Abstract
BACKGROUND:
Most treatment recommendations for hypertension are based on criteria that consider efficacy, safety and cost. Given the need for long-term use of antihypertensive agents, treatment compliance should also be taken into consideration in the selection process.
OBJECTIVE:
The purpose of the present study was to estimate persistence and adherence to antihypertensive agents in a real-life setting.
METHODS:
Persistence and adherence to treatment were estimated using data from the Regie de l’assurance maladie du Quebec.
RESULTS:
Data from a random sample of 4561 subjects with a diagnosis of hypertension covered by the Regie de l’assurance maladie du Quebec drug plan and using one of the antihypertensive agents reimbursed by the drug plan for the first time between January 2000 and December 2001 were analyzed. The persistence rate observed after a two-year period with diuretics was significantly lower (52.8%) than with any other classes of antihyperten-sive agent (P<0.01). Persistence rates for beta-blockers, calcium channel blockers, angiotensin-II receptor blockers and angiotensin-I converting enzyme inhibitors were 69.3%, 64.3%, 60.9% and 58.9%, respectively. After two years, the proportion of patients who were 80% adherent to their treatment was 64.9% for angiotensin-I converting enzyme inhibitors, 65.0% for angiotensin-II receptor blockers, 64.2% for calcium channel blockers, 60.3% for beta-blockers and 50.9% for diuretics. The proportion of patients who were 80% adherent to their treatment was significantly lower for diuretics than with any other antihypertensive agents (P<0.01).
CONCLUSION:
Persistence and adherence to treatment are essential to treatment success. Results of the present study indicate that, in a real-life setting, patients are significantly less compliant to diuretics than to any other antihypertensive agents.
Keywords: Compliance, Hypertension, Population health
Abstract
HISTORIQUE :
La plupart des recommandations de traitement contre l’hypertension découlent de critères qui tiennent compte de l’efficacité, l’innocuité et du coût. Étant donné la nécessité d’utiliser les antihypertensifs sur une période prolongée, il faut également tenir compte de la compliance au traitement dans le processus de sélection.
OBJECTIF :
La présente étude visait à estimer la persistance et l’observance aux antihypertensifs en situation réelle.
MÉTHODOLOGIE :
Les auteurs ont estimé la persistance et l’observance au traitement d’après les données de la Régie de l’assurance-maladie du Québec.
RÉSULTATS :
Les auteurs ont analysé les données tirées d’un échantillon aléatoire de 4 561 sujets dont l’hypertension était diagnostiquée, qui étaient couverts par la Régie de l’assurance-maladie du Québec et qui utilisaient l’un des antihypertensifs remboursés par le régime d’assurance-maladie entre janvier 2000 et décembre 2001. Le taux de persistance aux diurétiques au bout de deux ans était considérablement plus faible (52,8 %) qu’aux autres catégories d’antihypertensifs (P<0,01). Les taux de persistance aux béta-bloquants, aux inhibiteurs calciques, aux antagonistes de la réception de l’angiotensine II et aux inhibiteurs de l’enzyme de conversion de l’angiotensine I étaient de 69,3 %, de 64,3 %, de 60,9 % et de 58,9 %, respectivement. Au bout de deux ans, la proportion de patients qui adhéraient à 80 % à leur traitement était de 64,9 % dans le cas des inhibiteurs de l’enzyme de conversion de l’angiotensine I, de 65,0 % dans celui des antagonistes de la réception de l’angiotensine II, de 64,2 % dans celui des inhibiteurs calciques, de 60,3 % dans celui des béta-bloquants et de 50,9 % dans celui des diurétiques. La proportion de patients qui adhéraient à 80 % à leur traitement était considérablement plus faible dans le cas des diurétiques que des autres antihypertensifs (P<0,01).
CONCLUSION :
La persistance et l’observance au traitement sont essentielles pour que le traitement fonctionne. Selon les résultats de la présente étude, en situation réelle, les patients sont beaucoup moins compliants aux diurétiques qu’à toute autre forme d’antihypertensif.
