Abstract
BACKGROUND:
Changes within skeletal muscle, including augmentation of its capacity to elicit reflex increases in both efferent muscle sympathetic nerve activity (MSNA) and ventilation during work, contribute significantly to exercise intolerance in heart failure (HF). Previously, we demonstrated that peak oxygen uptake (pVO2) in HF relates inversely to MSNA at rest and during exercise.
OBJECTIVE:
To test the hypothesis that there is an independent positive relationship between resting MSNA and the ratio of ventilation to carbon dioxide output during exercise (VE/VCO2) that is augmented in HF.
METHODS:
MSNA at rest and VE/VCO2 during stationary cycling were measured in 30 patients (27 men) with HF (mean ± SD ejection fraction 20±6%) and in 31 age-matched controls (29 men).
RESULTS:
MSNA was higher in HF patients than in controls (51.5±14.3 bursts/min versus 33.0±11.1 bursts/min; P<0.0001). The VE/VCO2 slope was also higher in HF patients than in controls (33.7±5.7 versus 26.0±3.5; P<0.0001), whereas pVO2 was lower in HF patients than in controls (18.6±6.6 versus 31.4±8.4 mL/kg/min; P<0.0001). There were significant relationships between MSNA and VE/VCO2 in both HF (r=0.50; P=0.005) and control subjects (r=0.36; P=0.046). The slope of this regression equation was steeper in HF (0.20 versus 0.11 × MSNA; P=0.001). An analysis of covariance for main effects, including age and pVO2, identified a significant independent relationship between MSNA burst frequency and VE/VCO2 (P=0.013) that differed between HF and controls (P<0.01).
CONCLUSIONS:
The magnitude of resting sympathetic activity correlates positively with the VE/VCO2 slope. Augmentation of this relationship in HF patients is consistent with the concept that enhanced mechanoreceptor reflex activity exaggerates their ventilatory response to exercise.
Keywords: Chronic heart failure, Sympathetic nervous system, Ventilation
Abstract
HISTORIQUE :
Les changements affectant les muscles squelettiques, y compris l’augmentation de la capacité du réflexe thoracique lors de l’activité des nerfs sympathiques musculaires (ANSM) efférents et de la ventilation à l’effort, contribuent significativement à l’intolérance à l’effort en présence d’insuffisance cardiaque (IC). Nous avons déjà démontré que la consommation maximum d’oxygène (pVO2) de l’Hb dans l’IC est inversement proportionnelle à l’ANSM au repos et à l’effort durant l’exercice.
OBJECTIF :
Vérifier l’hypothèse selon laquelle il y a un lien positif indépendant entre l’ANSM au repos et le rapport ventilation:rejet de dioxyde de carbone à l’effort (VE/VCO2), accru en présence d’IC.
MÉTHODES :
L’ANSM au repos et le VE/VCO2 durant l’exercice sur bicyclette stationnaire ont été mesurée chez 30 patients (27 hommes) atteints d’IC (fraction d’éjection, 20 ± 6 % [moyenne ± É.-T.]) et chez 31 témoins assortis selon l’âge (29 hommes).
RÉSULTATS :
L’ANSM a été plus élevée chez les patients atteints d’IC que chez les témoins (51,5 ± 14,3 salves/minute, contre 33,0 ± 11,1 salves/minute; p < 0,0001). La courbe VE/VCO2 a aussi été plus élevée chez les patients atteints d’IC que chez les témoins (33,7 ± 5,7, contre 26,0 ± 3,5; p < 0,0001), tandis que la pVO2 a été plus faible chez les patients atteints d’IC que chez les témoins (18,6 ± 6,6 contre 31,4 ± 8,4 mL/kg/minute; p < 0,0001). On a noté des liens significatifs entre l’ANSM et le VE/VCO2 tant chez les sujets atteints d’IC (r = 0,50, p = 0,005) que chez les témoins (r = 0,36, p = 0,046). La courbe de cette équation de régression a été plus abrupte chez les sujets atteints d’IC (0,20, contre 0,11 × ANSM, p = 0,001). Une analyse de la covariance pour les effets principaux, y compris l’âge et la pVO2 a fait ressortir un lien indépendant et significatif entre la fréquence des salves d’ANSM et le VE/VCO2 (p = 0,013) qui différait entre les sujets atteints d’IC et les témoins (p < 0,01).
