Abstract
Background
Ejection fraction (EF) is an important method of mortality prediction among cardiac patients, and has been used to identify the highest risk patients for enrollment in the defibrillator primary prevention trials. Evidence suggests that measures of EF by different imaging modalities may not be equivalent. In the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) the type of imaging modality for EF assessment was not mandated.
Methods
Baseline assessment of EF was performed using either echocardiography, radionuclide angiography (RNA), or contrast angiography. Multivariable analysis using a Cox proportional hazards model was used to examine whether the modality of assessing EF affected the likelihood of survival.
Results
Among the 2,521 patients enrolled in SCD-HeFT, EF was measured by RNA in 616 (24%), echocardiography in 1,469 (58%), and contrast angiography in 436 (17%). Mean EF as measured by RNA was 25.1 ± 6.9%, by echocardiography was 23.8 ± 6.9%, by and by angiography 21.9 ± 6.9%. These measures were significantly different (p<0.001) and each pair-wise comparison differed significantly (p<0.001 for each). Multivariable analysis showed no significant difference in survival between patients enrolled based on RNA versus echocardiography (hazard ratio 1.06, 95% CI: 0.88,1.28), RNA versus angiography (hazard ratio 1.25, 95% CI: 0.97,1.62), or echocardiography versus angiography (hazard ratio 1.18, 95% CI: 0.94,1.48).
Conclusions
Among patients enrolled in SCD-HeFT the distribution of ejection fractions measured by radionuclide angiography differed from those measured by echocardiography or contrast angiograms. Survival did not differ according to modality of EF assessment.
Clinical trials have demonstrated that mortality is reduced by implanted cardioverter defibrillators (ICDs) among patients with poor left ventricular function1–5. Patient eligibility for these trials was determined, at least in part, by a reduced cardiac performance as measured by left ventricular ejection fraction (EF). Among cardiac patients the EF is among the best predictors of mortality6 and was intended to identify the highest risk patients for enrollment in the primary prevention trials. As the critical determinant of ICD provision, and the sole measure necessary for assessment of patient ICD eligibility according to national consensus guidelines7–10 and reimbursement for American Medicare patients11, accuracy and precision of EF determination seems critical. However, evidence suggests that measures of EF by different imaging modalities may not be equivalent12–14. In the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) the method of imaging modality for EF assessment was not mandated15. In selecting a method to assess EF in a potential ICD recipient, one must choose among imaging modalities which provide various degrees of quantitative versus qualitative assessment, and may not be similar in terms of precision and accuracy. Furthermore, this decision must be made without knowing what means of assessment was used for the original SCD-HeFT patients. To address these issues, the following is a report on the imaging modalities used for enrollment of patients in SCD-HeFT, and an assessment for association of imaging modality with survival.
Methods
The design and results of SCD-HeFT have been previously reported 1;15. In brief, 2,521 patients with New York Heart Association class II or III congestive heart failure and left ventricular (LV) ejection fraction ≤ 35%, as measured by nuclear imaging, echocardiography, or catheterization within 3 months of enrollment, were randomly assigned to treatment with ICD, amiodarone (double-blind), or placebo. The primary end-point was all-cause mortality. Adjunctive medical therapy was optimized according to contemporary clinical practice guidelines. ICD therapy consisted of single-lead devices programmed to detect rates exceeding 188 bpm for 18 of 24 intervals and to deliver shock therapies only.
Statistical analysis
Categorical variables are presented as percentages and were compared using the likelihood ratio χ2 test. Continuous variables are reported as means and standard deviations (sd) as well as median and interquartile ranges (IQR). These were compared using the non-parametric Wilcoxon rank sum tests for 2 groups or Kruskall-Wallis tests for 3 groups.
Multivariable analysis using a Cox proportional hazards model was used to examine whether the modality of assessing EF affected the likelihood of survival. The model was adjusted for potentially confounding covariates, including randomized therapy, ischemic or nonischemic etiology of heart failure, NYHA class, age, gender, EF, diabetes, mitral regurgitation, renal insufficiency, prior substance abuse, systolic BP, time since heart failure diagnosis, 6-minute walk distance, Duke Activity Status Index score, and use of digoxin and ACE-inhibitors. Tests for interactions between imaging modality and ejection fraction were included in the model to determine if the relationship of ejection fraction to survival differed according to method of EF assessment.
