Abstract
OBJECTIVE:
To examine the association of nonpain symptoms in men and women with exercise-related silent ischemia, as well as the independence of these findings from other clinical factors.
METHODS:
A prospective study of 482 women and 425 men (mean age 58 years) undergoing exercise stress testing with myocardial perfusion imaging. Analyses were performed on 60 women and 155 men with no angina but medical perfusion imaging evidence of ischemia during exercise.
MEASURES:
The presence of various non-pain-related symptoms. Ischemia is indicated by myocardial perfusion defects on exercise stress testing with single photon emission computed tomography.
RESULTS:
Women reported more nonangina symptoms than men (P<0.05). They experienced fatigue, hot flushes, tense muscles, shortness of breath and headaches more frequently (P<0.05). Symptoms relating to muscle tension and diaphoresis were associated with ischemia after controlling for pertinent clinical covariates. However, the direction of association differed according to sex and history of coronary artery disease events or procedures. Sensitivity of the detection models showed modest improvements with the addition of these symptoms.
CONCLUSIONS:
While patients who experience silent ischemia experience a number of nonpain symptoms, those symptoms may not be sufficiently specific to ischemia, nor sensitive in detecting ischemia, to be of particular help to physicians in the absence of other clinical information.
Keywords: Coronary artery disease, Sex, Silent ischemia, Symptoms
Abstract
OBJECTIF :
Examiner l’association des symptômes non douloureux chez les hommes et les femmes à l’ischémie silencieuse reliée à l’exercice, ainsi que l’indépendance de ces observations par rapport à d’autres facteurs cliniques.
MÉTHODOLOGIE :
Une étude prospective de 482 femmes et 425 hommes (âge moyen de 58 ans) subissant une épreuve à l’effort avec imagerie par perfusion myocardique. Les auteurs ont analysé 60 femmes et 155 hommes ne faisant pas d’angine mais démontrant une ischémie à l’effort d’après l’imagerie par perfusion médicale.
MESURES :
Présence de divers symptômes non douloureux. L’ischémie est indiquée par des anomalies de la perfusion myocardique pendant l’épreuve à l’effort avec tomographie d’émission monophotonique.
RÉSULTATS :
Les femmes ont déclaré plus de symptômes non angineux que les hommes (P<0,05). Elles ressentaient plus de fatigue, de bouffées de chaleur, de tensions musculaires, d’essoufflements et de céphalées (P<0,05). Les symptômes reliés aux tensions musculaires et à la diaphorèse s’associaient à l’ischémie après le contrôle des covariables cliniques pertinentes. Cependant, la direction de l’association différait selon le sexe et les antécédents d’événements ou d’interventions coronaropathiques. La sensibilité aux modèles de détection a révélé de modestes améliorations avec l’ajout de ces symptômes.
CONCLUSIONS :
Les patients qui souffrent d’ischémie ont des symptômes non douloureux, mais qui ne sont peut-être ni assez caractéristiques de l’ischémie ni assez sensibles à la déceler pour être utiles aux médecins en l’absence d’autre information clinique.
At a symptom level, ischemia is generally associated with angina pain. However, ischemic episodes are asymptomatic (silent) in as many as 80% of cases (1–3). Myocardial infarctions can also be silent in up to 68% of cases (1,4). Several studies have associated silent ischemia (SI) with an adverse prognosis, particularly in high-risk individuals (5–22). For example, patients experiencing SI during daily activities were four to 16 times more likely (in patients with a history of coronary artery disease [CAD]) to suffer from various fatal and non-fatal cardiac events than patients without SI (12).
Whether this outcome is related to the disease state or results from behavioural factors, such as delay in seeking medical attention, is not clear. It is possible that a lack of pain or recognition may reduce motivation to engage in preventive behaviours (19), decrease compliance with medical treatment or interfere with appropriate self-regulatory behaviours (eg, cessation of physical activity) during ischemic episodes. A lack of pain markedly contributes to the underdiagnosis of CAD in the general population, because medical tests and treatments are most frequently performed in patients complaining of angina (16), despite the fact that chest pain appears to be marker of ischemia with relatively low specificity (11,23).
It is possible that patients with SI experience subtler symptoms that can be useful in alerting physicians to the possibility of their CAD risk. However, Pennebaker and Watson (24) demonstrated that people attend to sensations that fit their expectations of a disease state. Thus, if a particular symptom profile does not correspond to a patient’s model of ischemia or CAD, the patient may disregard it and not report it. This is particularly true if the sensation reflects a normal bodily process or state. In support of this, Barsky et al (25) discovered a history of mild, subtle symptoms during careful clinical questioning of patients who had suffered from silent myocardial infarctions –symptoms that patients had dismissed as being irrelevant.
We conducted a prospective correlational investigation of the symptoms of men and women experiencing ischemia during exercise stress testing with nuclear imaging. A standardized approach to the measurement of pain and nonpain symptoms was used. We anticipated that patients with SI experience nonpain symptoms and that, based on findings in patients with angina (26–28), men and women differ in the frequency in which these symptoms are reported. Finally, we explored whether patients with and without ischemia can be distinguished from each other on the basis of these nonpain symptoms after controlling for pertinent clinical and psychosocial variables.
PATIENTS AND METHODS
The research protocol was accepted by the hospital scientific and ethics committees. Written, informed consent was obtained from all participants.