CONCLUSIONS :
L’ampleur de l’activité sympathique au repos est en corrélation positive avec la courbe VE/VCO2. L’augmentation de ce lien chez les patients atteints d’IC concorde avec la notion selon laquelle une activité réflexe mécanoréceptrice accrue accentue la réponse ventilatoire à l’exercice.
Keywords: Chronic heart failure, Sympathetic nervous system, Ventilation
Patients with chronic heart failure (HF) complain of breathlessness and fatigue (1). Their degree of exercise intolerance can be assessed by measuring peak oxygen uptake (pVO2) during incremental exercise testing (2). Patients with HF also demonstrate an increased ventilatory response to exercise, ie, a steeper relationship between ventilation (VE) and carbon dioxide output (VCO2), reported as the VE/VCO2 slope (3–5). VE/VCO2 slope in HF correlates inversely with pVO2 (3,4). Both low pVO2 and increased VE/VCO2 slope identify chronic HF patients with more severe HF symptoms and higher mortality rates (6–8).
The ‘muscle hypothesis’ of HF (9–12) proposes that changes within skeletal muscle, including augmentation of its capacity to elicit reflex increases in efferent muscle sympathetic nerve activity (MSNA) and ventilation, when stimulated by work, greatly contribute to exercise intolerance in this condition. Previous work from our laboratory (13) has focused on the efferent sympathoneural limb of this somatic pressor reflex. When the afferent nerve endings in skeletal muscle that are responsive to metabolic products of work (metaboreceptors, primarily type IV fibres) [14]) are stimulated by isometric or isotonic exercise, the reflex increase in MSNA is elicited at a lower workload in HF patients, and its magnitude is augmented (13). This sympathoexcitatory response is sustained when ischemic metabolites generated by exercise are trapped locally following post-handgrip ischemia by a proximal blood pressure cuff inflated to suprasystolic levels. HF patients with the lowest pVO2 exhibit the highest reflex sympathoneural responses to these stimuli (13). Similarly, if passive exercise is used to stimulate the afferent nerve endings that are responsive to stretch (mechanoreceptors, primarily type III fibres [14]) and remove the confounding influence of central command, MSNA increases reflexively in HF patients, but not in control subjects (15). Augmentation of these exercise pressor reflexes in HF could act as a peripheral neurogenic constraint to exercise in this condition.
Our group has previously reported (16,17) higher muscle sympathetic nerve firing rates recording during supine rest in individuals with chronic HF than in age- and sex-matched control subjects, as well as a significant inverse correlation between resting MSNA and pVO2 in HF (18). Although it is known that plasma catecholamines are highest in HF patients with reduced exercise capacity and an augmented ventilatory response to exercise (19,20), we did not explore potential relationships between resting MSNA and VE/VCO2 at the time of these experiments (18). To address this new question, we investigated an additional 13 HF patients and 14 healthy control subjects. The aim of the present investigation was to test three hypotheses: that there is a positive relationship between the magnitude of sympathetic activation at rest, as determined by MSNA, and VE/VCO2 during exercise; that this relationship is independent of the inverse relationship between resting MSNA and pVO2; and that the slope of the MSNA VE/VCO2 relationship is augmented in chronic HF.