Hypertension is a common condition affecting a large proportion of the Canadian population. Between 1986 and 1992, the prevalence of hypertension in Canada was estimated to be 27.4% (31.0% in men and 23.8% in women) (1). Hypertension is an important risk factor of morbidity and premature mortality due to cardiovascular and renal diseases (2,3). It is estimated to be the third leading cause of death, accounting for 6% of deaths worldwide (4,5). It is also the leading cause of visits to physician offices. In 2003, there were 20,274,000 visits made to Canadian office-based physicians as a result of hypertension (6). Because the lifetime risk for developing hypertension in the population is estimated to be 90% (7), the burden of uncontrolled hypertension will grow in parallel with an aging population unless effective treatment strategies are implemented. Unfortunately, hypertension continues to represent a significant public health concern, due to suboptimal prevention and management. A Canadian survey found that among patients with hypertension, only 16% were treated and controlled, 23% were treated but not controlled, 19% were not treated and 42% were unaware they had hypertension (8).
Despite the evidence supporting the efficacy of lifestyle interventions for reducing high blood pressure, most patients require medication for effective blood pressure control. In fact, many hypertensive patients, especially those with additional cardiovascular risk factors, require multiple antihypertensive (AHT) medications to achieve blood pressure treatment goals. Several classes of medication are used to treat hypertension: diuretics, beta-blockers, angiotensin-I converting enzyme (ACE) inhibitors, calcium channel blockers (CCBs) and angiotensin-II receptor blockers (ARBs). The decision regarding which AHT medication physicians select to treat hypertension is facilitated by clinical practice guidelines such as those of the Canadian Hypertension Education Program (9–11). These published recommendations are based on the best scientific evidence from clinical trials. For example, clinical trials such as the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALL-HAT) (12), the Losartan Intervention for Endpoint Reduction (LIFE) (13), the Second Australian National Blood Pressure Study (ANBP2) (14) and the Heart Outcomes Prevention Evaluation Trial (HOPE) (15) have all influenced the development of treatment recommendations and may be reflected in differences in prescribing habits among clinicians. Despite such recommendations, debate at the practice level regarding the choice of individual drugs for the treatment of hypertension is ongoing and could be related to a lack of adherence to guidelines among clinicians.
Results from clinical trials may not be generalizable to the clinical practice due to differences between the two settings (16). For example, the high level of persistence and adherence to medication that is observed in clinical trials of AHT medication is often not observed in clinical practice (17,18). In a recent study (17), persistence to treatment observed in the ALLHAT study was compared with persistence reported in other studies; these authors found that although persistence to therapy was very high in the ALLHAT study and similar between treatments (greater than 90% at one year), real-world studies reported much lower level of persistence (75% or less at one year). This lack of compliance in the real world impacts the effectiveness of AHT treatment. Consequently, treatment recommendations need to be based on both the results of clinical trials and experience in clinical practice.
The objective of the present study was to estimate the prescription choices, persistence and adherence to AHT agents, in the context of clinical practice in Quebec.
METHOD
The present retrospective cohort study was conducted using data from the pharmaceutical services database of the Quebec provincial drug plan (Regie de l’assurance maladie du Quebec [RAMQ]). Since 1977, this insurance program has covered every person aged 65 years or older and the beneficiaries of the social assistance program. In January 1997, those individuals who did not have access to a medication insurance plan became eligible for coverage under the RAMQ program. In 2003, there were a total of 3,155,594 persons covered by the program. The RAMQ pharmaceutical services database includes information from pharmacists’ claims for dispensed medications reimbursed by the program, but not medications received in a hospital.