Patients
Four hundred twenty-five men and 482 women were recruited who were referred for exercise stress assessment using myocardial perfusion imaging (MPI) with technetium-99m-sestamibi at a tertiary hospital specializing in cardiology between 2000 and 2003.
Exclusion criteria included inability to speak or read French or English, age younger than 18 years, inpatient status, documented cardiac event or procedure (eg, angioplasty) in the previous two months, another significant and incapacitating health problem (eg, cancer), substance abuse and cognitive deficit.
Fifty-five men and 58 women refused to participate because they lacked time (n=12), were not interested (n=38), were too tired, stressed or uncomfortable (n=10), or for no precise reason (n=53).Age could be obtained for 52 of them (Xage= 60 years).
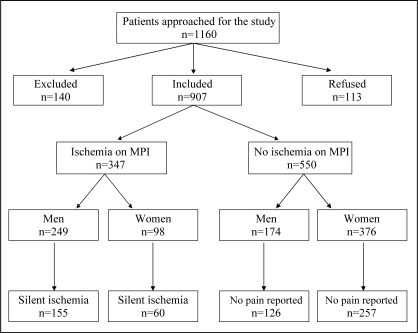
For the present manuscript, only patients who did not report angina pain on exercise testing were retained for analyses. Figure 1 shows the flow of patients from recruitment efforts to diagnosis of ischemia using MPI.
Figure 1).
Flow of patients from recruitment to diagnosis of ischemic status. Note that ischemia status could not be determined by myocardial perfusion imaging (MPI) for 10 of the patients in the study
Procedure
Patients scheduled to undergo exercise MPI testing were evaluated on two consecutive days. Information on the medical, sociodemographic and psychological status of patients was obtained at baseline (day 1), while pain and symptom data were obtained immediately following exercise (day 2). A more detailed description of the procedure and measures has been previously published (27).
Instruments
Symptom checklist
A 23-item checklist was designed to assess the more subtle symptoms associated with ischemia. It included symptoms typically associated with angina (eg, breathlessness, tight feeling in the chest) and symptoms reportedly more prevalent in women (eg, nausea, fatigue). Participants could add other symptoms if they were not found on the list.
Dermatome pain map (29,30)
A graphic that includes two line drawings of the human body and assesses anatomical pain location.
Short form of the McGill Pain Questionnaire (31)
The short form of the McGill Pain Questionnaire measures qualitative and quantitative aspects of pain.
Chest pain quality scale
A scale used to classify patients’ daily chest pain (if present) as typical, atypical or nonspecific angina.
Psychiatric symptom index (32)
A 29-item measure of psychological status.
Medical assessment
MPI results were used as the principal indicator of the presence of myocardial ischemia, because these have been shown to be more sensitive and specific in the diagnosis of CAD than standard electrocardiogram results (33). The images were reviewed by one of three trained nuclear imaging specialists, who based the decision on clinical impression.
The exercise stress tests were performed on a treadmill using the Bruce protocol. The protocol was ceased when the patient reported considerable pain, was out of breath, presented with significant anomalies on electrocardiogram (eg, greater than 3 mm ST segment deviation) or had achieved at least 90% of the maximal heart rate expected for his/her age. The metabolic estimated cost (number of metabolic equivalents) achieved during exercise was noted for each patient.
Statistical analysis
ANOVAs and χ2 analyses were performed to examine sociodemographic and medical differences among patients with and without MPI evidence of ischemia on exercise stress testing.
All primary analyses were performed separately for individuals with and those without a history of CAD events or procedures. This decision was based on evidence of clinical heterogeneity among these groups, and on preliminary analyses showing that patients with a history of CAD versus those without exhibited differences in symptom profile, as well as in the association of these symptoms with ischemia status.
Sex differences in symptom profile among patients with silent ischemia during exercise
To identify sex-related differences in symptoms reported by patients with SI during exercise, χ2 analyses were performed. Although not discussed in detail in the results section, these analyses were also performed in patients with neither pain nor ischemia to aid in the interpretation of the detection models described below.
Detection of ischemia from nonpain symptoms reported during exercise stress testing
Binary logistic regressions were performed to examine whether subtle nonpain symptoms experienced during exercise stress testing can aid in detecting patients who showed evidence of exercise-induced ischemia during testing.
To ensure greater stability of measure for the nonpain symptoms and to reduce problems with collinearity among related symptoms (eg, difference between tense muscles and stiff muscles), logistic regression analyses were performed on groupings of symptoms. A factor analysis with varimax rotation was performed on all symptoms reported by at least five men or women to identify which symptoms grouped together. Three factors were identified. Factor 1 (cardiovascular or neurological) accounted for 21% of the variance and included palpitations, throbbing in the head or neck, dizziness, fatigue, headache and shortness of breath. Factor 2 (diaphoresis) accounted for 12% of the variance and included perspiration and hot flushes. Factor 3 (muscle tension) accounted for 11% of the variance and included ‘tense’ and ‘stiff’ muscles. Weakness and trembling loaded poorly on all factors and thus were considered separately.
Clinical covariates were forced into block 1 of the detection models. A variable was considered to be covariate if its association with MPI status (indicating absence or presence of myocardial ischemia) reached P<0.20 in univariate logistic regressions. The symptom variables, on the other hand, were entered into a backward logistic regression in block 2. Only significant or near-significant symptom findings are reported. Because there was considerable heterogeneity among men and women, the detection models were performed separately by sex and, as mentioned above, by history of CAD.
