Abstract
Maltreated foster children are subjected to a range of early adverse experiences, including neglect, abuse, and multiple caregiver disruptions. Research suggests that such disturbances alter the development and subsequent functioning of the hypothalamic-pituitary-adrenocortical system. The current study was designed to investigate morning cortisol levels in 117 foster children and 60 low-income, nonmaltreated children. Maltreatment and foster care placement experiences were coded from official records. Analyses revealed that the foster children were significantly more likely than the nonmaltreated children to have low morning cortisol levels. Additionally, specific maltreatment experiences were significantly associated with the foster children’s morning cortisol levels. Foster children with low morning cortisol levels experienced more severe physical neglect than the other foster children. In contrast, foster children with high morning cortisol levels experienced more severe emotional maltreatment. These results suggest that specific early adverse experiences have differential effects on the functioning of the hypothalamic-pituitary-adrenocortical system.
Keywords: cortisol, preschool-aged children, foster care, physical neglect, emotional maltreatment
In the United States, there are 513,000 children in foster care and 311,000 children enter foster care each year (U.S. Department of Health and Human Services, 2006). Foster children are exposed to a host of early adverse experiences, including neglectful and/or abusive care and repeated caregiver disruptions. It is not surprising, therefore, that numerous studies have found foster children to be an exceptionally high-risk group. Foster children exhibit physical, cognitive, and socioemotional disparities compared to their peers (Leslie, Gordon, Ganger, & Gist, 2002; Pears & Fisher, 2005; Zima et al., 2000). These children are also at risk for anxiety disorders, affective disorders, and disruptive behavior disorders (Casey Family Programs, 2005; Clausen, Landsverk, Ganger, Chadwick, & Litrownik, 1998; Garland et al., 2001). Although there is considerable evidence of negative outcomes among foster children, little is known about the biological mechanisms underlying the association between early adverse experiences and later maladaptation in this population. Moreover, there is limited research examining the differential impact of specific dimensions of early adverse experiences on these mechanisms. The current study was aimed at examining the impact of maltreatment and foster care placement experiences on one possible biological mechanism, the hypothalamic-pituitary-adrenocortical (HPA) system.
The HPA system serves two distinct functions: maintaining the diurnal rhythmicity of hormone production and mounting responses to stressors. Cortisol, the hormonal end product of the HPA system, displays a diurnal rhythm characterized by relatively high levels at waking that steadily decrease to very low levels at bedtime (Schmidt-Reinwald et al., 1999). Elevated morning cortisol levels play a role in the metabolism of stored energy, the stimulation of appetite, and the promotion of processes involved in learning (de Kloet, 1991; de Kloet, reugdenhil, Oitzl, & Joels, 1998). The HPA system is also sensitive to a wide range of physically aversive and psychologically stressful situations. Cortisol facilitates the mobilization of the central and peripheral resources necessary to manage such challenges (Hennessy & Levine, 1979; Sapolsky, Romero, & Munck, 2000). Although the HPA system is critical to adaptive functioning, prolonged dysregulation of this system has deleterious effects on physical development, immune functioning, and cognitive functioning (Johnson, Kamilaris, Chrousos, & Gold, 1992; Lupien, Maheu, Tu, Fiocco, & Schramek, 2007; Sapolsky et al., 2000). Additionally, dysregulation of the HPA system has been implicated in the etiology of anxiety disorders, affective disorders, and disruptive behavior disorders (De Bellis et al., 1999; Heim & Nemeroff, 2001; Kaufman & Charney, 2001; Repetti, Taylor, & Seeman, 2002).
There is substantial evidence suggesting that early adverse experiences profoundly alter the development and subsequent functioning of the HPA system (Bremner & Vermetten, 2001; Gunnar & Vazquez, 2001; Sáchez, Ladd, & Plotsky, 2001). Acute stressors activate the HPA system and elevate cortisol levels. As such, atypically high morning cortisol levels have been observed among children who experienced multiple types of maltreatment, maltreated children with posttraumatic stress disorder, and adolescents exposed to maternal postnatal depression (Cicchetti & Rogosch, 2001; De Bellis et al., 1999; Halligan, Herbert, Goodyer, & Murray, 2004). In contrast, chronic stress and activation of the HPA system are believed to lead to down-regulation of the system and blunted cortisol levels at the expected peak of the diurnal rhythm and/or during stressors (Fries, Hesse, Hellhammer, & Hellhammer, 2005; Gunnar & Vazquez, 2001; Heim, Ehlert, & Hellhammer, 2000). For example, low morning cortisol levels have been observed in rhesus monkeys exposed to repeated maternal separations, children raised in institutions, and women with histories of childhood abuse (Carlson & Earls, 1997; Heim, Newport, Bonsall, Miller, & Nemeroff, 2001; Sáchez et al., 2005). Most germane to the current study, children placed in foster care as infants had lower morning cortisol levels than nonmaltreated children (Dozier et al., 2006). In sum, although there is evidence that early adverse experiences alter the development of the HPA system, high and low morning cortisol levels have been observed among different populations exposed to these experiences.
Given these findings, it might be fruitful to elucidate the specific dimensions of early adverse experiences that impact the HPA system. For example, prolonged maternal separations during the first weeks of life have been shown to alter stress reactivity in rodents (Rosenfeld, Wetmore, & Levine, 1992; van Oers, de Kloet, & Levine, 1997). However, these effects on stress reactivity were related to disruptions in specific maternal behaviors such as licking, grooming, and nursing behaviors during the separations (Suchecki, Rosenfeld, & Levine, 1993; van Oers, de Kloet, & Levine, 1999). Similarly, specifying the relevant dimensions of early adverse experiences in humans might shed light on the observation of high and low morning cortisol levels among different populations. In this regard, foster children might be an ideal population to study. Foster children’s early adverse experiences can be abstracted from detailed official records and can be quantified using standardized coding systems (e.g., Barnett, Manly, & Cicchetti, 1993). Additionally, foster children tend to be quite heterogeneous in terms of their maltreatment and foster care placement experiences (e.g., type and severity of maltreatment). Thus, the impact of specific dimensions of early adverse experiences can be examined.