Data were obtained for a random sample of 150,000 patients, all of whom were enrolled in the RAMQ drug reimbursement program between January 1 and December 31, 2003. Analyses were performed on patients who initiated treatment with AHT medications between January 1, 2000, and December 31, 2001 (index date). Patients were excluded from the analyses if they used AHT treatment during the 12 months preceding the index date. Finally, to be included, patients were required to be covered by the drug plan from the 12-month period preceding the index date of AHT treatment through the 24 months following the index date and should have had a diagnosis of hypertension (International Classification of Diseases – ninth revision codes 401.0 to 405.9)
Level of comorbidities was estimated by calculating a chronic disease score. This chronic disease score is an adaptation of the Von Korff score (19), which was updated to take into account the medications introduced since the initial development of the score. Number and severity of comorbidities were assessed based on the subject’s medication profile according to the subject’s prescription data in 2000. The score excluded medications used for treatment of acute problems (eg, infection) or common symptoms (eg, nasal congestion). Individual medications are assigned to the chronic diseases that medication would treat. The original chronic disease score has been validated as an indicator of comorbidity, and it correlated with future resource utilization. Higher scores indicate higher level of comorbidity.
Analyses of persistence and adherence were performed for each of the following classes of AHTs: diuretics (amiloride, ethacrynic acid, furosemide, hydrochlorothiazide, indepamide, metolazone and spironolactone), beta-blockers (acebutolol, atenolol, bisoprolol, labetalol, metoprolol, nadolol, oxprenolol, pindolol, propranolol, sotalol and timolol), ACE inhibitors (benazepril, captopril, cilazapril, enalapril, fosinopril, lisinopril, perindopril, quinapril, ramipril and trandolapril), CCBs (amlodipine, diltiazem, felodipine, nifedipine and verapamil) and ARBs (candesartan, eprosartan, irbesartan, losartan, telmisartan and valsartan).
Persistence to treatment was estimated at each month over the two-year period by calculating the proportion of patients at each month who had not abandoned their treatment. In some studies (20,21), treatment persistence is defined as nonrenewal of a medication for a period longer than an arbitrary ‘grace’ period, generally 45 to 60 days. With such a definition of persistence, patients who have temporarily ceased their treatment for longer than the grace period would be considered nonpersistent forever, even if after that cessation period, they restart their treatment and continue it for years. In the present study, there was no defined grace period. Patients were considered persistent as long as they had not completely ceased using their AHT. Gaps in medication use during the study period were captured through estimates of the treatment adherence. Persistence to any AHT was also estimated according to initial treatment. With this estimation, patients were considered persistent as long as they were using an AHT, even if they switched to different agents over time.
Adherence to treatment was estimated over the two-year period by a method referred to as the ‘continuous multiple interval measure of medication availability’, which is equivalent to ‘proportion of days covered’ (22). This corresponds to the sum of the days of supplied medication over a defined period, divided by the total days from the beginning to the end of the defined time period. The defined time period to estimate treatment adherence was 730 days (two years), except for cases where the initial AHT agent was discontinued but treatment was continued using a different AHT agent. In these cases, the end of the defined period corresponds to the time when the other AHT started. For example, if over a two-year period a patient only received an AHT for a total of 600 days, his adherence to treatment would be estimated at 82.2% (600/730). For a patient who received 500 days supplied of an initial AHT but after 630 days was switched to a different AHT, his adherence to the initial AHT would be estimated at 79.4% (500/630). For patients who switched to a different AHT, adherence to treatment was only estimated for the initial AHT. A patient was considered adherent to his treatment if the estimated adherence to treatment was 80% or more. Taking medication correctly for at least 80% of the time is the most commonly used definition of treatment adherence found in the literature (23). For each AHT, the proportion of patients who were at least 80% adherent to their treatment was then reported. Finally, the proportion of patients who were both adherent and persistent was estimated according to their initial treatment.
RESULTS
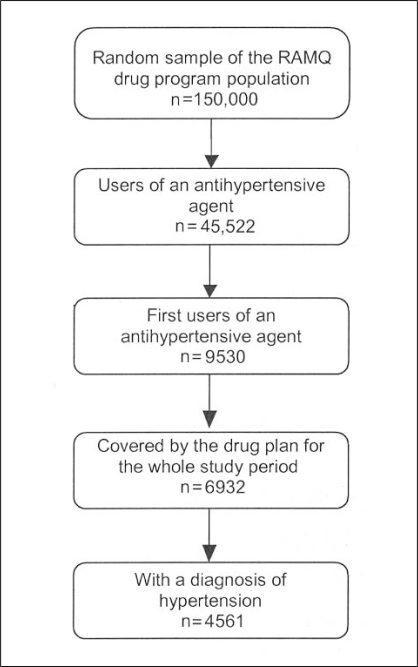
From the random sample of patients, 45,522 had used an AHT agent during the period from January 1, 2000, to December 31, 2001. Of these, 9530 (21%) initiated AHT treatment, and 6932 (73%) of these 9530 patients were covered by the provincial drug plan for the entire study period. Of these, 4561 (66%) had a diagnosis of hypertension (Figure 1).