Results
Patient Characteristics
Data on imaging modality for ejection fraction assessment were available for all 2,521 patients enrolled in SCD-HeFT and are summarized in Table 1. Ejection fraction was most commonly measured by echocardiography in 1,469 (58%), followed by radionuclide angiography (RNA) in 616 (24%), and contrast angiography in 436 (17%). Mean ejection fraction as measured by echocardiography was 23.8% +/− 6.9 (median 25, (IQR) 10), by RNA 25.1% +/− 6.9 (median 26, IQR 11) and by angiography 21.9% +/− 6.9 (median 20, IQR 13). The Kruskall-Wallis test, the non-parametric analogue of a one-way analysis of variance, showed that the differences in EF between modalities was statistically significant (p<0.001); moreover, each pair-wise comparison differed significantly (p<0.001 for each).
Table 1.
Ejection fraction measures according to imaging modality among all SCD-HeFT patients
| Median EF | ||||
|---|---|---|---|---|
| Method | N | Mean EF (SD) | (25th, 75th %iles) | Min, Max EF |
| Nuclear | 616 (24%) | 25.1 (6.9) | 26 (20, 31) | 5, 35 |
| Contrast | 436 (17%) | 21.9 (6.9) | 20 (15, 28) | 5, 35 |
| Echo | 1469 (58%) | 23.8 (6.9) | 25 (20, 30) | 5, 35 |
Contrast=Contrast angiography; Echo=Echocardiography; EF= Ejection fraction; Nuclear=Nuclear angiography
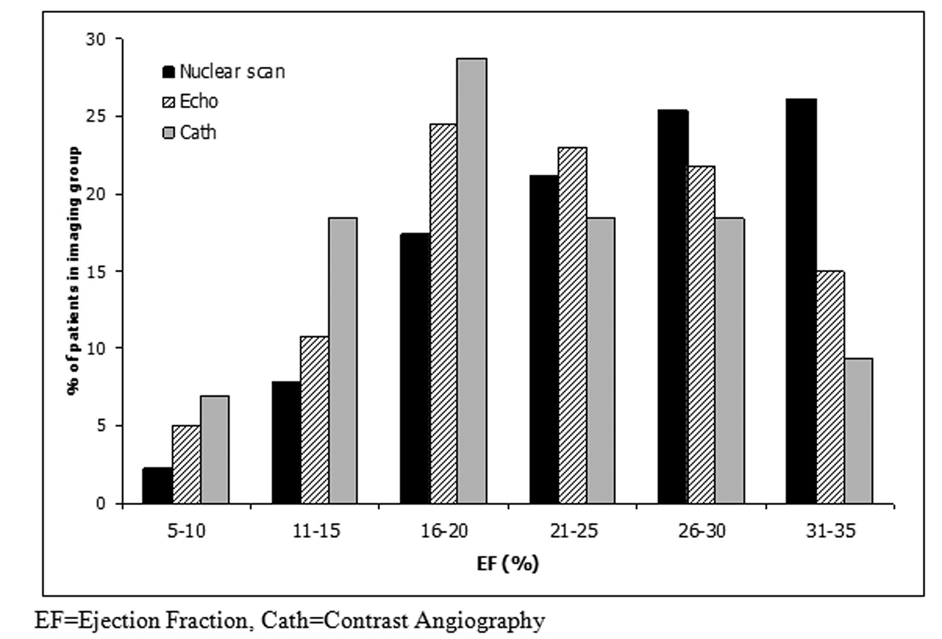
Shown in Figure 1 is a histogram of EF, in increments of 5%, for the 3 imaging modalities. Ejection fraction measures tended to be skewed toward higher values among patients who underwent a radionuclide scan, whereas echocardiography and contrast angiography appeared more symmetric.
Figure 1. Ejection Fraction by Imaging Modality.
Histogram of measured ejection fraction, in increments of 5%, according to imaging modality. Ejection fraction measures are skewed toward higher values among patients who underwent measurement by radionuclide scan, whereas echocardiography and contrast angiography have more symmetric distributions.