RESULTS
Three hundred forty-seven participants showed MPI evidence of ischemia. Results for the first set of analyses presented below are based on the 155 men and 60 women whose ischemia was silent during testing (representing 62% of all ischemic patients). The second set of analyses included all patients who did not experience angina pain during testing (including 126 men and 257 women with neither pain nor ischemia).
Patient characteristics
Mean scores and proportions for demographic and medical data for men and women as a function of ischemia status are presented in Tables 1 and 2.
TABLE 1.
Sociodemographic profile of men and women without pain during exercise single photon emission computed tomography
| Variable | Men
|
Women
|
||||
|---|---|---|---|---|---|---|
| Without ischemia (n=126) | With ischemia (n=155) | P | Without ischemia (n=257) | With ischemia (n=60) | P | |
| Marital status, n (%) | 0.233 | 0.978 | ||||
| Single | 11 (9) | 6 (4) | 32 (13) | 8 (13) | ||
| Married/living with someone | 98 (78) | 128 (83) | 166 (65) | 38 (63) | ||
| Separated/divorced/widowed | 17 (14) | 21 (14) | 59 (23) | 14 (23) | ||
| Education, n (%) | 0.167 | 0.23 | ||||
| Elementary school | 25 (20) | 31 (20) | 66 (26) | 22 (37) | ||
| High school | 52 (41) | 47 (30) | 88 (34) | 19 (32) | ||
| College/university | 49 (39) | 74 (48) | 101 (39) | 19 (32) | ||
| Smoking, n (%) | 20 (16) | 24 (16) | 0.929 | 35 (14) | 19 (32) | 0.001 |
| Psychological status (mean ± SD) | 12.4±9.75 | 11.9±8.96 | 0.635 | 17.5±12.45 | 18±12.23 | 0.806 |
TABLE 2.
Medical profile of men and women without pain during exercise single photon emission computed tomography
| Variable | Men
|
Women
|
||||
|---|---|---|---|---|---|---|
| Without ischemia (n=126) | With ischemia (n=155) | P | Without ischemia (n=257) | With ischemia (n=60) | P | |
| Age (mean ± SD) | 57.5±10.13 | 60.4±8.72 | 0.010 | 56.9±9.82 | 59.7±10.54 | 0.049 |
| Body mass index (kg/m2) (mean ± SD) | 27.3±3.83 | 27.5±4.07 | 0.796 | 26.1±4.41 | 26.7±5.56 | 0.324 |
| History of angina, n (%) | 27 (21) | 64 (41) | 0.002 | 52 (20) | 15 (25) | 0.518 |
| History of coronary events or procedures, n (%) | 48 (38) | 91 (59) | 0.001 | 30 (12) | 20 (33) | 0.000 |
| Hypertension, n (%) | 48 (38) | 71 (46) | 0.347 | 117 (46) | 29 (48) | 0.713 |
| Diabetes, n (%) | 17 (14) | 26 (17) | 0.774 | 30 (12) | 9 (15) | 0.698 |
| Hypercholesterolemia, n (%) | 74 (59) | 103 (67) | 0.346 | 105 (41) | 30 (50) | 0.364 |
| Family history of heart disease, n (%) | 83 (66) | 112 (72) | 0.157 | 209 (81) | 46 (77) | 0.639 |
| Family history of hypertension, n (%) | 43 (34) | 57 (37) | 0.593 | 155 (60) | 26 (43) | 0.030 |
| Family history of diabetes, n (%) | 51 (41) | 45 (29) | 0.058 | 108 (42) | 23 (38) | 0.707 |
| Beta-blockers, n (%) | 44 (35) | 63 (41) | 0.364 | 60 (23) | 25 (42) | 0.003 |
| Anticoagulants, n (%) | 67 (53) | 112 (72) | 0.001 | 100 (39) | 34 (57) | 0.009 |
| Lipid-lowering agents, n (%) | 72 (57) | 99 (64) | 0.294 | 76 (30) | 30 (50) | 0.002 |
| Type of daily angina pain, n (%) | 0.092 | 0.495 | ||||
| Typical pain | 2 (2) | 11 (7) | 20 (8) | 6 (10) | ||
| Atypical pain | 11 (9) | 20 (13) | 46 (18) | 14 (23) | ||
| Nonspecific pain | 22 (18) | 25 (16) | 53 (21) | 8 (13) | ||
| Patients tested in the morning, n (%) | 81 (64) | 105 (68) | 0.542 | 150 (59) | 44 (73) | 0.035 |
| Metabolic equivalents achieved during exercise testing (mean ± SD) | 8.8±1.82 | 8.2±1.59 | 0.003 | 7.2±1.50 | 6.6±1.56 | 0.008 |
Nonpain symptoms reported by patients with SI
The most common symptoms were shortness of breath (67%), fatigue (41%), perspiration (39%), palpitations (17%), dizziness (15%), hot flushes (14%), muscle tension (13%) and weakness (12%). Overall, women experienced significantly more symptoms during the exercise stress test compared with men (3.5±2.54 versus 2.2±1.96; F (1,211) = 15.230, P=0.000), irrespective of CAD history. Women reported fatigue, shortness of breath, throbbing in the head or neck, headaches, hot flushes and tense muscles more frequently than men. The direction of sex-related differences in symptoms relating to diaphoresis depended on history of CAD events or procedures (Tables 3 and 4).
