Abstract
Background
Withdrawal from an acute high ethanol dose induces behaviors reminiscent of withdrawal from chronic ethanol exposure. While such “hangover”-related anxiety has previously been shown to be considerably less pronounced in adolescent compared to adult male rats, ontogenetic studies are limited and few experiments have directly compared sex- and age-related differences in sensitivity to ethanol hangover.
Methods
The current experiments examined consequences of a previous ethanol challenge (4.0 g/kg i.p. injection, 20% v/v) on anxiety and exploratory behavior in the elevated plus-maze (EPM) and holeboard (HB) tests, respectively, in adolescent and adult male and female Sprague-Dawley rats.
Results
In Exp. 1, evidence of hangover-related anxiety and withdrawal-induced hypoactivity emerged at both ages and in both sexes. Since several procedural variables were changed in Exp. 1 relative to previous studies from our laboratory showing age-related differences in these hangover measures, Exp. 2 explored the possible contribution of two variables to ontogenetic expression of withdrawal-induced anxiogenesis: (1) isolation vs. social context during the post-challenge recovery period; and (2) EPM testing alone or immediately following a 5 min HB test. Results of Exp. 2 revealed few significant interactions of these variables with age- and ethanol exposure-related anxiety measures, although sequential testing (HB before EPM) notably suppressed activity in the EPM and altered the major underlying component of EPM behavior from anxiety to activity as revealed in factor analyses of these data. Additional analyses conducted on animals tested only in the EPM revealed attenuations in withdrawal anxiogenesis among adolescents, along with withdrawal-related decreases in activity at both ages.
Conclusions
Taken together, these results suggest that an attenuated sensitivity of adolescents to hangover-induced anxiogenesis does emerge in the EPM under certain test conditions, but can be masked by pretest circumstances, urging caution when using the EPM to index anxiety across age. The robustness of withdrawal-related hypoactivity at both ages suggests that adolescents may not be globally insensitive to the consequences of previous binge-like exposure to ethanol, but rather less likely to express certain hangover-related consequences.
Keywords: adolescence, ethanol hangover-induced anxiogenesis, elevated plus-maze, holeboard, factor analysis
Introduction
Adolescence is a time of considerable hormonal, neural, and psychosocial transformations across a variety of species (for review see Spear, 2000). Among the behavioral alterations reported in adolescents are increases in risk-taking and novelty seeking (Arnett, 1992) as well as frequent initiation of alcohol consumption in human adolescents, with as many as 75% o of high school seniors reporting that they have tried alcohol at least once during their lifetime (Johnston et al., 2005). Adolescents are not only trying alcohol, but are also frequently drinking relatively large amounts. For instance, in the 2005 Monitoring the Future study, as many as 30% of high school seniors reported drinking 5 or more drinks in a row within the last 2 weeks (Johnston et al., 2005), with this episodic drinking defined as “binge drinking.”
Animal models investigating ethanol exposure during adolescence have demonstrated age-related differences in ethanol sensitivity. For instance, adolescent animals have been shown to be less sensitive than adults to a number of ethanol effects, including hypnotic (Little et al., 1996; Silveri and Spear, 1998), motor impairing (White et al., 2002), anxiolytic (Varlinskaya and Spear, 2002) and certain withdrawal (Doremus et al., 2003; Varlinskaya and Spear, 2004) effects. In contrast, adolescents have been reported to exhibit conversely greater sensitivity than adults to a few restricted consequences of ethanol, namely ethanol-induced impairments in hippocampal long-term potentiation (LTP) and N-methyl-D-aspartate (NMDA) receptor function (Swartzwelder et al., 1995a; Swartzwelder et al., 1995b), ethanol-related disruption in performance on a spatial memory task (Markwiese et al., 1998), and the social facilitating effects of ethanol exposure (Varlinskaya and Spear, 2002; Varlinskaya and Spear, 2006). It has been hypothesized that an adolescent-related insensitivity to some of the negative consequences of alcohol consumption, together with an increased sensitivity to the social facilitating effects of ethanol, could serve as a permissive factor for excessive alcohol use both during the current drinking episode and on future occasions.
Acute ethanol withdrawal (or hangover) is of interest to researchers since withdrawal symptoms have been hypothesized to help curtail use on subsequent occasions (e.g. Smith and Barnes, 1983). Symptoms of hangover are observed following acute binge consumption and include headache, nausea, diarrhea, anorexia, fatigue and tremor, along with psychological signs such as anxiety, guilt and depression (Bogin et al., 1986; McKinney and Coyle, 2006; Smith and Barnes, 1983; Wiese et al., 2000). Among the common signs of acute withdrawal observed in both humans (Smith and Barnes, 1983) and rodents (Doremus et al., 2003; Gauvin et al., 1993; Varlinskaya and Spear, 2004) is a withdrawal-induced elevation in anxiety that is typically observed soon after ethanol has been eliminated from the body.
To date, there has been relatively little research examining the ontogeny of ethanol hangover. Human studies that rely on surveys and self-reports have observed that adolescents who commonly abuse alcohol report fewer withdrawal symptoms upon cessation of drinking, in contrast to reports from adults (Martin and Winters, 1998). Of the little research available using rodent models, adolescent rats have been reported to be less sensitive than adults to several hangover-related effects including attenuated hyperthermia (Brasser and Spear, 2002), ultrasonic vocalizations (USVs) (Brasser and Spear, 2002), and anxiogenesis when assessed in the elevated plus-maze (EPM) (Doremus et al., 2003) as well as social interaction tests (Varlinskaya and Spear, 2004). Most of the studies that have reported attenuated hangover severity in adolescent animals were conducted only in males, with few studies having compared males and females of both ages in terms of their susceptibility to hangover-induced behavioral alterations (Brasser and Spear, 2002; Varlinskaya and Spear, 2004). In these initial across-sex comparisons, interesting sex differences emerged, with few age differences in hangover-associated anxiogenesis emerging in females but adult males again showing more ethanol-related anxiogenesis during withdrawal than adolescent males (Varlinskaya and Spear, 2004).
In this series of experiments, therefore, both males and females were examined to assess potential sex differences in severity of ethanol hangover anxiogenesis in adolescents and adults when assessed in the EPM, as well as hangover-related reductions in exploratory behavior indexed using a holeboard test. In the course of these studies, to determine the locus of discrepancies between the results of the initial study in this series and prior work, the influence of several pretest manipulations on age and sex differences in the expression of hangover-related behaviors was also explored. This issue is of particular importance, given the sensitivity of the EPM to pretest perturbations (Carobrez and Bertoglio, 2005; Hogg, 1996; Rodgers et al., 1997; Rodgers and Cole, 1994) and evidence for differential sensitivity to these effects across age (Doremus-Fitzwater et al., in prep).
Materials and Methods
General Methods
Subjects
A total of 320 male and female Sprague-Dawley rats bred in our colony were used in these experiments. On the day after birth, postnatal day (P) 1, litters were culled to 8–10 pups, with 6 animals of one sex and 4 animals of the other being retained whenever possible. Pups were housed with their parents until weaning at P21, at which time they were housed in same-sex littermate pairs and given ad libitum access to food and water in a temperature-controlled vivarium maintained on a 14:10-hr light:dark cycle (lights on 0700). Only 1 animal per sex from a litter was placed into any given treatment condition. At all times rats used in these experiments were maintained and treated in accordance with guidelines for animal care established by the National Institute of Health (Institute of Laboratory Animal Resources, Commission on Life Sciences, 1996), using protocols approved by the Binghamton University Institutional Animal Care and Use Committee (IACUC).
Apparatuses and Testing Procedures
All behavioral testing was conducted under dim light (3 lux), with a white noise generator located in each testing room to attenuate extraneous sounds. All holeboard and plus-maze sessions were 5 min in duration and were conducted without the experimenter present. Sessions were videotaped via a camera mounted above each apparatus for later scoring. After each animal, apparatuses were cleaned with a 3% hydrogen peroxide solution and dried before testing of the next animal.
Elevated plus-maze (EPM)
Separate mazes were used for each age, with the adolescent EPM (30 × 8.9 cm open arms with 20.3 cm walls on closed arms) scaled to 3/4 of the adult dimensions (48.3 × 12.7 cm open arms with 29.2 cm walls on closed arms) based on crown: rump length and gait width analyses. Mazes were elevated to a height of 50 cm. Small plastic edges (0.6 and 1.3 cm for adolescents and adults, respectively) were located along each side and end of the open arms to reduce the likelihood of subjects falling during testing (Fernandes and File, 1996). Gaps of 4.0 cm (adolescent) and 4.5 cm (adult) at the junctions of these plastic edges provided easy access below the plane of the maze to allow for protected head-dipping behavior. Any animal that fell during testing was removed from the experiment and replaced with another subject.
