, 7, and 8 with NaOH.
Short-circuit current measurements.
Transwell inserts were mounted in a modified, vertical Ussing chamber (Costar), and the monolayers were voltage-clamped continuously to 0 mV after fluid resistance compensation using an automatic voltage clamp (VCC 600; Physiologic Instruments). Short-circuit current (ISC) was digitized at 1 Hz, and data were stored on a computer hard drive using Acquire and Analyze software build 2.2.0 (Physiologic Instruments). Transepithelial resistance (RT) was determined by stepping the clamp potential from 0 to ± 2 mV for 4 s every minute, recording the change in ISC, and calculating RT from Ohm's law (RT = ΔV/ΔI, where V is voltage and I is current).
ISC was allowed to stabilize at the beginning of each experiment and after each drug addition. By convention, an upward deflection in the ISC tracing represents net serosal-to-mucosal anion movement or mucosal-to-serosal cation movement. Mean data are presented as change in ISC from the stable ISC recorded before and after each drug addition. For example, in Fig. 1A, the change in ISC from points A to B represents the change in ISC in response to amiloride. Unless otherwise stated, X-axis labels on bar graphs represent the manipulation responsible for corresponding changes in ISC and are provided in chronological order from left to right.
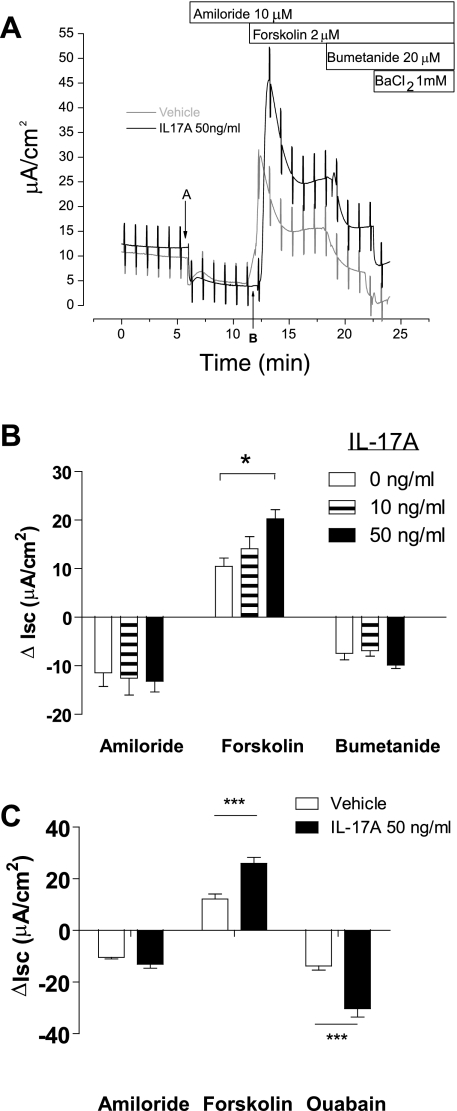
Fig. 1.
Dose-dependent increase in forskolin-stimulated short-circuit current (ISC) with interleukin (IL)-17A treatment. A: representative ISC traces demonstrating changes in ISC after sequential additions of amiloride, forskolin, and bumetanide. Points A and B indicate the times used to measure the change in ISC in response to amiloride. Note that there is enhanced forskolin-stimulated ISC in IL-17A-treated cells and that the additional ISC is not sensitive to bumetanide or barium. B: mean changes in ISC in response to amiloride, forskolin, and bumetanide after incubation of cells with IL-17A in the basolateral medium for 48 h. IL-17A induced a dose-dependent additional forskolin-stimulated ISC (n = 7–8 filters/group, 2 donors). C: IL-17A-induced forskolin-stimulated ISC is sensitive to ouabain (n = 6 filters/group, 1 donor). *P < 0.05 compared with 0 ng/ml. ***P < 0.001 compared with vehicle.
Low-light level, live-cell fluorescence microscopy.
Mature, well-differentiated HBE cells on Transwell supports were placed in a specially designed chamber (Bioptechs, Butler, PA) that allowed for perfusion of the serosal side of the cells. The chamber was mounted on the stage of an inverted fluorescence microscope (Nikon Eclipse TE2000-U) and imaged with a ×20 air objective (numeric aperture 0.75; Nikon S-Fluor). The serosal side of the cells was perfused continuously with normal bath solution that was held in an overhead, heated glass reservoir. Solution in the reservoir was gassed with 95% O2-5% CO2 and fed by gravity through glass tubing to glass heating coils close to the chamber inlet. Basolateral solution changes were made using an automated pinch-valve system (Warner Instruments, Hamden, CT). The entire chamber holding the Transwell insert was covered with a small box with an inlet for gas injection. SNARF dye was excited with light from a xenon arc lamp filtered with a 480/20 nm band-pass filter, and the ratio of fluorescence emissions at 640 and 580 nm (F640/F580) were captured every 10 s using a Hammamatsu ORCA-ER camera and Image Suite software (Perkin Elmer, Waltham, MA). Data were stored on a computer hard drive and subsequently analyzed using Origin 7.5 software (OriginLab, Northampton, MA).
HBE surface pH was measured using two separate protocols. The first protocol was used to measure resting mucosal surface pH based on the protocol of Jayaraman et al. (16). Briefly, SNARF-1 dextran (10,000 mol wt; Invitrogen) was dispersed by sonication in perfluorocarbon (FC-77; Sigma) at 1 mg/ml. SNARF-1 dextran in FC-77 (20 μl) was applied to the mucosal surface of mature, well-differentiated HBE cells. After a 5- to 10-min incubation at 37°C and 5% CO2, filters were mounted on the microscope stage and perfused on the serosal side with HCO3−-buffered solution. The F640-to-F580 (F640/F580) ratio was captured every minute. For calibration, cells were incubated in HEPES-buffered, high-K+ solution (pH 6.5, 7.0, or 7.5) for 2 h at 37°C. Mucosal high-K+ buffer was removed and replaced with 20 μl of 1 mg/ml SNARF-1 dextran in buffer of the same pH. F640/F580 was captured, and its relationship to pH was calculated by linear regression.
The second protocol was designed to measure HCO3− secretion in a small volume of HCO3−-free fluid added to the mucosal surface of polarized cells. The mucosal surface of the cells was submerged in solution containing (in mM): 120 NaCl, 25 sodium gluconate, 5 KCl, 1.2 MgCl2, 1.2 CaCl2, and 1 HEPES, pH 7.37. Amiloride hydrochloride (10 μM) and SNARF-5-free acid (1 μg/ml) (Invitrogen) were added to the solution immediately before its application to the mucosal surface of the cells. After mounting the chamber containing the cells on the microscope stage, the chamber was covered with a small box that was continuously infused with humidified 100% O2. F640/F580 was captured every min for 5 min after which the serosal solution was changed to one containing 2 μM forskolin. Data are reported as means ± SE of the rate of change in pH per min for each group.
