Abstract
We measured the intrinsic relative activity (RAi) of muscarinic agonists to detect possible selectivity for receptor subtypes and signaling pathways. RAi is a relative measure of the microscopic affinity constant of an agonist for the active state of a GPCR expressed relative to that of a standard agonist. First, we estimated RAi values for a panel of agonists acting at the M4 muscarinic receptor coupled to three distinct G-protein pathways: Gi inhibition of cAMP accumulation, Gs stimulation of cAMP accumulation, and Gα15 stimulation of phosphoinositide hydrolysis. Our results show similar RAi values for each agonist, suggesting that the same active state of the M4 receptor triggers the activation of the three G proteins. We also estimated RAi values for agonists across M1 to M4 muscarinic subtypes stably transfected in Chinese hamster ovary cells. Our results show selectivity of McN-A-343 [4-I-[3-chlorophenyl]carbamoyloxy)-2-butynyltrimethylammnonium chloride] for the M1 and M4 subtypes and selectivity of pilocarpine for the M1 and M3 subtypes. The other agonists tested lacked marked selectivity among M1 to M4 receptors. Finally, we estimated RAi values from published literature on M1, M2, and M3 muscarinic responses and obtained results consistent with our own studies. Our results show that the RAi estimate is a useful receptor-dependent measure of agonist activity.
Novel agonists for G protein coupled receptors (GPCRs) are often identified in high-throughput screens based on receptor coupling to alternative G proteins that mobilize Ca2+ (e.g., Gα15) (for review, see Milligan and Kostenis, 2006). In such a screen, the profile of an agonist may differ from how it behaves when the receptor is coupled to its native G protein (e.g., Gi). Furthermore, the Emax and EC50 values for triggering a response may vary, depending on the signaling pathway and response being measured. If the Emax values of a group of agonists differ within an assay, it is impossible to compare agonist activity accurately using potency ratios.
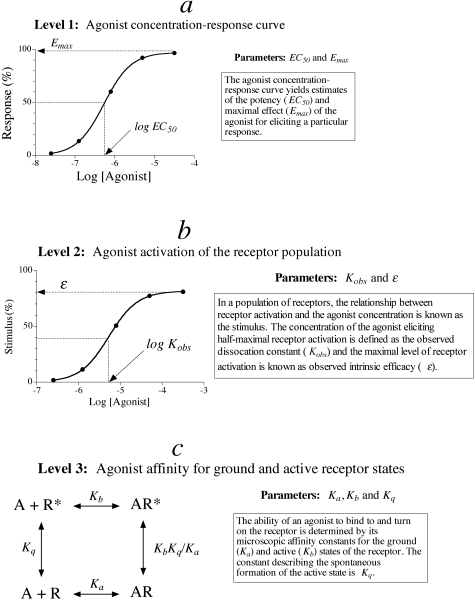
To understand how drug-receptor interactions influence the output of a functional assay, it is useful to consider different hierarchical levels of analysis of drug action (Fig. 1). On the surface (Fig. 1a), the behavior of an agonist in an assay can be characterized by its EC50 and Emax values, which depend not only on the receptor but on other elements in the signaling pathway as described. At a deeper level of analysis (Fig. 1b), one can examine the relationship between the agonist concentration and the activation state of a population of receptors. For instance, at a ligand-gated ion channel, this activation function represents the whole-cell current or ensemble average. At a GPCR, the corresponding function is known as the stimulus (Furchgott, 1966). The maximal stimulus is equivalent to observed intrinsic efficacy (ε), and the concentration of agonist eliciting a half-maximal stimulus is equivalent to the observed dissociation constant (Kobs). Observed affinity (1/Kobs) and intrinsic efficacy are more invariant than EC50 and Emax, yet nonetheless, these parameter are dependent on the G protein, the concentration of GTP, and other elements that physically interact with the receptor (Ehlert, 2000). It is possible to deduce the stimulus through the analysis of a downstream response using Furchgott's method of partial receptor inactivation (Furchgott, 1966). At an even deeper level of analysis (Fig. 1c), one can consider the microscopic affinity constants of the agonist for the ground and active states of the receptor (Colquhoun, 1998). These parameters are the ultimate determinants of agonist activity in different assays. It is possible to estimate these parameters at ligand-gated ion channels, in some instances, through single channel analysis (Colquhoun, 1998). At a GPCR, it is impossible to estimate microscopic constants from the concentration-response curve; however, it is possible to calculate a relative estimate of the microscopic affinity constant of an agonist for the active state of the receptor. Analysis of the results of a recent modeling study shows that the product of observed affinity (1/Kobs) and intrinsic efficacy (ε) of an agonist expressed relative to that of a standard agonist [ε′(1/Kobs′)] is also equivalent to the corresponding ratio of microscopic affinity constants for the active state of the receptor (Kb/Kb′) (Ehlert 2008). This ratio is termed, intrinsic relative activity (RAi).
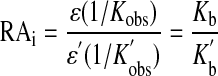 |
(1) |
Fig. 1.
Hierarchical levels of analysis of agonist action. The figure summarizes how agonist activity can be estimated at different, internally consistent levels of analysis. At the most superficial level (a, Level 1), agonist activity is estimated from the EC50 and Emax values of the measured response. These parameters depend on how the agonist interacts with the receptor as well as various elements in the signaling pathway. The second level of analysis (b) refers to the relationship between the agonist concentration and the proportion of the receptor population in the active state (i.e., stimulus). For a GPCR, this relationship depends on the agonist-receptor interaction as well as the concentration of GTP and proteins that physically interact with the receptor (e.g., G proteins). At the ultimate level of analysis (c, Level 3), activity is governed by the affinity of the agonist for ground and active states of the receptor. The goal of pharmacological analysis is to estimate these purely agonist-receptor-dependent parameters from more superficial measurements, such as the stimulus and response to an agonist.
In prior work, we showed how to estimate RAi from the concentration-response curves of the two agonists (Griffin et al., 2007). Thus, although observed affinity and efficacy are complex functions of microscopic constants, their product yields a simple constant proportional to the microscopic affinity constant of the agonist for the active state of the receptor.
Having a relative measure of the affinity of the agonist for the active state of a GPCR enables one to address several questions. For example, if different active states are involved in coupling to different G proteins, the estimate of the agonist RAi value should change depending upon the signaling pathway. In addition, if the agonist exhibits selectivity for different receptor subtypes, its RAi value should reflect this selectivity. Moreover, because all that is required for estimation of RAi is the agonist concentration-response curve, it should be possible to address these questions from previously published data. In the present report, we have tested these postulates in connection with the subtypes of the muscarinic receptor. Using a panel of agonists, we found little difference in agonist activity for triggering responses through the M4 receptor coupled to Gi, Gs, or Gα15. Upon investigating agonist activity at muscarinic subtypes using RAi analysis, we confirmed the selectivity of McN-A-343 for M1 and M4 receptors and also identified pilocarpine as an M1- and M3-selective agonist. Analysis of data from the literature also yielded a similar picture. Our results show that the RAi parameter is a simple and useful estimate for comparing agonist activity across assays.
Materials and Methods
Cell Culture. Chinese hamster ovary (CHO) cells stably expressing the human muscarinic M1 and M4 receptors were obtained from Acadia Pharmaceuticals (San Diego, CA). The expression levels of muscarinic receptors in these cells were approximately 0.1 pmol (CHO M4), 0.2 pmol/mg protein (CHO M2), 1.2 pmol/mg protein (CHO M3), and 1.3 pmol/mg protein (CHO M1). HEK-293T cells stably expressing Gα15 were provided by Dr. Olivier Civelli (University of California, Irvine, CA). CHO M1 cells were cultured in F-12K. CHO M4 and Gα15 HEK-293T cells were cultured in Dulbecco's modified Eagle's medium with high glucose plus l-glutamine. All media were supplemented with 10% fetal calf serum, penicillin-streptomycin (100 units/ml), and G418 (0.4 mg/ml), and cells were cultured at 37°C with 5% CO2. HEK-293T Gα15 cells were also supplemented with puromycin (0.625 μg/ml). A plasmid containing the human M4 receptor was obtained from the cDNA Resource Center (Missouri University of Science and Technology, Rolla, MO). An empty pcDNA3.1 vector was obtained from Invitrogen (Carlsbad, CA). HEK-293T Gα15 cells were transfected with 10 μg of the human M4 vector HEK Gα15 M4 or the empty plasmid HEK Gα15 null using Lipofectamine (5:1 Lipofectamine/DNA ratio) for 48 h before experimentation.
cAMP Accumulation. The effects of muscarinic agonists on forskolin-stimulated cAMP accumulation were measured in CHO M2 and M4 cells using a modification of the [3H]adenine-prelabeling method as described by Griffin et al. (2007). Pertussis toxin treatment was accomplished by first incubating the cells with the toxin for 16 h before the assay.