PATIENTS AND METHODS
Patients
Thirty patients with HF (27 men) and 31 age-matched control subjects (29 men) were studied. Chronic HF was defined as fatigue or breathlessness on exertion and reduced left ventricular ejection fraction (lower than 40%) on radionuclide ventriculography. Patients with neurological or pulmonary constraints to exercise capacity or inducible ischemia were excluded. Forced expiratory volume in the first second (FEV1) on baseline pulmonary function screening was greater than 80% of that predicted in all subjects. Seventeen patients (57%) were on beta-adrenoceptor blocking drugs (which do not alter resting MSNA when administered long-term to HF patients [21]), 28 (94%) were taking angiotensin-converting enzyme inhibitors, 17 (57%) were taking digitalis and 27 (90%) were taking diuretics. To avoid acute increases in MSNA and blood pressure due to bladder distension (22), loop diuretics (n=27; mean furosemide equivalent dose 84±25 mg) were withheld on the day of the microneurographic testing. Control subjects were recruited through local advertising, none of whom had a cardiac history or were taking any regular medication. Each subject provided informed written consent for these investigations following approval by the Institutional Research Ethics Boards.
Sympathetic nerve recordings
Subjects were studied at rest in the supine position. Blood pressure was monitored from the left arm every minute (Lifestat 200 model; Physio-Control, USA) and heart rate was measured continuously. Multiunit recordings of postganglionic MSNA were obtained with a unipolar tungsten electrode inserted selectively into a muscle nerve fascicle of the right or left peroneal nerve, posterior to the fibular head, using methods previously described (16,17,23). Acceptable recordings met the following four criteria: spontaneous bursts of neural discharge synchronous with the heart rate; no response to arousal stimuli or skin stroking; an increase in nerve burst frequency with apnea; and a signal to noise ratio of 3:1. Signals were recorded onto paper and exported to computer for real-time acquisition by LabVIEW software (National Instruments, USA). Subsequent analysis was performed by custom burst detection software with the capacity for postprocessing visual inspection. Editing of static noise and other artifacts was performed by a trained investigator blinded to the results of the exercise test.
After the setup and a stabilization period of 20 min, subjects lay quietly while a 7 min to 10 min baseline recording was acquired. The average resting heart rate, systolic and diastolic blood pressure and muscle sympathetic burst frequency were then determined.
Exercise testing
Following standard spirometry testing for FEV1 and forced vital capacity, each subject underwent peak exercise testing to exhaustion on a cycle ergometer according to an incremental protocol starting at 17 W and increasing by 17 W every minute until pedal speed could no longer be maintained. VO2, VCO2 and VE were measured continuously during exercise by means of open-circuit spirometry (Horizon MMC System or Vmax Series 229; Sensormedics, USA). Heart rate was monitored by a 12-lead electrocardiogram (Quinton, USA). A respiratory exchange ratio (VCO2/VO2) of 1.1 was taken to indicate maximal effort. The VE/VCO2 slope, which represents the relationship between VE and VCO2, was calculated for each subject by simple regression of data collected throughout exercise (5).
Statistical analysis
Results are reported as mean ± SD in text and tables. Variables were analyzed using a commercially available statistics program (Statview version 5; SAS Institute, USA). Unpaired Student’s t tests were used for comparisons between controls and HF patients, and between patients taking beta-blockers and those not taking beta-blockers. Linear regression was used to establish the slope of the relationship between resting MSNA burst frequency and VE/VCO2 in the two subject groups. Analysis of covariance was used to determine relationships between subject group, MSNA and pVO2, and their interactions with VE/VCO2. Multivariable linear regression with stepwise selection was used to identify the independent predictors of VE/VCO2. Variables tested in this model included age, HF, MSNA and pVO2.
RESULTS
Table 1 describes baseline and exercise variables in patients and control subjects. Mean left ventricular ejection fraction in patients was 20±6%. Thirteen patients had ischemic cardiomyopathy and 17 had dilated cardiomyopathy. Resting MSNA burst frequency and heart rate-adjusted MSNA burst incidence were higher in HF patients. Patients had significantly lower mean pVO2 and a steeper VE/VCO2 slope than controls.