To measure intracellular pH, mature HBE cells were loaded with acetoxymethyl ester SNARF-5 (SNARF-5-AM; Invitrogen) for 15–20 min at room temperature in the presence of 1 mg/ml water-soluble probenecid (Invitrogen). Cells were mounted on the microscope stage as described for extracellular pH measurements except the mucosal surface was submerged in 100 μl of normal, HCO3−-buffered solution with 10 μM amiloride. After mounting the chamber containing the cells on the microscope stage, the chamber was covered with a small box that was continuously infused with humidified 95% O2-5% CO2. F640/F580 was captured every minute as described above.
Chemicals.
Recombinant human IL-17A (R&D Systems) was dissolved in PBS and used at the indicated concentrations. Amiloride (Sigma, St. Louis, MO) and BaCl2 (Sigma) were dissolved in distilled, deionized water as 1,000× stocks. Bumetanide (Sigma) was dissolved in ethanol as a 1,000× stock. Forskolin (EMD; Calbiochem, San Diego, CA) was dissolved in dimethyl sulfoxide (DMSO) as a 5,000× stock. CFTRinh-172 (Calbiochem) was dissolved in DMSO as a 1,000× stock. DNDS (Invitrogen, Carlsbad, CA) was dissolved in normal buffer as a 100 mM stock and used at a final concentration of 3 mM. 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid (Invitrogen) was dissolved in DMSO as a 1,000× stock.
Statistical analysis.
Unless otherwise stated, values are means ± SE. Statistical comparisons were made with Prism 4 (GraphPad Software, San Diego, CA). Comparisons between groups were made with either Student's t-test or with two-way ANOVA followed by Bonferroni posttest analysis for significance between groups. Significance was defined as a P value ≤0.05.
RESULTS
A total of 213 HBE filters representing five nondisease donors were used for these studies. Each experiment was performed on cells from one to three donors. At baseline, the mean RT and ISC of untreated filters studied in normal bathing solution were 954 ± 555 Ω·cm2 and 8.6 ± 6.4 μA/cm2, respectively. For filters treated with 50 ng/ml IL-17A, the mean baseline RT and ISC were 755 ± 390 Ω·cm2 and 9.7 ± 5.8 μA/cm2, not different from the untreated control cells. CF cells were obtained from three different tissue samples.
Dose-response studies.
In an initial set of experiments, we examined the effects of increasing doses of IL-17A on basal, amiloride-sensitive, and forskolin-stimulated ion transport in HBE cells. HBE cells were incubated in the presence of 0, 10, or 50 ng/ml IL-17A in the basolateral medium for 48 h. We chose this time based on previous studies that showed effects of cytokines on ion transport in epithelial cells at 48 h (3, 31). Following IL-17A pretreatment, we studied ISC changes in response to sequential addition of amiloride, forskolin, bumetanide, and Ba2+ (Fig. 1A). Compared with vehicle, IL-17A pretreatment did not change baseline or amiloride-sensitive ISC. However, it augmented the magnitude of forskolin-stimulated ISC in a dose-dependent fashion (Fig. 1B), with a maximum effect at 50 ng/ml (20.3 ± 1.9 μA/cm2 for IL-17A vs. 10.5 ± 1.7 μA/cm2 for vehicle, P < 0.01). In contrast, the magnitude of the ISC responses to subsequent bumetanide and Ba2+ additions were not different between IL-17A-treated cells and controls (Fig. 1A). Consequently, the ISC in the IL-17A-treated cells was higher than that in control cells after forskolin stimulation and subsequent exposure to blockers of Cl− secretion. These data suggest that the additional, IL-17A-dependent, cAMP-activated current was not the result of Cl− secretion, because Cl− secretion depends on bumetanide-sensitive NKCC-1 mediated Cl− entry across the basolateral membrane, as well as Ba2+-sensitive basolateral K+ conductance for hyperpolarization of the cell and maintenance of a driving force for Cl− exit (12). Nonetheless, the additional forskolin-stimulated ISC in IL-17A-treated cells was completely inhibited by ouabain (Fig. 1C), suggesting that it depended on the maintenance of transmembrane ion gradients.
Time course studies.
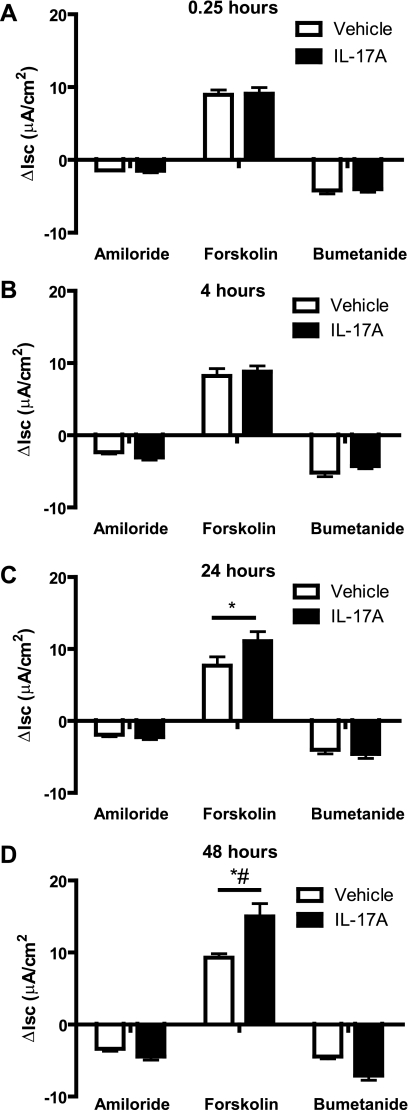
To investigate the time dependence of IL-17A-induced changes in ion transport, IL-17A either was added to the serosal bath in the Ussing chamber 0.25 h before addition of amiloride or was added to cells in differentiation media in the tissue culture incubator for 4, 24, or 48 h. No effects of IL-17A were observed at 0.25 or 4 h (Fig. 2, A and B); whereas additional forskolin-stimulated ISC was observed at 24 h and continued to increase during the subsequent 24 h (P < 0.05; Fig. 2, C and D). The appearance of an effect at 24 h, but not at 4 h, suggests that new protein production likely is required to produce the additional IL-17A-induced, forskolin-stimulated ISC. Because the most robust response to IL-17A was at 48 h at 50 ng/ml, we used these conditions for the remainder of the studies.