Phosphoinositide Hydrolysis. Muscarinic agonist-mediated stimulation of phosphoinositide hydrolysis was measured in adherent CHO cells and suspensions of HEK Gα15 cells using a modification of the [3H]inositol-prelabeling method of Berridge et al. (1982) and the extraction method of Kendall and Hill (1990). A detailed description of the method used for cell suspension experiments is described in Griffin et al. (2007). Confluent CHO M1 cell monolayers cultured in 24-well plates or 100-mm Petri dishes were washed in KRB before overnight incubation with [3H]inositol (2 μCi/well). On the morning of the experiment, the 24-well plates were washed twice with KRB. After 15-min incubation with KRB (270 μl) containing LiCl (10 mM), agonists (30 μl) were added for a subsequent 30-min incubation at 37°C in 5% CO2. The reaction was stopped with 5% perchloric acid (200 μl), and the samples were placed on ice. [3H]Inositol phosphates were isolated as described previously (Ehlert et al., 1996).
Analysis of Agonist Concentration Response Curves. Emax, EC50, and Hill slope were estimated from agonist concentration-response curves by nonlinear regression analysis using Prism (GraphPad Software, Inc., San Diego, CA) as described previously (Griffin et al., 2007).
Estimation of RAi. The RAi of test agonist B is defined as the product of its observed intrinsic efficacy (ε) and reciprocal of its Kobs divided by that of standard agonist A as described above in eq. 1. To avoid confusion, we have rewritten eq. 1 below with subscripts to observed intrinsic efficacy (ε) and observed affinity (K) to denote the parameters of the standard and test agonists.
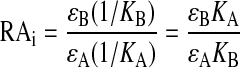 |
(2) |
The derivation of the RAi value and its estimation using either a null method or the operational model have been described in detail previously (Griffin et al., 2007), and step-by-step instructions for estimating RAi using Prism or a spreadsheet have also been described previously (Ehlert, 2008). A brief summary of the essential steps is given below. Because the RAi value is a relative measure of agonist activity, we always ran the standard agonist carbachol in each experiment.
Null Method. Pairs of equiactive log agonist concentrations were estimated for the standard (LOGA) and test (LOGB) agonists as described previously (Ehlert, 2008). The following equation was fitted to these data using nonlinear regression analysis.
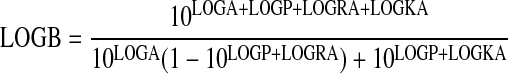 |
(3) |
In this equation, LOGRA denotes the logarithm of the RAi value, LOGKA denotes the logarithm of the observed dissociation constant of the standard agonist, and LOGP denotes the logarithm of the ratio of observed dissociation constants of the test agonist divided by that of the standard agonist (Log KB/KA). LOGKA was set to an arbitrarily high constant value of -1, and regression analysis yielded the best estimates of LOGRA and LOGP. It is possible to estimate the logarithm of KB from the estimate of LOGP and LOGKA, even though the latter is set as an arbitrarily high constant.
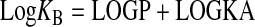 |
(4) |
Operational Method. For decreasing agonist concentration-response curves, like agonist-mediated inhibition of cAMP accumulation, the concentration-response curves of the standard agonist (A) and the various test agonists (B) were fitted simultaneously to eqs. 5 and 6, respectively, by nonlinear regression analysis.
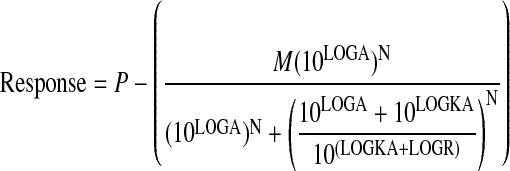 |
(5) |
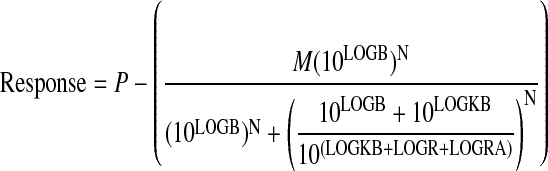 |
(6) |
In these equations, P denotes cAMP accumulation in the absence of agonist, N denotes the transducer slope factor in the operational model, LOGR denotes the ratio of the τ value of A divided by its observed dissociation constant (τA/KA), LOGKB denotes the logarithm of the observed dissociation constant of the test agonist (KB), and LOGRA denotes the logarithm of RAi, which is also a function of parameters in the operational model (Griffin et al., 2007).
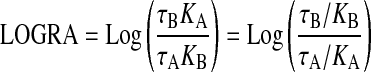 |
(7) |
Global nonlinear regression analysis is done sharing the estimates of N, M, P, and LOGR among the curves, and unique estimates of LOGRA and LOGKB are obtained for each test agonist. If the standard agonist is a full agonist, the parameter LOGKA is set as a constant at an arbitrarily high value during regression analysis (e.g., -1).
For increasing agonist concentration-response curves, such as agonist-mediated simulation of phosphoinositide hydrolysis, the concentration-response curve of the standard agonist and the various test agonists were fitted simultaneously to eqs. 8 and 9, respectively, by nonlinear regression analysis.
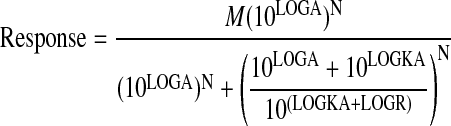 |
(8) |
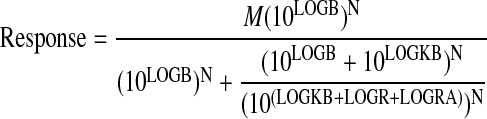 |
(9) |
Global nonlinear regression analysis is done as described above for decreasing concentration-response curves, with the exception that the regression equations lack the parameter P.
Operational Method for HEK Gα15 M4 Cells. As described below, HEK Gα15 M4 cells express low levels of an endogenous M3 receptor in addition to the transiently transfected M4 receptor, indicating that the muscarinic phosphoinositide response in these cells is caused by activation of both M3 and M4 muscarinic receptors. To estimate the RAi value corresponding to the M4 component, we analyzed the agonist concentration-response curves in HEK Gα15 M4 and HEK Gα15 null cells simultaneously according to the following two equations, respectively,
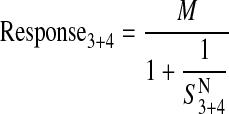 |
(10) |
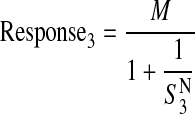 |
(11) |
in which S3+4 denotes a parameter proportional to the combined stimulus elicited by activation of both M3 and M4 receptors in HEK Gα15 M4 cells,
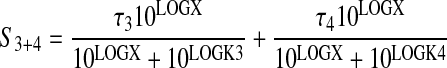 |
(12) |
and S3 denotes a parameter proportional to the stimulus elicited by activation of the M3 receptor in HEK Gα15 null cells.
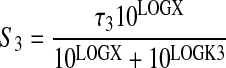 |
(13) |
The derivation of eqs. 10, 11, 12, 13 is given under Appendix. Regression analysis was done sharing the estimates of N, M, τ3, and LOGK3 between the curves and obtaining unique estimates of τ4 and LOGK4 for the data measured in HEK Gα15 M4 cells. With regard to full agonists in HEK Gα15 M4 cells, the estimates of K4 and τ4 are unreliable. Sometimes, it was necessary to set K4 as a constant at an arbitrarily high value to obtain a fit. Regardless, the ratio of τ4/K4 can be estimated accurately. Knowing the ratio of τ/K for the test agonist and standard agonist for a given response (i.e., M3 or M4), it is possible to estimate the corresponding RAi values using eq. 7 above.
Estimation of RAi from Published Studies. In most instances (11 of 19), we calculated RAi values from published concentration-response curves. To make this calculation, we carefully estimated the response values and agonist concentrations from published figures of agonist concentration-response curves. We then calculated the RAi values from these estimated concentration-response data using the operational method described above. In the remainder of the cases (8 of 19), only the EC50 and Emax values of the agonist were available from the literature. In these cases, we used the simple calculation for the estimation of RAi as described previously (Ehlert et al., 1999; Griffin et al., 2007),
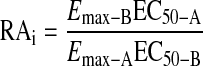 |
(14) |
in which the subscripts refer to the parameters of the standard (A) and test (B) agonists. This calculation is completely valid if the Hill slopes of the agonist concentration-response curves are equal to one or if the Emax values of the agonists are the same, in which case the RAi is equivalent to the potency ratio regardless of the Hill slopes. In six of the studies where the simple calculation (i.e., eq. 14) was used, the data were from studies on second messenger responses in cell lines transfected with subtypes of the muscarinic receptor. We have found that agonists typically exhibit Hill slopes close to one in these types of experiments, suggesting that the simple calculation was valid in these instances. In the remaining two cases, R-aceclidine in the rabbit vas deferens (Eltze et al., 1993) and McN-A-343 in guinea pig right atrium (Lambrecht et al., 1993), the Emax values of the agonists were 86 and 59% of the standard agonist, respectively. We expect the simple calculation of RAi to be valid in the case of R-aceclidine because its Emax is close to 100%. If the Hill slope of McN-A-343 differs from that of carbachol in the right atrium substantially, the simple estimate of RAi could be in error by 2- to 3-fold (see Ehlert et al., 1999).
Drug and Chemicals. Drugs and chemicals were obtained from the following sources: [3H]adenine and [3H]inositol (PerkinElmer Life and Analytical Sciences, Waltham, MA); F-12K, Dulbecco's modified Eagle's medium, trypsin-EDTA, and Lipofectamine (Invitrogen, Carlsbad, CA); G418 (Invivogen, San Diego, CA); arecoline, carbachol, McN-A-343, and oxotremorine-M (oxo-M), pilocarpine (Sigma-Aldrich, St. Louis, MO). The enantiomers of aceclidine were synthesized and resolved as described by Ringdahl et al. (1979).
