Abstract
OBJECTIVE:
Structural remodelling plays an important role in the genesis and maintenance of atrial fibrillation (AF). Although some studies that associate structural remodelling with atrial dilation have been reported, structural pulmonary venous (PV) remodelling due to chronic atrial dilation remains unclear.
METHODS:
Six sham dogs and five mitral regurgitation (MR) dogs (three months after partial mitral valve avulsion) were studied. Separate cryosections from the PV and left atrium (LA) were immunolabelled with antibodies against connexin (Cx) 40 and Cx43 and analyzed by confocal laser scanning microscopy. Tissue samples from the PV and LA were stained with hematoxylin and eosin, and Masson’s trichrome.
RESULTS:
In MR models, a decrease in Cx40 (0.57±0.2% versus 1.18±0.3%, P<0.05) and Cx43 (0.48±0.2% versus 1.56±0.5%, P<0.05) expression was observed compared with sham dogs. The distribution pattern of Cx40 and Cx43 changed from homogeneous to heterogeneous. Gap junction remodelling was not observed in the LA. In Masson’s trichrome-stained sections from MR dogs, regions with increased interstitial fibrosis were present in the PV. Thickness in the PV and the PV-LA junction did not change in the MR group.
CONCLUSION:
The present study demonstrated a decrease in Cx40 and Cx43 expression and increased interstitial fibrosis in PV due to MR. These changes may potentially be a mechanism that renders the dilated atria more susceptible to AF.
Keywords: Atrial fibrillation, Gap junction, Pulmonary vein, Remodelling
Abstract
OBJECTIF :
Le remodelage structurel joue un rôle important dans la genèse et le maintien de la fibrillation auriculaire (FA). Bien que certaines études aient associé le remodelage structurel à la dilatation auriculaire, on ne comprend pas vraiment le remodelage de la structure de la veine pulmonaire (VP) causé par la dilatation auriculaire chronique.
MÉTHODOLOGIE :
Les auteurs ont étudié six chiens fictifs et cinq chiens souffrant de régurgitation mitrale (RM) (trois mois après une avulsion partielle de la valvule mitrale). Ils ont immunomarqué des cryosections distinctes de la VP et de l’oreillette gauche (OG) avec des anticorps de la connexine (Cx) 40 et de la Cx43 et les ont analysées par microscope confocal à balayage laser. Ils ont coloré des échantillons tissulaires de la VP et de l’OG à l’hématoxyline, à l’éosine et au trichrome de Masson.
RÉSULTATS :
Dans les modèles de RM, les auteurs ont observé une diminution de l’expression de la Cx40 (0,57±0,2 % par rapport à 1,18±0,3 %, P<0,05) et de la Cx43 (0,48±0,2 % par rapport à 1,56±0,5 %, P<0,05) par rapport aux chiens fictifs. La séquence de distribution de la Cx40 et de la Cx43 est passée d’homogène à hétérogène. Les auteurs n’ont pas observé de remodelage de la jonction lacunaire dans l’OG. Dans les sections colorées au trichrome de Masson des chiens atteints de RM, les zones de fibrose interstitielle accrue dans l’épaisseur de la VP et la jonction de la VP et de l’OG n’étaient pas différentes au sein du groupe atteint de RM.
CONCLUSION :
La présente étude démontre une diminution de l’expression de la Cx40 et de la Cx43 et une augmentation de la fibrose interstitielle de la VP causées par la RM. Ces changements pourraient être un mécanisme pour rendre l’oreillette dilatée plus susceptible à la FA.
Atrial fibrillation (AF) is a common arrhythmia that occurs in a variety of clinical settings (hypertension, mitral valve disease, congestive heart failure, etc) (1). Among the risk factors predisposing to AF, atrial dilation is considered to be one of the most important. Structural remodelling plays an important role in the genesis and maintenance of AF (2). Among the various structural components of atrial myocardium, the determinants of passive conduction of the atrial impulse include the gap junctions, the size and packing geometry of individual myocytes, and the composition of the extracellular matrix (3). Intercellular coupling between myocytes is provided by gap junctional channels composed of connexins (4). These changes in connexin expression are important arrhythmogenic factors contributing to inhomogeneities in electrical impulse propagation and to local conduction block (5). In the atria, Cx43 and Cx40 are the principal connexins (6). Studies associating structural remodelling with atrial dilation have been reported (7–11), but the effect of chronic atrial dilation on structural remodelling in the pulmonary vein (PV) remains unclear. In the present paper, we report the effects of chronic atrial dilation due to mitral regurgitation (MR) on the histology and gap junctions of the canine PV.