TABLE 1.
Subject characteristics
| Characteristic | Patients (n=30) | Controls (n=31) | P |
|---|---|---|---|
| Age (years) | 50±13 | 46±12 | 0.25 |
| Height (cm) | 177±11 | 177±7 | 0.87 |
| Weight (kg) | 87.4±18.3 | 80.1±14.8 | 0.09 |
| FEV1 (% predicted) | 84±9 | 103±11 | 0.02 |
| Resting heart rate (beats/min) | 74±3 | 65±1 | 0.004 |
| Resting SBP (mmHg) | 125±40 | 119±28 | 0.84 |
| Resting DBP (mmHg) | 75±11 | 71±12 | 0.73 |
| Peak heart rate (beats/min) | 144±27 | 165±19 | 0.001 |
| Peak SBP (mmHg) | 149±41 | 196±26 | <0.0001 |
| Peak DBP (mmHg) | 77±11 | 83±13 | 0.02 |
| pVO2 (mL/min/kg) | 18.6±6.6 | 31.4±8.4 | <0.0001 |
| VE/VCO2 slope | 33.7±5.7 | 26.0±3.5 | <0.0001 |
| Peak VE (L/min) | 66±20 | 82±27 | 0.009 |
| Respiratory exchange ratio | 1.2±0.1 | 1.2±0.1 | 0.66 |
| MSNA (bursts/min) | 51.5±14.3 | 33.0±11.1 | <0.0001 |
| MSNA (bursts/100 beats) | 70.4±17.2 | 51.1±16.1 | <0.0001 |
Values are shown as mean ± SD. DBP Diastolic blood pressure; FEV1 Forced expiratory volume in the first second; SBP Systolic blood pressure; MSNA Muscle sympathetic nerve activity; pVO2 Peak oxygen consumption; VE/VCO2 Ratio of ventilation (VE) to carbon dioxide output
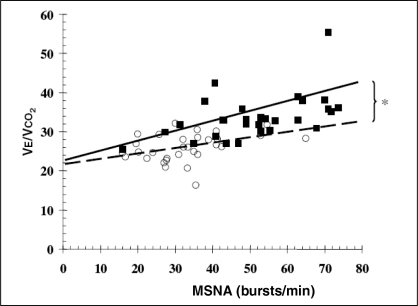
There was a significant positive correlation between MSNA and VE/VCO2 in the group as a whole (r=0.66; P<0.001). Similar significant correlations were present in HF patients (r=0.50; P=0.005) and in healthy control subjects (r=0.36; P=0.046). The slopes and intercepts for the regression equations describing these relationships were VE/VCO2 = 0.20 × MSNA + 23.4 for HF, and VE/VCO2 = 0.11 × MSNA + 22.3 for control subjects, indicating that patients had on average an 82% steeper slope than control subjects (0.20 versus 0.11; P=0.001) (Figure 1). There was no difference in the slope of this relationship between HF patients who were prescribed beta-blockers and those who were not.
Figure 1).
Regression equations (slope + intercept) describing the relationship between muscle sympathetic nerve activity (MSNA) and the slope of the ratio of ventilation to carbon dioxide output (VE/VCO2) in heart failure patients (filled squares and solid trendline) (VE/VCO2 = 0.20 × MSNA + 23.4; P=0.005, r=0.50) and controls (unfilled circles and dashed trendline) (VE/VCO2 = 0.11 × MSNA + 22.2; P=0.046, r=0.36). *P=0.001 for difference between subjects with and without heart failure
There was a significant inverse relationship between pVO2 and VE/VCO2 in HF patients (r=–0.48; P=0.007), but not in control subjects (r=–0.25; P=0.17). Analysis of covariance for main effects, including age and pVO2, identified a significant independent relationship between MSNA burst frequency and VE/VCO2 (P=0.013), which differed between HF and control subjects (P<0.01). Age (P=0.21) and pVO2 (P=0.30) did not contribute to this main effects model within this analysis.