Baseline characteristics of study patients, stratified by imaging modality, are shown in Table 2. Beta blocker and ACE-inhibitor use did not differ among groups. Use of aspirin or warfarin differed across groups (p=0.002) with decreasing use among patients in the radionuclide, echocardiography, and angiography groups, respectively.
Table 2.
Baseline characteristics of SCD-HeFT patients, stratified by imaging modality
| Baseline | Nuclear | Contrast | Echo |
|---|---|---|---|
| Characteristic | (N = 616) | (N = 436) | (N = 1469) |
| Randomized treatment | |||
| ICD | 34% (207) | 31% (137) | 33% (485) |
| Amiodarone | 33% (201) | 33% (146) | 34% (498) |
| Placebo | 34% (208) | 35% (153) | 33% (486) |
| Age (years) | 60 (53, 68) | 59 (52, 68) | 60 (51, 69) |
| Female | 21% (131) | 25% (111) | 24% (346) |
| Nonwhite race | 17% (104)* | 27% (118) | 25% (367)+ |
| Weight (lbs) | 190 (166, 221) | 193 (167, 222)! | 187 (162, 218)+ |
| NYHA Class | |||
| II | 71% (438) | 70% (304) | 69% (1019) |
| III | 29% (178) | 30% (132) | 31% (450) |
| HF etiology | |||
| Ischemic | 63% (391)* | 45% (198) | 49% (721)+ |
| Non-ischemic | 37% (225) | 55% (238) | 51% (748) |
| Ejection fraction | 26 (20, 31)* | 20 (15, 28)! | 25 (20, 30)+ |
| Prior CABG | 33.0% (203)* | 19.5% (85)! | 26.1% (384)+ |
| Prior PCI | 17.8% (171)* | 19.5% (85)! | 17.7% (260)+ |
| Any prior revascularization | 46.9% (289)* | 30.7% (134)! | 35.5% (521)+ |
| Diabetes | 35% (215) | 31% (134) | 28% (418)+ |
| Pulmonary disease | 15% (95)* | 22% (95) | 20% (290)+ |
| Hyperlipidemia | 60% (372)* | 47% (206) | 51% (751)+ |
| Hypertension | 53% (324) | 58% (253) | 56% (823) |
| Atrial Fib/Flutter | 13% (80) | 14% (60) | 17% (250)+ |
| Nonsustained VT | 18% (112)* | 25% (110) | 25% (361)+ |
| Syncope | 7% (42) | 4% (19)! | 7% (101) |
| EP study | 11% (67)* | 21% (91) | 17% (249)+ |
| QRSd>=120 ms | 44% (271)* | 36% (155)! | 41% (607) |
| Systolic BP | 117 (104, 130)* | 120 (108, 136)! | 118 (106, 130) |
| Diastolic BP | 70 (60, 78)* | 70 (62, 80) | 70 (62, 80)+ |
| Heart rate (bpm) | 72 (63, 80)* | 76 (65, 84) | 74 (64, 84)+ |
| Beta blocker | 72% (445) | 67% (293) | 68% (1,000) |
| ACE-Inhibitor | 82% (506) | 86% (373) | 85% (1,254) |
| Aspirin or warfarin | 85% (525)! | 77% (334)+ | 82% (1,199)* |
BP=Blood Pressure; BPM=Beats per Minute; CABG=Coronary artery bypass graft; Contrast=Contrast angiography; Echo=Echocardiography; HF=Heart Failure; ICD=Implanted Cardioverter-defbrillator; Nuclear=Nuclear angiography; NYHA=New York Heart Association; PCI=Percutaneous coronary intervention; QTSd=QRS duration; VT=Ventricular Tachycardia
Nuclear different from contrast angiography at p<0.05.
Contrast angiography different from Echo at p<0.05.
Echo different from Nuclear at p<0.05.
Total Mortality
Mortality rates at 2.5 years of follow-up, according to Kaplan-Meier method, were 16.3% in the RNA group, 16.9% in the contrast angiography group, and 17.7% in the echocardiography group. Multivariate analysis, with correction for factors related to survival, showed no significant differences in survival between patients enrolled based on RNA versus echocardiography (hazard ratio 1.06 [0.88,1.28]), RNA versus contrast angiography (hazard ratio 1.25 [0.97,1.62]), or echocardiography versus contrast angiography (hazard ratio 1.18 [0.94,1.48]). Results are summarized in Table 3.