Results
Analysis of Agonist Activity at the M4 Muscarinic Receptor Signaling through Different G Proteins. To investigate how the activity of specific agonists may be modified by the G protein through which the M4 receptor signals, we tested a panel of muscarinic agonists for their ability to elicit responses through M4 receptor coupling to Gi, Gs, and Gα15. The panel of compounds included agonists with varying structure, efficacy, and potency. The standard compound to which the RAi values of the other agonists were normalized was carbachol, selected because of its similar structure to the endogenous neurotransmitter, acetylcholine. Oxo-M was selected as an example of a highly efficacious muscarinic agonist (Fisher and Bartus, 1985). McN-A-343 was investigated as an example of a subtype-selective agonist. This compound was originally described as a sympathetic ganglionic stimulant (Roszkowski, 1961) and has been shown more recently to exhibit selectivity for M1 and M4 receptors (Lazareno and Birdsall, 1993). The enantiomers of aceclidine (Ringdahl et al., 1982) were selected as rigid analogs of acetylcholine. The racemate has been used as a treatment for glaucoma (Fechner et al., 1975). The partial agonist, pilocarpine, and arecoline, the natural alkaloid from betel nuts, also were tested.
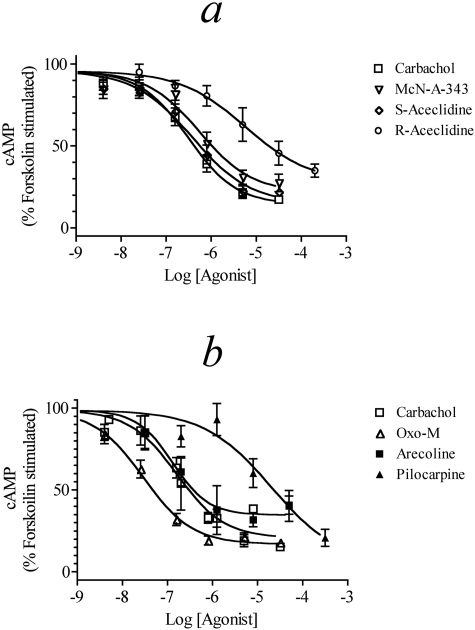
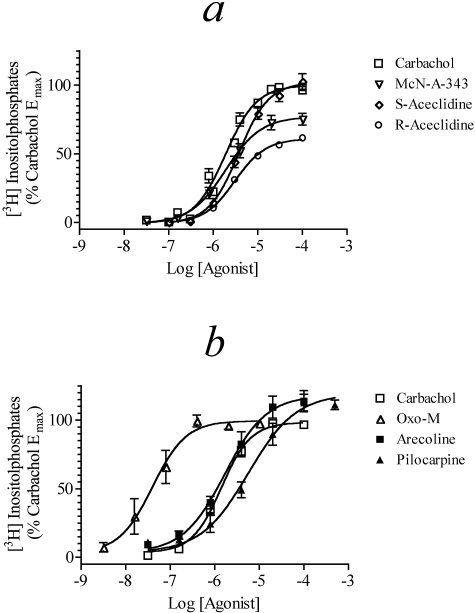
M4 Receptor-Mediated Inhibition of cAMP Accumulation. Agonist activity for signaling through the M4 receptor coupled to Gi was tested in CHO M4 cells by measuring inhibition of forskolin (10 μM)-stimulated cAMP accumulation (Fig. 2, a and b). Carbachol, S-aceclidine, and McN-A-343 all produced concentration-response curves with similar potency and maximal effect. Oxo-M was slightly more potent than carbachol but shared a similar maximal response, whereas arecoline had a similar potency as carbachol but a slightly decreased Emax. R-Aceclidine had a response both lower in potency and maximal effect compared with carbachol. Pilocarpine exhibited an EC50 at least two log units less potent than carbachol but displayed an increased maximal effect, although pilocarpine was not tested at higher concentrations. It is possible that pilocarpine causes a nonmuscarinic receptor-mediated inhibition of cAMP accumulation at high concentrations as has been previously seen with other agonists [e.g., R-acelidine in CHO cells (Griffin et al., 2007)]. The Emax, EC50, and Hill slope of each agonist are summarized in Table 1.
Fig. 2.
Muscarinic agonist-mediated inhibition of forskolin (10 μM) stimulated cAMP accumulation in CHO M4 cells. Concentration-response curves for carbachol, McN-A-343, S-aceclidine, and R-aceclidine (a) and carbachol, oxo-M, arecoline, and pilocarpine (b) are shown. The data represent the means ± S.E.M. of 4 to 10 experiments, each done in triplicate. The data are expressed relative to the level of stimulation caused by 10 μM forskolin alone.
TABLE 1.
Agonist activity for inhibiting forskolin stimulated cAMP accumulation in CHO M4 cells The data are from Fig. 2, a and b. The data represent the mean estimates ± S.E.M. The values in parentheses to the right of some of the estimates are the Log mean ± S.E.M.
|
Agonist
|
Emaxa
|
EC50
|
Hill Slope
|
RAi
|
|
|---|---|---|---|---|---|
| Null | Operational | ||||
| % | μM | ||||
| Oxotremorine-M | 82 ± 2.4 | 0.030 (–7.52 ± 0.07) | 0.84 ± 0.10 | 5.79 (0.76 ± 0.07) | 6.61 (0.82 ± 0.07) |
| Carbachol | 88 ± 2.1 | 0.23 (–6.63 ± 0.05) | 0.76 ± 0.06 | 1.0 (0.0) | 1.0 (0.0) |
| McN-A-343 | 80 ± 6.6 | 0.56 (–6.25 ± 0.17) | 0.65 ± 0.13 | 0.49 (–0.31 ± 0.12) | 0.63 (–0.20 ± 0.12) |
| S-Aceclidine | 87 ± 5.5 | 0.37 (–6.43 ± 0.14) | 0.60 ± 0.09 | 0.98 (–0.0074 ± 0.20) | 1.00 (0.0044 ± 0.10) |
| R-Aceclidine | 71 ± 9.1 | 5.74 (–5.24 ± 0.26) | 0.61 ± 0.15 | 0.071 (–1.50 ± 0.09) | 0.040 (–1.36 ± 0.04) |
| Arecoline | 65 ± 3.7 | 0.13 (–6.88 ± 0.09) | 1.10 ± 0.07 | 0.68 (–0.17 ± 0.10) | 0.73 (–0.14 ± 0.15) |
| Pilocarpine | 83 ± 5.8 | 9.75 (–5.01 ± 0.12) | 0.88 ± 0.10 | 0.013 (–1.89 ± 0.13) | 0.013 (–1.87 ± 0.15) |
Denotes the maximal inhibition of forskolin-stimulated cAMP accumulation
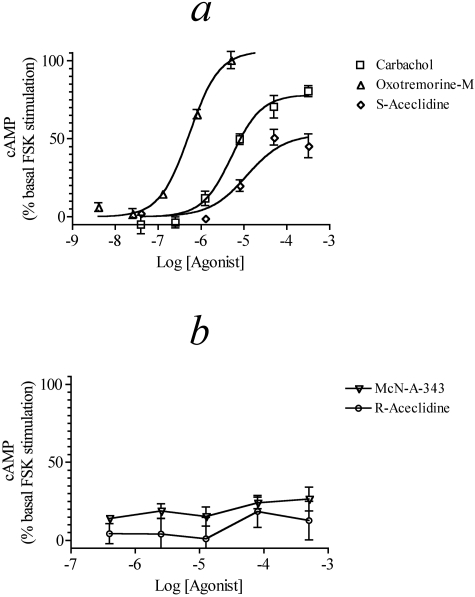
M4 Receptor-Mediated Stimulation of cAMP Accumulation. It has been shown that the cAMP response to muscarinic agonists in CHO M2 and M4 cells is biphasic. Low concentrations of agonist mediate inhibition of cAMP accumulation, whereas stimulation of cAMP accumulation occurs at higher concentrations of agonist (Mistry et al., 2005). The more potent inhibition of cAMP accumulation is prevented by pretreatment with pertussis toxin, which unmasks the Gs-dependent stimulation of cAMP accumulation. The role of Gs has been confirmed in small interference RNA studies (Michal et al., 2007). We investigated the ability of muscarinic agonists to enhance the cAMP accumulation elicited by a low concentration of forskolin (0.1 μM) in CHO M4 cells treated with pertussis toxin (see Figs. 3, a and b, and Table 2). Oxo-M stimulated an increase in cAMP accumulation with a maximal effect and potency significantly higher than those of carbachol, whereas the Emax and potency of S-aceclidine were lower than those of carbachol. Both McN-A-343 and R-aceclidine failed to produce substantial concentration-dependent increases in cAMP accumulation. The potency of carbachol for inhibiting cAMP accumulation is more than 1.3 log units higher than that for stimulating cAMP accumulation in pertussis toxin-treated cells, illustrating the low sensitivity of the CHO M4 Gs assay. This reduced sensitivity can account for the inability of the partial agonists to trigger a response in this assay, rather than inferring a selectivity based on the agonist-receptor-G protein interaction.