METHODS
Animals
All experiments conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication, revised 1996).
Eleven adult (one to two years of age) dogs of either sex weighing 17 kg to 28 kg were used in the study. Twelve-lead electrocardiograms were obtained to confirm sinus rhythm. Transthoracic echocardiograms (TTE) were performed to exclude any structural heart disease and to measure the left atrium (LA) size as well as the left ventricular ejection fraction. MR was produced in five of these dogs and the others were used as the control group.
MR model
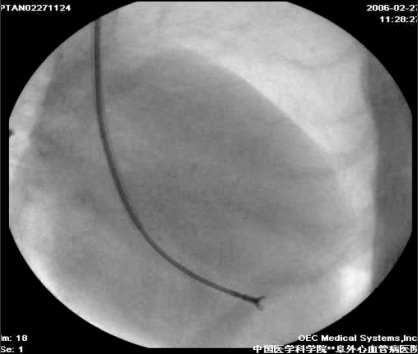
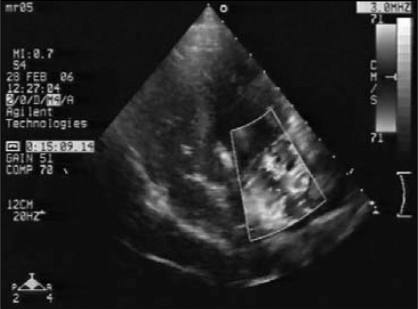
Dogs were anesthetized with an intravenous injection of pentobarbital (25 mg/kg). Anesthesia was maintained with halothane after intubation and they were mechanically ventilated with oxygen (2 L/min). The procedure was monitored with fluoroscopy and TTE. A custom Swarz sheath was inserted through the carotid artery and guided into the left ventricle (LV). A biopsy clamp was advanced through the sheath and attached to a mitral chorda (Figure 1). After the mitral chorda was ruptured by the clamp, the degree of MR was determined by TTE. This procedure was repeated until MR was moderate to severe, as judged by the size and flow velocity of the regurgitant jet (Figure 2). Twelve-lead electrocardiograms and physical examination were obtained in the week after the procedure and in subsequent weeks. All MR dogs were subjected to monthly TTE to measure LA size. These animals and the six sham dogs underwent studies after three months of MR.
Figure 1).
The custom Swarz sheath and biopsy clamp were positioned as the fluoroscopic right anterior oblique view. The mitral chordae were ruptured by the biopsy clamp
Figure 2).
Transthoracic echocardiographic image from the parasternal four-chamber view 15 min after the procedure. Colour Doppler revealed a moderate to severe regurgitant jet
Electrophysiological study
The sham and MR dogs were anesthetized with an intravenous injection of pentobarbital (25 mg/kg). The anesthesia was maintained with halothane after intubation and they were mechanically ventilated with oxygen (2 L/min). Then the inducibility of AF was tested by burst pacing. Sustained AF was defined as irregular, repetitive atrial responses lasting more than 5 min.
Immunohistochemistry
Longitudinal tissue samples from the middle of the PV and the LA from six control and five MR dogs were frozen rapidly in liquid nitrogen and stored at –80°C. Cryosections (10 μm) were immunolabelled after incubation in 0.1% trypsin. To avoid cross-reaction between the anti-Cx40 and the anti-Cx43 antibodies, labelling for each connexin was performed on separate section. Cryosections were blocked with 100% fetal calf serum for 30 min at 37°C and were washed with phosphate buffered saline containing 1% bovine serum albumin between successive incubation steps. Rabbit polyclonal anti-Cx43 (ZYMED Laboratories Inc, USA) and goat polyclonal anti-Cx40 (Santa Cruz Biotechnology, Inc, USA) were used to detect connexins. Cryosections were incubated with the primary antibodies overnight at 4°C. Subsequently, sections were incubated with the secondary antibodies goat-anti-rabbit TRITC and rabbit-anti-goat FITC. In negative controls, the primary antibodies were omitted. Three-channel scanning of single optical slices (less than 1 μm) by confocal laser scanning microscopy (Leica TCS SP2, Leica Microsystems Ltd, Germany) with appropriate filter blocks for the detection of FITC and TRITC fluorescence was used. Images were taken with the 40× objective lens. For each section, three randomly selected areas were labelled with Cx40 and Cx43 and examined. Sections containing blood vessels, which expressed Cx40 in their endothelial lining, were omitted. For quantification, the mean value of the densities from the three examined areas was taken. Connexin signal quantity was expressed per unit area of myocytic tissue. As previously described (12), the distribution was classified as heterogeneous when the SD of the three densities was greater than one. Images viewed with an 80× objective lens were used to confirm the location of connexins.