Multivariable linear regression identified three independent predictors of VE/VCO2: MSNA (partial r2 0.435; P<0.001), HF (partial r2 0.1; P=0.001) and age (partial r2 0.025; P=0.08). The equation for this model is: VE/VCO2 = 0.082 (age) + 4.87 (HF; 0=absent, 1=present) + 0.136 (MSNA) + 17.7; r2 = 0.56, P<0.001.
DISCUSSION
Recent interest in the origins of the exaggerated ventilatory response to exercise in HF, particularly the ‘muscle hypothesis’ of exercise hyperventilation (9–12), stimulated the present analysis of relationships between MSNA and the VE/VCO2 slope during exercise. In our previous experiments (13), which examined the efferent sympathetic limb of the exercise pressor reflex, increases in efferent MSNA elicited by isometric or isotonic exercise, or by posthandgrip forearm ischemia were augmented in patients with chronic HF compared with age- and sex- matched control subjects. Increases in MSNA were elicited by a lower threshold of work and were of greater magnitude in HF patients with compromised exercise capacity than in those with relatively preserved exercise capacity (13). Greater muscle metaboreceptor and mechanoreceptor stimulation may also contribute to the augmented ventilatory response to exercise in HF (10–12). In addition, we were the first to describe an inverse relationship between resting MSNA and pVO2 in HF, which is not present in healthy age-matched control subjects (17). The present investigations expanded on this previous series to test the hypothesis that there is an independent positive relationship between resting MSNA and VE/VCO2 that is augmented in HF.
The present investigation yielded three novel observations. First, in healthy middle-aged subjects, there was a significant positive relationship between sympathetic vasoconstrictor tone to muscle vascular beds at rest and the ventilatory response to exercise. Second, in chronic HF, there was a similar direct relationship, which was independent of the concurrent influences of either age, sex or pVO2. This is an important consideration, because both MSNA and VE/VCO2 increase with age in healthy subjects. Age-related increases in MSNA are more pronounced in women (24), and in general, VE/VCO2 is greater in women than in men (25). Third, HF patients exhibited a significantly steeper slope describing the relationship between MSNA and VE/VCO2 than age-matched healthy control subjects, indicating that the ventilatory response to exercise is not simply a function of the magnitude of a subject’s state of sympathetic activation at rest. Rather, this relationship is exaggerated in patients with left ventricular systolic dysfunction due to one or more HF-specific mechanisms.
Several afferent mechanisms may augment this ventilatory response to exercise in chronic HF. One of these includes sensitization or greater stimulation of muscle mechanoreceptors (15) and metaboreceptors (13) during work, perhaps exacerbated by conversion from type I (slow twitch, aerobic) to type II (fast twitch, anaerobic) muscle fibres (26,27). Other mechanisms include increased sensitivity of peripheral and central chemoreceptors (20,28,29), and amplification of signals within the central nervous system originating from these several inputs (30). The relative contribution of central command, an important stimulus to ventilation at the onset of exercise (31–33), diminishes as exercise is sustained (34,35).
Limitations
Limitations to the present analysis and interpretation should be acknowledged. First, the data do not permit definitive conclusions as to the precise afferent mechanism responsible for the exaggerated ventilatory response to exercise in chronic HF. Second, only resting values for MSNA were acquired; future experiments could investigate simultaneous changes in MSNA and VE/VCO2 slope elicited by upper arm or single-leg exercise. We do not consider the maintenance of prescribed HF therapies to be a limitation; a study of HF patients whose chronic homeostasis is perturbed by drug withdrawal would have less clinical relevance. However, because patients were managed with a combination of disease-modifying therapies, these observations might have underestimated the full impact of HF on the MSNA-VE/VCO2 relationship (36). For example, chronic beta-adrenoceptor blockade does not affect resting MSNA in HF (21), but it has been reported to reduce submaximal (37) and peak ventilation (38) during exercise. However, within the HF group, there was no difference between those who were taking taking beta-blockers and those who were not with respect to the regression equation relating MSNA and VE/VCO2.