TABLE 3.
Symptoms experienced by patients without a history of coronary artery disease
| Symptom | With ischemia, n (%)
|
Without ischemia, n (%)
|
||||
|---|---|---|---|---|---|---|
| Men (n=64) | Women (n=40) | P | Men (n=78) | Women (n=225) | P | |
| Weakness | 7 (11) | 8 (20) | NS | 6 (8) | 51 (23) | 0.004 |
| Trembling | 3 (5) | 4 (10) | NS | 4 (5) | 21 (9) | NS |
| Cardiovascular/neurological | ||||||
| Palpitations | 6 (9) | 9 (23) | 0.064 | 5 (6) | 60 (27) | 0.000 |
| Throbbing in head/neck | 1 (2) | 5 (13) | 0.020 | 5 (6) | 22 (10) | NS |
| Dizziness | 8 (13) | 9 (23) | NS | 18 (23) | 67 (30) | NS |
| Fatigue | 21 (33) | 21 (53) | 0.047 | 23 (30) | 101 (45) | 0.017 |
| Headache | 1 (2) | 5 (13) | 0.020 | 5 (6) | 22 (10) | NS |
| Shortness of breath | 36 (56) | 31 (78) | 0.028 | 40 (51) | 169 (75) | 0.000 |
| Diaphoresis | ||||||
| Perspiration | 35 (55) | 13 (33) | 0.027 | 36 (46) | 73 (32) | 0.030 |
| Hot flushes | 5 (8) | 8 (20) | 0.067 | 5 (6) | 37 (16) | 0.027 |
| Muscle tension | ||||||
| Tense muscles | 5 (8) | 11 (28) | 0.007 | 8 (10) | 36 (10) | NS |
| Stiff muscles | 6 (9) | 5 (13) | NS | 4 (5) | 16 (7) | NS |
NS Not significant
TABLE 4.
Symptoms experienced by patients with a history of coronary artery disease
| Symptom | With ischemia, n (%)
|
Without ischemia, n (%)
|
||||
|---|---|---|---|---|---|---|
| Men (n=91) | Women (n=20) | P | Men (n=48) | Women (n=30) | P | |
| Weakness | 8 (9) | 3 (15) | NS | 3 (6) | 6 (20) | 0.064 |
| Trembling | 4 (4) | 2 (10) | NS | 2 (4) | 3 (10) | NS |
| Cardiovascular/neurological | ||||||
| Palpitations | 16 (18) | 6 (30) | NS | 4 (8) | 7 (23) | 0.064 |
| Throbbing in head/neck | 2 (2) | 1 (5) | NS | 1 (2) | 3 (10) | NS |
| Dizziness | 12 (13) | 4 (20) | NS | 4 (8) | 8 (27) | 0.029 |
| Fatigue | 34 (37) | 12 (60) | 0.063 | 19 (40) | 13 (43) | NS |
| Headache | 1 (1) | 2 (10) | 0.026 | 1 (2) | 4 (13) | 0.048 |
| Shortness of breath | 62 (68) | 16 (80) | NS | 32 (67) | 25 (83) | NS |
| Diaphoresis | ||||||
| Perspiration | 25 (28) | 11 (55) | 0.017 | 22 (46) | 10 (33) | NS |
| Hot flushes | 10 (11) | 6 (30) | 0.028 | 5 (10) | 7 (23) | NS |
| Muscle tension | ||||||
| Tense muscles | 9 (10) | 2 (10) | NS | 11 (23) | 3 (10) | NS |
| Stiff muscles | 6 (7) | 1 (5) | NS | 6 (13) | 2 (7) | NS |
NS Not significant
Detection of ischemia from nonpain symptoms
Results can be seen in Tables 5 through 8. There was a lack of uniformity in symptoms and clinical factors associated with ischemia across the four groups. For example, age was significantly associated with ischemia in women with a history of CAD, but not for other groups. Smoking was significant for women (regardless of CAD history), but not for men. With respect to the main analyses, two groups of symptoms predominated: diaphoresis and muscle tension. Diaphoresis was significantly and positively associated with ischemia for both men and women; for men, it was among patients without a history of CAD, while for women, it was for those with a history of CAD. On the other hand, having muscle tension decreased the probability of having ischemia among men with a history of CAD, while for women with no history of CAD, it increased the probability of detecting ischemia.
TABLE 5.
Detection of ischemia in men with no history of coronary artery disease
| Variable entered | P | OR | 95% CI |
|---|---|---|---|
| Block 1 (forced in) | |||
| Hypertension | 0.408 | 0.694 | 0.291 to 1.651 |
| Family history of diabetes | 0.018 | 0.382 | 0.173 to 0.845 |
| METS achieved during exercise testing | 0.966 | 0.995 | 0.795 to 1.246 |
| Use of anticoagulants | 0.115 | 1.897 | 0.855 to 4.211 |
| Use of ACE inhibitors | 0.008 | 4.733 | 1.502 to 14.910 |
| Model χ2=20.403 | 0.001 | ||
| Block 2 (backward logistic regression) | |||
| Diaphoresis | 0.020 | 4.754 | 1.277 to 17.703 |
| Model χ2=5.722 | 0.017 | ||
| Full model χ 2=26.125 | 0.000 | ||
Block 1: sensitivity 46%; specificity 76%; positive predictive value 61%; negative predictive value 64%. Full model: sensitivity 62%; specificity 78%; positive predictive value 69%; negative predictive value 72%. ACE Angiotensinconverting enzyme; METS Metabolic equivalents
TABLE 8.