Histology
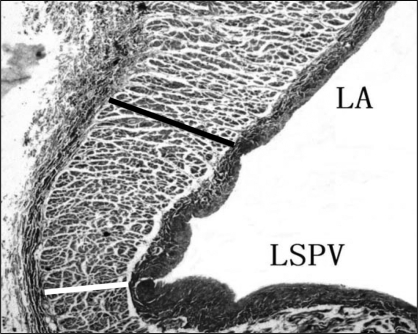
Longitudinal tissue samples (ie, parallel to the flow of blood) from the middle of the PV and LA of six control and five MR dogs were fixed in 10% neutral buffered formalin. The PVs were cut transversely, 3 mm to 5 mm above the PV-LA junction, and dissected into transverse blocks. At the PV-LA junctions, sections were cut longitudinally. Tissue was processed, embedded in paraffin, and sectioned in 4 μm to 5 μm thick sections. Serial sections were stained with hematoxylin and eosin, and Masson’s trichrome. According to methods presented in Figure 3, the thickness of the muscle sleeves proximate to the PV and the posterior PV-LA junction were measured.
Figure 3).
Longitudinal section stained with Masson’s trichrome at the left superior pulmonary vein (LSPV)-left atrium (LA) junction. The image was taken with 25× magnification. The solid black line indicates the thickness at posterior PV-LA junction. The white line indicates the thickness of the PV
Statistical analysis
Statistical analysis was performed by SPSS 10.5 software. All values were expressed as mean ± SD. An unpaired t test and χ2 test (AF inducibility) were used to evaluate differences between groups. P<0.05 was considered to be statistically significant.
RESULTS
MR model
Marked dilation of the LA was noted one month after the procedure. In the MR model, LA length was significantly greater than in the sham dogs after three months (3.8±0.6 cm versus 2.5±0.5 cm; P<0.05). In the following two months, the size of LA did not increase further. Although most of the dogs remained in sinus rhythm, two of the MR dogs developed atrial premature beats two months after the procedure. There was no significant difference in left ventricular ejection fraction between groups and no signs of heart failure were observed in MR models.
Sustained AF was not observed in any of the six sham dogs. In the MR group, sustained AF was inducible in four of five dogs (χ2, P<0.05)
Immunoconfocal assessment of gap junctions
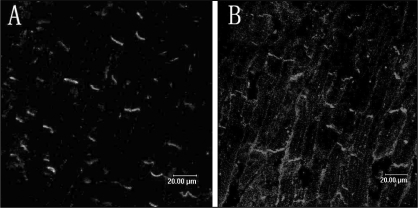
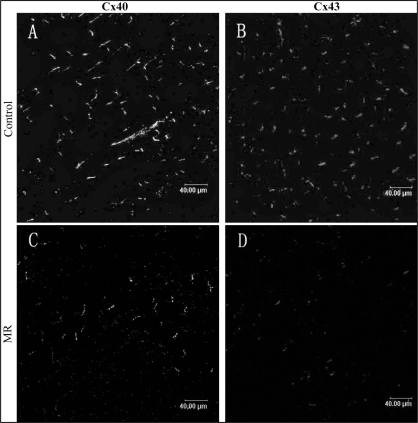
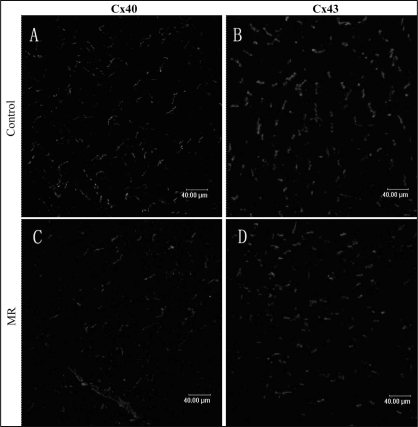
In longitudinally sectioned PV tissue from a sham dog, Cx40 and Cx43 are mainly located at the cell termini as short transverse lines representing intercalated discs (Figure 4). The distribution pattern of Cx40 and Cx43 was mostly homogeneous. Table 1 shows the expression of Cx40 and Cx43 in PV and LA in both groups. In PV, decreases in the expression of Cx40 (0.57±0.2% versus 1.18±0.3%, P<0.05) and Cx43 (0.48±0.2% versus 1.56±0.5%, P<0.05) were observed in MR dogs compared with control dogs (Figure 5). The distribution pattern of Cx40 and Cx43 was heterogeneous. In LA, no significant difference was found in the expression and distribution pattern of the connexins between the groups (Figure 6).