CONCLUSIONS
HF patients exhibit higher resting skeletal muscle sympathetic nerve discharge, an augmented ventilatory response to exercise and a steeper relationship between MSNA burst frequency and VE/VCO2. Augmentation of this relationship in HF patients is consistent with the concept that enhanced mechanoreceptor reflex activity exaggerates ventilatory response to exercise. Excessive ventilation may further compromise submaximal exercise tolerance by inducing dyspnea. Conversely, exercise training (29), which can attenuate muscle afferent feedback (39), may improve the ventilatory response to exercise.
Footnotes
FUNDING: Supported by an operating grant from the Heart and Stroke Foundation of Ontario (T4938). Dr Witte was supported by grants from Guidant Corp (Canada) and Medtronic (United Kingdom). Dr Floras is a Career Investigator of the Heart and Stroke Foundation of Ontario and holds the Canada Research Chair in Integrative Cardiovascular Biology.
REFERENCES
- 1.Clark AL, Sparrow JL, Coats AJ. Muscle fatigue and dyspnoea in chronic heart failure: Two sides of the same coin? Eur Heart J. 1995;16:49–52. doi: 10.1093/eurheartj/16.1.49. [DOI] [PubMed] [Google Scholar]
- 2.Clark AL, Poole-Wilson PA, Coats AJ. Exercise limitation in chronic heart failure: Central role of the periphery. J Am Coll Cardiol. 1996;28:1092–102. doi: 10.1016/S0735-1097(96)00323-3. [DOI] [PubMed] [Google Scholar]
- 3.Buller NP, Poole-Wilson PA. Mechanism of the increased ventilatory response to exercise in patients with chronic heart failure. Br Heart J. 1990;63:281–3. doi: 10.1136/hrt.63.5.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies SW, Emery TM, Watling MI, Wannamethee G, Lipkin DP. A critical threshold of exercise capacity in the ventilatory response to exercise in heart failure. Br Heart J. 1991;65:179–83. doi: 10.1136/hrt.65.4.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witte KK, Clark AL. Is the elevated slope relating ventilation to carbon dioxide production in chronic heart failure a consequence of slow metabolic gas kinetics? Eur J Heart Fail. 2002;4:469–72. doi: 10.1016/s1388-9842(02)00093-4. [DOI] [PubMed] [Google Scholar]
- 6.Davies LC, Francis DP, Piepoli M, Scott AC, Ponikowski P, Coats AJ. Chronic heart failure in the elderly: Value of cardiopulmonary exercise testing in risk stratification. Heart. 2000;83:147–51. doi: 10.1136/heart.83.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Francis DP, Shamim W, Davies LC, et al. Cardiopulmonary exercise testing for prognosis in chronic heart failure: Continuous and independent prognostic value from VE/VCO2 slope and peak VO2. Eur Heart J. 2000;21:154–61. doi: 10.1053/euhj.1999.1863. [DOI] [PubMed] [Google Scholar]
- 8.Witte KK, Cleland JG, Clark AL. Chronic heart failure, chronotropic incompetence, and the effects of beta blockade. Heart. 2006;92:481–6. doi: 10.1136/hrt.2004.058073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coats AJ. The “muscle hypothesis” of chronic heart failure. J Mol Cell Cardiol. 1996;28:2255–62. doi: 10.1006/jmcc.1996.0218. [DOI] [PubMed] [Google Scholar]
- 10.Piepoli M, Ponikowski P, Clark AL, Banasiak W, Capucci A, Coats AJ. A neural link to explain the “muscle hypothesis” of exercise intolerance in chronic heart failure. Am Heart J. 1999;137:1050–6. doi: 10.1016/s0002-8703(99)70361-3. [DOI] [PubMed] [Google Scholar]