Detection of ischemia in women with a history of coronary artery disease
| Variable entered | P | OR | 95% CI |
|---|---|---|---|
| Block 1 (forced in) | |||
| Use of beta-blockers | 0.178 | 2.526 | 0.656 to 9.730 |
| Diabetes | 0.241 | 2.435 | 0.549 to 10.790 |
| Smoking | 0.009 | 11.61 | 1.832 to 73.590 |
| Age | 0.292 | 1.041 | 0.966 to 1.123 |
| Model χ2=12.998 | 0.011 | ||
| Block 2 (backward logistic regression) | |||
| Diaphoresis | 0.069 | 4.985 | 0.883 to 28.151 |
| Model χ2=3.581 | 0.058 | ||
| Full model χ2=16.579 | 0.005 | ||
Block 1: sensitivity 45%; specificity 87%; positive predictive value 69%; negative predictive value 70%. Full model: sensitivity 60%; specificity 80%; positive predictive value 67%; negative predictive value 75%
Post hoc analyses
To understand why the direction of the sex-related difference in diaphoresis was dissimilar depending on whether patients had a history of coronary events, exploratory ANOVAs and χ2 analyses were performed on variables thought to have the potential to influence this symptom domain, in part, through sympathetic nervous system activation.
Among patients without a history of CAD events or procedures, men exerted more physical effort during exercise stress testing (P=0.000), they exhibited greater increases in systolic blood pressure (SBP) during testing (P=0.000), and they were more often diagnosed with diabetes (P=0.046) and hypercholesterolomia (P=0.024). Controlling for these variables eliminated the sex-related difference in diaphoresis.
However, among patients with a history of CAD, women were more frequently diagnosed with diabetes (P=0.045), more frequently smoked (P=0.008) and were more frequently diagnosed with hypertension. On the other hand, they showed smaller increases in SBP during exercise testing and achieved fewer metabolic equivalents than men. Controlling for these variables did not alter the results: women with a history of CAD more frequently reported diaphoresis than men.
DISCUSSION
The present study of a large, consecutive series of men and women referred for exercise-stress testing with MPI for evaluation of suspected or known CAD showed a high prevalence of SI (62%). This is in keeping with the literature on ischemia (1–3,6,10) and myocardial infarction (4).
Patients with SI reported a number of symptoms during exercise. Most common were shortness of breath (dyspnea), fatigue and perspiration. Consistent with the literature on angina (27,28), women experienced more symptoms during the exercise stress test than men. This was particularly true for fatigue, hot flushes, tense muscles, shortness of breath and headaches. Women visiting the emergency department for nontraumatic chest pain frequently present with more symptoms of fatigue than men, while men report diaphoresis more frequently in these settings (28). In the current study, however, the expected sex-related differences in perspiration were observed only among patients without a history of CAD. In the latter group, it is likely that greater comorbidity (particularly diabetes), exertion and sympathetic activation during exercise stress testing in men were responsible for the diaphoresis, because controlling for these variables eliminated sex-related differences. In patients with a history of CAD, however, perspiration was reported more frequently in women. These women also exhibited greater comorbidity (diabetes and hypertension) and smoked more – factors associated with sympathetic hyperactivity. On the other hand, during exercise-induced ischemia, they did not exhibit SBP hyper-reactivity, compared with men. Moreover, statistically controlling for these variables did not change the results in patients with a history of CAD – more women reported diaphoresis during exercise stress testing than men. The reasons for these differences remain to be elucidated.
Dyspnea is considered to be an anginal equivalent by many clinicians and researchers (1). In a study by Coodley (34), up to 25% of older patients with known CAD reported dyspnea as the primary symptom in the absence of angina. In the current study, 78% of women and 63% of men did. According to Gregoratos (35), dyspnea reflects elevated left ventricular end-diastolic pressure resulting from myocardial ischemia superimposed on reduced ventricular compliance. The latter is found frequently in hypertensive patients. Close to one-half of patients examined in the current study suffered from hypertension.
Patients with silent myocardial infarctions have also been reported to frequently present with neurological and gastrointestinal symptoms, including syncope, vertigo, fainting, abdominal pain and nausea (1,35). In the current sample of patients with SI, neurological symptoms (eg, weakness, fatigue, dizziness) were frequent, particularly in women. Gastrointestinal symptoms, on the other hand, were rarely reported.
Unfortunately, the reported symptoms were only partially useful in discriminating between patients with and without ischemia. Cardiovascular and neurological symptoms were reported to a similar extent, irrespective of ischemic status. On the other hand, diaphoresis was associated with close to a fivefold chance of experiencing exercise-related ischemia among women with a history of CAD and among men without CAD. Women without a CAD history who complained of muscle tension had a 3.4 greater chance of experiencing ischemia. We have previously reported (36) similar findings in women who also experienced angina pain on exercise stress testing. In men with a CAD history, however, muscle tension was related to a lower incidence of ischemia. In men with angina, atypical symptoms, such as muscle tension, have frequently shown limited or inverse relations with ischemia (12,36,37). The reasons for these group differences are unclear, but they likely reflect differences in clinical and pathological processes.