Fig. 2.
Time dependence of IL-17A-induced changes in ion transport. Human bronchial epithelial (HBE) cells were incubated with IL-17A (50 ng/ml) in the basolateral medium for 0.25 (A), 4 (B), 24 (C), or 48 (D) h (n = 4–6 filters/group, 2 donors). A significant response to IL-17A treatment was not seen until 24 h, which increased further by 48 h. *P < 0.05 compared with 0.25 h (*) and compared with 24 h (#).
HCO3− inhibition studies.
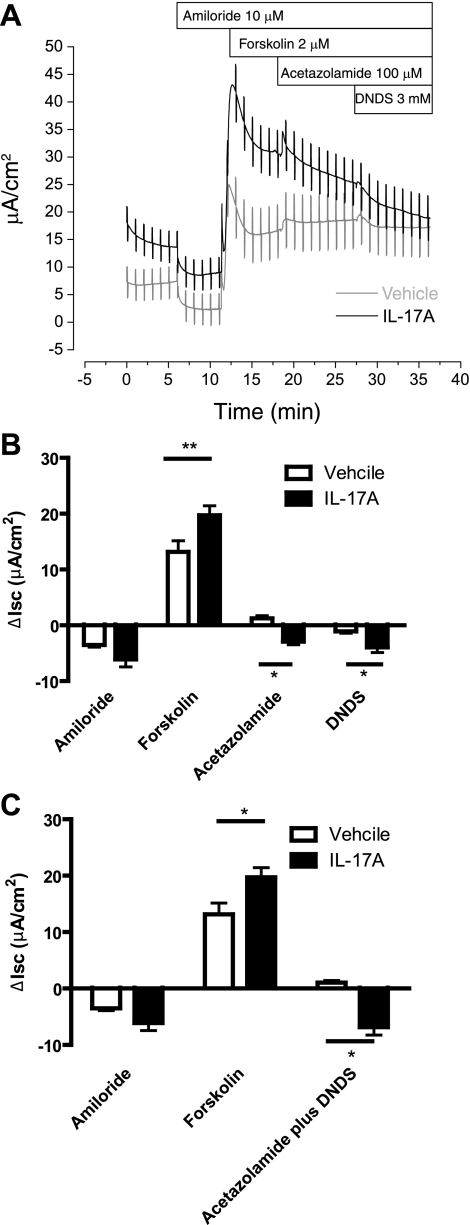
A previous demonstration of cAMP-activated, bumetanide-insensitive HCO3− secretion across human airways (15) suggested that the IL-17A-dependent, bumetanide-insensitive, forskolin-stimulated ISC could be associated with HCO3− secretion. To test this hypothesis, we sequentially exposed amiloride-inhibited, forskolin-stimulated HBE cells to acetazolamide, an inhibitor of carbonic anhydrase, and DNDS, an inhibitor of Na+/HCO3− cotransporters and Cl−/HCO3− exchangers (Fig. 3A). Acetazolamide increased ISC in control cells (P < 0.05 compared with no change by one-way t-test; Fig. 3B) but decreased ISC in IL-17A-treated cells (P < 0.05 when compared with no change or with the increase seen in controls). Serosal addition of DNDS decreased ISC in both controls and IL-17A-treated cells (Fig. 3B, DNDS), but the decrease in IL-17A-treated cells was larger (−1.1 ± 0.3 μA/cm2 for vehicle vs. −3.9 ± 0.9 μA/cm2 for IL-17A, P < 0.05, Mann-Whitney test). Therefore, the total inhibition of ISC by acetazolamide and DNDS was greater in the IL-17A-treated group than in controls (P < 0.05, 2-way ANOVA with Bonferroni posttests; Fig. 3C). Notably, the difference in mean total inhibition of ISC by acetazolamide and DNDS (8 μA/cm2) was close in magnitude to the increase in forskolin-stimulated ISC observed in IL-17A-treated HBE cells (6.5 μA/cm2) (Fig. 3C). These data indicate that the additional forskolin-stimulated ISC in IL-17A-treated cells was mediated by an acetazolamide- and DNDS-sensitive mechanism, consistent with HCO3− secretion. Of note, the IL-17A-dependent ISC was not inhibited by mucosal addition of DIDS, a nonspecific inhibitor of Cl−/HCO3− exchangers (22) (data not shown).
Fig. 3.
IL-17A-induced ISC is eliminated by inhibitors of HCO3− secretion. A: ISC trace demonstrating that IL-17A-induced ISC is inhibited by sequential addition of acetazolamide and DNDS. B: mean changes in ISC with sequential addition of amiloride, forskolin, acetazolamide, and DNDS (n = 7 filters/group, 2 donors). C: mean changes in ISC with acetazolamide and DNDS inhibition summed to demonstrate total inhibition of HCO3− secretion in control cells vs. IL-17A-treated cells. *P < 0.05 and **P < 0.001 compared with vehicle.
Ion substitution studies.