Table 3.
Mortality according to imaging modality among SCD-HeFT patients
| Patients with EF ≤ 30% | Patients with EF≤ 35% | |||
|---|---|---|---|---|
| Comparison | HR [95% CI] | p-value | HR [95% CI] | p-value |
| Nuclear vs Echo | 1.03 [0.84, 1.27] | 0.77 | 1.06 [0.88, 1.28] | 0.55 |
| Nuclear vs Contrast | 1.27 [0.96, 1.67] | 0.09 | 1.25 [0.97, 1.62] | 0.09 |
| Echo vs Contrast | 1.23 [0.97, 1.55] | 0.08 | 1.18 [0.94, 1.48] | 0.15 |
Contrast=Contrast angiography; Echo=Echocardiography; EF=Ejection fraction; HR=Hazard ratio; Nuclear=Nuclear angiography
Among patients with ejection fraction less than 20%, there was no detectable relationship between survival and ejection fraction. Among patients with an EF between 20 and 35%, a 5% increase in EF was associated with a lower mortality (hazard ratio 0.81 [0.75, 0.88]). This result was consistent for each initial EF imaging modality, as the interaction term combining imaging method and EF in the Cox model was not significant (p=0.71).
Of the total SCD-HeFT study population the vast majority (83%) had an EF ≤ 30%. We repeated our analyses among this group of patients and our results were similar (Table 3 and Table 4). Once again, survival did not differ according to EF assessment modality. Each 5% increase in EF between 20 and 30% was associated with a hazard ratio of 0.82 [0.73, 0.92]. Again, this result was uniform among patients regardless of initial imaging modality, as determined by a nonsignificant interaction term (p=0.71).
Table 4.
Ejection fraction measures according to imaging modality among SCD-HeFT patients with EF ≤ 30%
| Median EF | ||||
|---|---|---|---|---|
| Method | N | Mean EF (SD) | (25th, 75th %iles) | Min,Max EF |
| Nuclear | 455 (22%) | 22.3 (5.7) | 23 (19, 27) | 5, 30 |
| Contrast | 395 (19%) | 20.7 (6.0) | 20 (15, 25) | 5, 30 |
| Echo | 1249 (59%) | 22.0 (5.8) | 23 (19, 26) | 5, 30 |
Contrast=Contrast angiography; Echo=Echocardiography; EF= Ejection fraction; Nuclear=Nuclear angiography
Discussion
Primary prevention trials have demonstrated a survival benefit with ICD therapy among patients with impaired left ventricular function1–5. These trials have based enrollment on EF assessment by echocardiography, radionuclide scanning, or contrast angiography. Prior evidence suggests that measures of EF by different imaging modalities are not necessarily equivalent12–14. This may give rise to uncertainty as to whether the trial results apply uniformly to patients enrolled. For example, analysis of data from Studies Of Left Ventricular Dysfunction (SOLVD) has shown that mortality rates can differ among patients with similar ejection fractions measured by different imaging modalities16. Specifically, despite similarity of baseline EF (27% +/− 6), the adjusted all-cause mortality among SOLVD patients was higher among patients with echocardiographic assessment than those with radionuclide angiography (relative risk 1.15, 95% CI: 1.01 to 1.30). While the mechanism for this mortality difference is not clear, the authors postulated that patient characteristics and technical proficiency with echocardiography may be a factor, resulting in systematic measurement error.
Our analysis demonstrates that patients enrolled in SCD-HeFT on the basis of different imaging modalities had different means and distributions of EF (Figure 1). The underlying reason for these considerable differences is difficult to ascertain. One could hypothesize at least two possibilities – that patients with better LV function more often underwent nuclear assessment of EF, or that the imaging modalities measure EF differently in a systematic manner.