Figure 4).
Spatial distribution of connexin (Cx) 40 (A) and Cx43 (B) in the pulmonary vein (PV) in the control group. Connexins were mainly located at the cell termini as short transverse lines representing intercalated discs
TABLE 1.
The density of connexin (Cx) 40 and Cx43 in the pulmonary vein (PV) and left atrium (LA) from control and mitral regurgitation (MR) dogs
| Cx40 (μm2/μm2)
|
Cx43 (μm2/μm2)
|
|||
|---|---|---|---|---|
| Groups | PV | LA | PV | LA |
| Control (n=6) | 1.18±0.3% | 1.49±0.3% | 1.56±0.5% | 1.56±0.3% |
| MR (n=5) | 0.57±0.2%* | 1.43±0.4% | 0.48±0.2%* | 1.38±0.2% |
In dogs with MR, significant differences were found in the PV compared with control dogs
Figure 5).
Spatial distribution of connexin (Cx) 40 (A, C) and Cx43 (B, D) in the pulmonary vein in control (A, B) and mitral regurgitation (MR) (C, D) dogs. There was a marked decrease in the amount in the pulmonary vein in the MR dogs
Figure 6).
Spatial distribution of connexin (Cx) 40 (A, C) and Cx43 (B, D) in the left atrium in control (A, B) and mitral regurgitation (MR) (C, D). The amount and spatial distribution of the gap junction proteins did not appear to be markedly different between the groups
Histology
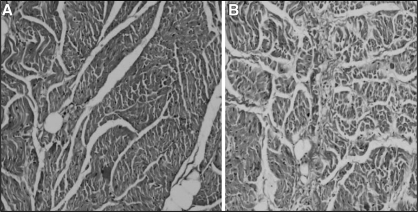
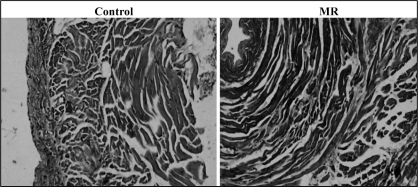
Sections from PV and LA were stained using hematoxylin and eosin, and Masson’s trichrome stain to compare tissue structure and the distribution of fibrous tissue in the control and MR groups. In Masson’s trichrome-stained PV sections from the MR dogs, regions with increased interstitial fibrosis were present in longitudinal and transverse section (Figures 7 and 8). However, the LA sections from the MR dogs only had a slight increase in fibre separation. No significant between-group difference was found in the thickness of the muscle in the proximal PV (control: 0.68±0.23 mm; MR: 0.76±0.35 mm, P=not significant). The thickness in the PV-LA junction did not change due to MR (control: 1.24±0.29 mm; MR: 1.37±0.43 mm, P=not significant), either. Also, there were no significant signs of chronic inflammation or signs of cellular necrosis.
Figure 7).
Longitudinal section (parallel to the blood flow) stained with Masson’s trichrome in control pulmonary veins at original magnification ×100 (A) and mitral regurgitation pulmonary veins at original magnification ×100 (B)
Figure 8).
Transverse section (perpendicular to the blood flow) stained with Masson’s trichrome in control pulmonary veins at original magnification ×100 and mitral regurgitation (MR) pulmonary veins at original magnification ×100
DISCUSSION
In the present study, we described changes in PV structure in a canine model of chronic atrial dilation. We found a decrease in Cx40 and Cx43 expression and changes in the distribution pattern of gap junctions in the PV in MR. The PVs of MR dogs displayed areas of increased interstitial fibrosis. Such structural remodelling was not observed in the LA. We did not find any significant difference between groups in the thickness of the muscle in the proximal PV or the PV-LA junction.