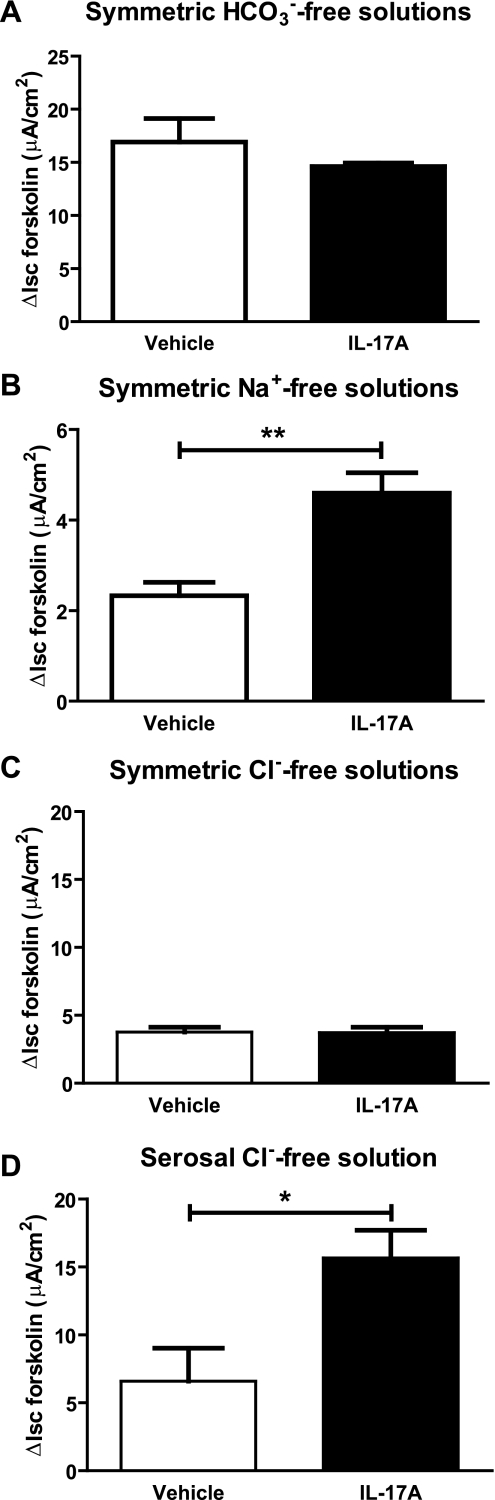
To verify that the additional forskolin-stimulated ISC in IL-17A-treated cells was primarily the result of HCO3− secretion, we performed ISC measurements in HCO3−-free solutions buffered to pH 7.4 with HEPES/NaOH and gassed with air. In these nominally HCO3−-free conditions, there was no difference in forskolin-stimulated ISC between vehicle and IL-17A-treated cells [16.9 ± 2.2 μA/cm2 vs. 14.6 ± 0.3 μA/cm2, respectively, P = not significant (Fig. 4A)]. These results support the hypothesis that IL-17A promotes HCO3− secretion in HBE cells under voltage-clamped conditions. To investigate the Na+ dependence of IL-17A-induced HCO3− secretion, ISC was measured in HBE cells bathed in Na+-free solutions. Under these conditions, both vehicle and IL-17A-treated cells had reduced forskolin-activated ISC. However, IL-17A treated cells had greater ISC than vehicle-treated cells (2.3 ± 0.3 μA/cm2 for vehicle vs. 4.6 ± 0.5 μA/cm2 for IL-17A, P < 0.01, 2-way ANOVA with Bonferroni posttests) (Fig. 4B), suggesting that the IL-17A-induced ISC was composed of both Na+-dependent and Na+-independent mechanisms.
Fig. 4.
IL-17A-induced ISC is dependent on the presence of both HCO3− and Cl−. A: HBE filters were mounted in HCO3−-free solutions buffered with HEPES and gassed with air. In the absence of soluble HCO3−, there was no difference in forskolin-stimulated ISC between non-IL-17A-treated and IL-17A-treated cells (n = 6 filters/group, 1 donor). B: HBE filters were mounted in symmetric Na+-free solutions and treated sequentially with amiloride and forskolin. Data demonstrate mean forskolin-stimulated ISC ± SE, which is significantly greater in IL-17A-treated cells compared with control. Note the different y-axis scale because the total amount of forskolin-stimulated ISC is significantly reduced in Na+-free solutions when compared with normal conditions. C: HBE filters were mounted in symmetric Cl−-free solutions and treated sequentially with amiloride and forskolin. Data represent mean forskolin-stimulated ISC under Cl−-free conditions, which is not different between IL-17A-treated cells and controls (n = 6 filters/group, 2 donors). D: HBE cells were mounted in asymmetric conditions with Cl−-free solution on the serosal side and normal Cl−- and HCO3−-containing solution on the mucosal side (n = 4 filters/group, 2 donors). Removal of serosal Cl− does not inhibit the effect of IL-17A on forskolin-stimulated ISC. *P < 0.05 by unpaired t-test. **P < 0.01 by Mann-Whitney test.
To investigate the Cl− dependence of IL-17A-induced HCO3− secretion, ISC measurements were performed in HBE cells bathed in Cl−-free, HCO3−-containing solutions. Forskolin-stimulated ISC in symmetrical Cl−-free conditions was the same for vehicle and IL-17A-treated cells (3.8 ± 0.4 vs. 3.7 ± 0.4 μA/cm2, respectively) (Fig. 4C). However, when ISC measurements were performed in the absence of serosal Cl− and presence of mucosal Cl−, IL-17A-treated cells had higher forskolin-stimulated ISC (P < 0.05, unpaired t-test with Welch's correction; Fig. 4C). Taken together, these findings suggest that the ISC induced by IL-17A requires the presence of HCO3− and Cl− in the mucosal bath solution.
Chloride addition studies.
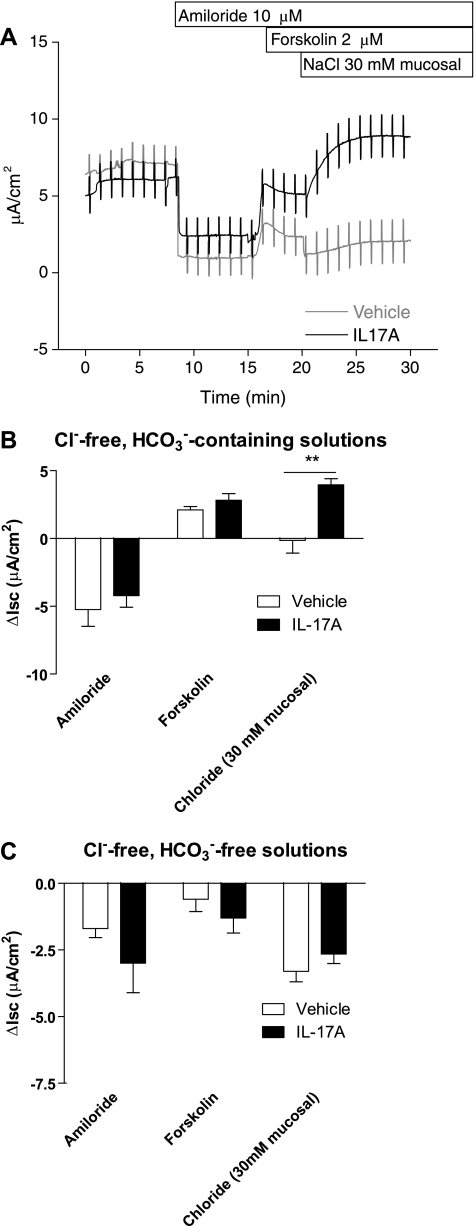
To test the hypothesis that IL-17A promoted Cl−-dependent HCO3− secretion, we performed experiments in which Cl− was added to the mucosal bath as a transport stimulus. In the first set of experiments, HBE cells were mounted in Ussing chambers in symmetrical Cl−-free solutions. After addition of amiloride and forskolin, 30 mM NaCl was added to the mucosal solution, and 30 mM sodium gluconate was added to the serosal solution. Under these conditions, there is a driving force for movement of Cl− across the epithelium from the mucosal to the serosal solution. The predicted negative ISC resulting from mucosal-to-serosal movement of Cl− down its concentration gradient was observed in control cells (−0.15 μA/cm2; Fig. 5A). In IL-17A-treated cells, however, addition of Cl− to the mucosal bath caused a sustained increase in ISC of 4.0 ± 0.4 μA/cm2 (Fig. 5A), different from the change seen in control cells (P < 0.01; Fig. 5B). To test the hypothesis that the ISC observed in IL-17A-treated cells under these conditions was the result of Cl−-dependent HCO3− secretion, we performed the same NaCl addition experiment in the absence of HCO3−. In the absence of HCO3−, both untreated and IL-17A-treated cells responded to the addition Cl− to the mucosal bath with a decrease in ISC (Fig. 5C). Therefore, in the absence of HCO3−, the polarity of the observed ISC changes is consistent with the direction of the imposed Cl− gradient. Taken together, these results strongly support a model of a novel mucosal Cl−-dependent HCO3− secretion in IL-17A-treated HBE cells.