Figure 1).
Selection of study patients from the Regie de l’assurance maladie du Quebec (RAMQ) database
The mean age of the study population was 68.6 years. Fifty-nine per cent of patients were women. Among the AHT agents, diuretics and ACE inhibitors were the most commonly used, followed by CCBs, beta-blockers and ARBs (Table 1). The proportion of women using diuretics was higher than with other AHT agents (P<0.01) while the proportion of women using CCBs, ACE inhibitors and beta-blockers was lower (P<0.01). Compared with other agents, beta-blockers were used less often by patients older than 60 years of age (P<0.01) but CCBs and diuretics were used more often by these patients (P<0.01). Over the two-year study period, 39% of patients had only used one AHT agent, 32% had used two agents, 19% had used three and 10% had used four or more.
TABLE 1.
Description of study population (n=4561)
| Characteristic | n (%)* |
|---|---|
| Sex | |
| Female | 2792 (61.2) |
| Male | 1769 (38.8) |
| Age groups | |
| <40 years | 97 (2.1) |
| 40 to 59 years | 859 (18.8) |
| 60 to 79 years | 2841 (62.3) |
| ≥80 years | 764 (16.8) |
| Age, years (mean ± SD) | 68.6±12.4 |
| Chronic diseases score (mean ± SD) | 2.9±2.7 |
| Antihypertensive agents† | |
| Calcium channel blockers | 1219 (26.7) |
| Diuretics | 1741 (38.2) |
| Angiotensin-I converting enzyme inhibitors | 1731 (38.0) |
| Angiotensin-II receptor blockers | 962 (21.1) |
| Beta-blockers | 1143 (25.1) |
Data are presented as n (%) unless otherwise specified;
†Total is more than 100% because some patients used more that one agent
The persistence rate observed after a two-year period with diuretics was significantly lower (52.8%) than with any other classes of AHT agent (P<0.001). Persistence rates for beta-blockers, CCBs, ARBs and ACE inhibitors were 69.3%, 64.3%, 60.9% and 58.9%, respectively (Table 2). Persistence rates of dihydropyridine (DHP) CCBs (DHP CCBs: amlodipine, felodipine, nifedipine) was 65.9% compared with 59.5% persistence rates on non-DHP CCBs (non-DHP CCBs: diltiazem, verapamil) (P<0.01). After two years, the proportion of patients who were 80% adherent to their treatment was 65.0% for ARBs, 64.9% for ACE inhibitors, 64.2% for CCBs, 60.3% for beta-blockers and 50.9% for diuretics. The proportion of patients who were 80% adherent to their treatment was significantly lower for diuretics than with any other AHT agents (P<0.01). The proportion of patients who after two years were both adherent and persistent to their initial treatment was significantly lower with diuretics than with nondiuretic AHTs (34.2% versus 46.5%; χ2 P<0.01).
TABLE 2.
Persistence and adherence to antihypertensive agents according to initial treatment
| Initial treatment | Persistence to initial treatment, % | Adherence to initial treatment, % | Persistence and adherence to initial treatment, % | Persistence to any antihypertensive treatment according to initial treatment, % | Persistence and adherence to any antihypertensive treatment according to initial treatment, % |
|---|---|---|---|---|---|
| Calcium channel blockers | 64.3 | 64.2 | 48.3 | 91.7 | 64.0 |
| Diuretics | 52.8 | 50.9 | 34.2 | 88.7 | 50.7 |
| ACE inhibitors | 58.9 | 64.9 | 43.9 | 91.2 | 64.8 |
| ARBs | 60.9 | 65.0 | 44.1 | 92.9 | 64.7 |
| Beta-blockers | 69.3 | 60.3 | 50.6 | 88.6 | 60.1 |
ACE Angiotensin-I converting enzyme; ARBs Angiotensin-II receptor blockers
Adherence was higher in patients in the 60 to 79 year age group. The number of AHT agents used had a negative impact on adherence to treatment. Treatment adherence was lower for patients who had used three agents or more than for those who used one or two agents. Finally, the level of comorbidities estimated with the adapted Von Korff score had no significant impact on treatment adherence.