Although there has been limited research on the HPA system with foster children, prior research has suggested that specific maltreatment and foster care experiences are associated with differential effects on the HPA system. For example, severe sexual abuse was related to high morning cortisol levels in school-aged maltreated children (Cicchetti & Rogosch, 2001). In contrast, it has been argued that the absence of a responsive caregiver, as is seen in cases of severe neglect, leaves young children vulnerable to stressors and chronic activation of the HPA system, ultimately leading to blunted morning cortisol levels (Gunnar, Fisher, & the Early Experience, Stress, and Prevention Network, 2006). Indeed, low morning cortisol levels have been observed among children placed in foster care primarily due to neglect and children raised in neglectful institutions (Carlson & Earls, 1997; Dozier et al., 2006). Finally, because repeated maternal separations resulted in low diurnal cortisol levels in nonhuman primates (Dettling, Feldon, & Pryce, 2002; Sáchez et al., 2005), the number of caregiver disruptions might be associated with low morning cortisol levels among foster children. However, although the number of caregiver disruptions has not been examined in foster children, two other foster care placement variables, age at entry into foster care and length of time since entering foster care, were found to be unrelated to morning cortisol levels (Dozier et al., 2006).
The current study was designed to examine patterns of morning cortisol production in foster children and their nonmaltreated peers and to explore associations between foster children’s morning cortisol levels and their maltreatment and foster care experiences. The study was focused on morning cortisol levels because diurnal cortisol levels can be assessed noninvasively in young children and because prior research has suggested that early adverse experiences impact morning cortisol levels (Carlson & Earls, 1997; Cicchetti & Rogosch, 2001; Dozier et al., 2006). In addition to using morning cortisol levels as a continuous variable, a trichotomous variable was created to permit the examination of low, average, and high morning cortisol levels separately. Furthermore, prior research has shown that adverse experiences broaden the distribution of cortisol levels by increasing the frequency of extreme values (Kaspers & Scholz, 2004), which distorts the sample mean and violates the distributional assumptions of most statistical tests. It was hypothesized that foster children, as a group, would be more likely to display low morning cortisol levels than nonmaltreated children. However, it was predicted that specific early adverse experiences would be differentially associated with morning cortisol levels. In particular, foster children who had experienced more severe sexual abuse were expected to exhibit high morning cortisol levels and foster children who had experienced more severe physical neglect were expected to exhibit low morning cortisol levels. It was difficult to make specific hypotheses regarding the relations between morning cortisol levels and foster care placement experiences; thus, these analyses were exploratory.
Methods
Participants
The sample included a foster care (FC) group of 117 maltreated 3- to 6-year-olds residing in foster care and a community comparison (CC) group of 60 low-income, nonmaltreated 3- to 6-year-olds living with their biological parents. For the FC group, children who were within the selected age range and were entering a new foster care placement were referred to the study by the county branch of the state child welfare system office. Referrals included children being placed in foster care for the first time, reentering foster care following failed reunifications with biological parents, or transitioning to a new foster care placement. The CC children were recruited via flyers posted throughout the community and advertisements in local newspapers. Inclusion criteria for the CC group were as follows: child consistently lived with at least one biological parent, household income was less than $30,000, parental education was less than a 4-year college degree, and family did not have any previous involvement with child welfare services as verified by child welfare services records.
All of the children were part of a randomized efficacy trial of a preventive intervention program for preschool-aged foster children and their caregivers (Multidimensional Treatment Foster Care for Preschoolers; Fisher, Ellis, & Chamberlain, 1999), in which the FC children were randomly assigned to the intervention condition or services-as-usual comparison condition. However, because the measures in the current paper were collected prior to the intervention, foster care treatment condition was not considered in the analyses. (The impact of the intervention on the children’s diurnal cortisol levels over a 12-month period is presented elsewhere [Fisher & Stoolmiller, in press; Fisher, Stoolmiller, Gunnar, & Burraston, 2007].)
Measures
Salivary cortisol
The caregivers were trained by staff members to collect saliva samples from their children. The samples were obtained on 2 consecutive, typical weekday mornings (30 min after waking but before eating breakfast). To stimulate salivation, the children chewed Trident Original sugarless gum, which prior research has shown to not affect cortisol levels (Schwartz, Granger, Susman, Gunnar, & Laird, 1998). Salivettes (Sarstedt, Inc., Newton, NC) were then placed in the children’s mouths. Once saturated, the Salivettes were placed in prelabeled plastic vials. The caregivers were instructed to freeze the samples until transported to the laboratory by staff members. The samples were then stored at −5°F (−20°C) until assayed. The samples were assayed for cortisol determination using the High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit (Salimetrics, LLC, State College, PA). Samples from each child were included in the same assay batch to minimize within-subject variability. The samples were assayed in duplicate and were averaged. Duplicates varying by more than 15% were reassayed. The intraassay and interassay coefficients of variance were 2.70% and 10.98%, respectively.
Certain medications, general health, food intake, and sleep patterns have been shown to affect cortisol levels. Thus, children who used steroid-based medications (e.g., steroidal asthma inhalers) on a regular basis were excluded from the study. Caregivers were reminded during daily phone conversations to avoid sampling when their children were using steroid-based medications or were ill. Caregivers completed a brief questionnaire regarding sampling times and their children’s eating and sleeping behaviors on the sampling days. The questionnaires were inspected to ensure compliance with sampling guidelines; two samples (i.e., both samples from one CC child) were excluded from analyses due to incorrect sampling times.