Fig. 3.
Muscarinic agonist-mediated stimulation of cAMP accumulation in CHO M4 cells treated with pertussis toxin. Concentration-response curves for carbachol, oxo-M, and S-aceclidine (a) and McN-A-343 and R-aceclidine (b) are shown. The data represent the means ± S.E.M. of four experiments, each performed in triplicate. The data are expressed as a percentage above the level of stimulation caused by 0.1 μM forskolin.
TABLE 2.
Agonist activity for stimulation of cAMP accumulation in CHO M4 cells previously treated with pertussis toxin The data are from Fig. 3a. The data represent the mean estimates ± S.E.M. The values in parentheses beneath some of the estimates are the Log mean ± S.E.M.
|
Agonist
|
Emaxa
|
EC50
|
Hill Slope
|
RAi
|
|
|---|---|---|---|---|---|
| Null | Operational | ||||
| % | μM | ||||
| Oxotremorine-M | 106 ± 3.8 | 0.54 (–6.27 ± 0.06) | 1.25 ± 0.21 | 11.80 (1.07 ± 0.04) | 10.67 (1.03 ± 0.10) |
| Carbachol | 78 ± 3.2 | 5.3 (–5.28 ± 0.07) | 1.26 ± 0.24 | 1.0 (0.0) | 1.0 (0.0) |
| S-Aceclidine | 53 ± 5.7 | 11.8 (–4.93 ± 0.23) | 1b | 0.39 (–0.41 ± 0.37) | 0.45 (–0.35 ± 0.14) |
Denotes the maximal stimulation of cAMP accumulation expressed as a percentage of basal cAMP accumulation, which is the amount of accumulation in the presence of forskolin (0.1 μM)
Hill slope constrained to 1
M4 Receptor-Mediated Phosphoinositide Hydrolysis via Gα15. Offermanns et al. (2001) have described how Gα15 can couple a wide variety G protein-coupled receptors to phospholipase C-ß. As a consequence, we investigated the ability of muscarinic agonists to stimulate the production of inositol phosphates in HEK Gα15 cells transiently transfected with the M4 receptor (HEK Gα15 M4 cells). Figure 4a shows the concentration-response curves of the five agonists tested in the HEK Gα15 M4 cells. Carbachol and S-aceclidine displayed full agonism with similar potency and Emax. Oxo-M also behaved as a full agonist but showed increased potency with its concentration-response curve located over one log unit to the left of carbachol. McN-A-343 was as potent as carbachol at stimulating phosphoinositide hydrolysis but had a decreased Emax, whereas R-aceclidine exhibited both lower potency and Emax. Table 3 lists the Emax, EC50, and Hill slope values of agonists for these responses.
Fig. 4.
Muscarinic agonist-mediated phosphoinositide hydrolysis in HEK Gα15 cells. Agonist-mediated phosphoinositide hydrolysis was measured in HEK Gα15 M4 cells (a) and HEK Gα15 cells (b). The data represent the mean values ± S.E.M. of four experiments, each done in triplicate. The data are expressed relative to the Emax for carbachol.
TABLE 3.
Agonist activity for stimulating phosphoinositide hydrolysis in HEK Gα15 M4 cells The data are from Fig. 4. The data represent the mean estimates ± S.E.M. The values in parentheses to the right of some of the estimates are the Log mean ± S.E.M.
|
Agonist
|
Emaxa
|
EC50
|
Hill Slope
|
RAi
|
|
|---|---|---|---|---|---|
| Null | Operationalb | ||||
| % | μM | ||||
| Oxotremorine-M | 97 ± 1.1 | 0.078 (–7.11 ± 0.04) | 0.82 ± 0.06 | 8.01 (0.90 ± 0.04) | 10.67 (1.028 ± 0.12) |
| Carbachol | 97 ± 0.9 | 0.90 (–6.05 ± 0.03) | 0.82 ± 0.03 | 1.0 (0.0) | 1.0 (0.0) |
| McN-A-343 | 70 ± 1.4 | 1.1 (–5.98 ± 0.06) | 0.87 ± 0.08 | 0.65 (–0.19 ± 0.07) | 0.44 (–0.36 ± 0.05) |
| S-Aceclidine | 91 ± 1.7 | 0.62 (–6.21 ± 0.05) | 0.82 ± 0.07 | 1.42 (0.15 ± 0.06) | 1.24 (0.09 ± 0.06) |
| R-Aceclidine | 61 ± 1.9 | 2.7 (–5.56 ± 0.08) | 0.92 ± 0.13 | 0.22 (–0.65 ± 0.04) | 0.13 (–0.87 ± 0.08 |
Denotes the maximum stimulation of phosphoinositide hydrolysis by carbachol
The operational RAi values for oxotremorine-M and carbachol were estimated using eqs. 10, 11, 12, 13, whereas those for McN-A-343, S-aceclidine, and R-aceclidine were estimated using eqs. 8 and 9. In each analysis the concentration-response curve of carbachol was analyzed simultaneously as the standard
In a prior study, we showed that an endogenous M3 muscarinic receptor elicits a weak phosphoinositide response in the HEK Gα15 cell (Griffin et al., 2007). Therefore, muscarinic responses measured in HEK Gα15 cells transiently transfected with the M4 receptor should represent the sum of M3 and M4 responses. To quantify the magnitude of the M3 component, we measured agonist-stimulated phosphoinositide hydrolysis in HEK Gα15 cells transfected with an empty pcDNA3.1 vector (HEK Gα15 null, see Fig. 4b). In general, the activities of all of the agonists were much less in these cells. Oxo-M produced a maximal response similar to that of carbachol but exhibited 10-fold greater potency. S-Aceclidine exhibited similar potency to carbachol but had a lower Emax value. Neither McN-A-343 nor R-aceclidine produced measurable concentration-dependent agonism in the HEK Gα15 null cells. Control experiments with HEK Gα15 M4 cells treated with pertussis toxin show a lack of contribution of Gi/o signaling to the phosphoinositide hydrolysis measured upon stimulation by the muscarinic agonists (data not shown).
Estimation of Agonist RAi Values for M4 Responses Elicited through Gi, Gs, and Gα15. The RAi values of agonists for eliciting responses through Gi (Fig. 2), Gs (Fig. 3), and Gα15 (Fig. 4) were estimated using both the operational and null methods as described under Materials and Methods. An additional analysis was done using the operational model for those agonists that elicited a significant response in both the HEK Gα15 M4 and HEK Gα15 null cells (carbachol and oxo-M). The concentration-response curves from both cell lines were analyzed simultaneously, sharing the estimates of the M3 parameters between both curves and using only the HEK Gα15 M4 cells for estimation of the M4 parameters. In this manner, the M4 component of the phosphoinositide response in HEK Gα15 M4 cells was determined. Further details of the calculations are given under Materials and Methods. This analysis enabled us to estimate two RAi values for an agonist: one for the M3 response and one for the M4 response. This careful analysis ultimately showed that the estimate of RAi value for the M4 component in the HEK Gα15 M4 cells was practically the same as that estimated assuming that the entire response was elicited by the M4 receptor. Presumably, the endogenous M3 response was too insensitive to influence the M4 response significantly.
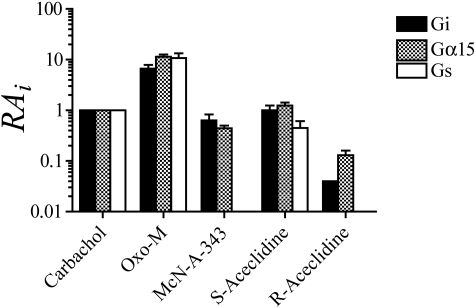
A summary of the RAi estimates is shown in Fig. 5, and the corresponding RAi values are also listed in Tables 1, 2, 3. Oxo-M exhibited the highest RAi values, whereas carbachol, S-aceclidine, and McN-A-343 all exhibited values similar to each other but somewhat lower than those of oxo-M. R-Aceclidine exhibited the lowest RAi values. None of the agonists exhibited a marked difference in activity for eliciting M4 responses through the three different G proteins. No RAi value was calculated for McN-A-343 and R-aceclidine in the CHO M4 Gs assay because of the immeasurable response to these agonists.
Fig. 5.
Comparison of the RAi values of muscarinic agonists for eliciting different responses through the M4 receptor via different G proteins. The estimates are from Tables 1, 2, 3.
Comparison of Agonist Activity across M1 to M4 Muscarinic Receptors. We used our RAi estimates to compare the activity of agonists across the M1 to M4 subtypes of the muscarinic receptor. For this analysis, we used data generated from our laboratory in which the test and standard agonists were assayed in the same experiment to minimize variation between experiments. Most of the RAi estimates for the M2 receptor were taken from Griffin et al. (2007) in which M2 receptor-mediated inhibition of forskolin-stimulated cAMP accumulation was measured. We ran additional experiments with arecoline and pilocarpine, and the combined results are given in Table 5. The data for the M3 receptor are from Ehlert et al. (1999). Additional data on the M1 receptor were obtained and are described below to give a complete picture of activity across the M1 to M4 subtypes.
TABLE 5.