At the start of all EPM sessions, each animal was placed on the center platform facing a closed arm. Behavioral measures scored from the videotape records included: open arm entries/time, closed arm entries/time, number of protected and unprotected head dips, number of protected and unprotected stretched attends, number of rears, and time spent in the central square. An animal was considered to have entered an arm when all four paws were placed in the arm and to have exited an arm when at least its two front paws moved outside of that arm. Protected head dips included dipping the head over the sides of the maze when in the center platform or an enclosed arm, while head dips were considered unprotected when the same behavior was observed on an open arm. Protected stretched attends were defined as when the animal’s two hind feet remained in a closed arm or the center platform while the animal elongated its head and shoulders, followed by subsequent retraction. Similarly, an unprotected stretched attend posture was defined as the same behavior, but when the animal was on an open platform.
Percentage of time spent in the open arms (%OAT) and percentage of open arm entries (%OAE) have repeatedly been shown to be reliable inverse measures of anxiety (i.e., lower percentages reflecting greater anxiety) on the EPM in adult (Lal et al., 1991; Pellow et al., 1985) as well as adolescent animals (Doremus et al., 2006). Both of these measures were calculated as the percentage of open arm time/entries relative to the total time spent/entries in either the open or closed arms (i.e. amount of time spent in the central hub was not included). Animals that never sampled the open arms were included in the analysis and given a percentage score of zero for these two measures. In addition to these traditional anxiety indices, percent protected head dips (%PHD) and percent protected stretched attend postures (%PSAP) have been suggested to be even more sensitive measures of anxiety, based on ethological analysis and pharmacological manipulations (Espejo, 1997; Rodgers and Cole, 1994; Rodgers and Dalvi, 1997). These percentages were also calculated in a manner parallel to %OAE and %OAT, although with these measures higher scores (i.e. those approaching 100%) reflect greater anxiety. For this reason, animals that failed to exhibit either the protected and unprotected forms of these behaviors, and hence were considered to be the most anxious using these measures, were given a score of 100% for data analysis on that measure. Closed arm entries (CAE) and number of rears were used as indices of activity, since they have been validated as activity measures in previous reports of EPM behaviors (Cruz et al., 1994; Rodgers and Dalvi, 1997).
Holeboard (HB) test
Exploration and activity levels were assessed using the HB test in age-scaled apparatuses constructed from wood and covered with a light, gray, gloss paint (adolescents: 43 × 50 × 34.5 cm; adults 56 × 66 × 45 cm). The floor of each chamber was divided into 12 equally sized squares, with 4 evenly spaced (18 × 21 cm vs. 21 × 28 cm apart, respectively) and sized (3 cm for adolescents and 4 cm for adults) holes located in the floor. Legs (4 cm) on the bottom of the apparatus raised the HB floor above the testing room floor to allow for head dips below the plane of the apparatus.
Animals were placed in a center square at the start of the 5-min HB test. Behavioral measures recorded included: frequency of outer and inner square crossings (a crossing was scored when the animal’s two forepaws crossed a demarcation line); frequency of and time spent rearing (defined as balancing on the haunches with both forepaws off the ground with or without using the walls for support); and frequency of and time spent head dipping (defined as poking the head into a hole to a depth that the eyes were below the plane of the floor). Previous research has shown that head dipping frequency and duration are reliable measures of exploration, whereas time and frequency of rearing and line crosses are valid measures of activity within this behavioral test (File and Wardill, 1975a; File and Wardill, 1975b).
Ethanol Challenge
In all experiments, animals received an intraperitoneal (i.p,) injection of either ethanol (20% v/v; diluted with a 0.9% saline solution at a dose of 4 g/kg) or saline (2.52% of body weight; isovolumetric to ethanol injection) at room temperature.
Twelve hr after injection for both male and female adolescents, 13 hr post-administration for adult females, and 18 hr post-challenge for adult males, animals were placed into their pretest conditions. These post-injection time points were selected so that manipulations would begin approximately 3 hr post-clearance at each age, based on data from our lab estimating ethanol clearance times in adolescent males and females at 9 hr, adult females at 10 hr, and adult males at 15 hr (see Fig. 3 from (Varlinskaya and Spear, 2004). The 3 hr post-clearance-to-test interval was chosen given that evidence for hangover-related anxiety and hypoactivity is more robust at this interval than at later post-clearance intervals in both adolescent and adult animals of both sexes. Specifically, robust withdrawal-induced anxiogenesis and hypoactivity was seen 3 hr after alcohol clearance in adult male rats tested in both the EPM (Doremus et al., 2003) and social interaction (Varlinskaya & Spear, 2004) tests, and in both adolescent and adult females tested in the social interaction test (Varlinskaya & Spear, 2004). In contrast, only limited evidence of anxiogenesis in the social interaction test was seen in adult males or in females of either age when testing was delayed until 7.5–9 hr post-clearance, with hypoactivity only evident at this time in adults (Varlinskaya &, Spear, 2004). Although adolescent males failed to show convincing evidence of withdrawal-related anxiogenesis at any post-clearance-to-test interval in either test, they did exhibit robust withdrawal-related hypoactivity at the 3 hr clearance interval (similar to their female counterparts), with less pronounced hypoactivity when testing was delayed until 7.5–9 hr post-clearance (Doremus et al., 2003; Varlinskaya & Spear, 2004).
Figure 3.
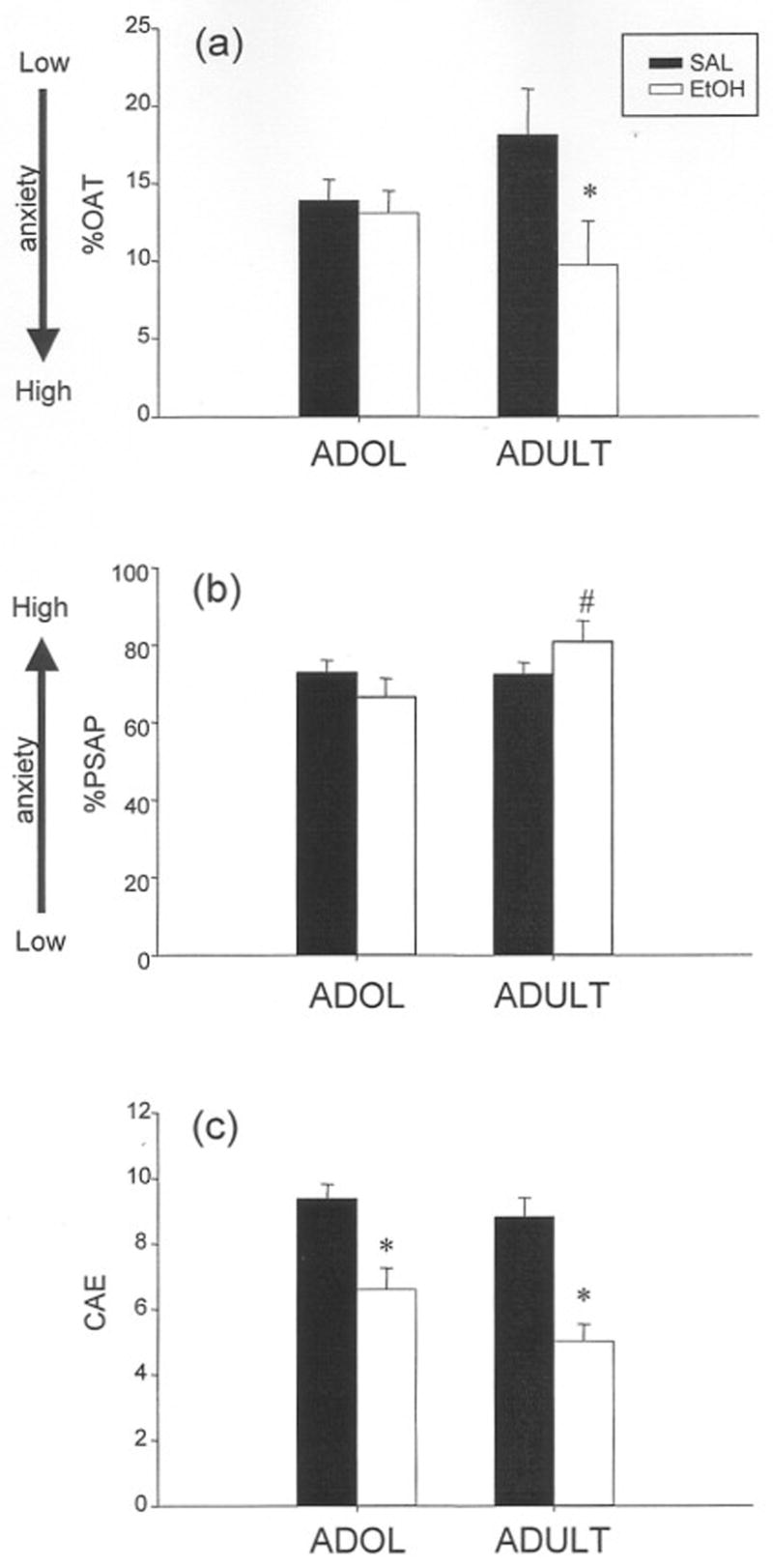
Elevated plus-maze behavior of adolescent and adult rats from Exp. 2 that were tested only in the plus-maze and that entered the open arms at least once. Data are collapsed over sex given that this variable did not interact with age-related differences in withdrawal anxiogenesis. Rats were administered either an ethanol (EtOH) or saline (SAL) challenge and were tested 3 hr post-clearance of the ethanol injection, with saline-exposed animals tested at an equivalent time point. (a) An age × exposure interaction for percent open arm time (%OAT) revealed that adults but not adolescents exhibited significant reductions in open arm time (as shown by the asterisk), (b) An age × exposure interaction for percent protected stretched attend postures (%PSAP) showed that ethanol-exposed adults were significantly more anxious than their ethanol-exposed adolescent counterparts (as indicated by the pound sign, #) (c) Significant reductions in closed arm entries (CAE) were again exhibited during hangover, regardless of age (as marked with the asterisks).