Cortisol level and cortisol classification
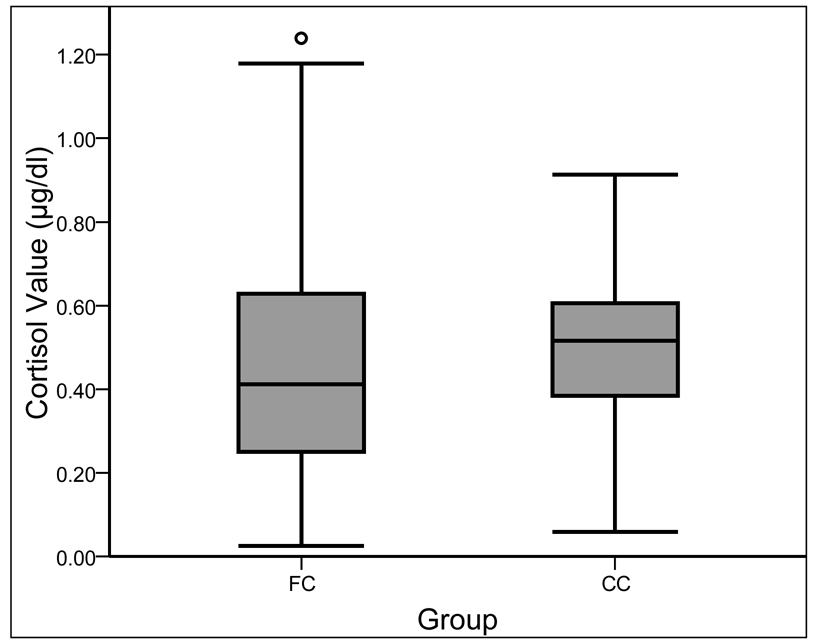
Because cortisol values (the term used to refer to the untransformed cortisol data) from the 2 sampling days were significantly correlated, r(169) = .37, p < .001, they were averaged to create a more reliable measure. For the continuous variable referred to as cortisol level, the cortisol values were subjected to a square root transformation and two outliers (i.e., more than 2.5 standard deviations above the mean) were replaced with less extreme cortisol values (i.e., next highest cortisol value in the sample) to normalize the distribution. The cortisol values were also transformed into a trichotomous variable referred to as cortisol classification. Cortisol values corresponding with the lower and upper quartile for the sample were used to classify the cortisol values as low (< .30 µg/dl), average (.30–.60 µg/dl), or high (> .60 µg/dl). The whole sample was used to establish these cutoffs to avoid biasing the analyses examining the group difference in cortisol classification. (It is difficult to compare cortisol values across studies due to different sampling and assaying techniques. However, as an informal comparison, Dozier and colleagues (2006) classified morning cortisol values less than .21 µg/dl as low and greater than .84 µg/dl as high.) Although the children’s cortisol values are displayed in Figure 1, the transformed cortisol level and cortisol classification variables were used in all analyses.
Figure 1.
Distributions of the untransformed, uncensored cortisol values for the foster care (FC) and community comparison (CC) children. (The circle indicates a cortisol value more than 3 times the interquartile range away from the upper quartile.)
Maltreatment experiences
The FC children’s maltreatment experiences were coded from child welfare services records using the Maltreatment Classification System (MCS; Barnett et al., 1993), which allows for the classification of each incident according to type and severity. The types of maltreatment defined in the MCS comprise physical abuse, sexual abuse, physical neglect (i.e., parental failure to provide adequate food, clothing, shelter, or medical care), supervisory neglect (i.e., parental failure to provide age-appropriate supervision), and emotional maltreatment (i.e., parental rejection, abandonment, or failure to protect child from witnessing traumatic events). Maltreatment severity in the MCS is coded on a 5-point scale ranging from less serious incidents to potentially life-threatening incidents.
Training in the use of this coding system was provided by one of the MCS authors (J. T. Manly). Twenty percent of the records were coded by two coders to compute interrater agreement. Coders first examined the child welfare services records to identify incidents of maltreatment. To be considered an incident, the situation had to match the provided definitions of maltreatment and had to be reported by a mandated reporter or founded by a child welfare services caseworker. Agreement on the identification of incidents of maltreatment was high (80%). Similarly, interrater agreement for the severity of each type of maltreatment was high (average κ = .72, range for individual categories = .82–.65). Number of maltreatment incidents, number of types of maltreatment, and mean severity for each type of maltreatment were calculated for each FC child.
Foster care placement experiences
The FC children’s foster care placement experiences were also coded from child welfare services records. These highly detailed records include the date of first entry into the foster care system and the date of entry and exit from every foster care placement thereafter. From these records, age at first entry into foster care, number of caregiver disruptions (i.e., due to placements in foster care, transitions within the foster care system, and eunifications with biological parents), and length of time since entering the current foster care placement were calculated for each FC child.
Statistical Analysis
Preliminary analyses were conducted to investigate the associations between cortisol level and cortisol classification and a number of potentially confounding demographic variables. Group differences in cortisol level and cortisol classification were then examined using an independent-samples t test and chi-square analyses, respectively. Pearson product-moment correlations were subsequently conducted to investigate the relations between the FC children’s cortisol levels and their early adverse experiences, and one-way analyses of variance (ANOVAs) and post hoc paired comparisons using Fisher’s least significant difference were conducted to examine the associations between the FC children’s cortisol classifications and their early adverse experiences. Because the CC children did not have any involvement with child welfare services, they were not included in the analyses exploring early adverse experiences. An alpha level of .05 was used for all statistical tests.