Agonist activity for inhibiting forskolin-stimulated cAMP accumulation in CHO M2 cells The data represent the mean estimates ± S.E.M. The values in parentheses to the right of some estimates are the Log mean ± S.E.M. NC denotes not calculated because the Emax for the test agonist was the same as the standard carbachol; therefore, RAi values were calculated as the potency ratio.
|
Agonist
|
Emaxa
|
EC50
|
Hill Slope
|
RAi
|
|
|---|---|---|---|---|---|
| Null | Operational | ||||
| % | μM | ||||
| Oxotremorine-Mb | 73 ± 2.3 | 0.047 (–7.32 ± 0.07) | 1.02 ± 0.06 | NC | 4.7 (0.67 ± 0.03) |
| Carbacholb | 73 ± 2.3 | 0.22 (–6.65 ± 0.07) | 0.90 ± 0.08 | 1.0 (0.0) | 1.0 (0.0) |
| McN-A-343b | 32 ± 5.1 | 38 (–4.42 ± 0.13) | 1.17 ± 0.28 | 0.0022 (–2.66 ± 0.23) | 0.0024 (–2.62 ± 0.12) |
| S-Aceclidineb | 73 ± 2.3 | 0.41 (–6.39 ± 0.10) | 0.89 ± 0.07 | NC | 0.55 (–0.26 ± 0.06) |
| R-Aceclidineb | 73 ± 2.3 | 2.6 (–5.59 ± 0.08) | 0.83 ± 0.08 | NC | 0.087 (–1.06 ± 0.04) |
| Arecoline | 45 ± 1.9 | 0.83 (–6.08 ± 0.07) | 1.20 ± 0.16 | 0.43 (–0.36 ± 0.05) | 0.35 (–0.45 ± 0.08) |
| Pilocarpine | 40 ± 1.4 | 17 (–4.76 ± 0.06) | 1.11 ± 0.16 | 0.014 (–1.86 ± 0.03) | 0.015 (–1.85 ± 0.07) |
Denotes the maximal inhibition of forskolin-stimulated cAMP accumulation
Data are from Griffin et al., 2007
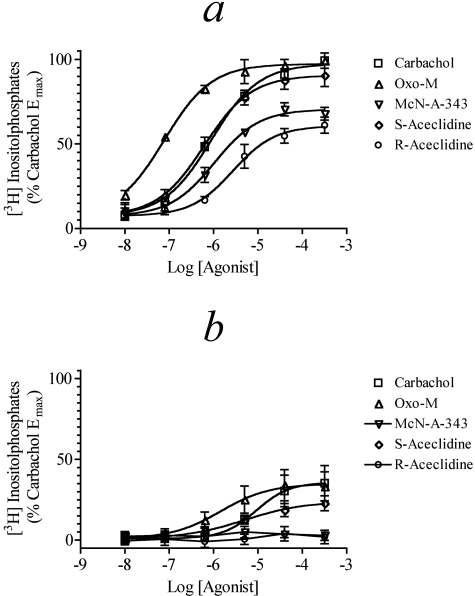
Our data on agonist-mediated stimulation of phosphoinositide hydrolysis in CHO M1 cells are shown in Fig. 6, a and b. Most of the agonists exhibited a similar maximal response, with the exception of the enantiomers of aceclidine whose Emax values were moderately lower. In addition, most of the agonists exhibited similar potency with the striking exception of oxo-M, which exhibited approximately 10-fold greater potency than carbachol. The potency of pilocarpine was approximately one-fourth that of carbachol. These data are summarized in Table 4.
Fig. 6.
Muscarinic agonist-mediated phosphoinositide hydrolysis in M1 CHO cells. Agonist-mediated phosphoinositide hydrolysis was measured in CHO cells stably transfected with the human M1 receptor. Concentration-response curves are shown for carbachol, McN-A-343, S-aceclidine, and R-aceclidine (a) and carbachol, oxo-M, arecoline, and pilocarpine (b). Mean values ± S.E.M. of three experiments are shown with each done in triplicate. The data are expressed relative to the Emax for carbachol.
TABLE 4.
Agonist activity for stimulating phosphoinositide hydrolysis in CHO M1 cells The data are from Fig. 6, a and b. The data represent the mean estimates ± S.E.M. The values in parentheses to the right of some of the estimates are the Log mean ± S.E.M.
|
Agonist
|
Emaxa
|
EC50
|
Hill Slope
|
RAi
|
|
|---|---|---|---|---|---|
| Null | Operational | ||||
| % | μM | ||||
| Oxotremorine-M | 100 ± 2.3 | 0.041 (–7.39 ± 0.05) | 1.16 ± 0.13 | 32 (1.51 ± 0.28) | 30 (1.48 ± 0.04) |
| Carbachol | 98 ± 1.1 | 1.4 (–5.86 ± 0.02) | 1.29 ± 0.07 | 1.0 (0.0) | 1.0 (0.0) |
| McN-A-343 | 77 ± 1.7 | 1.9 (–5.72 ± 0.04) | 1.09 ± 0.09 | 0.69 (–0.16 ± 0.06) | 0.64 (–0.20 ± 0.07) |
| S-Aceclidine | 102 ± 1.5 | 3.8 (–5.42 ± 0.03) | 1.29 ± 0.07 | 0.72 (–0.14 ± 0.11) | 0.66 (–0.18 ± 0.04) |
| R-Aceclidine | 61 ± 0.46 | 3.1 (–5.51 ± 0.01) | 1.25 ± 0.03 | 0.31 (–0.51 ± 0.11) | 0.22 (–0.66 ± 0.04) |
| Arecoline | 118 ± 2.9 | 1.7 (–5.77 ± 0.05) | 0.93 ± 0.08 | 1.51 (0.18 ± 0.04) | 1.22 (0.09 ± 0.04) |
| Pilocarpine | 119 ± 3.1 | 5.7 (–5.24 ± 0.05) | 0.81 ± 0.06 | 0.48 (–0.32 ± 0.06) | 0.49 (–0.31 ± 0.04) |
Denotes the maximal stimulation of phosphoinositide hydrolysis by carbachol
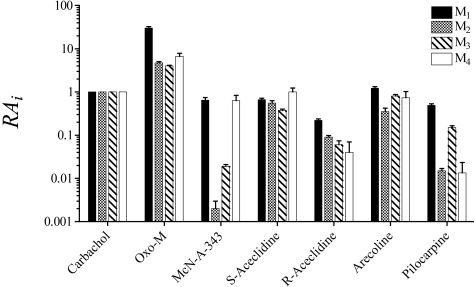
Agonist RAi Values at M1 to M4 Muscarinic Receptors. A summary of the RAi estimates for agonists across M1 to M4 muscarinic receptors is shown in Fig. 7. All values were estimated using the operational method. For this analysis, RAi values were estimated from phosphoinositide assays in CHO M1 and CHO M3 cells and from cAMP assays on CHO M2 and CHO M4 cells in which the inhibition of cAMP accumulation elicited by forskolin was measured. Oxo-M displayed increased agonist activity relative to carbachol across the M1 to M4 muscarinic subtypes, with an especially high RAi value of 30 at the M1 receptor and values of 4.0 to 6.6 at the other subtypes. S-Aceclidine, arecoline, and R-aceclidine exhibited approximately uniform activity at the M1 to M4 subtypes. The former two compounds had activity similar to carbachol, whereas R-aceclidine exhibited approximately one-tenth the activity of carbachol. The most selective compounds were McN-A-343 and pilocarpine. McN-A-343 exhibited high selectivity for the M1 and M4 subtypes and much lower activity at the M2 (0.0020) and M3 (0.019) subtypes. The RAi values of McN-A-343 at the M1 and M4 subtypes were comparable with those of carbachol. Pilocarpine exhibited activity less than carbachol but showed selectivity between the muscarinic subtypes; its RAi values for the M1 (0.49) and M3 (0.15) subtypes were much higher than those for the M2 (0.015) and M4 (0.013) receptors. One way analysis of variance showed no significant differences among the log RAi values of S-aceclidine (F3,12 = 1.16; P = 0.37) and R-aceclidine (F3,12 = 2.40; P = 0.12) across the M1 to M4 receptor subtypes. In contrast, oxo-M (F3,12 = 43.29; P = 1.03 × 10-6), McN-A-343 (F3,12 = 105.9; P = 8.6 × 10-10), pilocarpine (F3,10 = 18.30; P = 2.2 × 10-4), and arecoline (F3,10 = 4.76 P = 0.0260) exhibited significant differences in their log RAi values across receptor subtypes. Post hoc comparisons using T tests with the Bonferroni adjustment showed that oxo-M exhibited selectivity for M1 receptors relative to M2 to M4 (P < 0.001), McN-A-343 exhibited selectivity for M1 and M4 relative to M2 and M3 (P < 0.001), and pilocarpine exhibited selectivity for M1 and M3 relative to M2 and M4 (P < 0.01). Post hoc comparisons failed to identify significant differences among the log RAi values of arecoline at the M1 to M4 subtypes.
Fig. 7.
Comparison of the RAi values of agonists for eliciting responses in CHO cells transfected with M1 to M4 muscarinic receptors. The estimates are from Tables 1, 4, and 5 and Ehlert et al. (1999).
