Abstract
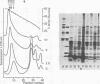
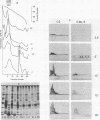
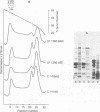
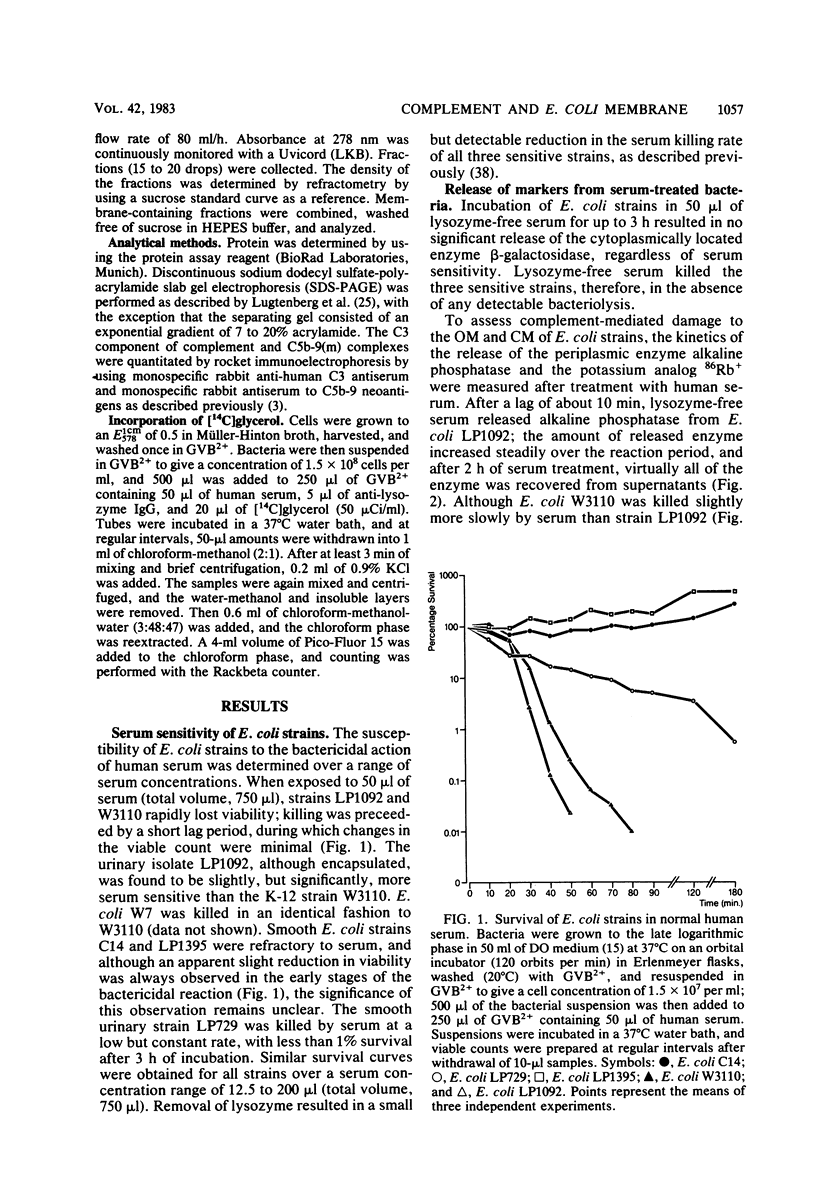
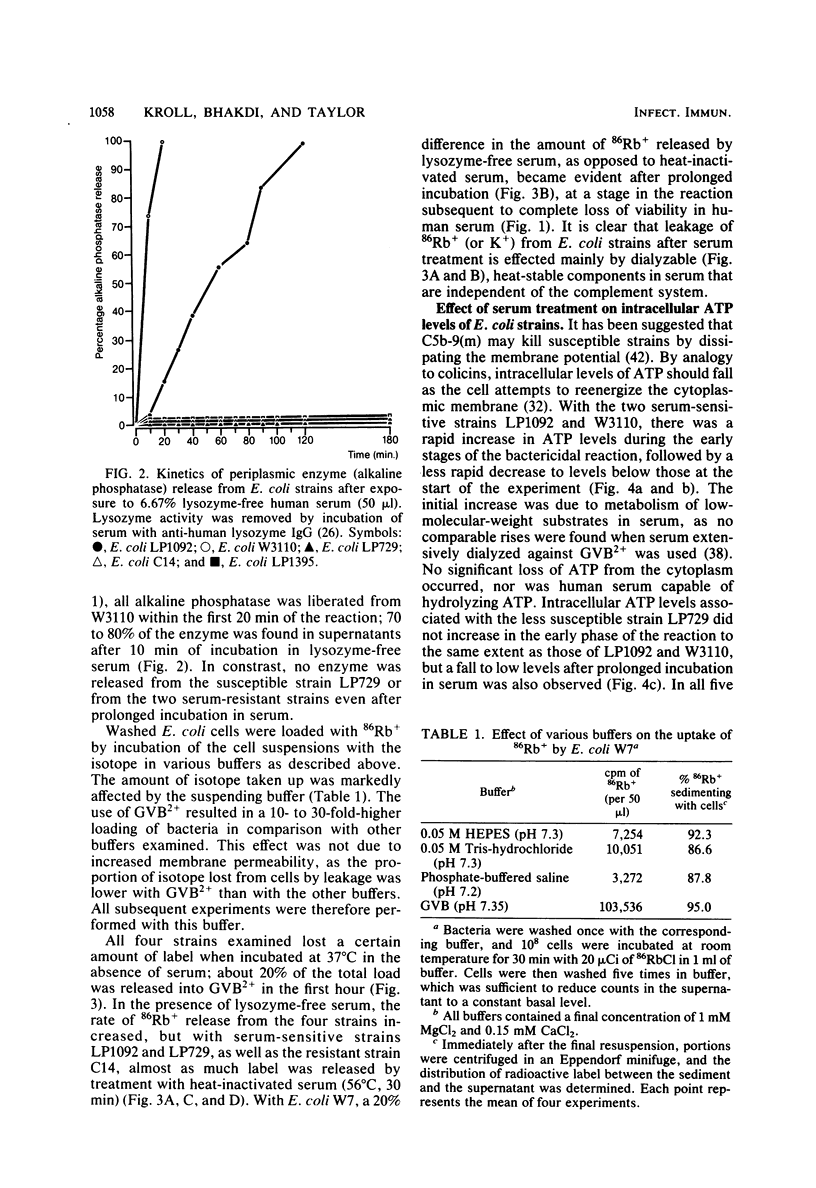