Fig. 5.
IL-17A induces Cl−-dependent HCO3− secretion. HBE cells were mounted in Ussing chambers with Cl−-free solutions on both the serosal and mucosal sides (n = 3 - 6 filters/group, 3 donors). After addition of amiloride and forskolin, 30 mM NaCl was added to the mucosal bath, and 30 mM sodium gluconate was added to the serosal bath. A: representative ISC traces demonstrating the effect of addition of Cl− to the mucosal bath. In the presence of HCO3−, IL-17A-treated cells respond to mucosal Cl− addition with an increase in ISC (solid line). This ISC change in is opposite to the expected ISC change for movement of Cl− down its concentration gradient. B: mean ISC changes for control and IL-17A-treated cells with mucosal Cl− addition. C: mean ISC changes for control and IL-17A-treated cells with mucosal Cl− addition in the absence of HCO3−. In the absence of HCO3−, addition of mucosal Cl− results in a decrease in ISC in both control and IL-17A-treated cells. This ISC change is consistent with the expected ISC generated by movement of Cl− down its concentration gradient. **P < 0.01 by 2-way ANOVA.
Dependence of HCO3− secretion on cystic fibrosis transmembrane conductance regulator.
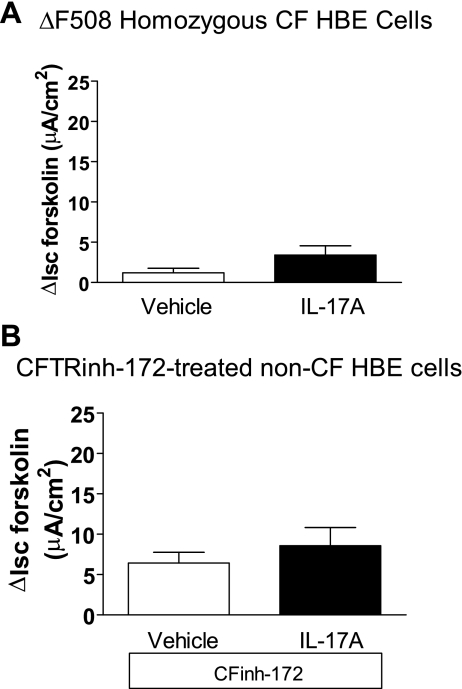
Because the IL-17A-enhanced ISC was dependent on cAMP, we assessed the possible role of cystic fibrosis transmembrane conductance regulator (CFTR) in the IL-17A-induced, forskolin-stimulated ISC. Primary bronchial epithelial cells from CF patients with ΔF508 homozygous genotype were treated with IL-17A and studied using the same drug addition protocols as above. As anticipated, the magnitude of the forskolin-stimulated ISC was much smaller in CF cells than in non-CF cells. Notably, IL-17A was without effect on forskolin-stimulated ISC in CF cells (Fig. 6A). This result demonstrates a requirement for functional CFTR expression in IL-17A-induced Cl−-dependent HCO3− secretion. To test this further, normal HBE cells were treated with the CFTR inhibitor CFTRinh-172 (30 μM for 90 min) before study in the Ussing chamber. CFTRinh-172 pretreatment inhibited forskolin-stimulated ISC in control cells by 55%. Importantly, it eliminated the difference in forskolin-stimulated ISC between controls and IL-17A-treated cells (Fig. 6B). These data further support the idea that the ISC response to IL-17A pretreatment depends on CFTR function.
Fig. 6.
Cystic fibrosis transmembrane conductance regulator (CFTR) dependence of IL-17A-induced HCO3− secretion. A: HBE cells from cystic fibrosis (CF) patients were stimulated with IL-17A at 0 or 50 ng/ml in the basolateral medium for 48 h before study (n = 6 filters/group, 3 donors). We observed very little forskolin-stimulated ISC in either group, and there was no difference between IL-17A-treated cells and controls (P = 0.09). B: normal HBE cells were incubated with vehicle (dimethyl sulfoxide) or CFinh-172 (30 μM) in normal bath solution applied to the apical surface for 90 min before study (n = 5 filters/group, 2 donors). Pretreatment with CFTRinh-172 eliminated the ability of IL-17A to augment forskolin-stimulated ISC.
Low-light level, live-cell fluorescence microscopy.
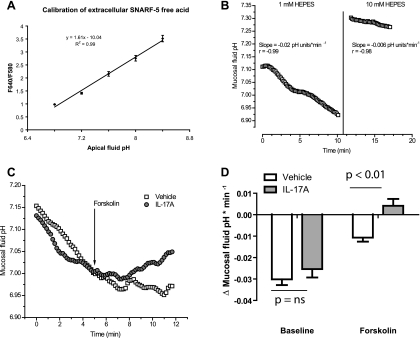
To confirm that the additional forskolin-stimulated ISC seen in IL-17A-treated HBE cells reflected HCO3− secretion, we measured pH of the apical solution bathing control and IL-17A-treated cells using low-light level fluorescence microscopy of the pH indicator dye SNARF-5. The relationship of the F640/F580 emission ratio of SNARF-5-free acid to mucosal fluid pH was found to be linear over the pH range examined (Fig. 7A). To monitor changes in mucosal solution pH over time, we placed 100 μl of a HCO3−-free solution with low buffering capacity on the mucosal surface of the cells while normal buffer was perfused across the serosal surface at a rate of ∼1–2 ml/min. Under these conditions, HBE cells acidified the mucosal solution at a constant rate over a 10-min observation period (Fig. 7B, left). The rate of acidification was attenuated when the mucosal solution was replaced by one with stronger buffering capacity (10 mM HEPES, pH 7.4) (Fig. 7B, right), suggesting that the technique specifically measured H+ equivalent secretion in the mucosal solution.
Fig. 7.