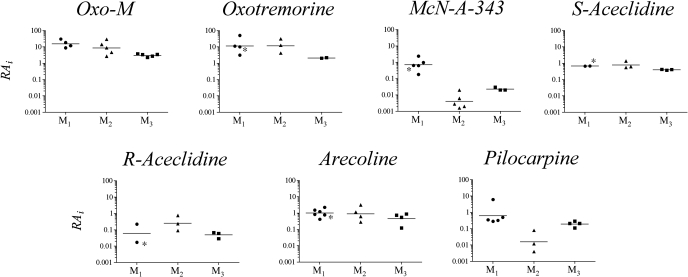
Estimation of RAi Values from Published Data. Because the estimation of RAi only requires the agonist concentration-response curve, it should be possible to estimate RAi values from previously published data for a variety of responses and determine how invariant the estimate is for a given agonist at a given receptor subtype. To investigate this issue, we calculated the RAi values of selected agonists for eliciting responses through M1, M2, and M3 muscarinic receptors. Five published studies were used to compare agonist activity at the M1 receptor in addition to our own just described. Agonist-stimulated phosphoinositide hydrolysis was analyzed from studies by Richards and van Giersbergen (1995) (CHO M1), Schwarz et al. (1993) (CHO M1), and Mei et al. (1991) (B82 fibroblasts transfected with the M1 receptor); agonist-stimulated GTPase activity in CHO M1 cells (Lazareno and Birdsall, 1993) was also analyzed. We also examined the data of Eltze et al. (1993) on M1 receptor-mediated inhibition of electrically stimulated contraction in rabbit vas deferens. However, there is some question that this response may be mediated by the M4 receptor as described under Discussion. Four studies on cell lines, three on myocardial homogenates, and two on the isolated left atrium were selected for comparison of M2 RAi values. The studies on cell lines included experiments on the inhibition of cAMP accumulation in CHO M2 cells by Griffin et al. (2007), Mistry et al. (2005), McKinney et al. (1991), and Wang and El-Fakahany (1993). The studies on inhibition of adenylate cyclase activity in myocardial homogenates included those of Ehlert (1985), Keen and Nahorski (1988), and Ehlert et al. (1996). The studies on the isolated, guinea pig left atrium were from Christopoulos and Mitchelson (1997) and Lambrecht et al. (1993). RAi values for the M3 receptor were estimated from studies measuring contraction in the guinea pig ileum and phosphoinositide hydrolysis in cells and tissues. The data on phosphoinositide hydrolysis were from Ek and Nahorski (1988) (parotid gland and ileum), Matsumoto et al. (1994) (ciliary muscle), and Ehlert et al. (1999) (CHO M3 cells). The data on the contractility of the ileum was from Ringdahl et al. (1982), Hanin et al. (1966), and Ehlert et al. (1999). RAi values were calculated as described under Materials and Methods and plotted as scatter plots for comparison in Fig. 8. We have indicated those values that were calculated from the rabbit vas deferens with an asterisk because this tentative M1 response may actually be an M4 response. If the Emax of the standard and reference agonist were the same, the RAi would be estimated as the potency ratio (see Griffin et al., 2007).
Fig. 8.
Comparison of the RAi values of agonists for eliciting different responses in assays for M1, M2, and M3 muscarinic receptors. The estimates were calculated from the published concentration-response curves of Lazareno et al. (1993), Eltze et al. (1993), Mei et al. (1991), Richards and van Giersbergen (1995), Schwarz et al. (1993), Griffin et al. (2007), Ehlert (1985), McKinney et al. (1991), Ehlert et al. (1996), Keen and Nahorski (1998), Christopoulos and Mitchelson (1997), Ehlert et al. (1999), Matsumoto et al. (1994), Ek and Nahorski (1998), Hanin et al. (1966) and Ringdahl et al. (1982), Wang and El-Fakahany (1993), and Lambrecht et al. (1993). Asterisks are used to indicate the RAi values estimated from the study of Eltze et al. (1993) on the rabbit vas deferens because there is a question whether this response is M1 (as indicated in the figure) or M4.
The greatest variation in RAi values was noted at the M2 receptor (standard deviation of Log RAi = 0.45; 2.8-fold), the least variation at the M3 receptor (standard deviation of Log RAi = 0.14; 1.4-fold), and intermediate variation at the M1 receptor (standard deviation of Log RAi = 0.39; 2.5-fold). One-way analysis of variance revealed no significant differences in the Log RAi values of oxotremorine (F2,6 = 2.31; P = 0.18), S-aceclidine (F2,5 = 3.77; P = 0.10), R-aceclidine (F2,5 = 1.93; P = 0.24), and arecoline (F2,11 = 1.26; P = 0.32) at the M1 to M3 subtypes. In contrast, oxo-M (F2,11 = 5.78; P = 0.017), McN-A-343 (F2,10 = 43.69; P = 1.14 × 10-5), and pilocarpine (F2,9 = 9.81; P = 0.005) exhibited significant differences at the M1 to M3 subtypes. Oxo-M had geometric mean RAi values of 15.5, 8.6, and 3.0 at the M1, M2, and M3 subtypes, respectively, suggesting increased activity at the M1 and M2 receptors relative to M3. McN-A-343 exhibited the greatest variation in RAi values across subtypes (172-fold) with a geometric mean of 0.70 at the M1 receptor and lower values of 0.0041 and 0.023 at M2 and M3 receptors, respectively. The corresponding RAi values for pilocarpine at the M1 to M3 subtypes are 0.63, 0.012, and 0.19, suggesting selectivity primarily for M1 and M3 receptors over M2.
Assessment of RAi values for an agonist within the same receptor type highlights differences between studies. RAi values for R-aceclidine at the M1 receptor vary from 0.017 in the rabbit vas deferens of Eltze et al. (1993) to 0.22 in CHO M1 cell data from this study. The variation in RAi estimates at the M1 receptor is also seen for oxotremorine, with RAi values ranging from 3.2 at the M1 receptor in murine fibroblasts by Mei et al. (1991) to 50 calculated from GTPase activity in CHO M1 cells by Lazareno et al. (1993). As shown in Fig. 8, the RAi value (6.1) for pilocarpine from Lazareno et al. (1993) was also much higher than that estimated for pilocarpine in four other studies analyzed (0.49, 0.33, 0.35, and 0.28), in which phosphoinositide hydrolysis was measured in either CHO M1 or B82 M1 cells. Oxo-M, McN-A-343, S-aceclidine, and arecoline show less variation in RAi values between the M1 studies evaluated.
The RAi values for oxo-M at the M2 receptor vary from 2.7 (Ehlert et al., 1996) to 30 (Ehlert 1985). These studies both investigated cardiac adenylate cyclase activity but in different species (rat and rabbit, respectively). The RAi values for McN-A-343, oxo-M, arecoline, R-aceclidine, and pilocarpine also show greater than a log unit range across M2 studies. S-Aceclidine has the least difference in RAi estimates of all compounds illustrated in Fig. 8, with a standard deviation of log RAi values of 0.21. As described above, the variance in agonist RAi values is substantially decreased when surveying M3-based assays.
Discussion
The RAi value is a relative measure of the microscopic affinity constant of an agonist for the active state of the receptor. Therefore, if different active states are involved in the coupling of a GPCR to different G proteins, different RAi values might be expected. A panel of muscarinic agonists, carbachol, oxo-M, McN-A-343, S-aceclidine, and R-aceclidine, were assessed for possible selectivity for different active states of the M4 receptor coupling to Gi, Gs, or Gα15. Our data with the M4 receptor provide no evidence for different active states of the M4 receptor. This result may suggest that measurement of M4 activation via Gα15 is an appropriate substitute for estimating agonist activity at the M4 receptor signaling through Gi, but it is conceivable that other novel agonists may preferentially direct signaling at the M4 receptor through one G protein more than another. For example, at the M2 receptor, it has been shown that McN-A-343 has 10-fold greater activity when activating M2 receptor signaling via Gα15 versus Gi (Griffin et al., 2007). Therefore, before implementation of a cellular screen based on alternative G protein signaling, it would seem prudent to use RAi in conjunction with as many well characterized agonists as possible to evaluate potential differences in signaling caused by alternative G protein coupling. Agonist concentrations required to increase cAMP via M4 signaling through Gs were much higher than those required to inhibit forskolin-stimulated cAMP via M4 signaling through Gi, suggesting a possible physiological irrelevance of the M4 activation via Gs. Nonetheless, this pathway does provide an additional example of the use of RAi in alternative screening paradigms.
Because the RAi value is a relative measure of the microscopic affinity constant of an agonist for the active state of the receptor, its use represents an improvement in prior characterizations of the M1 to M4 subtypes requiring the two parameters, EC50 and Emax. RAi also presents an advantage over the use of potency ratios because RAi can be calculated in assays in which the agonists elicit different maximal responses. A rank order of agonist activity, based on selectivity for the active state, is given in Table 6. Our data on CHO M1 cells generally agrees with published data. Two moderate differences are with regard to arecoline and pilocarpine, which gave a higher level of maximal stimulation (118 and 119%, respectively) than previously shown in studies on the phosphoinositide response in CHO M1 cells by Schwarz et al. (1993) (87 and 66%, respectively) and Richards and van Giersbergen (1995) (85 and 76%, respectively).