Results
Preliminary Analyses
The FC and CC children did not significantly differ in terms of age, F(1, 175) = 0.56, ns, sex, Pearson χ2(1, N = 177) = 0.01, ns, or ethnicity, Pearson χ2(1, N = 177) = 3.20, ns. The mean age was 4.41 years (SD = 0.84) for the FC children and 4.31 years (SD = 0.79) for the CC children. Males made up 54% (n = 63) of the FC group and 53% (n = 32) of the CC group. The ethnicity of the FC children was 87% (n = 102) European American, 7% (n = 8) Latino, 5% (n = 6) Native American, and 1% (n = 1) African American. The ethnicity of the CC children was 77% (n = 46) European American, 8% (n = 5) Latino, 7% (n = 4) Native American, 7% (n = 4) African American, and 2% (n = 1) Pacific Islander. Preliminary analyses revealed that cortisol level was not significantly related to sex, r(174) = .02, ns, age, F(1, 174) = 0.01, ns, or ethnicity F(1, 174) = 0.47, ns. Similarly, cortisol classification was not predicted by age, F(2, 173) = 0.25, ns, sex, Pearson χ2(2, N = 176) = 1.46, ns, or ethnicity, Pearson χ2(2, N = 176) = 1.45, ns.
Most of the FC children remained in foster care over the course of the study, which made it difficult to obtain information about the FC children’s biological families and to compare the family characteristics of the FC and CC children. However, this information was obtained for a subsample of FC children who returned to their biological parents’ care (n = 39). The income level for these FC families and the CC families was significantly different, F(1, 97) = 10.62, p < .005, with median income levels of $10,000–14,999 for the FC families and $15,000–19,999 for the CC families. The groups also differed on the highest level of education attained by the FC and CC parents, F(1, 97) = 16.98, p < .001 (FC Mdn = completed high school; CC Mdn = completed some college or vocational school courses). Preliminary analyses with the children for whom this information was available indicated that cortisol level was not associated with income, r(96) = .12, ns, or parental education, r(96) = .14, ns. Additionally, cortisol classification was not related to income, F(2, 95) = 0.96, ns, or parental education, F(2, 95) = 1.43, ns.
There were 24 sibling dyads (14 FC and 10 CC), 3 sibling triads (3 FC), and 1 sibling tetrad (1 FC) in the study. Preliminary analyses, which included only one randomly selected child from each sibling group, were conducted to determine the effect of these sibling groups on the results. The pattern of these results was the same as the results obtained from the analyses that included all of the children. Thus, to preserve power, all of the children were retained for subsequent analyses.
Group Differences in Cortisol Level and Cortisol Classification
An independent-samples t test was conducted to explore the difference between the FC and CC children’s cortisol levels. Levene’s Test for Equality of Variances indicated that the variances of the groups differed, F(1, 174) = 10.42, p < .001. Thus, the statistics for the t test that does not assume equality of variances are reported. The cortisol levels of the FC and CC children were significantly different, t(149.73) = −1.99, p < .05, with lower cortisol levels observed in the FC children (M = 0.64, SD = 0.20) than in the CC children (M = 0.69, SD = 0.15). The distributions of cortisol values for each group are presented in Figure 1.
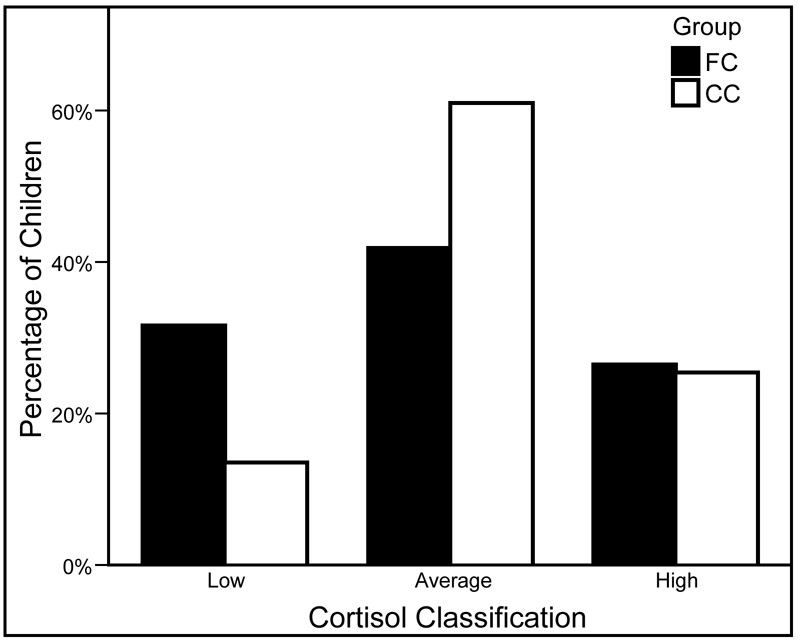
Consistent with the results for cortisol level, the results from a chi-square analysis with the FC and CC children revealed that there was a significant group difference in cortisol classification, Pearson χ2(2, N = 176) = 8.00, p < .05. As is shown in Figure 2, follow-up pairwise comparisons indicated that the FC children were more likely to be in the low cortisol classification group than the CC children, Pearson χ2(1, N = 176) = 6.73, p < .01, and that the CC children were more likely to be in the average cortisol classification group than the FC children, Pearson χ2(1, N = 176) = 5.75, p < .05. The percentage of children in the high cortisol classification group did not differ across groups, Pearson χ2(1, N = 176) = 0.02, ns.
Figure 2.
Percentage of foster care (FC) and community comparison (CC) children in the low (FC n = 37; CC n = 8), average (FC n = 49; CC n = 36), and high (FC n = 31; CC n = 15) cortisol classification groups.