DISCUSSION
Hypertension represents a significant economic burden, absorbing a large and growing share of health resources. The economic burden of hypertension is complex and related primarily to the consequences of undertreatment (and the resultant cardiovascular outcomes including stroke and coronary artery disease), as well as the cost of medications and physician visits for management. Obviously, much of the economic burden of hypertension could be avoided by adequate control of blood pressure levels (24). Undertreatment of hypertension is due, in part, to the failure to diagnose but also to patients not achieving recommended treatment targets. Failure to achieve target blood pressure may be related to physician prescribing habits, and patient adherence and persistence to their AHT medications. In fact, some studies (25–28) have shown a direct correlation between noncompliance to AHT medications and increased health care expenditure.
It is paradoxical that despite the unequivocal evidence supporting the treatment of hypertension in large clinical trials, there is a failure of treatment in the real-world clinical setting. The artificial setting of the clinical trial ensures standardization of treatment and compliance among the study cohort, yet the transference to the clinical setting is not addressed through any formal implementation guideline. This is not only an issue of the homogeneity of clinical trial cohorts through inclusion and exclusion criteria, but more importantly, a delivery issue including changing physician and patient persistence behaviour. Consequently, to be more applicable in the context of current practice, treatment recommendations and guidelines should consider the results of clinical trials in the context of their implementation in a real life setting. According to large trials, such as ALLHAT, and even more when economic considerations are taken into account, diuretics can be seen as ideal treatment option. But in a real-life setting, poor compliance with diuretics can lead to lack of effectiveness and increases in number of physician visits to adjust the treatment, thus limiting the potential economic benefits of using diuretics.
Treatment compliance to AHT agents has been previously estimated using administrative databases from drug reimbursement programs (18,21,29–31). Estimation of treatment persistence is dependent on the methods used to measure it. In studies using a grace period, the level of persistence is associated with the duration of the grace period. For example, with a grace period of 15 days, McCombs et al (32) found a 5% persistence with diuretics after one year; while using a 45-day grace period, Bloom (33) estimated a 44% persistence to diuretics over the same period. It can also be argued that using the grace period method somehow reflects adherence to treatment more than persistence to treatment. Therefore, differences in methodological approaches make it difficult to make meaningful comparisons across studies.
A recent review by Lopatriello et al (34) pointed out the difficulty in comparing results across different compliance studies. In addition to methodological differences, compliance studies vary in how terms used to describe patterns of patient behaviour toward treatment are defined. That is, compliance, adherence and persistence are defined differently across studies. Finally, studied populations also vary from one study to another. Some studies are performed with newly diagnosed patients or new users of AHT medication, while others are performed with prevalent users.
Patients in the present study were all covered by the provincial drug plan and therefore did not have to pay for their medication, or only partially. Because the cost of treatment can affect patients’ compliance, these results may be different than those for populations that pay for their medications.
The results of the present study are consistent with these previous studies showing that over time, there are a substantial number of patients who abandon their AHT treatment, and that compliance to therapy varies among AHT choices. Furthermore, and in concordance with the present study, other Canadian studies on persistence of AHT agents using the Saskatchewan (35), British Columbia (36) or the Quebec (31) provincial drug database also found a lower persistence or adherence with diuretics compared with other classes of AHT agents.
In the present study, compliance to treatment was measured by two specific dimensions: persistence and adherence. Treatment persistence reflected the duration over which a patient had not ceased their medication and treatment adherence was represented as the degree of prescription filling in a defined period of time. As in many other studies, we estimated adherence to treatment allowing for a 20% deviation from optimal (100%) adherence (37–40). This percentage was arbitrarily selected but was based on the assumption that patients who took at least 80% of their prescribed medication would benefit. Hence, we believe our definition is relevant to clinical practice goals for treatment.