TABLE 6.
Rank order of agonist activity based upon RAi values calculated via the operational method Data were taken from Tables 1, 4, and 5 and Fig. 7.
| Receptor | RAi Rank Order |
|---|---|
| M1 | Oxo-M > >arecoline = carbachol > S-aceclidine = McN-A-343 > pilocarpine > R-aceclidine |
| M2 | Oxo-M > carbachol > S-aceclidine > arecoline > R-aceclidine > pilocarpine > McN-A-343 |
| M3 | Oxo-M > carbachol = arecoline > S-aceclidine > pilocarpine > R-aceclidine = McN-A-343 |
| M4 | Oxo-M > carbachol = S-aceclidine > arecoline > McN-A-343 > R-aceclidine = pilocarpine |
In this study, McN-A-343 displayed increased RAi values at the M1 and M4 receptors compared with M2 and M3. This pattern correlates with previous data indicating selectivity of McN-A-343 for both M1 and M4 muscarinic receptors (Lazareno et al., 1993). Roszkowski (1961) first described the press- or effect of McN-A-343 in cats and suggested that this response was mediated by activation of a neuronal muscarinic receptor (M1) in sympathetic ganglia triggering catecholamine release. In the rabbit vas deferens, McN-A-343 inhibits the contractile response to electrical field stimulation, and this response is blocked potently by the M1-selective antagonist pirenzepine (Eltze, 1988). It is conceivable that this response is mediated by the M4 receptor because pirenzepine exhibits moderately high affinity for the M4 receptor (pKD = 7.23) in addition to its high affinity for the M1 receptor (pKD = 7.77) (Ehlert et al., 1997). In cell lines, McN-A-343 exhibits greater potency and maximal effect at stimulating GTPase activity in CHO M4 cells, compared with that observed in CHO M1 cells, but exhibits much lower activity at the M2 and M3 subtypes (Lazareno et al., 1993).
Pilocarpine exhibited RAi values of 0.49, 0.015, 0.21, and 0.01 across M1 to M4 receptors, respectively, indicating selectivity for M1 and M3 receptors compared with M2 and M4. Pilocarpine has previously been shown to exhibit selectivity for the M1 receptor based on its activation of GTPase activity in CHO M1 cells (Lazareno and Birdsall, 1993). More recently, Fox et al. (2001) showed that the salivating effect of pilocarpine is due to its selective stimulation of M1 and M3 receptors present on salivary glands, and Gautam et al. (2004) described how the salivary effect of pilocarpine is prevented in M1/M3 receptor double-knockout mice. These data and those of Hammer et al. (1980) and Buckley and Burnsock (1986), showing high-affinity binding sites for pirenzepine in the rat submaxillary gland, are consistent with the expression of both M1 and M3 receptors in this tissue. Our demonstration of the M1 and M3 selectivity of pilocarpine may explain its utility in Sjogren's syndrome for the treatment of dry mouth. Selectivity of pilocarpine has also been investigated centrally; Bymaster et al. (2003) showed that seizures were induced in mice by pilocarpine activation of the M1 receptor.
The final section of this report compared RAi values for selected agonists in 19 previously published studies dating from Hanin et al. (1966) to Griffin et al. (2007). If two assays are based upon the same receptor but provide significantly different RAi values for a compound, it may indicate a difference in the active state of the G protein-receptor complex between the two assays or simply variability. It should be noted that, in evaluating historical data, there is no knowledge of whether the control agonist was tested within the same experiment as the test agonist and, hence, whether control for possible interassay variability was adequate. There is a distinct lack of variation in the estimates of RAi by different investigators for agonist activity at the M3 receptor (Fig. 7). With the exception of the RAi of arecoline in the ciliary muscle of the rabbit (Matsumoto et al., 1994), all agonists presented very similar RAi values across the different studies, which investigated phosphoinositide hydrolysis in cell lines, glands, and smooth muscle and contraction in the guinea pig ileum (Hanin et al., 1966; Ringdahl et al., 1979; Ek and Nahorski, 1988; Ehlert et al., 1996, 1999). Variability in agonist activity across assays was evident in the M1 and M2 sets of data. At the M1 receptor, pilocarpine showed greater than a 20-fold difference between the high value from Lazareno et al. (1993) and the low value from Schwarz et al. (1993). These studies both used CHO M1 cells; however, Lazareno et al. (1993) measured GTPase activity, whereas Schwarz et al. (1993) measured phosphoinositide hydrolysis. The high RAi value of pilocarpine in the GTPase assay might be attributed to a particularly low potency response of carbachol, the standard to which other agonists were compared. RAi values for all other agonists, with the exception of oxo-M, tested in the study of Lazareno et al. (1993) are higher than those calculated from other M1-based studies, suggesting unusually low activity for carbachol. The pattern of selectivity that we observed in our studies (Fig. 6) is generally consistent with data from the literature on the M1 to M3 subtypes (Fig. 7). That is, both sets of data show that oxotremorine, S-aceclidine, R-aceclidine, and arecoline lack selectivity, whereas McN-A-343 exhibits selectivity for M1 relative to M2 and M3, pilocarpine exhibits selectivity for M1 and M3 relative to M2, and oxo-M exhibits selectivity for the M1 relative to M3. The data from the literature, however, do not support an M1 selectivity of oxo-M relative to M2, perhaps because of variation in RAi estimates at the M2 receptor. The RAi value calculated for R-aceclidine in the M1 rabbit vas deferens assay (Eltze et al., 1993) is more than 10-fold lower than that calculated here in the CHO M1 cells. R-Aceclidine is a good substrate for acetylcholinesterase (Pyttel and Robinson, 1973). Therefore, its activity may be reduced in the isolated tissue because of cholinesterases but not in CHO M1 cells, which lack these enzymes.
The potency and ability of an agonist to turn on a GPCR depends on its microscopic affinity constants for ground and active states of the receptor (Colquhoun, 1998; Kenakin, 2007; Ehlert, 2008). It is currently impossible to determine each microscopic affinity constant from the kinds of data we have analyzed; nonetheless, RAi does provide a relative estimate of the microscopic affinity constant of the active state of the receptor. This parameter is completely dependent on the properties of the agonist and the receptor and is completely independent of G proteins and other elements in the signaling cascade. If there are multiple active conformations of the receptor available to the agonist, as well as multiple G proteins or effectors, the RAi estimate represents a weighted average, depending on the receptor conformations selected by the ligand and attendant effectors (e.g., G proteins). Although it may seem that the G protein has an influence on the estimate of RAi, G proteins actually provide a window for detecting different active conformations of the receptor. With defined experimental systems, it should be possible to estimate RAi for specific GPCR-G protein pairs, similar to our results shown in Fig. 4, making RAi analysis a powerful tool for quantifying ligand-directed signaling.
Appendix
This appendix describes the derivation of eqs. 10, 11, 12, 13, which were used for the analysis of the concentration-response curves in HEK Gα15 M4 and HEK Gα15 null cells. These equations are based on the operational model (Black and Leff, 1983), which describes the agonist concentration-response curve as a logistic function of the stimulus (s).
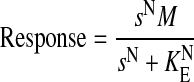 |
(15) |
In this equation, N denotes the transducer slope factor, M denotes the maximal response of the system, and KE denotes a constant related to the sensitivity of the stimulus-response function. Equation 15 can be rearranged into the following form.
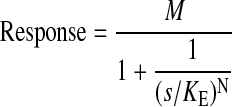 |
(16) |
Substituting in a parameter (S) for s/KE yields eqs. 10 and 11 under Materials and Methods. The stimulus is defined according to Furchgott (1966).
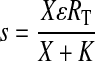 |
(17) |
In this equation, X denotes the concentration of agonist, ε denotes the observed intrinsic efficacy of the agonist-receptor complex, RT denotes the total receptor concentration, and K denotes the observed dissociation constant of the agonist-receptor complex. Dividing both sides of eq. 17 by KE yields
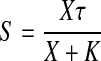 |
(18) |
in which
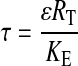 |
(19) |
Equations 18 and 19 provide the basis for eqs. 12 and 13 under Materials and Methods.
This work was supported by National Institutes of Health [Grant GM 69829].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.145219.
ABBREVIATIONS: GPCR, G protein-coupled receptor; RAi, intrinsic relative activity; CHO, Chinese hamster ovary; KRB, Krebs Ringer bicarbonate; HEK, human embryonic kidney; McN-A-343, 4-I-[3-chlorophenyl]carbamoyloxy)-2-butynyltrimethylammnonium chloride; oxo-M, oxotremorine-M; F-12K, F-12 medium Kaighn's modification.