Given the rapid decrease in cortisol levels across the morning, it was important to verify that the observed group differences in cortisol level and cortisol classification did not reflect differential sampling procedures. Although cortisol level was not significantly related to the length of time between waking up and collecting the sample, r(174) = −.13, ns, it was correlated with the time of sample collection, r(174) = −.17, p < .05. Similarly, cortisol classification was significantly associated with the time of sample collection, F(2, 173) = 3.92, p < .05, but not the length of time between waking up and collecting the sample, F(2, 173) = 1.92, ns. However, one-way ANOVAs revealed that the groups did not significantly differ in terms of the length of time between waking up and collecting the sample, F(1, 174) = 0.84, ns, or the time of sample collection, F(1, 174) = 0.45, ns. Thus, the differences between the FC and CC children in morning cortisol levels were not merely manifestations of differential sampling procedures.1
Associations Between Cortisol Level and Cortisol Classification and Early Adverse Experiences
Descriptive information about the FC children’s maltreatment and foster care placement experiences suggested that these children encountered a range of early adverse experiences: 95% (n = 111) of the FC children experienced multiple types of maltreatment. In terms of the specific types of maltreatment, 32% (n = 38) experienced physical abuse, 26% (n = 30) experienced sexual abuse, 82% (n = 96) experienced physical neglect, 89% (n = 104) experienced supervisory neglect, and 90% (n = 105) experienced emotional maltreatment. Additionally, the FC children experienced an average of 3.74 caregiver disruptions (SD = 2.25), with 85% (n = 100) of the FC children having experienced multiple caregiver disruptions.
Relations between the FC children’s cortisol levels and maltreatment and foster care experiences were examined using Pearson product-moment correlations. As was expected, cortisol level was negatively correlated with severity of physical neglect, r(115) = −.22, p < .05. In addition, cortisol level was positively related to severity of emotional maltreatment, r(115) = .25, p < .01. Cortisol level was not associated with the number of maltreatment incidents, r(115) = −.06, ns, number of types of maltreatment, r(115) = −.06, ns, severity of physical abuse, r(115) = −.05, severity of sexual abuse, r(115) = −.07, ns, or severity of supervisory neglect, r(115) = − .03, ns. Cortisol level was also not significantly correlated with age at entry into foster care, r(115) = .17, ns, number of caregiver disruptions, r(115) = −.14, ns, or length of time since entering the current foster care placement, r(115) = −.06, ns.
Paralleling the results for cortisol level, the results from a one-way ANOVA indicated that the severity of physical neglect was significantly related to cortisol classification, F(2, 114) = 4.01, p < .05. As is shown in Table 1, the children in the low cortisol classification group experienced more severe physical neglect than the children in the average and high cortisol classification groups, t(84) = 2.15, p < .05, and t(66) = 2.68, p < .01, respectively. In contrast, the children in the average and high cortisol classification groups did not differ on severity of physical neglect, t(78) = −.80, ns. Additionally, cortisol classification was significantly associated with the severity of emotional maltreatment, F(2, 114) = 3.98, p < .05. Post hoc paired comparisons showed that children in the high cortisol classification group had experienced more severe emotional maltreatment than children in the low and average cortisol classification groups, t(66) = 2.23, p < .05, and t(78) = 2.70, p < .01, respectively. Children in the low cortisol classification group did not significantly differ from children in the average cortisol classification group on severity of emotional maltreatment, t(84) = 0.35, ns. Cortisol classification was not related to the number of maltreatment incidents, F(2, 114) = 0.03, ns, number of types of maltreatment, F(2, 114) = 0.03, ns, severity of physical abuse, F(2, 114) = 0.29, ns, severity of sexual abuse, F(2, 114) = 0.10, ns, or severity of supervisory neglect, F(2, 114) = 0.04, ns. Cortisol classification was also not significantly related to age at entry into foster care, F(2, 114) = 1.60, ns, number of caregiver disruptions, F(2, 114) = 0.95, ns, or length of time since entering the current foster care placement, F(2, 114) = 0.49, ns.2
Table 1.
Descriptive Statistics for Early Adverse Experiences for Foster Care Children in Each Cortisol Classification Group
| Low | Average | High | Total | |||||
|---|---|---|---|---|---|---|---|---|
| Early adverse experiences | M | SD | M | SD | M | SD | M | SD |
| Number of maltreatment incidents | 6.95 | 3.17 | 6.88 | 4.61 | 7.10 | 4.40 | 6.96 | 4.11 |
| Number of types of maltreatment | 3.35 | 0.98 | 3.31 | 1.18 | 3.29 | 1.24 | 3.32 | 1.13 |
| Mean severity of physical abuse | 0.73 | 1.29 | 0.86 | 1.27 | 0.65 | 1.12 | 0.76 | 1.23 |
| Mean severity of sexual abuse | 0.73 | 1.40 | 0.71 | 1.29 | 0.60 | 1.14 | 0.69 | 1.28 |
| Mean severity of physical neglect*,a | 2.21 | 1.05 | 1.70 | 1.00 | 1.51 | 1.23 | 1.81 | 1.11 |
| Mean severity of supervisory neglect | 2.88 | 1.42 | 2.86 | 1.16 | 2.79 | 1.14 | 2.85 | 1.23 |
| Mean severity of emotional maltreatment*,b | 2.61 | 1.44 | 2.52 | 1.35 | 3.32 | 1.02 | 2.76 | 1.34 |
| Age at first entry into foster care (years) | 3.11 | 1.66 | 3.46 | 1.41 | 3.72 | 1.06 | 3.42 | 1.42 |
| Number of caregiver disruptions | 3.89 | 2.13 | 3.92 | 2.41 | 3.26 | 2.11 | 3.74 | 2.25 |
| Time since entering current foster care placement (days) | 32.32 | 10.45 | 33.86 | 7.58 | 32.29 | 6.68 | 32.96 | 8.36 |
Low > Average and High.
High > Low and Average.
p < .05.