While the present study was the first, to our knowledge, to systematically and prospectively examine symptom profiles in both men and women with SI, several limitations merit mention. In women, the smaller sample size might have led to insufficient power of the analyses to detect weaker predictors of ischemia. There is concern that the models may be overfit and should therefore be seen as hypothesis-generating at this time. SI was defined based on a single event. Nonetheless, because the goal of the manuscript was to identify nonpain symptoms associated with SI, measured simultaneously, it was not considered to be essential that patients should be excluded if they had experienced angina in the past. An additional limitation concerns the fact that the participants were mainly French-speaking and white. Further research in other ethnic groups would be helpful in assessing the applicability of these results beyond Caucasians. Finally, patients attended a cardiac tertiary hospital, where the prevalence of CAD is higher than in other institutions. Indeed, a large proportion of participants (particularly among the men) had a history of coronary events or procedures and significant comorbidities (eg, hypertension). The high-risk profile of most of the recruited patients may have obscured findings. Research has shown that as the a priori likelihood of developing myocardial ischemia and heart disease increases, chest pain loses its significance as an additional predictive factor (15). This may also be true of other symptoms. We addressed this issue by analyzing the results separately for patients with and without a CAD history, and did, indeed, show clinical and symptomatic heterogeneity among these groups.
The impacts of these nonpain symptoms are not negligible. First, sensitivity of the detection models improved by 16% in men with no history of CAD, 8% in women with no history of CAD and 15% in women with a history of CAD. When used in conjunction with other clinical data, the presence of these symptoms may aid to alert physicians to the possible presence of ischemic events in these patients. On the other hand, patients who present with nonspecific symptoms, such as diaphoresis, fatigue or muscle tension, may be more likely to be misdiagnosed than patients who report more typical angina. Most physicians will evaluate and treat their patients based on the presence of typical angina symptoms, rather than rely on documentation of myocardial ischemia (18). Similarly, patients may delay longer in seeking treatment for nonspecific symptoms, such as occasional muscle tension or diaphoresis. This may place those with unrecognized CAD at greater risk for morbidity and mortality (37,38).
Given studies indicating a similar or worse prognosis in patients with silent compared with painful myocardial ischemia or infarcts (6,7,9,10,14,17,20), particularly among high-risk individuals (15,18,21,36,39,40), continued research on the origin and detection of SI are clearly needed to improve patient care and education. In the meantime, it is suggested that patients reporting perspiration and/or muscle tension (in women) on physical effort be followed up for testing, particularly if other symptoms commonly associated with CAD or ischemic processes, such as dyspnea and fatigue, are also present. In the long-term, the costs of testing false-positives are likely to be outweighed by those associated with the late diagnosis and treatment of true-positives. Similarly, it may be cautious to teach patients in whom SI has been detected to temporarily cease or reduce the intensity of ongoing physical activity when they experience these symptoms.
TABLE 6.
Detection of ischemia in men with a history of coronary artery disease
| Variable entered | P | OR | 95% CI |
|---|---|---|---|
| Block 1 (forced in) | |||
| Type of daily angina pain* | 0.004 | 0.435 | 0.247 to 0.765 |
| Family history of hypertension | 0.282 | 1.624 | 0.671 to 3.932 |
| Use of lipid-lowering agents | 0.074 | 0.346 | 0.108 to 1.106 |
| Time of day tested | 0.311 | 0.648 | 0.280 to 1.501 |
| Smoking | 0.593 | 0.75 | 0.261 to 2.154 |
| METS achieved during exercise testing | 0.487 | 1.126 | 0.806 to 1.572 |
| Age | 0.006 | 1.085 | 1.023 to 1.152 |
| Model χ2=27.208 | 0.000 | ||
| Block 2 (backward logistic regression) | |||
| Muscle tension | 0.042 | 0.184 | 0.032 to 0.944 |
| Model χ2=4.315 | 0.038 | ||
| Full model χ2=31.523 | 0.000 | ||
*A smaller number indicates more typical angina symptoms. Block 1: sensitivity 86%; specificity 53%; positive predictive value 77%; negative predictive value 68%. Full model: sensitivity 85%; specificity 57%; positive predictive value 78%; negative predictive value 68%. METS Metabolic equivalents
TABLE 7.
Detection of ischemia in women with no history of coronary artery disease
| Variable entered | P | OR | 95% CI |
|---|---|---|---|
| Block 1 (forced in) | |||
| Time of day tested | 0.002 | 0.226 | 0.086 to 0.596 |
| Age | 0.174 | 1.030 | 0.987 to 1.074 |
| Diabetes | 0.247 | 0.379 | 0.073 to 1.963 |
| METS achieved during exercise testing | 0.039 | 0.743 | 0.560 to 0.985 |
| Family history of hypertension | 0.094 | 0.530 | 0.252 to 1.115 |
| Family history of diabetes | 0.325 | 0.671 | 0.303 to 1.486 |
| Smoking | 0.018 | 3.109 | 1.215 to 7.953 |
| Model χ2=29.485 | 0.000 | ||
| Block 2 (backward logistic regression) | |||
| Muscle tension | 0.053 | 3.410 | 0.986 to 11.797 |
| Model χ2=3.564 | 0.059 | ||
| Full model χ2=33.049 | 0.000 | ||
Block 1: sensitivity 8%; specificity 99%; positive predictive value 50%; negative predictive value 86%. Full model: sensitivity 16%; specificity 98%; positive predictive value 60%; negative predictive value 87%. METS Metabolic equivalents
Footnotes
FUNDING: This research was supported in part by the Canadian Institutes for Health Research (CIHR, MOP 53242) and the Heart and Stroke Foundation of Canada. A Chercheur-boursier clinicien scholarship was also awarded to the first author by the Fonds de la recherche en santé du Québec.