In the myocardium, gap junctions are responsible for the rapid and coordinated propagation of excitation (13,14). Gap junction channels are formed by the docking of two connexons (hemichannel); each connexon consists of six connexin subunits. The role of gap junction abnormalities in AF is actively studied. Several investigators have reported changes in the expression of atrial connexins during AF, but the results are not consistent (15–19). Various underlying etiologies have been suggested as explanations for the differences between these studies. Recently, alterations in tissue structure due to atrial dilation have been reported (7–11), but the role of chronic atrial dilation on structural PV remodelling remains unclear. In the present study, we described the decrease of Cx40 and Cx43 in PV for the first time, which is similar to the changes seen in the gap junction in atrial tissue due to chronic atrial dilation (8). We also showed that the distribution pattern of Cx40 and Cx43 changed from homogeneous to heterogeneous. However, gap junction remodelling did not appear in the LA, which agrees with the results of the study by Guerra et al (20). The increased interstitial fibrosis may be the underlying mechanism for alteration in the gap junction. Interstitial fibrosis may lead to alterations of the cytoskeleton network and adhesion proteins that contribute to normal gap junction organization (21). Some studies (22) have reported that increased interstitial fibrosis is always associated with a decrease in gap junction expression.
Structural PV remodelling as we described may be a potential mechanism that renders the chronically dilated atria more susceptible to AF. The attenuation of connexins could lead to abnormal electrical coupling between cardiomyocytes and slow conduction velocity, which could facilitate microreentry. (23) The change in gap junction distribution pattern may promote dispersion of action-potential duration and alternans, which may also increase the vulnerable window for reentry. (24) Moreover, these changes were present in sinus rhythm, which suggests that structural remodelling and alteration of gap junctions is an early event in the development of the AF substrate.
Limitation
To avoid cross-reaction between the anti-Cx40 and the anti-Cx43 antibodies, labelling for each connexin was performed on separate sections; therefore, we could not evaluate the co-localization of Cx40 and Cx43. We cannot assign causality and can only speculate on the relationship between connexins and conduction. Although our study showed that there was structural remodelling due to atrial dilation in PV, it remains to be determined how much these effects pertain to the clinical situation.
REFERENCES
- 1.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population based cohort: The Framingham Heart Study. JAMA. 1994;271:840–4. [PubMed] [Google Scholar]
- 2.Schoonderwoerd BA, Van Gelder IC, Van Veldhuisen DJ, Van den Berg MP, Crijns HJ. Electrical and structural remodeling: Role in the genesis and maintenance of atrial fibrillation. Prog Cardiovasc Dis. 2005;48:153–68. doi: 10.1016/j.pcad.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431–88. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- 4.Gros DB, Jongsma HJ. Connexins in mammalian heart function. Bioessays. 1996;18:719–30. doi: 10.1002/bies.950180907. [DOI] [PubMed] [Google Scholar]
- 5.Nattel S, Li D, Yue L. Basic mechanisms of atrial fibrillation – very new insights into very old ideas. Annu Rev Physiol. 2000;62:51–77. doi: 10.1146/annurev.physiol.62.1.51. [DOI] [PubMed] [Google Scholar]
- 6.Davis LM, Kanter HL, Beyer EC, Saffitz JE. Distinct gap junction protein phenotypes in cardiac tissues with disparate conduction properties. J Am Coll Cardiol. 1994;24:1124–32. doi: 10.1016/0735-1097(94)90879-6. [DOI] [PubMed] [Google Scholar]