Discussion
The current study was designed to examine patterns of morning cortisol production among maltreated foster children compared to their nonmaltreated peers. Overall, there were two noteworthy findings. First, the results of this study provide corroborating evidence that early adverse experiences have a significant impact on the HPA system. As was hypothesized, the foster children displayed significantly lower morning cortisol levels than the nonmaltreated children. Furthermore, when the children’s morning cortisol levels were classified as low, average, or high, nearly one third of the foster children (more than twice the percentage of nonmaltreated children) were in the low cortisol classification group. These results are consistent with research involving nonhuman primates exposed to repeated maternal separations, children raised in institutions, and children placed in foster care as infants (Carlson & Earls, 1997; Dozier et al., 2006; Sáchez et al., 2005). Taken in combination, these studies support the conceptualization that blunted morning cortisol levels are a likely consequence of chronic stress and chronic activation of the HPA system (Fries et al., 2005; Gunnar & Vazquez, 2001; Heim, Ehlert, et al., 2000). Although the exact mechanism is not known, it has been proposed that chronic elevations in corticotrophin-releasing factor (CRF), a hypothalamic secretogogue that begins the hormonal cascade of the HPA system, result in an adaptive down-regulation of CRF receptors in the pituitary and ultimately blunted cortisol levels (Heim, Ehlert, et al., 2000; Heim et al., 2001). However, there is a need for research examining the counterregulatory mechanisms underlying the low morning cortisol levels observed among these populations.
Second, the results from the current study suggest that specific early adverse experiences differentially affect the HPA system. That is, although the foster children were more likely to exhibit low morning cortisol levels, there was a great deal of heterogeneity within the foster sample. Notably, individual differences in morning cortisol levels were associated with different maltreatment experiences. Consistent with prior research with neglected children (Carlson & Earls, 1997; Dozier et al., 2006), foster children with histories of severe physical neglect were more likely to have low morning cortisol levels. In contrast, foster children who had experienced severe emotional maltreatment were more likely to have high morning cortisol levels. This result is consistent with prior research findings that depressed, school-aged children who were experiencing ongoing, severe emotional maltreatment demonstrated increased activation of the HPA system in response to pharmacological manipulation (Kaufman et al., 1997). In the current study, morning cortisol levels were not significantly associated with severity of physical abuse, sexual abuse, or supervisory neglect or with number of caregiver disruptions.
A possible explanation for the differential effects of physical neglect and emotional maltreatment is that these experiences represent different types of stressors. That is, it has been suggested that chronic stress results in decreased cortisol production, whereas acute stress results in increased cortisol production (Fries et al., 2005). In the current study, severe physical neglect involved parental failure to meet the children’s physical needs and to provide responsive caregiving. Such unresponsive caregiving might also fail to buffer young children from stressors and chronic activation of the HPA system (Gunnar & Donzella, 2002; Gunnar et al., 2006). As such, physical neglect might be considered a pervasive, chronic stressor. In contrast, emotional maltreatment included parental rejection and failure to protect children from witnessing traumatic events. Emotional maltreatment of this nature might be characterized as a periodic, acute stressor. Although this explanation is consistent with the conceptualization formulated by Fries and colleagues (2005), additional research is needed to understand the nature of these maltreatment experiences. Such research might prove beneficial in moving toward more defined conceptual models with specific early adverse experiences contributing to specific outcomes.
There are a number of potential implications of the different patterns of morning cortisol production observed among foster children. Because elevated morning cortisol levels mobilize energy resources, children with low morning cortisol levels might lack the needed resources to undertake the processes involved in learning and socialization (Gunnar & Vazquez, 2001). In addition, because cortisol has an immunosuppressive action, low cortisol levels might result in increased vulnerability to autoimmune disorders (Heim, Ehlert, et al., 2000). Finally, low diurnal cortisol levels have been observed in adolescent males with externalizing behaviors and adults with posttraumatic stress disorder (Shirtcliff, Granger, Booth, & Johnson, 2005; Yehuda, Halligan, & Bierer, 2002). In contrast, elevated cortisol levels have been shown to impair cognitive processes, particularly vigilance and memory (Lupien et al., 2007). Elevated cortisol levels also suppress immune and inflammatory reactions, leaving individuals vulnerable to illnesses and infections (Sapolsky et al., 2000). Finally, a number of studies have reported elevated diurnal cortisol levels among individuals with affective disorders (Gold, Goodwin, & Chrousos, 1988; Plotsky, Owens, & Nemeroff, 1998). Thus, both low and high morning cortisol levels might be dysfunctional and might place foster children at risk for a host of difficulties.
Conversely, elevated diurnal cortisol levels among foster children might be an adaptive response to neglectful and/or abusive environments (Tarullo & Gunnar, 2006). That is, the capacity to mobilize the HPA system might reflect an active attempt to cope with stressful living conditions. Indeed, Cicchetti and Rogosch (2007) found high morning cortisol levels among physically abused children to be associated with higher resilient functioning. Similarly, high morning cortisol levels might be an adaptive response to the unpredictable, acute stressors experienced within emotionally maltreating environments. However, it is likely that this initially adaptive pattern of cortisol production would be less adaptive in new environments and would be detrimental to the children over time.
It is notable that severity of sexual abuse was not significantly related to the foster children’s morning cortisol levels in the current study. This result diverges from a prior study that found a positive association between severity of sexual abuse and morning cortisol levels (Cicchetti & Rogosch, 2001). There were, however, several differences between the two studies. Specifically, the sample for the current study included preschool-aged maltreated foster children rather than school-aged maltreated children and the cortisol samples for the current study were collected at the children’s homes rather than at a day camp. Thus, future research should explore whether the discrepant results were due to developmental or contextual differences. For example, the morning cortisol levels in the prior study might reflect the children’s responses to a novel environment and peer group rather than their typical diurnal pattern.
In the current study, the foster children’s morning cortisol levels were also not related to their foster care placement experiences. Similarly, Dozier and colleagues (2006) reported a lack of association between morning cortisol levels and foster care placement experiences. Taken together, these results suggest that specific maltreatment experiences impact the development of the HPA system more strongly than foster care placement experiences.