Data Analysis
Experimenters blind to the treatment of the subjects scored all behaviors. Behaviors were compared across test conditions using between-groups analysis of variance (ANOVA) procedures, with post hoc comparisons made with Fischer’s LSD tests (p ≤ .05). In Exp. 1, one ethanol-exposed adolescent female died shortly after testing and was therefore not included in those analyses. In Exp. 2, two animals fell from the EPM apparatus and were replaced. The videotape records of two additional animals were lost due to equipment difficulties and hence were not included in the data analyses for Exp. 2.
Data from the EPM and HB in each experiment also were subjected to factor analysis (Fernandes and File, 1996; Rodgers and Johnson, 1995). Prior to each analysis, test variables were checked for violations of normality. Skewness and kurtosis statistics were evaluated, with data resulting in a statistic of 2 times or less the standard error considered acceptable for analysis. When a particular variable violated this criterion, that measure was subject to transformation (e.g., square root, log (n + 1), are sine), using the transform producing the most normal distribution for that variable within the given data set. Before factor analysis, the correlation matrix was checked for highly correlated and redundant measures which were removed when necessary. A principal components analysis was then conducted, with an orthogonal (varimax) rotation used on the factor matrix. Only components with an Eigenvalue of 1 or greater were retained for final rotation. Additionally, the Kaiser-Meyer-Olkin (KMO) measure of sampling adequacy and Bartlett’s test of sphericity were analyzed to ensure that these data were adequate for use in the analyses.
Experiment 1
Previous results from our lab demonstrated a relative insensitivity to anxiogenesis following withdrawal from an acute ethanol challenge in adolescent males compared to adult males when indexed in the EPM (Doremus et al., 2003) and social interaction (Varlinskaya & Spear, 2004) tests. However, when both males and females were examined in the social interaction test, few age differences emerged in females (Varlinskaya & Spear, 2004). Therefore, the purpose of this experiment was to determine whether the sex differences in withdrawal seen in the social interaction test following acute administration of ethanol would also be evident in EPM and HB tests, given that the EPM and social interaction tests seemingly index different forms of anxiety (Cheeta et al., 2001; File, 1995; Johnston and File, 1991). In our earlier work, we also have observed consistent hangover-related hypoactivity in males and females of both ages, at least when assessed indirectly in the EPM and social interaction tests via measurement of closed arm entries and crosses between the two compartments, respectively (Doremus et al., 2003; Varlinskaya & Spear, 2004). Consequently, in this study we also extended our prior work by examining hangover-related hypoactivity directly in the HB prior to EPM testing. This sequential testing strategy has been used previously (Fernandes et al., 1999; File et al., 1991; Morato and Brandao, 1996; Pellow et al., 1985; Pellow and File, 1986; for review see Hogg, 1996) and provides the opportunity to examine activity and exploration measures in a test situation not explicitly designed to measure anxiety, as well as the advantage of subjecting combined HB and EPM data to factor analyses (Fernandes et al., 1999).
Methods
A total of 64 Sprague-Dawley rats were used across the 8 experimental conditions defined by the 2 (age) × 2 (sex) × 2 (drug challenge) factorial design of this experiment. On days 33–35 for adolescents and P70–75 for adults, each animal was injected with either saline or 4 g/kg ethanol between 1800 and 2000 hr and isolated in a novel cage with ad libitum access to food and water.
The pretest isolation period consisted of placing each animal alone in a novel holding cage for 25 min prior to the onset of testing. This pretest isolation period was included because data from our laboratory has suggested that rats isolated in novel holding cages prior to EPM testing exhibited greater open arm activity than rats tested directly from their home cage (Doremus et al., 2004). Following the isolation period, each animal was placed into the HB test, followed immediately by testing in the EPM.
Results
HB Testing
Exploration in the HB, as measured by frequency (Fig. 1a) and time spent head dipping, was generally decreased during the hangover period following prior ethanol exposure. Total crosses (Fig. 1b) and rears in the HB also were significantly lower in animals previously challenged with a large dose of ethanol than in animals exposed to saline, confirming previous reports that hypoactivity is a robust and consistent measure of hangover evident across age and sex (Doremus et al., 2003; Ristuccia and Spear, 2005; Varlinskaya and Spear, 2004). The variables of age and sex did not interact with the ethanol exposure variable for any of these HB behavioral measures. Table 1 provides a complete listing of all results from the HB in Exp. 1.
Figure 1.
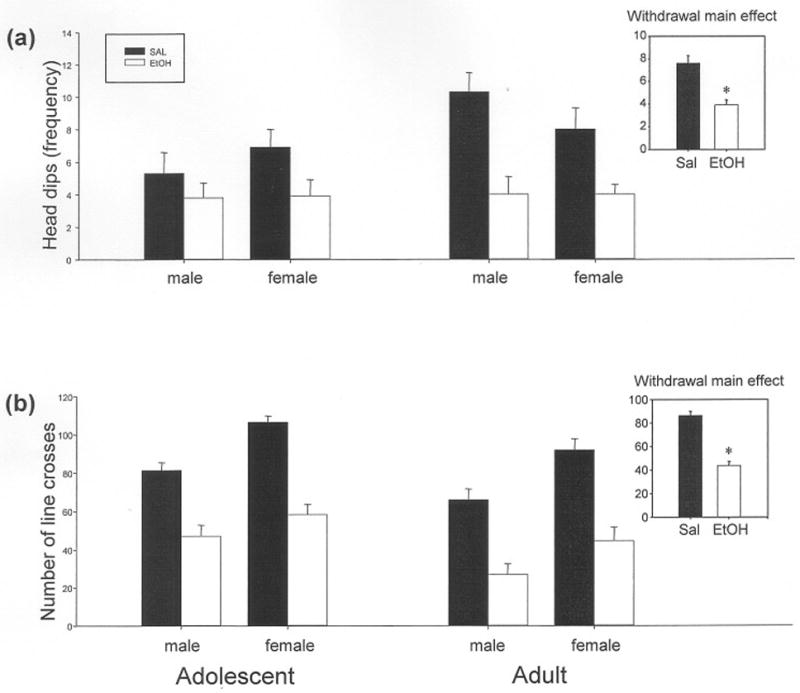
Holeboard behaviors of both adolescent and adult male and female rats from Exp. 1 following prior challenge with either ethanol (EtOH, white bars) or saline (SAL, black bars). Although there were no significant interactions for any of the variables, main effects of both ethanol exposure and age were observed for both (a) frequency of head dips and (b) total number of line crosses during the 5 min session, with a main effect of sex also observed for line crosses. Inserts are included for each behavior (collapsed across age and sex) in order to show the main effect of ethanol pre-exposure. There was a general reduction in head dips and line crosses in the ethanol compared to the saline group, as shown by the asterisks. In this, and in all subsequent figures, bars depict the mean value for each group, with vertical lines representing standard error of the mean.
Table 1.