With the first hypothesis, if one assumes that the each modality measures EF accurately and identically, there appears to be a systematic difference in the modality used for entry assessment. Ejection fraction testing strategy may have resulted in such a bias. As an example, investigators may have performed an initial EF assessment by echocardiography, enrolling to the study if the EF was clearly <35%, but repeating assessment using an RNA if the initial results were equivocal. If the more quantitative RNA result was then used for SCD-HeFT entry criteria, this would result in a bias toward radionuclide results among patients with higher ejection fractions. A similar result would be seen if the RNA was avoided among sicker patients, with poorer EF, although motivation for such clinical bias seems unclear. In such a scenario, one would expect greater survival among the healthier patients – those with RNA assessment – an expectation that is not evident in our results. With the second hypothesis, one assumes that EF determinations based on the three modalities are not identical, and that the true distribution of the three groups is similar, with radionuclide scanning providing a higher estimate of EF than the other imaging modalities12. Figure 1 could, indeed, be consistent with a systematic overestimation of ejection fraction by RNA at higher ejection fractions (or systematic underestimation by echocardiography and contrast angiography). Under such a scenario one would expect that if RNA patients had EF reassessed by the other two modalities, the measured ejection fraction would tend to be lower. This would manifest as no difference in survival between imaging modality groups, but a survival disadvantage to RNA patients at any given EF measure (as the true EF is either lower than the RNA measure and more accurate with other modalities, or higher than the echo and angiogram measures and more accurate with RNA). One would also expect no difference in survival in spite of higher measured ejection fractions in RNA patients – an expectation that is in keeping with our results.
Among each of the pair-wise comparisons, there was a trend toward greater survival with the imaging modality that demonstrated the lower mean EF. The most pronounced example is that of angiography (mean EF 21.9%) and RNA (mean EF 25.1%), where RNA was associated with a 25% higher risk of death although this did not achieve conventional levels of statistical significance (p=0.091). While this appears counterintuitive, in that one might expect the group with higher ejection fraction to have the higher survival rate, there are several possible explanations. First, apparent differences in mortality may simply reflect the play of chance. The proximity to the conventional 5% level may be illusory as we did not adjust our level of significance for the multiple hypotheses tested. Second, the population with higher EF may (and presumably does) have a higher rate of survival, but the study was underpowered to detect this difference. Third, the survival results may be confounded by an unknown factor which is associated with greater survival, and incrementally distributed among the angiography, echocardiography, and RNA groups, respectively. One such candidate factor is revascularization. However, the incidence of prior angioplasty did not differ among the imaging groups. In contrast, distribution of prior coronary artery bypass grafting (CABG) among the three imaging groups is not uniform (see Table 2), but survival among the treatment groups was not correlated with prior CABG, indicating that this is not a confounding factor. Revascularization rates after enrollment in SCD-HeFT did not differ among the imaging groups, as illustrated in Table 5. Although it is impossible to discern the true underlying reason for the unexpected survival trend, it almost certainly involves some combination of the three factors described above.
Table 5.
Revascularization post enrollment among SCD-HeFT patients
| Nuclear | Contrast | Echo | |
|---|---|---|---|
| (N=616) | (N=436) | (N=1469) | |
| CABG | 1.5% (9) | 1.2% (5) | 1.5% (22) |
| PCI | 5.1% (31) | 4.2% (18) | 4.1% (60) |
| Any revascularization | 6.3% (39) | 5.3% (23) | 5.5% (80) |
CABG=Coronary artery bypass graft; Contrast=Contrast angiography; Echo=Echocardiography; Nuclear=Nuclear angiography; PCI=Percutaneous coronary intervention
Some limitations of our study should be considered. This was not a randomized comparison of survival based on modality of EF assessment. As such, there were significant differences in the baseline characteristics of the three EF assessment groups. Although we used statistical methods to adjust for these differences, residual confounding may have remained and it is possible that the groups differed in other ways. As only a single measure of EF was submitted for each patient on entry into SCD-HeFT, data is not available from this study to explore the important question of comparative results among multiple imaging modalities in single patients. Assessment of EF was performed locally by investigators without involvement of a central core laboratory. As such, it is possible that for each technique, different algorithms (including subjective EF assessment by echocardiography) may have been used. It is possible that EF estimates were originally reported as ranges from echocardiographic assessment, and converted to a single number for the purpose of study entry. This could potentially introduce measurement bias. Finally, while this analysis includes a large number of patients and substantial follow-up, our study remains underpowered to exclude the possibility of moderate survival differences between modalities of EF determination.
Conclusions
Among patients enrolled in SCD-HeFT, the distribution of ejection fractions measured by radionuclide angiography differed from those measured by echocardiography or contrast angiograms. We were unable to detect a difference in survival according to modality of EF assessment.