IL-17A augments forskolin-stimulated HCO3− secretion by HBE cells. A: calibration of mucosal SNARF-5-free acid fluorescence ratio [ratio of fluorescence emissions at 640 and 580 nm (F640/F580)] to pH is linear from pH 6.8 to pH 8.4. B: mucosal fluid pH acidifies over time (left), and the rate of acidification can be attenuated by increasing the buffering capacity of the mucosal fluid by increasing HEPES concentration to 10 mM (right). C: representative traces of mucosal fluid pH vs. time in controls and IL-17A-treated cells. D: mean change in rate of mucosal fluid pH change at baseline and after forskolin stimulation (P < 0.01 by Mann-Whitney).
To examine the effects of IL-17A, we compared the rates of mucosal solution pH changes in vehicle-treated and IL-17A-treated cells. For these experiments, we measured a basal rate of acidification during an initial 5-min period before adding 2 μM forskolin to the serosal solution. After 2–3 min to allow for forskolin-mediated activation of cAMP signaling, the rate of pH change was measured over the subsequent 5 min. Representative traces are shown in Fig. 7C. The baseline rate of mucosal acidification was not different between vehicle and IL-17A-treated cells. Both vehicle- and IL-17A-treated cells responded to forskolin stimulation by decreasing the rate of mucosal fluid acidification (P < 0.01 for both vehicle and IL-17A-treated cells). However, whereas vehicle-treated cells continued to acidify the mucosal fluid after forskolin stimulation, IL-17A-treated cells alkalinized the mucosal fluid after forskolin stimulation (P < 0.01 compared with vehicle by Mann-Whitney, n = 6 filters total representing 3 donors; Fig. 7D).
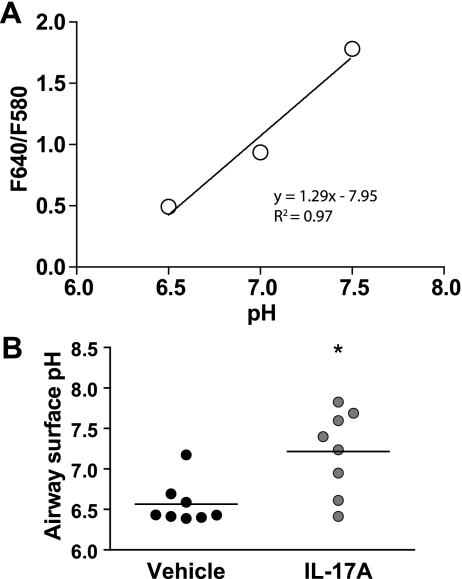
We also examined the effect of IL-17A on resting airway surface pH in vehicle and IL-17A-treated cells using SNARF-labeled dextran dispersed in PFC-77. The application of 20 μl of SNARF-dextran in PFC labeled the mucosal surface of the cells in the absence of additional buffer. The F640-to-F580 ratio of SNARF-1 dextran is linear between pH 6.5 and 7.5 (Fig. 8A). The surface pH of IL-17A-treated cells (7.2 ± 0.2) was more alkaline than that of controls [6.6 ± 0.1, P < 0.05 (Fig. 8B)].
Fig. 8.
IL-17A alkalinizes surface pH in HBE cells. SNARF-1 dextran (1 mg/ml in perfluorocarbon) was added to the mucosal surface of mature HBE cells, and the F640-to-F580 ratio was measured every min. A: calibration of SNARF-1 dextran in aqueous solutions of pH 6.5, 7, and 7.5. B: surface pH measurements from 8 vehicle (open circles) and IL-17A-treated (filled circles) filters representing 3 donors. Note that resting surface pH is more alkaline in IL-17A-treated cells than controls. Horizontal bar represents group mean. *P < 0.05 by Mann-Whitney.
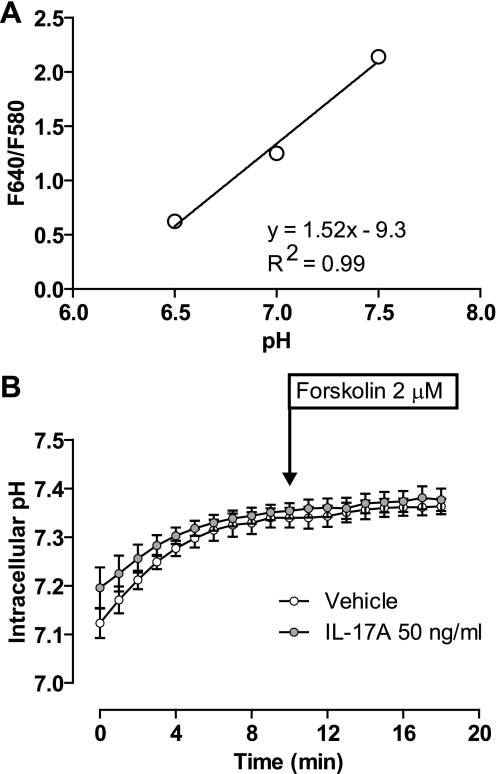
To examine whether IL-17A affected intracellular pH, we loaded cells with SNARF-5-AM. For these experiments, we followed a procedure similar to that described for measurement of mucosal fluid pH except that the mucosal surface of the cells was submerged in 100 μl of HCO3−-buffered solution in the presence of 95% O2-5% CO2, and all solutions contained 1 mM probenicid. The F640-to-F580 ratio of intracellular SNARF-5 is linear between pH 6.5 and 7.5 (Fig. 9A). Both control and IL-17A-treated cells displayed a stable baseline 5–7 min after initiating perfusion, with an intracellular pH of 7.33 ± 0.02 and 7.34 ± 0.02, respectively. After forskolin stimulation, intracellular pH in control and IL-17A-treated cells was 7.36 ± 0.02 and 7.38 ± 0.02, respectively. These results reveal no difference in intracellular pH between control and IL-17A-treated cells.
Fig. 9.
Intracellular pH is not different in IL-17A-treated cells vs. controls. Mature HBE cells were loaded with SNARF-5-AM before mounting on the microscope stage. Cells were overlayed with 100 μl of HCO3−-buffered solution and kept in the presence of 95% O2-5% CO2. Cells were perfused on the serosal side with CO2-gassed HCO3−-buffered solution. F640/F580 was measured every min until stability after which the serosal solution was changed to one containing 2 μM forskolin. A: calibration of intracellular pH using SNARF-5-AM after equilibration in solutions of pH 6.5, 7, and 7.5. B: mean intracellular pH ± SE in controls (open circles) vs. IL-17A-treated cells (filled circles) (n = 8 filters from 3 donors). There is no difference between IL-17A-treated cells and controls in either baseline pH or change in pH with forskolin.