Holeboard and plus-maze ANOVA results for Exp. 1
| Test | Behavior | Results | Significant effects | F value; p value |
|---|---|---|---|---|
|
HB
| ||||
| Crosses | EtOH < Sal | Exposure | F(l,55)=114.31; p ≤ .00001 | |
| M<F | Sex | F(1,55)=25.60; p ≤ .00001 | ||
| Adult < Adol | Age | F(1,55)=16.35; p ≤ .001 | ||
|
|
||||
| Rears | EtOH < Sal | Exposure | F(1,55)=31. 81; p ≤ .00001 | |
| M<F | Sex | F(1,55)=16.73; p ≤ .00l | ||
| Adult < Adol | Age | F(1,55)=5.24; p ≤ .05 | ||
|
|
||||
| No.HD | EtOH < Sal | Exposure | F(1,55)=22.06; p ≤ .0001 | |
| Adol < Adult | Age | F(1,55)=4.30; p ≤ .05 | ||
|
|
||||
| HD time | EtOH < Sal | Exposure | F(1,55)=15.92; p ≤ .001 | |
|
| ||||
|
EPM
| ||||
| %OAE | EtOH < Sal | Exposure | F(1,55)=15.47; p ≤ .001 | |
|
|
||||
| %OAT | EtOH < Sal | Exposure | F(1,55)=17.85; p ≤ .0001 | |
|
|
||||
| %PHD | Sal< EtOH (Adol only) | Age × Exposure | F(1,55)=5.13; p ≤ .05 | |
|
|
||||
| %PSAP | Sal < EtOH | Exposure | F(1,55)=8.17; p ≤ .01 | |
|
|
||||
| CAE | EtOH < Sal | Exposure | F(1,55)=25.27; p ≤ .00001 | |
| M<F | Sex | F(1,55)=15.05; p ≤ .001 | ||
|
|
||||
| Rears | EtOH < Sal | Exposure | F(1,55)=17.49; p ≤ .001 | |
| M<F | Sex | F(1,55)=15.01; p ≤ .001 | ||
All statistical results from both the elevated plus-maze (EPM) and holeboard (HB) tests in Exp. 1 are shown in Table 1. Adolescent (Adol) and adult male (M) and female (F) rats were previously challenged with a large bolus of either saline (SAL) or ethanol (EtOH) and then tested sequentially in the holeboard, followed by the plus-maze test. Main effects of prior ethanol exposure, age, and sex, as well as interactions of these variables are shown. Holeboard behaviors examined were; total number of line crosses (Crosses); total number of rears (Rears); frequency of head dips (No. HD); time spent head-dipping (HD time). Plus-maze behaviors included: percent open arm entries (%OAE); percent open arm time (%OAT); percent protected head dips (%PHD); percent protected stretched attend postures (%PSAP); number of closed arm entries (CAE); and number of rears (Rears).
EPM Testing
Overall, when indexed by the anxiety measures %OAE, %OAT and %PSAP, prior ethanol administration increased anxiety with no significant interactions involving age and sex. These data are illustrated in Figs. 2a and b for the measures %OAT and %PSAP. Examination of %PHD revealed that while adult animals previously exposed to ethanol (69.8 ± 9.3) were comparable to their saline-exposed counterparts (67.6 ± 7.2), ethanol-injected adolescents (77.2 ± 9.2} were significantly more anxious than their adolescent saline controls (37.1 ± 7.0). Activity measures in the EPM (CAE and rears) were also significantly decreased by previous ethanol challenge (Fig. 2c), with no significant interactions involving age or sex for these measures as well. For a complete listing of all EPM results from this experiment, refer to Table 1.
Figure 2.
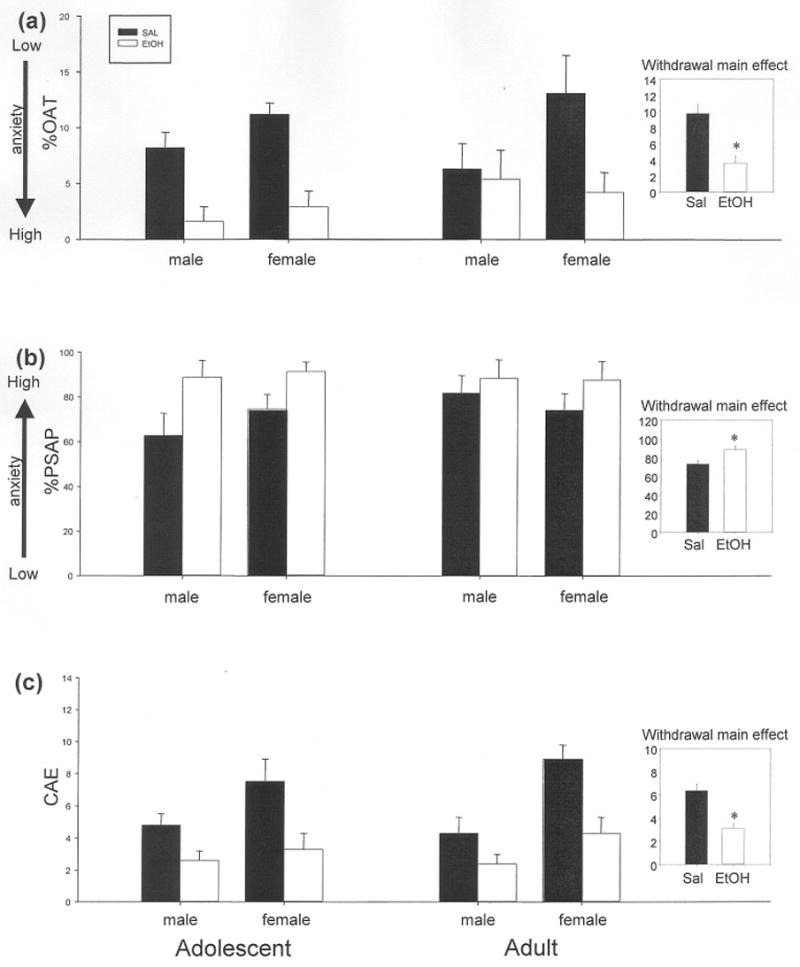
Elevated plus-maze behaviors of both adolescent and adult male and female rats from Exp. 1 following prior challenge with either ethanol (EtOH) or saline (SAL). For both (a) percent open arm time (%OAT) and (b) percent protected stretched attend postures (%PSAP), only a main effect of ethanol exposure emerged in these analyses, with elevated anxiety observed in ethanol-exposed compared to saline-exposed animals. A main effect of ethanol exposure was also observed for (c) number of closed arm entries (CAE), along with a main effect of sex. Inserts for each behavior are included to emphasize the overall withdrawal effect (collapsed across age and sex), with asterisks noting the increase in anxiety behaviors and decrease in activity following prior ethanol exposure.
Factor Analyses
The lack of notable age or sex differences in hangover-related anxiogenesis in the EPM was unexpected, given that we have previously observed less hangover-related anxiety among adolescents than adults in this test, at least when testing males (Doremus et al., 2003). Several procedural variables were different in this study, however, when compared to our earlier EPM experiments, with the sequential testing strategy being one of the more prominent changes. Even though we have formerly used factor analyses to confirm similar principle behavioral components in the EPM in both adolescents and adults (Doremus et al., 2006), it was possible that insertion of a HB test before EPM testing may have altered expression of the underlying components of behavior in this test. Consequently, factor analyses were conducted on the EPM and HB data from this experiment.
When the correlation matrix on the combined EPM and HB data set was checked for highly correlated and redundant measures, the HB measures of number of head dips and rears were highly correlated with the measures of time spent head dipping and rearing, respectively. Therefore, only the frequency data for these measures were included in factor analyses involving HB data. Results from the factor analysis of the combined data produced a 4-component solution, which had a sampling adequacy of .80 and comprised 75% of the total variance. The first factor accounted for 32.9% of the variance and was primarily comprised of activity measures such as closed arm entries, total arm entries, rears in the EPM, rears in the HB, and total line crosses in the HB, Variables that loaded highly on factor 2 consisted predominantly of anxiety measures from the EPM (%OAE, square root %OAT, and arcsine of %PSAP) and accounted for 23.5% of the variance. Factor 3 accounted for 11.2% of the total variance, with the exploratory/risk assessment behaviors of square root of total stretched attend postures in the EPM and frequency of head dips in the HB loading highly on this factor. Finally, percentage of inner square crossings in the HB was the only factor to load highly on the fourth component, equaling 7.8% of the variance (data not shown).
These findings contrast with previous factor analyses of EPM data where anxiety-related measures typically loaded on the primary component and accounted for the greatest percentage of the variance (Bertoglio and Carobrez, 2000; Cruz et al., 1994; Doremus et al., 2006). To determine whether this predominance of activity measures on factor 1 might have been driven largely by inclusion of HB measures in this analysis, data from only the EPM test was subjected to factor analysis. The factor analysis of these data (Table 3) yielded a 2-component solution, which accounted for 72.0% of the total variance and had a sampling adequacy of .81. Despite the general comparability of this data set to that used in prior factor analyses of EPM data, however, factor 1 (42.2% of the variance) again was comprised of a mixture of activity and risk-assessment measures, whereas factor 2 (29.8% of the variance) consisted of variables thought to index anxiety.