The results of the current study raise many questions for future research. Because of ethical and methodological issues, most research with young foster children has focused on measures of diurnal cortisol. Although chronic stress appears to result in low morning cortisol levels, it is not appropriate to assume a similar effect on the system’s response to psychological or pharmacological challenges. For example, although women with histories of childhood abuse have been shown to exhibit low diurnal and pharmacologically stimulated cortisol levels, they have also been shown to demonstrate more typical cortisol responses to a psychological stressor (Heim et al., 2001; Heim, Newport, et al., 2000). Additionally, it will be important to examine the malleability of the HPA system following a change in the environment. Interestingly, research with rodents has shown that environmental enrichment later in life ameliorates the effect of maternal separations on stress reactivity (Francis, Diorio, Plotsky, & Meaney, 2002). Furthermore, as is noted above, the children in the current study participated in a randomized efficacy trial of a family-based preventive intervention program. Results of this efficacy trial have demonstrated that the intervention might be effective in preventing the increasingly dysregulated morning cortisol levels observed among the foster children who did not receive the intervention (Fisher & Stoolmiller, in press; Fisher et al., 2007).
In summary, maltreated foster children showed significant alterations in their morning cortisol production compared to nonmaltreated children. Although low morning cortisol levels were the characteristic pattern observed in the foster children, morning cortisol levels varied depending upon their maltreatment experiences. Specifically, the foster children with low morning cortisol levels had experienced more severe physical neglect, whereas the foster children with high morning cortisol levels had experienced more severe emotional maltreatment. Physical abuse, sexual abuse, and supervisory neglect did not appear to impact morning cortisol levels. Overall, these results suggest that the HPA system responds differentially to specific types of early adverse experiences.
Acknowledgements
Support for this research was provided by the following grants: MH059780 and MH065046, NIMH, U.S. PHS; HD045894, NICHD, U.S. PHS; and DA021424, NIDA, U.S. PHS. The authors thank the staff and families of the Multidimensional Treatment Foster Care for Preschoolers program, Kristen Greenley for project management, and Matthew Rabel for editorial assistance.
Footnotes
Although the current study focused on morning cortisol levels, evening cortisol levels were also assessed. The majority of the FC and CC children displayed very low evening cortisol values. Indeed, the cortisol values corresponding with the lower and upper quartile for the sample were .02 µg/dl and .05 µg/dl, respectively. Thus, the evening cortisol values were only examined as a continuous variable. These cortisol values were subjected to a common logarithmic transformation, and four outliers were replaced with less extreme cortisol values to normalize the distribution. The evening cortisol level variance was larger for the FC children than the CC children, F(1, 174) = 5.81, p < .05. However, there was not a significant group difference in evening cortisol level, t(145.50) = 1.53, ns. Of the FC children’s maltreatment and foster care experiences, evening cortisol level was only significantly correlated with severity of sexual abuse, r(115) = −.19, p < .05.
A prior publication (Fisher et al., 2007) noted that the current study found that the foster children who entered foster care before the age of 2 years or who had experienced more than 4 caregiver disruptions were more likely to display low morning cortisol levels than the other foster children and the nonmaltreated children. Subsequent analyses suggested that these associations were not as robust as the associations between morning cortisol levels and maltreatment experiences. Thus, these separate analyses are not presented in the current study.
Contributor Information
Jacqueline Bruce, Email: jackieb@oslc.org, Oregon Social Learning Center, Eugene, OR and Center for Research to Practice, Eugene OR.
Philip A. Fisher, Email: philf@oslc.org, Oregon Social Learning Center, Eugene, OR and Center for Research to Practice, Eugene OR.
Katherine C. Pears, Email: katherinep@oslc.org, Oregon Social Learning Center, Eugene, OR and Center for Research to Practice, Eugene OR.
Seymour Levine, Center for Neuroscience, University of California, Davis, CA.
References
- Barnett D, Manly JT, Cicchetti D. Defining child maltreatment: The interface between policy and research. In: Cicchetti D, Toth SL, editors. Child abuse, child development, and social policy. Vol. 8. Norwood, NJ: Ablex; 1993. pp. 7–73. [Google Scholar]
- Bremner JD, Vermetten E. Stress and development: Behavioral and biological consequences. Development and Psychopathology. 2001;13:473–489. doi: 10.1017/s0954579401003042. [DOI] [PubMed] [Google Scholar]
- Carlson M, Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Annals of the New York Academy of Sciences. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- Casey Family Programs. Improving family foster care: Findings from the Northwest Foster Care Alumni Study. Seattle, WA: Author; 2005. [Google Scholar]
- Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology. 2001;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Personality, adrenal steroid hormones, and resilience in maltreated children: A multilevel perspective. Development and Psychopathology. 2007;19:787–809. doi: 10.1017/S0954579407000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen JM, Landsverk J, Ganger W, Chadwick D, Litrownik A. Mental health problems of children in foster care. Journal of Child and Family Studies. 1998;7:283–296. [Google Scholar]
- De Bellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, et al. Developmental traumatology: I. Biological stress systems. Biological Psychiatry. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- de Kloet ER. Brain corticosteroid receptor balance and homeostatic control. Frontiers in Neuroendocrinology. 1991;12:95–164. doi: 10.1016/j.yfrne.2018.02.003. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Vreugdenhil E, Oitzl MS, Joels A. Brain corticosteroid receptor balance in health and disease. Endocrine Reviews. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Feldon J, Pryce CR. Early deprivation and behavioral and physiological responses to social separation/novelty in the marmoset. Pharmacology,Biochemistry, and Behavior. 2002;73:259–269. doi: 10.1016/s0091-3057(02)00785-2. [DOI] [PubMed] [Google Scholar]