REFERENCES
- 1.Aronow WS, Silent MI. Prevalence and prognosis in older patients diagnosed by routine electrocardiograms. Geriatrics. 2003;58:24–40. [PubMed] [Google Scholar]
- 2.Cohn PF, Fox KM, Daly C. Silent myocardial ischemia. Circulation. 2003;108:1263–77. doi: 10.1161/01.CIR.0000088001.59265.EE. [DOI] [PubMed] [Google Scholar]
- 3.Meiltz A, Ciaroni S. [Silent myocardial ischaemia: A deafening silence] Rev Med Suisse. 2005;1:613–6. [PubMed] [Google Scholar]
- 4.Kannel WB, Abbott RD. Incidence and prognosis of unrecognized myocardial infarction. An update on the Framingham study. N Engl J Med. 1984;311:1144–7. doi: 10.1056/NEJM198411013111802. [DOI] [PubMed] [Google Scholar]
- 5.Amsterdam EA. Treatment and outcome in silent myocardial ischemia: More pieces of the puzzle. Am J Cardiol. 1997;29:1474–5. doi: 10.1016/s0002-9149(97)82784-1. [DOI] [PubMed] [Google Scholar]
- 6.Biagini E, Schinkel AF, Bax JJ, et al. Long term outcome in patients with silent versus symptomatic ischaemia during dobutamine stress echocardiography. Heart. 2005;91:737–42. doi: 10.1136/hrt.2004.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohn PF. Silent myocardial ischemia: Recent developments. Curr Atheroscler Rep. 2005;7:155–63. doi: 10.1007/s11883-005-0039-8. [DOI] [PubMed] [Google Scholar]
- 8.Deedwania PC, Carbajal EV. Is silent myocardial ischemia clinically important? Does it have prognostic significance? Postgraduate Med. 1989;86:99–110. doi: 10.1080/00325481.1989.11700776. [DOI] [PubMed] [Google Scholar]
- 9.de Marchena E, Asch J, Martinez J, et al. Usefulness of persistent silent myocardial ischemia in predicting a high cardiac event rate in men with medically controlled, stable angina pectoris. Am J Cardiol. 1994;73:390–2. doi: 10.1016/0002-9149(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 10.Elhendy A, Schinkel AF, van Domburg RT, Bax JJ, Poldermans D. Comparison of late outcome in patients with versus without angina pectoris having reversible perfusion abnormalities during dobutamine stress technetium-99m sestamibi single-photon emission computed tomography. Am J Cardiol. 2003;91:264–8. doi: 10.1016/s0002-9149(02)03152-1. [DOI] [PubMed] [Google Scholar]
- 11.Gottlieb SO, Gottlieb SH, Achuff SC, et al. Silent ischemia on Holter monitoring predicts mortality in high-risk postinfarction patients. JAMA. 1988;259:1030–5. [PubMed] [Google Scholar]
- 12.Hedblad B, Juul-Möller S, Svensson K, et al. Increased mortality in men with ST segment depression during 24 h ambulatory long-term ECG recording. Results from prospective population study ‘Men born in 1914’, from Malmö, Sweden. Eur Heart J. 1989;10:149–58. doi: 10.1093/oxfordjournals.eurheartj.a059455. [DOI] [PubMed] [Google Scholar]
- 13.Kowalchuk GJ, Nesto RW. Silent myocardial ischemia. Mechanisms and rationale for therapy. Am J Med. 1989;86(Suppl 1A):9–13. doi: 10.1016/0002-9343(89)90004-1. [DOI] [PubMed] [Google Scholar]
- 14.Kurl S, Laukkanen JA, Tuomainen TP, et al. Association of exercise-induced, silent ST-segment depression with the risk of stroke and cardiovascular diseases in men. Stroke. 2003;34:1760–5. doi: 10.1161/01.STR.0000078564.46376.0A. [DOI] [PubMed] [Google Scholar]
- 15.Laukkanen JA, Kurl S, Lakka TA, et al. Exercise-induced silent myocardial ischemia and coronary morbidity and mortality in middle-aged men. J Am Coll Cardiol. 2001;38:72–9. doi: 10.1016/s0735-1097(01)01311-0. [DOI] [PubMed] [Google Scholar]
- 16.Rosen SD, Paulesu E, Nihoyannopoulos P, et al. Silent ischemia as a central problem: Regional brain activation compared in silent and painful myocardial ischemia. Ann Intern Med. 1996;124:939–49. doi: 10.7326/0003-4819-124-11-199606010-00001. [DOI] [PubMed] [Google Scholar]
- 17.Sajadieh A, Nielson OW, Rasmussen V, et al. Prevalence and prognostic significance of daily-life silent myocardial ischaemia in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J. 2005;26:1402–9. doi: 10.1093/eurheartj/ehi169. [DOI] [PubMed] [Google Scholar]
- 18.Solomon H, DeBusk RF. Contemporary management of silent ischemia: The role of ambulatory monitoring. Review. Int J Cardiol. 2004;96:311–9. doi: 10.1016/j.ijcard.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Torosian T, Lumley MA, Pickard SD, et al. Silent versus symptomatic myocardial ischemia: The role of psychological and medical factors. Health Psychol. 1997;16:123–30. doi: 10.1037//0278-6133.16.2.123. [DOI] [PubMed] [Google Scholar]