- 7.Verheule S, Wilson E, Everett T, 4th, Shanbhag S, Golden C, Olgin J. Alterations in atrial electrophysiology and tissue structure in a canine model of chronic atrial dilation due to mitral regurgitation. Circulation. 2003;107:2615–22. doi: 10.1161/01.CIR.0000066915.15187.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rucker-Martin C, Milliez P, Tan S, et al. Chronic hemodynamic overload of the atria is an important factor for gap junction remodeling in human and rat hearts. Cardiovasc Res. 2006;72:69–79. doi: 10.1016/j.cardiores.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi S, Akita T, Takagishi Y, et al. Disorganization of gap junction distribution in dilated atria of patients with chronic atrial fibrillation. Circ J. 2006;70:575–82. doi: 10.1253/circj.70.575. [DOI] [PubMed] [Google Scholar]
- 10.Neuberger HR, Schotten U, Verheule S, et al. Development of a substrate of atrial fibrillation during chronic atrioventricular block in the goat. Circulation. 2005;111:30–7. doi: 10.1161/01.CIR.0000151517.43137.97. [DOI] [PubMed] [Google Scholar]
- 11.Ohara K, Miyauchi Y, Ohara T, et al. Downregulation of immunodetectable atrial connexin40 in a canine model of chronic left ventricular myocardial infarction: Implications to atrial fibrillation. J Cardiovasc Pharmacol Ther. 2002;7:89–94. doi: 10.1177/107424840200700205. [DOI] [PubMed] [Google Scholar]
- 12.Wilhelm M, Kirste W, Kuly S, et al. Atrial distribution of connexin 40 and 43 in patients with intermittent, persistent, and postoperative atrial fibrillation. Heart Lung Circ. 2006;15:30–7. doi: 10.1016/j.hlc.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–8. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- 14.Rohr S. Role of gap junctions in the propagation of the cardiac action potential. Cardiovasc Res. 2004;62:309–22. doi: 10.1016/j.cardiores.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 15.Dupont E, Ko Y, Rothery S, et al. The gap-junctional protein connexin40 is elevated in patients susceptible to postoperative atrial fibrillation. Circulation. 2001;103:842–9. doi: 10.1161/01.cir.103.6.842. [DOI] [PubMed] [Google Scholar]
- 16.Kostin S, Klein G, Szalay Z, Hein S, Bauer EP, Schaper J. Structural correlate of atrial fibrillation in human patients. Cardiovasc Res. 2002;54:361–79. doi: 10.1016/s0008-6363(02)00273-0. [DOI] [PubMed] [Google Scholar]
- 17.Nao T, Ohkusa T, Hisamatsu Y, et al. Comparison of expression of connexin in right atrial myocardium in patients with chronic atrial fibrillation versus those in sinus rhythm. Am J Cardiol. 2003;91:678–83. doi: 10.1016/s0002-9149(02)03403-3. [DOI] [PubMed] [Google Scholar]
- 18.Polontchouk L, Haefliger JA, Ebelt B, et al. Effects of chronic atrial brillation on gap junction distribution in human and rat atria. J Am Coll Cardiol. 2001;38:883–91. doi: 10.1016/s0735-1097(01)01443-7. [DOI] [PubMed] [Google Scholar]
- 19.van der Velden HM, Ausma J, Rook MB. Gap junctional remodeling in relation to stabilization of atrial fibrillation in the goat. Cardiovasc Res. 2000;46:476–86. doi: 10.1016/s0008-6363(00)00026-2. [DOI] [PubMed] [Google Scholar]
- 20.Guerra JM, Everett TH, 4th, Lee KW, Wilson E, Olgin JE. Effects of the gap junction modifier rotigaptide (ZP123) on atrial conduction and vulnerability to atrial fibrillation. Circulation. 2006;114:110–8. doi: 10.1161/CIRCULATIONAHA.105.606251. [DOI] [PubMed] [Google Scholar]
- 21.Giepmans BN. Gap junctions and connexin-interacting proteins. Cardiovasc Res. 2004;62:233–45. doi: 10.1016/j.cardiores.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Luo MH, Li YS, Yang KP. Fibrosis of collagen I and remodeling of connexin 43 in atrial myocardium of patients with atrial fibrillation. Cardiology. 2006;107:248–53. doi: 10.1159/000095501. [DOI] [PubMed] [Google Scholar]
- 23.van der Velden HM, Jongsma HJ. Cardiac gap junctions and connexins: Their role in atrial fibrillation and potential as therapeutic targets. Cardiovasc Res. 2002;54:270–9. doi: 10.1016/s0008-6363(01)00557-0. [DOI] [PubMed] [Google Scholar]
- 24.Qu Z, Karagueuzian HS, Garfinkel A, Weiss JN. Effects of Na(+) channel and cell coupling abnormalities on vulnerability to reentry: A simulation study. Am J Physiol Heart Circ Physiol. 2004;286:H1310–21. doi: 10.1152/ajpheart.00561.2003. [DOI] [PubMed] [Google Scholar]