Observational studies are considered to provide a lower level of evidence compared with randomized clinical trials. Observational studies such as this one provide evidence in the context of standard clinical practices but are subject to bias that can only be avoided with a randomized controlled design. For example, it is possible that physicians and/or patients’ characteristics that led to the selection of a specific AHT agent may also be the same characteristics influencing adherence to treatment. There are limitations to the present study, as with any study using administrative claims databases. We assumed that reimbursed medications retrieved from the databases were taken by the patient. Moreover, samples received by the patient from his/her physician could not be taken into account. Additionally, medications received during hospitalization were not included in the database and could have impacted our estimation of adherence. Because of the lack of clinical end points or observation of actual data at the point of care in the database, the clinical impact of compliance was not assessed.
Finally, compliance was estimated for medications used for the treatment of hypertension, although in some cases they could have been used for another indication because some of these medications have other indications. By limiting the study population to only those with a diagnosis of hypertension, this potential bias has been minimized. In some instances, the choice of a given AHT medication could have been made primarily for another indication, but this should have a limited impact on the overall results.
CONCLUSION
Persistence and adherence to treatment are essential for treatment success and may vary substantially across different therapeutic options. This is particularly important in the treatment of hypertension, which is an important risk factor for cardiovascular disease in Canada and is the leading cause for visits to primary care physicians. Results of this study indicate that in a real-life setting, patients are significantly less persistent and adherent to diuretics than to any other AHT agent. Treatment recommendations should not only be based on results of clinical trials, but should also take into consideration treatment performance in real-life setting.
Acknowledgments
This study was supported by Pfizer Canada Inc, Kirkland, Quebec. The choice of the study design was decided by the principal investigator, Jean Lachaine, and data analyses were performed at the Faculty of Pharmacy, University of Montreal and were conducted independently of Pfizer Canada.
Footnotes
COMPETING INTERESTS: Jean Lachaine has received past research funding from the following industrial partners: Pfizer Canada Inc, Merck Frosst Canada Ltd, Novartis Canada Inc, and AstraZeneca Canada Inc. Robert Petrella has received past research funding from the following industrial partners: Pfizer Canada Inc, Solvay Canada, Biovail Canada, AstraZeneca Canada Inc, Servier Canada and Takeda. Elizabeth Merikle and Farzad Ali are employees of Pfizer Canada Inc.
REFERENCES
- 1.Wolf-Maier K, Cooper RS, Banegas JR, et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada and the United States. JAMA. 2003;289:2363–9. doi: 10.1001/jama.289.18.2363. [DOI] [PubMed] [Google Scholar]
- 2.Berenson GS, Srinivasan SR, Bao W, Newman WP, III, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–6. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 3.Thompson DW, Furlan AJ. Clinical epidemiology of stroke. Neurol Clin. 1996;14:309–15. doi: 10.1016/s0733-8619(05)70258-9. [DOI] [PubMed] [Google Scholar]
- 4.Murray CJ, Lopez AD. Evidence based health policy – lessons from the Global Burden of Disease Study. Science. 1996;274:740–3. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- 5.Murray CJ, Lopez AD. Global mortality, disability and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–46. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 6.IMS Health, Canadian Disease and Therapeutic Index (CDTI), 2004