- Dozier M, Manni M, Gordon MK, Peloso E, Gunnar MR, Stovall-McClough KC, et al. Foster children’s diurnal production of cortisol: An exploratory study. Child Maltreatment. 2006;11:189–197. doi: 10.1177/1077559505285779. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Ellis H, Chamberlain P. Early Intervention Foster Care: A model for preventive risk in young children who have been maltreated. Children's Services: Social Policy, Research, and Practice. 1999;2:159–182. [Google Scholar]
- Fisher PA, Stoolmiller M. Intervention effects on foster parent stress: Associations with children’s cortisol levels. Development and Psychopathology. doi: 10.1017/S0954579408000473. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007;32:892–905. doi: 10.1016/j.psyneuen.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. Journal of Neuroscience. 2002;22:7840–7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Garland AF, Hough RL, McCabe KM, Yeh M, Wood PA, Aarons GA. Prevalence of psychiatric disorders in youths across five sectors of care. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:409–418. doi: 10.1097/00004583-200104000-00009. [DOI] [PubMed] [Google Scholar]
- Gold PW, Goodwin FK, Chrousos GP. Clinical and biochemical manifestations of depression: Relation of the neurobiology of stress. New England Journal of Medicine. 1988;319:413–420. doi: 10.1056/NEJM198808183190706. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Fisher PA, the Early Experience, Stress, and Prevention Network Bringing basic research on early experience and stress neurobiology to bear on preventive interventions for neglected and maltreated children. Development and Psychopathology. 2006;18:651–677. [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Halligan SL, Herbert J, Goodyer IM, Murray L. Exposure to postnatal depression predicts elevated cortisol in adolescent offspring. Biological Psychiatry. 2004;55:376–381. doi: 10.1016/j.biopsych.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biological Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. American Journal of Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. Journal of American Medical Association. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Hennessy JW, Levine S. Stress, arousal, and the pituitary-adrenal system: A psychoendocrine hypothesis. In: Sprague JM, Epstein AN, editors. Progress in psychobiology and physiological psychology. Vol. 8. New York: Academic Press; 1979. pp. 133–178. [Google Scholar]
- Johnson EO, Kamilaris TC, Chrousos GP, Gold PW. Mechanisms of stress: A dynamic overview of hormonal and behavioral homeostasis. Neuroscience and Biobehavioral Reviews. 1992;16:115–130. doi: 10.1016/s0149-7634(05)80175-7. [DOI] [PubMed] [Google Scholar]
- Kaspers FA, Scholz OB. Stress-induced increase in morning cortisol variance. Stress and Health. 2004;20:127–139. [Google Scholar]
- Kaufman J, Birmaher B, Perel J, Dahl RE, Moreci P, Nelson B, et al. The corticotropin-releasing hormone challenge in depressed abused, depressed nonabused, and normal control children. Biological Psychiatry. 1997;42:669–679. doi: 10.1016/s0006-3223(96)00470-2. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Charney D. Effects of early stress on brain structure and function: Implications for understanding the relationship between child maltreatment and depression. Development and Psychopathology. 2001;13:451–471. doi: 10.1017/s0954579401003030. [DOI] [PubMed] [Google Scholar]
- Leslie LK, Gordon JN, Ganger W, Gist K. Developmental delay in young children in child welfare by initial placement type. Infant Mental Health Journal. 2002;23:496–516. [Google Scholar]
- Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain and Cognition. 2007;65:209–237. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Pears KC, Fisher PA. Developmental, cognitive, and neuropsychological functioning in preschool-aged foster children: Associations with prior maltreatment and placement history. Developmental and Behavioral Pediatrics. 2005;26:112–122. doi: 10.1097/00004703-200504000-00006. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Owens MJ, Nemeroff CB. Psychoneuroendocrinology of depression: Hypothalamic-pituitary-adrenal axis. Psychiatric Clinics of North America. 1998;21:293–307. doi: 10.1016/s0193-953x(05)70006-x. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–366. [PubMed] [Google Scholar]
- Rosenfeld P, Wetmore JB, Levine S. Effects of repeated maternal separations on the adrenocortical response to stress of preweanling rats. Physiology and Behavior. 1992;52:787–791. doi: 10.1016/0031-9384(92)90415-x. [DOI] [PubMed] [Google Scholar]
- Sánchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Development and Psychopathology. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sánchez MM, Noble PM, Lyon CK, Plotsky PM, Davis M, Nemeroff CB, et al. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biological Psychiatry. 2005;57:373–381. doi: 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero M, Munck A. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schmidt-Reinwald A, Pruessner JC, Hellhammer DH, Federenko I, Rohleder N, Schürmeyer TH, et al. The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sciences. 1999;64:1635–1660. doi: 10.1016/s0024-3205(99)00103-4. [DOI] [PubMed] [Google Scholar]
- Schwartz EB, Granger DA, Susman EJ, Gunnar MR, Laird B. Assessing salivary cortisol in studies of child development. Child Development. 1998;69:1503–1513. [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Development and Psychopathology. 2005;17:167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Rosenfeld P, Levine S. Maternal regulation of the hypothalamic-pituitary-adrenal axis in the infant rat: The roles of feeding and stroking. Developmental Brain Research. 1993;75:185–192. doi: 10.1016/0165-3806(93)90022-3. [DOI] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Hormones and Behavior. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Washington, DC: Author; The AFCARS report: Preliminary FY 2005 estimates as of September 2006. 2006
- van Oers HJJ, de Kloet ER, Levine S. Persistent, but paradoxical, effects on HPA regulation of infants maternally deprived at different ages. Stress: The International Journal on the Biology of Stress. 1997;1:249–261. doi: 10.3109/10253899709013745. [DOI] [PubMed] [Google Scholar]
- van Oers HJJ, de Kloet ER, Levine S. Persistent effects of maternal deprivation on HPA regulation can be reversed by feeding and stroking, but not by dexamethasone. Journal of Neuroendocrinology. 1999;11:581–588. doi: 10.1046/j.1365-2826.1999.00329.x. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, Bierer LM. Cortisol levels in adult offspring of Holocaust survivors: Relation to PTSD symptom severity in the parent and child. Psychoneuroendocrinology. 2002;27:171–180. doi: 10.1016/s0306-4530(01)00043-9. [DOI] [PubMed] [Google Scholar]
- Zima BT, Bussing R, Freeman S, Yang X, Belin TR, Forness SR. Behavior problems, academic skill delays and school failure among school-aged children in foster care: Their relationship to placement characteristics. Journal of Child and Family Studies. 2000;9:87–103. [Google Scholar]