- 11.Piepoli M, Clark AL, Coats AJ. Muscle metaboreceptors in hemodynamic, autonomic, and ventilatory responses to exercise in men. Am J Physiol. 1995;269:H1428–36. doi: 10.1152/ajpheart.1995.269.4.H1428. [DOI] [PubMed] [Google Scholar]
- 12.Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P, Coats AJ. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: Effects of physical training. Circulation. 1996;93:940–52. doi: 10.1161/01.cir.93.5.940. [DOI] [PubMed] [Google Scholar]
- 13.Notarius CF, Atchison DJ, Floras JS. Impact of heart failure and exercise capacity on sympathetic response to handgrip exercise. Am J Physiol Heart Circ Physiol. 2001;280:H969–76. doi: 10.1152/ajpheart.2001.280.3.H969. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res. 2002;12:429–39. doi: 10.1007/s10286-002-0059-1. [DOI] [PubMed] [Google Scholar]
- 15.Middlekauff HR, Chiu J, Hamilton MA, et al. Muscle mechanoreceptor sensitivity in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1937–43. doi: 10.1152/ajpheart.00330.2004. [DOI] [PubMed] [Google Scholar]
- 16.Hara K, Floras JS. After-effects of exercise on haemodynamics and muscle sympathetic nerve activity in young patients with dilated cardiomyopathy. Heart. 1996;75:602–8. doi: 10.1136/hrt.75.6.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Notarius CF, Ando S, Rongen GA, Floras JS. Resting muscle sympathetic nerve activity and peak oxygen uptake in heart failure and normal subjects. Eur Heart J. 1999;20:880–7. doi: 10.1053/euhj.1998.1447. [DOI] [PubMed] [Google Scholar]
- 18.Notarius CF, Azevedo ER, Parker JD, Floras JS. Peak oxygen uptake is not determined by cardiac noradrenaline spillover in heart failure. Eur Heart J. 2002;23:800–5. doi: 10.1053/euhj.2001.2942. [DOI] [PubMed] [Google Scholar]
- 19.Francis GS, Cohn JN, Johnson G, Rector TS, Goldman S, Simon A. Plasma norephinephrine, plasma renin activity and congestive heart failure: Relation to survival and the effects of therapy in V-HeFT II follow-up. Circulation. 1993;87(Suppl VI):40–8. [PubMed] [Google Scholar]
- 20.Kinugawa T, Tomikura Y, Ogino K, et al. Relation between neurohormonal activation and enhanced ventilatory response to exercise in patients with chronic congestive heart failure. Am J Cardiol. 2002;89:604–7. doi: 10.1016/s0002-9149(01)02306-2. [DOI] [PubMed] [Google Scholar]
- 21.Azevedo ER, Kubo T, Mak S, et al. Nonselective versus selective beta-adrenergic receptor blockade in congestive heart failure: Differential effects on sympathetic activity. Circulation. 2001;104:2194–9. doi: 10.1161/hc4301.098282. [DOI] [PubMed] [Google Scholar]
- 22.Fagius J, Karhuvaara S. Sympathetic activity and blood pressure increases with bladder distension in humans. Hypertension. 1989;14:511–7. doi: 10.1161/01.hyp.14.5.511. [DOI] [PubMed] [Google Scholar]
- 23.Ando S, Dajani HR, Floras JS. Frequency domain characteristics of muscle sympathetic nerve activity in heart failure and healthy humans. Am J Physiol Reg Integrative Comp Physiol. 1997;273(1 Pt 2):R205–12. doi: 10.1152/ajpregu.1997.273.1.R205. [DOI] [PubMed] [Google Scholar]
- 24.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension. 2005;45:522–5. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]
- 25.Habedank D, Reindl I, Vietzke G, et al. Ventilatory efficiency and exercise tolerance in 101 healthy volunteers. Eur J Appl Physiol Occup Physiol. 1998;77:421–6. doi: 10.1007/s004210050354. [DOI] [PubMed] [Google Scholar]