- 7.Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D’Agostino RB, Levy D. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Sludy. JAMA. 2002 Feb 27;287(8):1003–10. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 8.Joffres MR, Ghadirian P, Fodor JG, et al. Awareness, treatment and control of hypertension in Canada. Am J Hypertens. 1997;10:1097–102. doi: 10.1016/s0895-7061(97)00224-0. [DOI] [PubMed] [Google Scholar]
- 9.Hemmelgarn BR, Zarnke KB, Campbell NR, et al. Canadian Hypertension Education Program, Evidence-Based Recommendations Taskforce The 2004 Canadian Hypertension Education Program recommendations for the management of hypertension: Part I. Blood pressure measurement, diagnosis and assessment of risk. Can J Cardiol. 2004;20:31–40. [PubMed] [Google Scholar]
- 10.Khan NA, McAlister FA, Campbell NR, et al. Canadian Hypertension Education Program The 2004 Canadian recommendations for the management of hypertension: Part II-Therapy. Can J Cardiol. 2004;20:41–54. [PubMed] [Google Scholar]
- 11.Touyz RM, Campbell N, Logan A, Gledhill N, Petrella R, Padwal R, Canadian Hypertension Education Program The 2004 Canadian recommendations for the management of hypertension: Part III-Lifestyle modifications to prevent and control hypertension. Can J Cardiol. 2004;20:55–9. [PubMed] [Google Scholar]
- 12.The ALLHAT Collaborative Research Group Major outcomes in high risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–97. doi: 10.1001/jama.288.23.2981. (Errata in 2003;289:178; 2004;291:2196.) [DOI] [PubMed] [Google Scholar]
- 13.Dahlof B, Devereux RB, Kjeldsen SE, et al. for the LIFE study group Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomized trial against atenolol. Lancet. 2001;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 14.Wing LM, Reid CM, Ryan P, et al. Second Australian National Blood Pressure Study (ANBP2). Australian Comparative Outcome Trial of ACE inhibitor- and diuretic-based treatment of hypertension in the elderly. Management Committee on behalf of the High Blood Pressure Research Council of Australia. Clin Exper Hypertens. 1997;19:779–91. doi: 10.3109/10641969709083186. [DOI] [PubMed] [Google Scholar]
- 15.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–53. doi: 10.1056/NEJM200001203420301. (Errata in 20004;342:1376; 2000;342:748.) [DOI] [PubMed] [Google Scholar]
- 16.Birnbaum HG, Cremieux PY, Greenberg PE, LeLorier J, Ostrander JA, Venditti L. Using healthcare claims data for outcomes research and pharmacoeconomic analyses. Pharmacoeconomics. 1999;16:1–8. doi: 10.2165/00019053-199916010-00001. [DOI] [PubMed] [Google Scholar]
- 17.Cardinal H, Monfared AA, Dorais M, Lelorier J. A comparison between persistence to therapy in ALLHAT and in everyday clinical practice: A generalizability issue. Can J Cardiol. 2004;20:417–21. [PubMed] [Google Scholar]
- 18.Caro JJ, Speckman JL, Salas M, Raggio G, Jackson JD. Effect of initial drug choice on persistence with antihypertensive therapy: The importance of actual practice data. CMAJ. 1999;160:41–6. [PMC free article] [PubMed] [Google Scholar]
- 19.Von Korff M, Wagner EH, Saunders K. A chronic disease score from automated pharmacy data. J Clin Epidemiol. 1992;45:197–203. doi: 10.1016/0895-4356(92)90016-g. [DOI] [PubMed] [Google Scholar]
- 20.Bourgault C, Rainville B, Suissa S. Antihypertensive drug therapy in Saskatchewan: Patterns of use and determinants in hypertension. Arch Intern Med. 2001;161:1873–9. doi: 10.1001/archinte.161.15.1873. [DOI] [PubMed] [Google Scholar]
- 21.Sheehy O, LeLorier J. Patterns of amlodipine and felodipine use in an elderly Quebec population. Can J Cardiol. 2000;16:1109–17. [PubMed] [Google Scholar]
- 22.Morningstar BA, Sketris IS, Kephart GC, Sclar DA. Variation in pharmacy prescription refill adherence measures by type of oral antihyperglycaemic drug therapy in seniors in Nova Scotia, Canada. J Clin Pharm Ther. 2002;27:213–20. doi: 10.1046/j.1365-2710.2002.00411.x. [DOI] [PubMed] [Google Scholar]
- 23.Hill MN. Adherence to antihypertensive therapy. In: lzzo JL, Black HR, editors. Hypertension Primer Dallas. 2nd edn. Texas: American Heart Association; 1999. pp. 348–51. [Google Scholar]
- 24.Institute of Health Economics. Hypertension and economic burden of illness in Canada Report submitted to the Laboratory for Disease Control. June 2000.