TABLE 3.
Factor analysis results of Exp. 1 EPM data only
| Component | ||
|---|---|---|
| Behavior | 1 | 2 |
| %OAE | .11 | .91 |
| √%OAT | .43 | .87 |
| %PHD | .32 | −.57 |
| arcsine %PSAP | −.31 | −.79 |
| CAE | .94 | .12 |
| TAE | .88 | .38 |
| rears | .78 | .08 |
| THD | .69 | .45 |
| √TSAP | .58 | .17 |
| √%Hubt | .86 | −.21 |
|
| ||
| % variance | 42.2% | 29.8% |
|
| ||
| Sampling adequacy | .81 | |
Factor loadings for Fxp. 1 plus-maze data (collapsed across sex and age) are shown, with loadings higher than 0.5 (or less than −0.5) enlarged and boldfaced for emphasis. Plus-maze behaviors analyzed in this data set were: percentage of open arm entries (%OAE), square root of percent open arm time (√%OAT), percentage of protected head dips (%PHD) and arcsine of percent protected stretched attend postures (arcsine %PSAP), number of closed arm entries (CAE), number of total arm entries (TAE), number of rears (rears), total number of head dips (THD), square root of total number of stretched attend postures (√TSAP), and square root of percent time spent in the central hub (√%THUB).
Experiment 2
In Exp. 1, when both age- and sex-related differences in ethanol withdrawal–induced anxiogenesis were directly compared within the same study, male and female animals of both ages were generally found to exhibit comparable increases in anxiety during the withdrawal phase following ethanol challenge, a lack of age-related differences that contrast with the relative resistance of adolescent animals to hangover effects evident in male animals in our earlier EPM work (Doremus et al., 2003). Also surprising in Exp. 1 were the results of factor analyses showing that activity, and not anxiety, was the factor accounting for the greatest amount of variance in the EPM. There were a number of changes, however, in the EPM pretest conditions of Exp. 1 compared to our prior studies. Among these changes were the inclusion of a sequential testing design, with all animals tested in the HB apparatus immediately prior to testing in the EPM; animals also were socially isolated during the overnight intoxication and recovery interval, rather than being placed with a similarly exposed age-mate during this overnight period. Since either or both of these methodological changes could have contributed to the unanticipated results in Exp. 1, the influence of these pretest variables on the expression of withdrawal-induced anxiogenesis was explored in Exp. 2.
Methods
A total of 256 Sprague-Dawley rats were used across the 32 experimental conditions defined by the 2 (age) × 2 (sex) × 2 (drug challenge) × 2 (pretest social context: isolated or paired during intoxication/recovery) × 2 (test: EPM test alone or EPM testing immediately following a HB test) factorial design of this experiment. At 1900–2000 hr, adolescent (P33–35) and adult (P70–P75) animals received an injection of either saline or ethanol (detailed in General Methods). Directly after the injection, animals were placed either alone or with their cage mate in a clear plastic tub with ad libitum food and water access as in our previous experiments (Doremus et al., 2003; Varlinskaya and Spear, 2004). For the paired social context condition, cage partners always received the same drug challenge and remained together until the time of pretest isolation.
As outlined in the General Methods, the overnight interval between drug administration and pretest isolation was determined on the basis of clearance times for each age and sex group, with pretest isolation beginning 3 hr post-clearance in each group (i.e. 12 hr post-injection for adolescents of both sexes, and 13 and 18 hr for adult females and males, respectively). After the 30 min pretest isolation period, half of the animals in each group were tested only in the EPM, whereas the remaining subjects were first given a 5 min HB test immediately prior to testing in the EPM.
Measures analyzed were the same as those in Exp. 1. In the initial ANOVAs of the EPM data, all variables and animals were considered. Additional analyses were also conducted on a subset of the data: animals that received testing only in the EPM and that sampled the open arms at least once—test conditions similar to those of Doremus et al. (2003), where age-related differences in withdrawal-related anxiety were observed.
Results
Holeboard
No significant effects of the pretest social context variable emerged in the analysis of the HB data. In other respects, the results were similar to those observed in Exp. 1, with significant withdrawal-related suppressions in crosses, rears and head dips observed, as shown in the ANOVA summaries in Table 2. These effects did not interact with sex or age, although adolescent-typical elevations in activity relative to adults and significantly greater activity levels in females than males were again evident.
Table 2.
Holeboard and plus-maze ANOVA results for Exp. 2
| Test | Behavior | Results | Significant effects | F value; p value |
|---|---|---|---|---|
|
HB
| ||||
| Crosses | EtOH < Sal | Exposure | F(1,110)=129.93; p ≤ .0001 | |
| M<F | Sex | F(1,110)=7.69; p ≤ .01 | ||
| Adult < Adol | Age | F(1,110)=3.98; p ≤ .05 | ||
|
|
||||
| Rears | EtOH < Sal | Exposure | F(1,110)=68.02; p ≤ .00001 | |
| Adult < Adol | Age | F(1,110)=8.29; p ≤ .01 | ||
|
|
||||
| No. HD | EtOH < Sal | Exposure | F(1,110)=16.88; p ≤ .0001 | |
| F < M (adults only) | Age × Sex | F(1,110)=6.17; p ≤ .05 | ||
|
|
||||
| HD time | EtOH < Sal | Exposure | F(1,110)=9.92; p ≤ .01 | |
|
| ||||
|
EPM
| ||||
| %OAE | EtOH < Sal | Exposure | F(1,222)=11.85; p ≤ .001 | |
| M<F | Sex | F(1,222)=5.20; p ≤ .05 | ||
|
|
||||
| %OAT | EtOH < Sal | Exposure | F(1,222)=26.44; p ≤ .00001 | |
| HB+EPM < EPM alone | Test | F(1,222)=4.44; p ≤ .05 | ||
| Adol M & Adult F < Adol F | Age × Sex | F(1,222)=5.27; p ≤ .05 | ||
|
|
||||
| %PHD | Sal < EtOH | Exposure | F(1,222)=13.24; p ≤ .001 | |
| Adol < Adult | Age | F(1,222)=6.52; p ≤ .05 | ||
| F<M | Sex | F(1,222)=5.70; p ≤ .05 | ||
|
|
||||
| %PSAP | Sal < EtOH (HB+EPM Adol M & Adult F only) | Age × Sex ×Test × Exposure | F(1,222)=4.13; p ≤ .05 | |
| Adol isolated M > all Adol groups; Adol isolated F < Adult isolated F | Age × Sex × Pretest | F(1,222)=4.82; p ≤ .05 | ||
|
|
||||
| CAE | EtOH < Sal | Exposure | F(1,222)=88.55; p ≤ .00001 | |
| Adult < Adol | Age | F(1,222)=12.54; p ≤ .001 | ||
| HB+EPM < EPM alone | Test | F(1,222)=16.5; p ≤ .0001 | ||
|
|
||||
| Rears | EtOH < Sal; | Exposure | F(1,220)=54.82; p ≤ .00001 | |
| HB+EPM < EPM alone; Adult < Adol (HB+EPM only) | Age × Test | F(1,220)=3.90; p ≤ .05 | ||
All statistical results from both the elevated plus-maze (EPM) and holeboard (HB) tests in Exp. 2 are shown in Table 2. Adolescent (Adol) and adult male (M) and female (F) rats were previously challenged with a large bolus of either saline (SAL) or ethanol (EtOH). Half of the animals were isolated during the overnight interval between drug challenge and test (isolated), whereas the remaining animals were placed in a social context during this interval. Additionally, the influence of sequential testing was explored, with half of the animals tested sequentially in the holeboard followed by the EPM test (HB+EPM) and the other half of the animals tested only in the EPM apparatus (EPM alone). Main effects of prior ethanol exposure, age, sex, testing procedure (test) and pretest social context (pretest), as well as interactions of these variables, are shown. Holeboard and plus-maze behaviors examined were the same as those listed in Table 1.
Elevated Plus-Maze
Overall, the pretest social context likewise exerted few effects on EPM behavior. Percent PSAP was the only measure affected by the pretest social context. Adolescent males that were isolated after injection were more anxious with this measure (84.7 ± 3.0} than their female counterparts (70.2 ± 4.0) and than adolescent males placed in a social context post-injection (71.4 ± 5.8). With this measure, isolated adult females (86.3 ± 2.9) were found to be more anxious than their adolescent female counterparts (70.2 ±4.0). None of these effects interacted with the ethanol exposure variable, however.