DISCUSSION
Our data demonstrate that IL-17A treatment of mature, well-differentiated HBE cells specifically augments forskolin-stimulated ISC that likely represents a novel HCO3− secretion. The conclusion that the IL-17A-enhanced ISC is the result of HCO3− secretion is supported by the sensitivity of the additional current to inhibitors of HCO3− production and transport, acetazolamide and DNDS (Fig. 3, B and C), and by the absence of the IL-17A-induced current when HCO3−/CO2 were removed from the mucosal and serosal solutions (Fig. 4A). This conclusion also is supported by direct measurements of mucosal fluid pH (Fig. 7D) that demonstrated enhanced forskolin-stimulated secretion of base equivalents in IL-17A-treated cells. By pharmacological and ion substitution experiments, we conclude that this IL-17A-induced HCO3− secretion mechanism is CFTR-dependent, mucosal Cl−-dependent, partially Na+-dependent, and inhibitable by serosal DNDS but not by mucosal DIDS. Accordingly, the HCO3− secretion pathway likely involves HCO3− entry across the basolateral membrane on a stilbene-sensitive Na+-HCO3− cotransporter and possibly an electrogenic Cl−/HCO3− exchanger. HCO3− exit across the apical membrane takes place via a CFTR-dependent mechanism that may represent direct conductance of HCO3− through CFTR or exit of HCO3− on a Cl−/HCO3− exchanger that is dependent on CFTR.
HCO3− can enter epithelial cells across the basolateral membrane via Na+-dependent or Na+-independent mechanisms, or be generated intracellularly through metabolic production via carbonic anhydrase activity (6). HCO3− secretion by HBE cells requires a sufficient electrochemical driving force and a HCO3− exit pathway across the apical membrane. Under the ionic conditions used in the present study, the apical membrane equilibrium potentials for Cl− and HCO3− are approximately −30 and −15 mV, respectively, based on previous studies in Calu-3 and HBE cells (4, 20). Blocking Na+ conductance at the apical membrane with amiloride hyperpolarizes the apical membrane, creating an enhanced driving force for anion exit across the apical membrane (29). With the application of forskolin, anion conductance is activated, and the apical membrane potential is expected to approach the Cl− equilibrium potential (20), remaining sufficiently hyperpolarized to provide a driving force for HCO3− secretion. CFTR may provide an exit pathway for HCO3− in HBE cells, and, therefore, HBE cells can secrete HCO3− in response to forskolin (27).
In IL-17A-treated HBE cells, simultaneous Cl− and HCO3− secretion may occur by permeation of both anions through CFTR. In support, absence of CFTR anion conductance, either in ΔF508 homozygous bronchial epithelial cells (Fig. 6A) or by pharmacological inhibition of CFTR in normal HBE cells (Fig. 6B), abolished the effect of IL-17A on ISC. Alternatively, it is possible that HCO3− is secreted via a non-CFTR pathway in IL-17A-treated HBE cells, but the driving force for HCO3− secretion across the apical membrane is unfavorable in the absence of CFTR. However, this explanation seems unlikely since the presence of CFTR in the apical membrane would be expected to be a depolarizing force on apical membrane potential. Finally, CFTR dependence could reflect IL-17A-induced activation of an anion exchanger that is dependent on CFTR for its activity or expression (19, 28).
In support of the idea that IL-17A activated an anion exchange mechanism, the IL-17A-induced change in ISC was dependent on the presence of both Cl− and HCO3− (Fig. 4). Also, under initially Cl−-free conditions, addition of Cl− to the mucosal bath evoked a HCO3−-dependent increase in ISC in IL-17A-treated HBE cells (Fig. 5). This result is consistent with a model in which Cl− moving down its electrochemical gradient is exchanged at either the apical or basolateral membrane for HCO3− in a stoichiometry of >1 HCO3−:1 Cl−. Together these data suggest the involvement of Cl−/HCO3− exchange as one possible mechanism responsible for IL-17A-induced HCO3− secretion across HBE cells.
IL-17A-enhanced forskolin-stimulated ISC was observed only after >4 h of exposure to IL-17A, suggesting that new protein expression was required. Accordingly, we hypothesize that IL-17A increases the expression, trafficking, or activation of a HCO3− transport pathway. This hypothesis is supported by our measurements of a more alkaline surface pH in IL-17A-treated cells compared with controls (Fig. 8). Moreover, the mucosal surface pH values that we measured are consistent with previous in vitro and in vivo measurements of airway surface pH in normal and inflamed states (reviewed in Ref. 6). Despite enhanced HCO3− secretion in IL-17A-treated cells, intracellular pH was not different in IL-17A-treated cells and controls (Fig. 9B). This result suggests that HCO3− transport at the apical and basolateral membrane is coordinated such that intracellular HCO3− concentration remains constant during enhanced secretion.
Our results do not address the molecular identity of the transport pathway responsible for the effect of IL-17A on ISC. Members of the SLC26 sulfate transporter family function as Cl−/HCO3− exchangers that interact with CFTR (19). Of note, previous studies performed under noninflammatory conditions demonstrated a role for SLC26a3 in HBE cells (28), whereas stimulation of HBE cells with IL-4 was associated with upregulation of SLC26a4 activity (26). Together, these data suggest that airway epithelial cells are capable of responding to external stimuli, including inflammatory cytokines, with alterations in the expression of HCO3− transporters, including Cl−/HCO3− exchangers.
Inflammatory cytokines from both the Th1 and Th2 families can affect the ion transport phenotype of HBE cells. For example, IL-1β increases CFTR activity, expression, and HCO3− concentration in samples of mucosal fluid from HBE cell cultures (10). Interferon-γ, another Th1 cytokine, decreases Na+ absorption and increases UTP-stimulated anion secretion by HBE cells (7). Both IL-4 and IL-13, Th2 cytokines, decrease Na+ absorption and increase anion secretion in HBE cells in response to cAMP and purinergic agonists (3, 9). Here, we demonstrated that IL-17A, a Th17 cytokine, increased forskolin-stimulated HCO3− secretion by HBE cells. Therefore, there may be a common signaling pathway through which these cytokines act in HBE cells to augment both physical and immunological innate defense mechanisms through altered ion transport.
Many physiological processes of airway epithelia are upregulated by IL-17A, including mucin production (2), chemokine elaboration (18, 23), and neutrophil recruitment (30). Because mucin secretion and neutrophil degranulation would be expected to acidify the airway surface, epithelial cells may also increase HCO3− secretion in response to IL-17A to maintain pH homeostasis (10). Therefore, in diseases in which CFTR conductance is impaired, such as CF and possibly chronic obstrucive pulmonary disease (1), the innate defense mechanisms of the airways may be altered by inadequate epithelial responses to inflammatory stimuli.