References
- Berridge MJ, Downes CP, and Hanley MR (1982) Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J 206 587-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JW and Leff P (1983) Operational models of pharmacological agonism. Proc R Soc Lond B Biol Sci 220 141-162. [DOI] [PubMed] [Google Scholar]
- Buckley NJ and Burnstock G (1986) Autoradiographic localization of peripheral M1 muscarinic receptors using [3H]pirenzepine. Brain Res 375 83-91. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Carter PA, Yamada M, Gomeza J, Wess J, Hamilton SE, Nathanson NM, McKinzie DL, and Felder CC (2003) Role of specific muscarinic receptor subtypes in cholinergic parasympathomimetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine-induced seizure activity. Eur J Neurosci 17 1403-1410. [DOI] [PubMed] [Google Scholar]
- Christopoulos A and Mitchelson F (1997) Pharmacological analysis of the mode of interaction of McN-A-343 at atrial muscarinic M2 receptors. Eur J Pharmacol 339 153-156. [DOI] [PubMed] [Google Scholar]
- Colquhoun D (1998) Binding, gating, affinity and efficacy: the interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br J Pharmacol 125 924-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlert FJ (2008) On the analysis of ligand-directed signaling at G protein-coupled receptors. Naunyn Schmiedebergs Arch Pharmacol 377 549-577. [DOI] [PubMed] [Google Scholar]
- Ehlert FJ (1985) The relationship between muscarinic receptor occupancy and adenylate cyclase inhibition in the rabbit myocardium. Mol Pharmacol 28 410-421. [PubMed] [Google Scholar]
- Ehlert FJ (2000) Ternary complex model, in Biomedical Applications of Computer Modeling (Christopoulos A ed) pp 21-85, CRC Press, Boca Raton.
- Ehlert FJ, Griffin MT, and Glidden PF (1996) The interaction of the enantiomers of aceclidine with subtypes of the muscarinic receptor. J Pharmacol Exp Ther 279 1335-1344. [PubMed] [Google Scholar]
- Ehlert FJ, Griffin MT, Sawyer GW, and Bailon R (1999) A simple method for estimation of agonist activity at receptor subtypes: comparison of native and cloned M3 muscarinic receptors in guinea pig ileum and transfected cells. J Pharmacol Exp Ther 289 981-992. [PubMed] [Google Scholar]
- Ehlert FJ, Thomas EA, Gerstin EH, and Griffin MT (1997) Muscarinic receptors and gastrointestinal smooth muscle, in Muscarinic Receptor Subtypes in Smooth Muscle (Eglen RM ed) pp 92-147, CRC Press, Boca Raton.
- Ek B and Nahorski S (1988) Muscarinic receptor coupling to inositol phospholipid metabolism in guinea-pig cerebral cortex, parotid gland and ileal smooth muscle. Biochem Pharmacol 37 4461-4467. [DOI] [PubMed] [Google Scholar]
- Eltze M (1988) Muscarinic M1- and M2-receptors mediating opposite effects on neuromuscular transmission in rabbit vas deferens. Eur J Pharmacol 151 205-221. [DOI] [PubMed] [Google Scholar]
- Eltze M, Ullrich B, Mutschler E, Moser U, Bungardt E, Friebe T, Gubitz C, Tacke R, and Lambrecht G (1993) Characterization of muscarinic receptors mediating vasodilation in rat perfused kidney. Eur J Pharmacol 238 343-355. [DOI] [PubMed] [Google Scholar]
- Fechner PU, Teichmann KD, and Weyrauch W (1975) Accommodative effects of aceclidine in the treatment of glaucoma. Am J Ophthalmol 79 104-106. [DOI] [PubMed] [Google Scholar]
- Fisher SK and Bartus RT (1985) Regional differences in the coupling of muscarinic receptors to inositol phospholipid hydrolysis in guinea pig brain. J Neurochem 45 1085-1095. [DOI] [PubMed] [Google Scholar]
- Fox RI, Konttinen Y, and Fisher A (2001) Use of Muscarinic Agonists in the Treatment of Sjögren's Syndrome. Clin Immunol 101 249-263. [DOI] [PubMed] [Google Scholar]
- Furchgott RF (1966) The use of β-haloalkylamines in the differentiation of receptors and in the determination of dissociation constants of receptor-agonist complexes. Adv Drug Res 3 21-55. [Google Scholar]
- Gautam D, Heard TS, Cui Y, Miller G, Bloodworth L, and Wess J (2004) Cholinergic stimulation of salivary secretion studied with M1 and M3 muscarinic receptor single- and double-knockout mice. Mol Pharmacol 66 260-267. [DOI] [PubMed] [Google Scholar]
- Griffin MT, Figueroa KW, Liller S, and Ehlert FJ (2007) Estimation of agonist activity at G protein-coupled receptors: analysis of M2 muscarinic receptor signaling through Gi/o, Gs, and Gq. J Pharmacol Exp Ther 321 1193-1207. [DOI] [PubMed] [Google Scholar]
- Hammer R, Berrie CP, Birdsall NJ, Burgen AS, and Hulme EC (1980) Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature 283 90-92. [DOI] [PubMed] [Google Scholar]
- Hanin I, Jenden DJ, and Cho AK (1966) The influence of pH on the muscarinic action of oxotremorine, arecoline, pilocarpine, and their quaternary ammonium analogs. Mol Pharmacol 2 352-359. [PubMed] [Google Scholar]
- Keen M and Nahorski SR (1988) Muscarinic acetylcholine receptors linked to the inhibition of adenylate cyclase activity in membranes from the rat striatum and myocardium can be distinguished on the basis of agonist efficacy. Mol Pharmacol 34 769-778. [PubMed] [Google Scholar]
- Kenakin T (2007) Collateral efficacy in drug discovery: taking advantage of the good (allosteric) nature of 7TM receptors. Trends Pharmacol Sci 28 407-415. [DOI] [PubMed] [Google Scholar]
- Kendall D and Hill S (1990) Measurement of [3H]inositol phospholipid turnover, in Methods in Neurotransmitter Receptor Analysis (Yamamura H ed) pp 68-87, Raven Press, New York.
- Lambrecht G, Moser U, Grimm U, Pfaff O, Hermanni U, Hildebrandt C, Waelbroeck M, Christophe J, and Mutschler E (1993) New functionally selective muscarinic agonists. Life Sci 52 481-488. [DOI] [PubMed] [Google Scholar]
- Lazareno S and Birdsall NJ (1993) Pharmacological characterization of acetylcholin [35S]-GTPγS binding mediated by human muscarinic m1–m4 receptors: antagonist studies. Br J Pharmacol 109 1120-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S, Yorio T, DeSantis L, and Pang IH (1994) Muscarinic effects on cellular functions in cultured human ciliary muscle cells. Invest Ophthalmol Vis Sci 35 3732-3738. [PubMed] [Google Scholar]
- McKinney M, Miller JH, Gibson VA, Nickelson L, and Aksoy S (1991) Interactions of agonists with M2 and M4 muscarinic receptor subtypes mediating cyclic AMP inhibition. Mol Pharmacol 40 1014-1022. [PubMed] [Google Scholar]
- Mei L, Lai J, Yamamura HI, and Roeske WR (1991) Pharmacologic comparison of selected agonists for the M1 muscarinic receptor in transfected murine fibroblast cells (B82). J Pharmacol Exp Ther 256 689-694. [PubMed] [Google Scholar]
- Michal P, El-Fakahany EE, and Dolezal V (2007) Muscarinic M2 receptors directly activate Gq/11 and Gs G-proteins. J Pharmacol Exp Ther 320 607-614. [DOI] [PubMed] [Google Scholar]
- Milligan G and Kostenis E (2006) Heterotrimeric G-proteins: a short history. Br J Pharmacol 147 (Suppl. 1): S46-S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry R, Dowling MR, and Challiss RAJ (2005) An investigation of whether agonist-selective receptor conformations occur with respect to M2 and M4 muscarinic acetylcholine receptor signalling via Gi/o and Gs proteins. Br J Pharmacol 144 566-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermanns S, Negulescu P, Hu YH, and Simon MI (2001) Conditionally expressed Ga15 couples to endogenous receptors in GH3 cells. Naunyn Schmiedebergs Arch Pharmacol 364 140-148. [DOI] [PubMed] [Google Scholar]
- Pyttel R and Robinson JB (1973) Interaction of 3-quinuclidinol and its derivatives with acetylcholinesterase. J Pharm Sci 62 684-685. [DOI] [PubMed] [Google Scholar]
- Richards MH and van Giersbergen PL (1995) Human muscarinic receptors expressed in A9L and CHO cells: activation by full and partial agonists. Br J Pharmacol 114 1241-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringdahl B, Ehlert FJ, and Jenden DJ (1982) Muscarinic activity and receptor binding of the enantiomers of aceclidine and its. Mol Pharmacol 21 594-599. [PubMed] [Google Scholar]
- Ringdahl B, Resul B, and Dahlbom R (1979) Facile preparation of the enantiomers of 3-acetoxyquinuclidine and 3-quinuclidinol. Acta Pharm Suec 16 281-283. [PubMed] [Google Scholar]
- Roszkowski AP (1961) An unusual type of sympathetic ganglionic stimulant. J Pharmacol Exp Ther 132 156-170. [PubMed] [Google Scholar]
- Schwarz RD, Davis RE, Jaen JC, Spencer CJ, Tecle H, and Thomas AJ (1993) Characterization of muscarinic agonists in recombinant cell lines. Life Sci 52 465-472. [DOI] [PubMed] [Google Scholar]
- Wang SZ and El-Fakahany EE (1993) Application of transfected cell lines in studies of functional receptor subtype selectivity of muscarinic agonists. J Pharmacol Exp Ther 266 237-243. [PubMed] [Google Scholar]