Sequential testing (i.e. inclusion of a HB test prior to testing on the EPM) decreased activity on the EPM relative to testing only the EPM, and altered expression of some anxiety measures (see Table 2). Sequentially tested animals were less active, as indexed by CAE (5.26 ± .27) and rears (6.89 ± .40), when compared to animals tested only in the EPM (6.71 ± .32 for CAE; 10.19 ± .44 for rears). With the %OAT measure, sequentially tested animals were more anxious and spent significantly less time on the open arms (7.78 ± .75) than animals tested only in the EPM (10.32 ± .99). When anxiety was assessed via %PSAP, sequential testing also tempered the expression of withdrawal-related anxiogenesis, with significant anxiogenic effects of ethanol withdrawal only emerging in sequentially tested adolescent males and adult females (data not shown).
As in Exp. l, withdrawal-induced anxiogenesis and hypoactivity were evident at both ages and were largely unaffected by either of the pretest variables examined in this study. In terms of age and sex effects in the EPM (see Table 2), several significant effects did emerge, with adolescents again generally more active and less anxious than their adult counterparts, and female rats less anxious than males.
The results of these initial analyses did not support our hypothesis that the variables of pretest social context and sequential testing would affect age- or sex-related differences in the expression of ethanol withdrawal anxiogenesis. Examination of the raw data, however, revealed that a notable number of animals in this experiment failed to enter the open arms at all during the EPM test, an occurrence particularly common among sequentially tested animals. It is difficult to interpret anxiety levels in data sets when a significant number of animals fail to fully enter the open arms, since failure to do so obscures the effects of anxiogenic manipulations and reduces the likelihood of detecting withdrawal-related elevations in anxiety. Consequently, an additional set of ANOVAs was conducted on data from animals that were tested only in the EPM and that had entered the open arms at least once (N = 93; ×̄ sample size/group = 5.8; range: 2–9).
Despite the more restricted size of this data set, the results examining age-related differences in withdrawal anxiogenesis obtained in these analyses were more reminiscent of our previous findings (see Doremus et al., 2003), with an adolescent-associated attenuation in the expression of hangover anxiogenesis that was observed in adults with some anxiety measures. For the measure %OAT, adults exposed to a prior ethanol challenge exhibited significantly greater anxiety than their saline-exposed counterparts, while ethanol-exposed adolescents did not reveal these elevations in anxiety (Fig. 3a) [age × exposure interaction: F(1,77) = 3.88, p ≤ .05]. Likewise, with the anxiety measures %OAE (data not shown) and %PSAP (Fig. 3b), ethanol-exposed adults were found to be significantly more anxious than their adolescent counterparts during the hangover period [age × exposure interaction for %OAE: F(1,77) = 4.56, p ≤ .05; for %PSAP: F(1,77) = 4.45, p ≤ .05]. Percent PHD was the only anxiety-like measure in which these age differences in the expression of withdrawal-induced anxiogenesis were not observed (data not shown). Withdrawal-induced hypoactivity again emerged for both CAE (Fig. 3c) and rears and was not influenced by age or sex [exposure main effect for CAE: F(1,77) = 32.7, p ≤ .000001; for rears: F(1, 76) = 11.1, p ≤ .01].
Factor Analyses
In the factor analysis results from Exp. 1, anxiety-like behaviors did not load highly on the primary underlying behavioral component, as is usually the case. To determine whether the sequential testing procedure used in that study may have contributed to these atypical factor loadings, EPM data from Exp. 2 were subjected to factor analysis separately for animals tested in the EPM alone versus those tested in both the HB and EPM apparatuses.
Reminiscent of Exp. 1, the factor analysis of data from animals tested in both the HB and EPM tests (see Table 4, left side) revealed that activity and risk assessment behaviors again loaded primarily on component 1, while factor 2 generally consisted of anxiety measures. Importantly, when data from animals that were tested in the EPM alone were subjected to factor analysis (see Table 4, right side), more typical findings emerged, with factor 1 primarily consisting of anxiety measures [%OAE, square root of %OAT, square root of (%PHD + 1), arcsine of %PSAP, but also square root of total head dips and total arm entries] and factor 2 showing high factor loadings for activity and risk-assessment-related behaviors (closed arm entries, total arm entries, square root of rears, square root of TSAP, and square root of percentage of time spent in the center square).
TABLE 4.
Factor analysis results of EPM data from animals in Exp. 2
| HB + EPM | EPM alone | |||
|---|---|---|---|---|
| Component | Component | |||
| Behavior | 1 | 2 | 1 | 2 |
| %OAE | .12 | .94 | .95 | .12 |
| √%OAT | .44 | .86 | .86 | .40 |
| √(%PHD+1) | −.15 | −.79 | −.84 | −.04 |
| Arcsine %PSAP | −,24 | −.86 | −.86 | −.22 |
| √CAE | .93 | .24 | .28 | .86 |
| √TAE | .85 | .47 | .51 | .79 |
| √rears | .73 | .24 | .00 | .74 |
| √THD | .54 | .68 | .73 | .49 |
| TSAP | .73 | .18 | .12 | .69 |
| √%Hubt | .86 | .10 | .26 | .76 |
|
| ||||
| % variance | 39.7% | 38.2% | 40.3% | 34.4% |
|
| ||||
| Sampling adequacy | .80 | .79 | ||
Factor loadings for Exp. 2 plus-maze data (collapsed across sex and age) are shown, with animals tested in both the plus-maze and holeboard (HB + EPM) or animals tested only in the EPM (EPM alone) analyzed separately. Loadings higher than 0.5 (or less than −0.5) are enlarged and boldfaced for emphasis. Behaviors analyzed in this data set are the same as those listed in Table 3, except that several additional behavioral measures also were subjected to a square root transformation [closed arm entries, total arm entries, rears, total head dips, and percent protected head dips (%PHD) + 1)].
General Discussion
In previous work examining hangover-related increases in anxiety in adolescent and adult rats, adult males demonstrated significant anxiogenesis in the EPM test—an increase in anxiety that was not observed in male adolescents (Doremus et al., 2003). When both male and female adolescent and adult animals were directly compared using the EPM test in the first experiment of this series, however, neither age- nor sex-related differences in the expression of withdrawal anxiogenesis were observed. Experiment 2 was then conducted to assess the impact of several pretest procedural conditions used in Exp. 1 on the ontogenetic expression of withdrawal-induced anxiogenesis in the plus-maze. Results of this study revealed that testing in the HB prior to the EPM test suppressed activity and elevated anxiety during the hangover period in both adolescents and adults, as well as altered the underlying components of EPM behavior that emerged from principle component analyses. Indeed, when only examining animals from Exp. 2 with test conditions similar to those used previously in our laboratory (Doremus et al., 2006; Doremus et al., 2003) (i.e. animals that were tested only in the EPM and at least sampled the open arms), some evidence for age-related differences again emerged, with adolescents occasionally showing attenuation in withdrawal-associated increases in anxiety relative to adults, an age effect that was not moderated by sex.
The results from this experimental series add to the growing body of literature that supports anxiogenesis as part of the withdrawal response seen in adults following acute ethanol exposure using several different anxiety assays (Brasser and Spear, 2002; Doremus et al., 2003; Gauvin et al., 1997; Gauvin et al., 1992; Morse et al., 2000; Varlinskaya and Spear, 2004). The developmental expression of withdrawal-induced anxiety, however, has received less attention. Studies focusing on anxiogenesis following an acute ethanol challenge (Brasser and Spear, 2002; Doremus et al., 2003; Varlinskaya and Spear, 2004) have generally reported attenuated sensitivity during adolescence to this particular sign of hangover. In the current experiments, adolescents were generally found to express adult-typical hangover-related anxiogenesis, although evidence for the previously observed adolescent attenuation in sensitivity to this withdrawal effect also emerged under specific test circumstances.
Indeed, in the current experiments, alterations in baseline levels of anxiety and activity in the EPM following particular pretest manipulations influenced EPM assessments of withdrawal-related anxiety. Most notably, inclusion of a HB test prior to EPM testing reduced subsequent activity levels in the EPM, thereby creating a situation in which animals were less likely to enter the open arms. Without ever completely entering the open arms, it is at best questionable as to whether this test is an accurate index of anxiety. The inclusion of a sequential testing procedure also elevated baseline anxiety levels relative to testing only in the EPM, with the expression of withdrawal-related anxiogenesis more pronounced as well in some sequentially tested groups. Isolation during the overnight interval had only a minor impact on EPM behavior, and did not influence expression of withdrawal-related effects.