- 25.Paramore LC, Halpern MT, lapuerta P, et al. Impact of poorly controlled hypertension on healthcare resource utilization and cost. Am J Manag Care. 2001;7:389–98. [PubMed] [Google Scholar]
- 26.Rizzo JA, Simons WR. Variations in compliance among hypertensive patients by drug class: Implications for health care costs. Clin Ther. 1997;19:1446–57. doi: 10.1016/s0149-2918(97)80018-5. [DOI] [PubMed] [Google Scholar]
- 27.McCombs JS, Nichol MB, Newman CM, Sclar DA. The costs of interrupting antihypertensive drug therapy in a Medicaid population. Med Care. 1994;32:214–26. doi: 10.1097/00005650-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Caro JJ, Speckman JL. Existing treatment strategies: Does noncompliance make a difference? J Hypertens Suppl. 1998;16:S31–4. [PubMed] [Google Scholar]
- 29.Marentette MA, Gerth WC, Billings OK, Zarnke KB. Antihypertensive therapy persistence and drug class. Can J Cardiol. 2002;18:649–56. [PubMed] [Google Scholar]
- 30.Caro JJ, Salas M, Speckman JL, Raggio G, Jackson JD. Persistence with treatment for hypertension in actual practice. CMAJ. 1999;160:31–7. [PMC free article] [PubMed] [Google Scholar]
- 31.Perreault S, Lamarre D, Blais L, et al. Persistence with treatment in newly treated middle-aged patients with essential hypertension. Ann Pharmacother. 2005;39:1401–8. doi: 10.1345/aph.1E548. [DOI] [PubMed] [Google Scholar]
- 32.McCombs JS, Nichol MB, Newman CM, Sclar DA. The costs of interrupting antihypertensive drug therapy in a Medicaid population. Med Care. 1994;32:214–26. doi: 10.1097/00005650-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Bloom B. Continuation of initial antihypertensive mediction after 1 year of therapy. Clin Ther. 1998;20:671–81. doi: 10.1016/s0149-2918(98)80130-6. [DOI] [PubMed] [Google Scholar]
- 34.Lopatriello S, Berto P, Cramer J, Bustacchini S, Ruffo P. Different aspects of adherence to antihypertensive treatments. Expert Rev Pharmacoeconomics Outcomes Res. 2004;4:317–33. doi: 10.1586/14737167.4.3.317. [DOI] [PubMed] [Google Scholar]
- 35.Marentette MA, Gerth WC, Billings DK, Zarnke KB. Antihypertensive persistence and drug class. Can J Cardiol. 2002;18:649–56. [PubMed] [Google Scholar]
- 36.Morgan SG, Yan L. Persistence with hypertension treatment among community-dwelling BC seniors. Can J Clin Pharmacal. 2004;11:e267–e273. [PubMed] [Google Scholar]
- 37.Hamilton GA. Measuring adherence in a hypertension clinical trial. Eur J Cardiovasc Nurs. 2003;2:219–28. doi: 10.1016/S1474-5151(03)00058-6. [DOI] [PubMed] [Google Scholar]
- 38.Chapman RH, Benner JS, Petrilla AA, et al. Predictors of adherence with antihypertensive and lipid-lowering therapy. Arch Intern Med. 2005;165:1147–52. doi: 10.1001/archinte.165.10.1147. [DOI] [PubMed] [Google Scholar]
- 39.Wang PS, Benner JS, Glynn RJ, Winkelmayer WC, Mogun H, Avorn J. How well do patients report noncompliance with antihypertensive medications?: A comparison of self-report versus filled prescriptions. Pharmacoepidemiol Drug Saf. 2004;13:11–9. doi: 10.1002/pds.819. [DOI] [PubMed] [Google Scholar]
- 40.Schectman JM, Bovbjerg VE, Voss JO. Predictors of medicationrefill adherence in an indigent rural population. Med Care. 2002;40:1294–300. doi: 10.1097/00005650-200212000-00016. [DOI] [PubMed] [Google Scholar]