- 20.Yano K, MacLean CJ. The incidence and prognosis of unrecognized myocardial infarction in the Honolulu, Hawaii, Heart Program. Arch Intern Med. 1989;149:1528–32. [PubMed] [Google Scholar]
- 21.Zellweger MJ, Hachamovitch R, Kang X, et al. Prognostic relevance of symptoms versus objective evidence of coronary artery disease in diabetic patients. Eur Heart J. 2004;25:543–50. doi: 10.1016/j.ehj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Zellweger MJ, Weinbacher M, Zutter AW, et al. Long-term outcome of patients with silent versus symptomatic ischemia six months after percutaneous coronary intervention and stenting. J Am Coll Cardiol. 2003;42:33–40. doi: 10.1016/s0735-1097(03)00557-6. [DOI] [PubMed] [Google Scholar]
- 23.Krantz DS, Hedges SM, Gabbay FH, et al. Triggers of angina and ST-segment depression in ambulatory patients with coronary artery disease: Evidence for an uncoupling of angina and ischemia. Am Heart J. 1994;128:703–12. doi: 10.1016/0002-8703(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 24.Pennebaker JW, Watson D. Blood pressure estimation and beliefs among normotensives and hypertensives. Health Psychol. 1988;7:309–28. doi: 10.1037//0278-6133.7.4.309. [DOI] [PubMed] [Google Scholar]
- 25.Barsky AJ, Hochstrasser B, Coles NA, Zisfein J, O’Donnell C, Eagle KA. Silent myocardial ischemia. Is the person or the event silent? JAMA. 1990;264:1132–5. doi: 10.1001/jama.264.9.1132. [DOI] [PubMed] [Google Scholar]
- 26.D’Antono B, Dupuis G, Fleet R, et al. Sex differences in chest pain and prediction of exercise-induced ischemia. Can J Cardiol. 2003;19:515–22. [PubMed] [Google Scholar]
- 27.D’Antono B, Dupuis G, Fortin C, et al. Angina symptoms in men and women with stable coronary artery disease and evidence of exercise-induced myocardial perfusion defects. Am Heart J. 2006;151:813–9. doi: 10.1016/j.ahj.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 28.Devon HA, Johnson Zerwic J. Symptoms of acute coronary syndromes: Are there gender differences? A review of the literature. Heart Lung. 2002;31:235–45. doi: 10.1067/mhl.2002.126105. [DOI] [PubMed] [Google Scholar]
- 29.Fleet RP, Dupuis G, Marchand A, et al. Detecting panic disorder in emergency department chest pain patients: A validated model to improve recognition. Ann Behav Med. 1997;19:124–31. doi: 10.1007/BF02883329. [DOI] [PubMed] [Google Scholar]
- 30.Margolis RB, Tait RC, Krause SJ. A rating system for use with patient pain drawings. Pain. 1986;24:57–65. doi: 10.1016/0304-3959(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–7. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 32.Ilfeld FW. Further validation of a psychiatric symptom index in a normal population. Psychol Reports. 1976;39:1215–28. [Google Scholar]
- 33.Shepherd J. Identifying patients at risk for coronary heart disease: Treatment implications. Eur Heart J. 1998;19:1776–83. doi: 10.1053/euhj.1998.1122. [DOI] [PubMed] [Google Scholar]
- 34.Coodley EL. Coronary artery disease in the elderly. Postgrad Med. 1990;87:223–7. doi: 10.1080/00325481.1990.11704568. [DOI] [PubMed] [Google Scholar]
- 35.Gregoratos G. Clinical presentation of coronary artery disease in the elderly: How does it differ from younger populations. Am J Ger Cardiol. 1998;7:35–40. [PubMed] [Google Scholar]
- 36.D’Antono B, Dupuis G, Fortin C, et al. Detection of exercise-induced myocardial ischemia from symptomatology experienced during testing in men and women. Can J Cardiol. 2006;22:411–7. doi: 10.1016/s0828-282x(06)70927-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milner KA, Funk M, Arnold A, et al. Typical symptoms are predictive of acute coronary syndromes in women. Am Heart J. 2002;143:283–8. doi: 10.1067/mhj.2002.119759. [DOI] [PubMed] [Google Scholar]
- 38.Deedwania PC. Silent myocardial ischaemia in the elderly. Drugs Aging. 2000;16:381–9. doi: 10.2165/00002512-200016050-00007. [DOI] [PubMed] [Google Scholar]
- 39.Aronow WS, Epstein S. Usefulness of silent myocardial ischemia detected by ambulatory electrocardiographic monitoring in predicting new coronary events in elderly patients. Am J Cardiol. 1988;62:1295–6. doi: 10.1016/0002-9149(88)90277-9. [DOI] [PubMed] [Google Scholar]
- 40.Valensi P. La maladie coronarienne silencieuse chez les patients diabétiques: Les nouvelles recommendations. Rev Med Liege. 2005;60:531–5. [PubMed] [Google Scholar]