The observation that alterations in pretest procedural variables can influence subsequent assessments of anxiety in the EPM has been well documented (for review see Carobrez and Bertoglio, 2005; Hogg, 1996; Rodgers et al., 1997; Rodgers and Dalvi, 1997). For instance, variables such as housing condition (Haller and Halasz, 1999; Karim and Arslan, 2000), time of day (Griebel et al., 1993), prior handling (Schmitt and Hiemke, 1998), cohort removal (Cheeta et al., 2001; Rodgers et al., 1994), transportation via cart to the testing situation (Morato and Brandao, 1996) and even construction of the maze itself (Morato and Castrechini, 1989, but see also Falter et al., 1992) have all been shown to impact anxiety levels during the EPM test. Several researchers have cautioned that special attention must be given when designing pharmacological studies using the EPM, since changes in baseline anxiety due to pretest conditions can alter the conclusions reached regarding the efficacy of pharmacological manipulations for altering anxiety levels. To our knowledge, the current study is first to report that procedural variables can obscure the expression of age-related differences in hangover-related anxiogenesis in the EPM.
In Exp. 2, sequential testing of animals was found to not only affect levels of activity and anxiety exhibited during the EPM test, but also to influence the primary underlying components of EPM behavior. Under many test circumstances, adult EPM behavior has been shown to be driven primarily by anxiety measures, with these variables accounting for the greatest amount of the variance in this behavioral test (Cruz et al., 1994; Doremus et al., 2006; Fernandes and File, 1996; Wall and Messier, 2000). Studies from our lab have also verified anxiety as the primary behavioral component in the EPM in both male and female adolescent and adult rats (Doremus et al., 2006). In the current studies, however, when a sequential testing strategy was used, activity emerged as the most prominent underlying behavioral component, with typical EPM factor analysis results only emerging when conducted on data from animals tested in the EPM alone. These results contrast with other studies that have reported the predominance of the anxiety component in factor analyses of EPM data collected following pretest exposure to a novel environment such as an open field, HB or social interaction tests (Fernandes et al., 1999; File et al., 1991; Morato and Brandao, 1996; Fellow et al., 1985; Fellow and File, 1986).
Importantly, even in the factor analysis of Exp. 2 with animals tested only in the EPM, the amount of variance accounted for by the first (anxiety) factor did not vary notably from the second (activity) factor, findings that contrast with the greater dominance of the anxiety component in previous analyses of non-drug-exposed animals from our lab (Doremus et al., 2006) and others (Cruz et al., 1994; Rodgers and Johnson, 1995; Wall and Messier, 2000). Ethanol-exposed animals were included, however, in the current factor analyses and thus it is possible that the pronounced withdrawal-related hypoactivity in these animals might have induced a greater than usual contribution of activity-like behaviors to the profile of behaviors generated during the test. The inclusion of hypoactive ethanol-exposed animals may explain why the factor loadings of sequentially tested animals in the present studies contrast with prior analyses conducted in non-drug-exposed animals (Fernandes et al., 1999; File et al., 1991; Morato and Brandao, 1996; Fellow et al., 1985; Pellow and File, 1986).
Overall, few sex differences in the expression of withdrawal-related anxiogenesis and hypoactivity emerged in these experiments. The lack of compelling evidence for differences between male and female rats in their sensitivity to hangover effects are reminiscent of other studies that also failed to observe sex differences in withdrawal anxiogenesis in both the EPM (Overstreet et al., 2004) and social interaction tests (Overstreet et al., 2002; Overstreet et al., 2004) following chronic exposure to ethanol. There are some reports, however, of sex-related differences in withdrawal, with a number of studies observing attenuated sensitivity to ethanol withdrawal anxiogenesis in females compared to males across a variety of paradigms (Gatch et al., 1999; Jung et al., 1999; Jung et al., 2000; Overstreet et al., 2004). Inconsistencies across studies in expression of sex differences in withdrawal-associated anxiogenesis following acute challenge with ethanol are likely due, in part, to differences in procedural variables, including method of administration, dosing regimen, and testing procedures.
In contrast to hangover anxiogenesis, withdrawal-related reductions in activity levels were consistently observed in these experiments under all test conditions, and in animals of both ages and sexes. Ethanol withdrawal-related hypoactivity has also been observed among adolescent animals in other studies from our laboratory and elsewhere (Doremus et al,, 2003; Ristuccia and Spear, 2005; Slawecki and Roth. 2004; Slawecki et al., 2006; Varlinskaya and Spear, 2004), further documenting the robust and ubiquitous nature of the activity suppression associated with withdrawal. Given the consistency with which this component of ethanol withdrawal is observed, there is surprisingly little evidence regarding its underlying neural mechanisms. Studies examining the efficacy of pharmacological manipulations for alleviating anxiogenic behavior during acute withdrawal have reported little effect of these anxiolytic drugs on the reversal of withdrawal-associated hypoactivity (File et al., 1991; Gatch et al., 1999; Gauvin et al., 1993; Jung et al., 2000; Lal et al., 1991). Regardless of its origin, the robust activity suppression seen during the withdrawal phase presents potential challenges for assessment of other withdrawal-related behaviors, particularly when further exacerbated by various pretest and test conditions, therefore rendering possible age- and sex-related differences in the expression of other ethanol withdrawal-induced behavioral alterations more difficult to detect.
The results of these studies have shown that while age-related differences in hangover-associated anxiogenesis are evident under certain circumstances, the particular pretest manipulations and testing conditions used may considerably influence subsequent EPM behavior. Given these results, caution should be exerted when using behavioral tests, such as the EPM, which are highly susceptible to alterations in baseline levels of activity and anxiety, perhaps differentially across age. Without careful consideration of these limitations, it is possible that age- or sex-related differences in the effects of pharmacological manipulations may be potentially masked or inappropriately attributed.
Acknowledgments
This research was supported by grant R37 AA12525 to Linda P. Spear
References
- Arnett J. Reckless behavior in adolescence: A developmental perspective. Developmental Review. 1992;12(4):339–373. [Google Scholar]
- Bertoglio LJ, Carobrez AP. Previous maze experience required to increase open arms avoidance in rats submitted to the elevated plus-maze model of anxiety. Behav Brain Res. 2000;108(2):197–203. doi: 10.1016/s0166-4328(99)00148-5. [DOI] [PubMed] [Google Scholar]
- Bogin RM, Nostrant TT, Young MJ. Propranolol for the treatment of the alcoholic hangover. Am J Drug Alcohol Abuse. 1986;12(3):279–84. doi: 10.3109/00952998609007397. [DOI] [PubMed] [Google Scholar]
- Brasser SM, Spear NE. Physiological and behavioral effects of acute ethanol hangover in juvenile, adolescent, and adult rats. Behav Neurosci. 2002;116(2):305–20. doi: 10.1037//0735-7044.116.2.305. [DOI] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29(8):1193–205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Cheeta S, Irvine E, File SE. Social isolation modifies nicotine's effects in animal tests of anxiety. Br J Pharmacol. 2001;132(7):1389–95. doi: 10.1038/sj.bjp.0703991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994;49(1):171–6. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya El, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75(2):411–8. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Varlinskaya El, Spear LP. Age-related differences in elevated plus maze behavior between adolescent and adult rats. Ann N Y Acad Sci. 2004;1021:427–30. doi: 10.1196/annals.1308.057. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Varlinskaya El, Spear LP. Factor analysis of eJevated plus-maze behavior in adolescent and adult rats. Pharmacol Biochem Behav. 2006;83(4):570–7. doi: 10.1016/j.pbb.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Espejo EF. Selective dopamine depletion within the medial prefrontal cortex induces anxiogenic-like effects in rats placed on the elevated plus maze. Brain Res. 1997;762(1–2):281–4. doi: 10.1016/s0006-8993(97)00593-3. [DOI] [PubMed] [Google Scholar]
- Falter U, Gowcr AJ, Gobert J. Resistance of baseline activity in the elevated plus-maze to exogenous influences. Behavioural Pharmacology. 1992;3:123–8. [PubMed] [Google Scholar]