Acknowledgments
SCD-HeFT was supported by grants (UO1 HL55766, UO1 HL55297, and UO1 HL55496) from the National Heart, Lung, and Blood Institute, NIH, and by Medtronic, Wyeth-Ayerst Laboratories. This substudy was supported by a grant from Medtronic of Canada.
Author Disclosures:
Dr. Bardy: Research funding from Medtronic and Wyeth–Ayerst Pharmaceuticals; Founder of, board member of, consultant to, and equity holder in Cameron Health.
Dr. Gula: Grant support from Medtronic of Canada
Dr. Klein: Consultant fees from Medtronic
Dr. Krahn: Consultant fees from Boston Scientific and Transoma
Dr. Lee: Research funding from Medtronic and Wyeth–Ayerst Pharmaceuticals
Dr. Mark: Grant support and speaking fees from Medtronic.
Dr. Poole: Speaking fees from Boston Scientific, Medtronic and Biotronic
Dr. Yee: Consultant fees from Medtronic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N.Engl.J.Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 2.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N.Engl.J.Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N.Engl.J.Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 4.Buxton AE, Lee KL, Fisher JD, et al. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N.Engl.J.Med. 1999;341:1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 5.Hohnloser SH, Kuck KH, Dorian P, et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N.Engl.J.Med. 2004;351:2481–2488. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 6.Solomon SD, Anavekar N, Skali H, et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112:3738–3744. doi: 10.1161/CIRCULATIONAHA.105.561423. [DOI] [PubMed] [Google Scholar]
- 7.Tang AS, Ross H, Simpson CS, et al. Canadian Cardiovascular Society/Canadian Heart Rhythm Society position paper on implantable cardioverter defibrillator use in Canada. Can.J.Cardiol. 2005;21 Suppl A:11–18. [PubMed] [Google Scholar]
- 8.Armstrong PW, Bogaty P, Buller CE, et al. The 2004 ACC/AHA Guidelines: a perspective and adaptation for Canada by the Canadian Cardiovascular Society Working Group. Can.J Cardiol. 2004;20:1075–1079. [PubMed] [Google Scholar]
- 9.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction; A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction) J Am.Coll.Cardiol. 2004;44:E1–E211. doi: 10.1016/j.jacc.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death--executive summary: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Eur.Heart J. 2006;27:2099–2140. doi: 10.1093/eurheartj/ehl199. [DOI] [PubMed] [Google Scholar]
- 11.CMMS; Centers for Medicare & Medicaid Services. Pub 100-03 Medicare National Coverage Determinations. 2005 Ref Type: Electronic Citation http://www.cms.hhs.gov/transmittals/downloads/R29NCD.pdf.
- 12.Bellenger NG, Burgess MI, Ray SG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? ur.Heart J. 2000;21:1387–1396. doi: 10.1053/euhj.2000.2011. [DOI] [PubMed] [Google Scholar]
- 13.Naik MM, Diamond GA, Pai T, et al. Correspondence of left ventricular ejection fraction determinations from two-dimensional echocardiography, radionuclide angiography and contrast cineangiography. J Am.Coll.Cardiol. 1995;25:937–942. doi: 10.1016/0735-1097(94)00506-L. [DOI] [PubMed] [Google Scholar]
- 14.Bernard Y, Meneveau N, Boucher S, et al. Lack of agreement between left ventricular volumes and ejection fraction determined by two-dimensional echocardiography and contrast cineangiography in postinfarction patients. Echocardiography. 2001;18:113–122. doi: 10.1046/j.1540-8175.2001.00113.x. [DOI] [PubMed] [Google Scholar]
- 15.Bardy GH. The Sudden Cardiac Death-Heart Failure Trial (SCD-HeFT), Arrhythmia treatment and therapy: Evaluation of clnical trial evidence. New York: Marcel Dekker; 2000. pp. 323–342. [Google Scholar]
- 16.Rashid H, Exner DV, Mirsky I, et al. Comparison of echocardiography and radionuclide angiography as predictors of mortality in patients with left ventricular dysfunction (studies of left ventricular dysfunction) Am.J Cardiol. 1999;84:299–303. doi: 10.1016/s0002-9149(99)00280-5. [DOI] [PubMed] [Google Scholar]