- 26.Asmussen E, Nielsen M. Experiments on nervous factors controlling respiration and circulation during exercise employing blocking of the blood flow. Acta Physiol Scand. 1964;60:103–11. doi: 10.1111/j.1748-1716.1964.tb02873.x. [DOI] [PubMed] [Google Scholar]
- 27.Hambrecht R, Fiehn E, Yu J, et al. Effects of endurance training on mitochondrial ultrastructure and fiber type distribution in skeletal muscle of patients with stable chronic heart failure. J Am Coll Cardiol. 1997;29:1067–73. doi: 10.1016/s0735-1097(97)00015-6. [DOI] [PubMed] [Google Scholar]
- 28.Griffiths TL, Henson LC, Whipp BJ. Influence of inspired oxygen concentration on the dynamics of the exercise hyperpnoea in man. J Physiol. 1986;380:387–403. doi: 10.1113/jphysiol.1986.sp016292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ueno H, Asanoi H, Yamada K, et al. Attenuated respiratory modulation of chemoreflex-mediated sympathoexcitation in patients with chronic heart failure. J Card Fail. 2004;10:236–43. doi: 10.1016/j.cardfail.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Yamada K, Asanoi H, Ueno H, et al. Role of central sympathoexcitation in enhanced hypercapnic chemosensitivity in patients with heart failure. Am Heart J. 2004;148:964–70. doi: 10.1016/j.ahj.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 31.Krogh A, Lindhard J. The regulation of respiration and circulation during the initial stages of muscular work. J Physiol. 1913;42:112–36. doi: 10.1113/jphysiol.1913.sp001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strange S, Secher NH, Pawelczyk JA, et al. Neural control of cardiovascular responses and of ventilation during dynamic exercise in man. J Physiol. 1993;470:693–704. doi: 10.1113/jphysiol.1993.sp019883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spengler CM, von Ow D, Boutellier U. The role of central command in ventilatory control during static exercise. Eur J Appl Physiol Occup Physiol. 1994;68:162–9. doi: 10.1007/BF00244030. [DOI] [PubMed] [Google Scholar]
- 34.Adams L, Frankel H, Garlick J, Guz A, Murphy K, Semple SJ. The role of spinal cord transmission in the ventilatory response to exercise in man. J Physiol. 1984;355:85–97. doi: 10.1113/jphysiol.1984.sp015408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brice AG, Forster HV, Pan LG, et al. Is the hyperpnea of muscular contractions critically dependent on spinal afferents? J Appl Physiol. 1988;64:226–33. doi: 10.1152/jappl.1988.64.1.226. [DOI] [PubMed] [Google Scholar]
- 36.Reindl I, Kleber FX. Exertional hyperpnea in patients with chronic heart failure is a reversible cause of exercise intolerance. Basic Res Cardiol. 1996;91(Suppl 1):37–43. doi: 10.1007/BF00810522. [DOI] [PubMed] [Google Scholar]
- 37.Witte KK, Thackray SD, Nikitin NP, Cleland JG, Clark AL. The effects of alpha and beta blockade on ventilatory responses to exercise in chronic heart failure. Heart. 2003;89:1169–73. doi: 10.1136/heart.89.10.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Witte KK, Thackray S, Nikitin N, Cleland JGF, Clark AL. The effects of long-term beta-blockade on the ventilatory responses to exercise in chronic heart failure. Eur J Heart Fail. 2005;7:612–7. doi: 10.1016/j.ejheart.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Fisher JP, White MJ. Muscle afferent contributions to the cardiovascular response to isometric exercise. Exp Physiol. 2004;89:639–46. doi: 10.1113/expphysiol.2004.028639. [DOI] [PubMed] [Google Scholar]