- Fernandes C, File SE. The influence of open arm ledges and maze experience in the elevated plus-maze. Pharmacol Biochem Behav. 1996;54(1):31–40. doi: 10.1016/0091-3057(95)02171-x. [DOI] [PubMed] [Google Scholar]
- Fernandes C, Gonzalez MI, Wilson CA, File SE. Factor analysis shows that female rat behaviour is characterized primarily by activity, male rats are driven by sex and anxiety. Pharmacol Biochem Behav. 1999;64(4):731–8. doi: 10.1016/s0091-3057(99)00139-2. [DOI] [PubMed] [Google Scholar]
- File SE. Animal models of different anxiety states. In: Biggio G, Sanna E, Costa E, editors. GABAAReceptors and Anxiety: From Neurobiology to Treatment. Raven Press; New York: 1995. pp. 93–113. [PubMed] [Google Scholar]
- File SE, Wardill AG. The reliability of the hole-board apparatus. Psychopharmacologia. 1975a;44(1):47–51. doi: 10.1007/BF00421183. [DOI] [PubMed] [Google Scholar]
- File SE, Wardill AG. Validity of head-dipping as a measure of exploration in a modified hole-board. Psychopharmacologia. 1975b;44(1):53–9. doi: 10.1007/BF00421184. [DOI] [PubMed] [Google Scholar]
- File SE, Zharkovsky A, Gulati K. Effects of baclofen and nitrendipine on ethanol withdrawal responses in the rat. Neuropharmacology. 1991;30(2):183–90. doi: 10.1016/0028-3908(91)90202-m. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Wallis CJ, Lal H. Effects of NMDA antagonists on ethanol-withdrawal induced “anxiety” in the elevated plus maze. Alcohol. 1999;19(3):207–11. doi: 10.1016/s0741-8329(99)00045-2. [DOI] [PubMed] [Google Scholar]
- Gauvin DV, Briscoe RJ, Baird TJ, Vallett M, Holloway FA. The paradoxical hedonic valence of acute ethanol withdrawal (hangover) states in rats: place and taste conditioning. Alcohol. 1997;14(3):261–8. doi: 10.1016/s0741-8329(96)00151-6. [DOI] [PubMed] [Google Scholar]
- Gauvin DV, Goulden KL, Holloway FA. State-dependent stimulus control: cueing attributes of ethanol “hangover” in rats. Alcohol Clin Exp Res. 1993;17(6):1210–4. doi: 10.1111/j.1530-0277.1993.tb05231.x. [DOI] [PubMed] [Google Scholar]
- Gauvin DV, Youngblood BD, Holloway FA. The discriminative stimulus properties of acute ethanol withdrawal (hangover) in rats. Alcohol Clin Exp Res. 1992;16(2):336–4l. doi: 10.1111/j.1530-0277.1992.tb01387.x. [DOI] [PubMed] [Google Scholar]
- Griebel G, Moreau J, Jenck F, Martin JR, Misslin R. Some critical determinants of the behaviour of rats in the elevated plus-maze. Behavioural Processes. 1993;29:37–48. doi: 10.1016/0376-6357(93)90026-N. [DOI] [PubMed] [Google Scholar]
- Haller J, Halasz J. Mild social stress abolishes the effects of isolation on anxiety and chlordiazepoxide reactivity. Psychopharmacology (Berl) 1999;144(4):311–5. doi: 10.1007/s002130051012. [DOI] [PubMed] [Google Scholar]
- Hogg S. A review of the validity arid variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54(1):21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Johnston AL, File SE. Sex differences in animal tests of anxiety. Physiol Behav. 1991;49(2):245–50. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2005. National Institute on Drug Abuse; Bethesda, MD: 2005. p. 66. (NIH Publication No. 06-5882), vol NIH Publication No. 05-5726. [Google Scholar]
- Jung ME, Wallis CJ, Catch MB, Lal H. Sex differences in the pentylenetetrazol-like stimulus induced by ethanol withdrawal. J Pharmacol Exp Ther. 1999;291(2):576–82. [PubMed] [Google Scholar]
- Jung ME, Wallis CJ, Catch MB, Lal H. Sex differences in nicotine substitution to a pentylenetetrazol discriminative stimulus during ethanol withdrawal in rats. Psychopharmacology (Berl) 2000;149 (3 ):235–40. doi: 10.1007/s002130000392. [DOI] [PubMed] [Google Scholar]
- Karim A, Arslan MI. Isolation modifies the behavioural response in rats. Bangladesh Med Res Counc Bull. 2000;26(1):27–32. [PubMed] [Google Scholar]
- Lal H, Prather PL, Rezazadeh SM. Anxiogenic behavior in rats during acute and protracted ethanol withdrawal: reversal by buspirone. Alcohol. 1991;8(6):467–71. doi: 10.1016/s0741-8329(91)90153-n. [DOI] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20(8):1346–51. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22(2 ):416–21. [PubMed] [Google Scholar]
- Martin CS, Winters KC. Diagnosis and assessment of alcohol use disorders among adolescents. Alcohol Health Res World. 1998;22(2):95–105. [PMC free article] [PubMed] [Google Scholar]
- McKinney A, Coyle K. Alcohol hangover effects on measures of affect the morning after a normal night’s drinking. Alcohol Alcohol. 2006;41(1):54–60. doi: 10.1093/alcalc/agh226. [DOI] [PubMed] [Google Scholar]
- Morato S, Brandao ML. Transporting rats to the test situation on a cart can modify rat exploratory behavior in the elevated plus-maze. Psychobiology. 1996;24(3):247–252. [Google Scholar]
- Morato S, Castrechini P. Effects of floor surface and environmental illumination on exploratory activity in the elevated plus-maze. Braz J Med Biol Res. 1989;22(6):707–10. [PubMed] [Google Scholar]
- Morse AC, Schulteis G, Holloway FA, Koob GF. Conditioned place aversion to the “hangover” phase of acute ethanol administration in the rat. Alcohol. 2000;22(1):19–24. doi: 10.1016/s0741-8329(00)00099-9. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res. 2002;26(8):1259–68. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Similar anxiety-like responses in male and female rats exposed to repeated withdrawals from ethanol. Pharmacol Biochem Behav. 2004;78(3):459–64. doi: 10.1016/j.pbb.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14(3):149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24(3):525–9. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- Ristuccia RC, Spear LP. Sensitivity-and tolerance lo autonomic effects of ethanol in adolescent and adult rats during repeated vapor inhalation sessions. Alcohol Clin Exp Res. 2005;29(10):1809–20. doi: 10.1097/01.alc.0000183010.72764.cd. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Cao B-J, Dalvi A, Holmes A. Animal models of anxiety: an ethological perspective. Brazilian Journal of Medical and Biological Research. 1997;30:289–304. doi: 10.1590/s0100-879x1997000300002. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Cole JC. The Elevated Plus-maze: Pharmacology, Methodology, and Ethology. In: Cooper SJ, Hendrie CA, editors. Ethology and PsychopharmacoIogy. John Wiley & Sons. Ltd; Chichester: 1994. pp. 9–44. [Google Scholar]
- Rodgers RJ, Cole JC, Harrison-Phillips DJ. “Cohort removal” induces hyperthermia but fails to influence plus-maze behaviour in male mice. Physiol Behav. 1994;55(1):189–92. doi: 10.1016/0031-9384(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997;21(6):801–10. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Johnson NJ. Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol Biochem Behav. 1995;52(2):297–303. doi: 10.1016/0091-3057(95)00138-m. [DOI] [PubMed] [Google Scholar]
- Schmitt U, Hiemke C. Strain differences in open-field and elevated plus-maze behavior of rats without and with pretest handling. Pharmacol Biochem Behav. 1998;59(4):807–11. doi: 10.1016/s0091-3057(97)00502-9. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22(3):670–6. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Roth J. Comparison of the onset of hypoactivity and anxiety-like behavior during alcohol withdrawal in adolescent and adult rats. Alcohol Clin Exp Res. 2004;28(4):598–607. doi: 10.1097/01.alc.0000122767.69206.1b. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Roth J, Gilder A. Neurobehavioral profiles during the acute phase of ethanol withdrawal in adolescent and adult Sprague-Dawley rats. Behav Brain Res. 2006;170(1):41–51. doi: 10.1016/j.bbr.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Smith CM, Barnes GM. Signs and symptoms of hangover: prevalence and relationship to alcohol use in a general adult population. Drug Alcohol Depend. 1983;11(3–4):249–69. doi: 10.1016/0376-8716(83)90017-0. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4 ):417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Age-dependent inhibition of long-term potential ion by ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res. 1995a;19(6):1480–5. doi: 10.1111/j.1530-0277.1995.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Differential sensitivity of NMDA receptor-mediated synaptic potentials to ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res. 1995b;19(2):320–3. doi: 10.1111/j.1530-0277.1995.tb01509.x. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26(10):1502–11. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcohol Clin Exp Res. 2004;28(1):40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Differences in the social consequences of ethanol emerge daring the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Dev Psychobiol. 2006;48(2):146–61. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- Wall PM, Messier C. Ethological confirmatory factor analysis of anxiety-like behaviour in the murine elevated plus-maze. Behav Brain Res. 2000;114(1–2):199–212. doi: 10.1016/s0166-4328(00)00229-1. [DOI] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder US. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002;73(3):673–7. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Wiese JG, Shlipak MG, Browner WS. The alcohol hangover. Ann Intern Med. 2000;132(11):897–902. doi: 10.7326/0003-4819-132-11-200006060-00008. [DOI] [PubMed] [Google Scholar]