GRANTS
This work was supported by National Institutes of Health Grants K08 HL-081080 (J. L. Kreindler), R01 HL-079142, (J. K. Kolls), P50 HL-084932 (J. K. Kolls, R. A. Frizzell), P30 DK-72506, and RO1 DK-68196 (R. A. Frizzell) and CFF RDP to the University of Pittsburgh. R. J. Lee was supported by predoctoral fellowships from the National Science Foundation and the Cystic Fibrosis Foundation.
Acknowledgments
We thank Dr. J. Kevin Foskett, Professor of Physiology at the University of Pennsylvania, for help in the preparation of this manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Cantin AM, Hanrahan JW, Bilodeau G, Ellis L, Dupuis A, Liao J, Zielenski J, Durie P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med 173: 1139–1144, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem 278: 17036–17043, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Danahay H, Atherton H, Jones G, Bridges RJ, Poll CT. Interleukin-13 induces a hypersecretory ion transport phenotype in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 282: L226–L236, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Devor DC, Singh AK, Lambert LC, DeLuca A, Frizzell RA, Bridges RJ. Bicarbonate and chloride secretion in Calu-3 human airway epithelial cells. J Gen Physiol 113: 743–760, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubin PJ, Kolls JK. IL-23 mediates inflammatory responses to mucoid Pseudomonas aeruginosa lung infection in mice. Am J Physiol Lung Cell Mol Physiol 292: L519–L528, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer H, Widdicombe JH. Mechanisms of acid and base secretion by the airway epithelium. J Membr Biol 211: 139–150, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galietta LJ, Folli C, Marchetti C, Romano L, Carpani D, Conese M, Zegarra-Moran O. Modification of transepithelial ion transport in human cultured bronchial epithelial cells by interferon-γ. Am J Physiol Lung Cell Mol Physiol 278: L1186–L1194, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Galietta LJV, Folli C, Caci E, Pedemonte N, Taddei A, Ravazzolo R, Zegarra-Moran O. Effect of inflammatory stimuli on airway ion transport. Proc Am Thorac Soc 1: 62–65, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Galietta LJV, Pagesy P, Folli C, Caci E, Romio L, Costes B, Nicolis E, Cabrini G, Goossens M, Ravazzolo R, Zegarra-Moran O. IL-4 is a potent modulator of ion transport in the human bronchial epithelium in vitro. J Immunol 168: 839–845, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Gray T, Coakley R, Hirsh A, Thornton D, Kirkham S, Koo JS, Burch L, Boucher R, Nettesheim P. Regulation of MUC5AC mucin secretion and airway surface liquid metabolism by IL-1beta in human bronchial epithelia. Am J Physiol Lung Cell Mol Physiol 286: L320–L330, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Gray TE, Guzman K, Davis CW, Abdullah LH, Nettesheim P. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol 14: 104–112, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Halm DR, Frizzell RA. Intestinal chloride secretion. In: Textbook of Secretory Diarrhea, edited by Lebenthal E and Duffey M. New York, NY: Raven, 1990, p. 47–58.
- 13.Happel KI, Zheng M, Young E, Quinton LJ, Lockhart E, Ramsay AJ, Shellito JE, Schurr JR, Bagby GJ, Nelson S, Kolls JK. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol 170: 4432–4436, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6: 1123–1132, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Illek B, Yankaskas JR, Machen TE. cAMP and genistein stimulate HCO3− conductance through CFTR in human airway epithelia. Am J Physiol Lung Cell Mol Physiol 272: L752–L761, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Jayaraman S, Song Y, Verkman AS. Airway surface liquid pH in well-differentiated airway epithelial cell cultures and mouse trachea. Am J Physiol Cell Physiol 281: C1504–C1511, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Kao CY, Chen Y, Thai P, Wachi S, Huang F, Kim C, Harper RW, Wu R. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J Immunol 173: 3482–3491, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Kao CY, Huang F, Chen Y, Thai P, Wachi S, Kim C, Tam L, Wu R. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK/NF-kappaB-dependent signaling pathway. J Immunol 175: 6676–6685, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol 6: 343–350, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreindler JL, Jackson AD, Kemp PA, Bridges RJ, Danahay H. Inhibition of chloride secretion in human bronchial epithelial cells by cigarette smoke extract. Am J Physiol Lung Cell Mol Physiol 288: L894–L902, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol 177: 4662–4669, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Lohi H, Lamprecht G, Markovich D, Heil A, Kujala M, Seidler U, Kere J. Isoforms of SLC26A6 mediate anion transport and have functional PDZ interaction domains. Am J Physiol Cell Physiol 284: C769–C779, 2003. [DOI] [PubMed] [Google Scholar]
- 23.McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, Steele C, Finder JD, Pilewski JM, Carreno BM, Goldman SJ, Pirhonen J, Kolls JK. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-α and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol 175: 404–412, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myerburg MM, Butterworth MB, McKenna EE, Peters KW, Frizzell RA, Kleyman TR, Pilewski JM. Airway surface liquid volume regulates ENaC by altering the serine protease-protease inhibitor balance: a mechanism for sodium hyperabsorption in cystic fibrosis. J Biol Chem 281: 27942–27949, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 6: 1133–1141, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedemonte N, Caci E, Sondo E, Caputo A, Rhoden K, Pfeffer U, Di Candia M, Bandettini R, Ravazzolo R, Zegarra-Moran O, Galietta LJV. Thiocyanate transport in resting and IL-4-stimulated human bronchial epithelial cells: role of pendrin and anion channels. J Immunol 178: 5144–5153, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Smith JJ, Welsh MJ. cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. J Clin Invest 89: 1148–1153, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wheat VJ, Shumaker H, Burnham C, Shull GE, Yankaskas JR, Soleimani M. CFTR induces the expression of DRA along with Cl−/HCO3− exchange activity in tracheal epithelial cells. Am J Physiol Cell Physiol 279: C62–C71, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Willumsen NJ, Davis CW, Boucher RC. Intracellular Cl- activity and cellular Cl− pathways in cultured human airway epithelium. Am J Physiol Cell Physiol 256: C1033–C1044, 1989. [DOI] [PubMed] [Google Scholar]
- 30.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 194: 519–528, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zund G, Madara JL, Dzus AL, Awtrey CS, Colgan SP. Interleukin-4 and interleukin-13 differentially regulate epithelial chloride secretion. J Biol Chem 271: 7460–7464, 1996. [DOI] [PubMed] [Google Scholar]