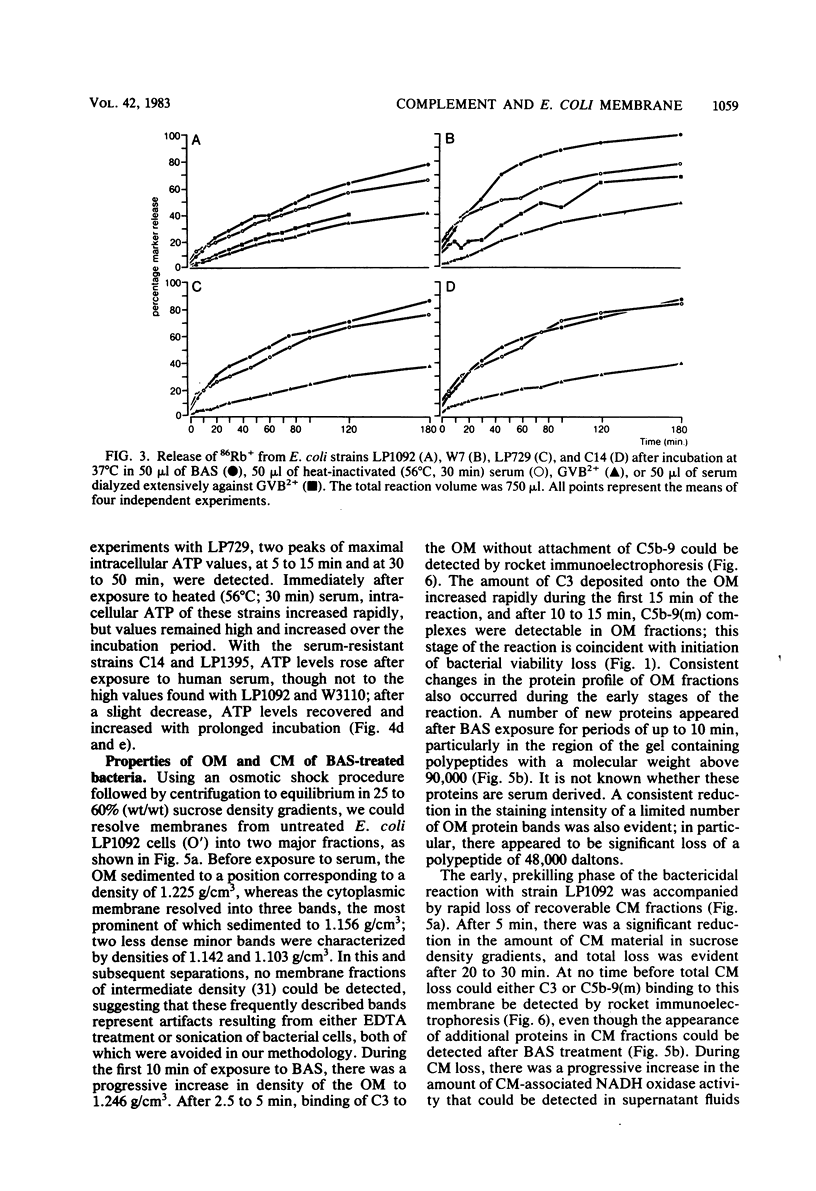
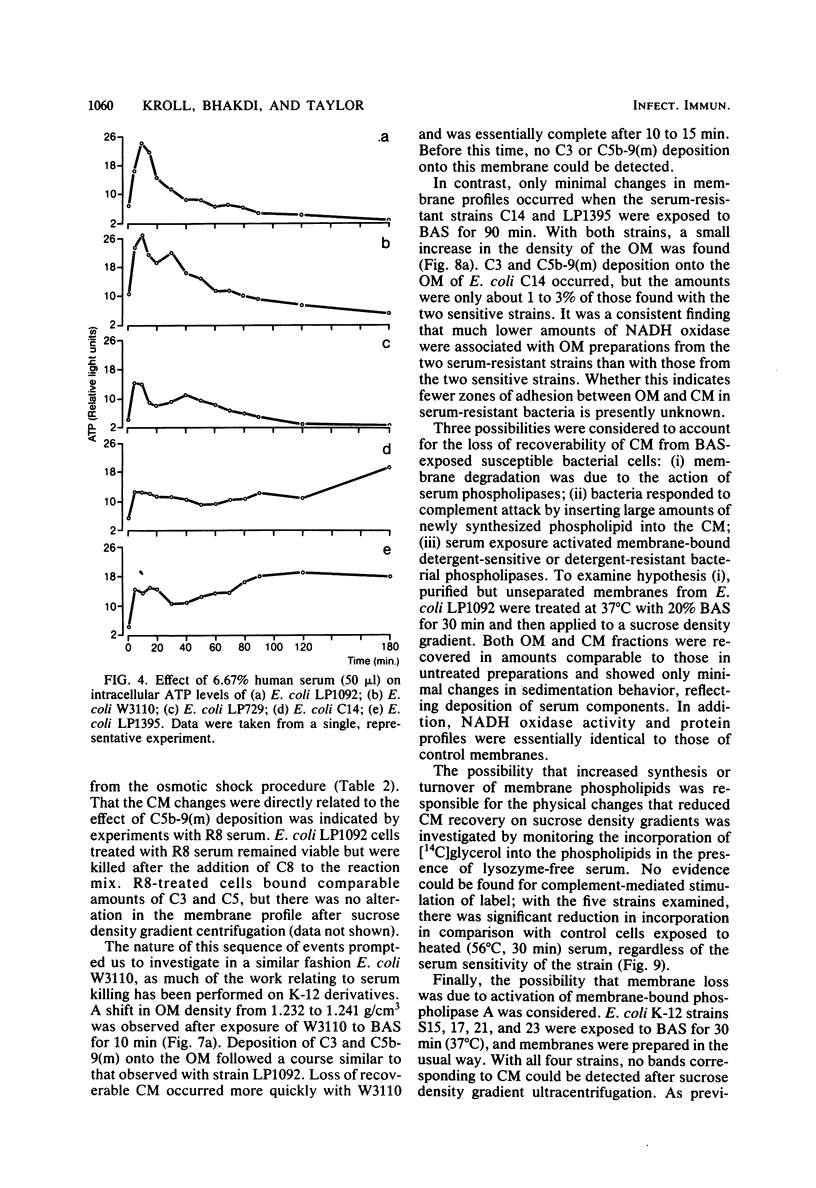
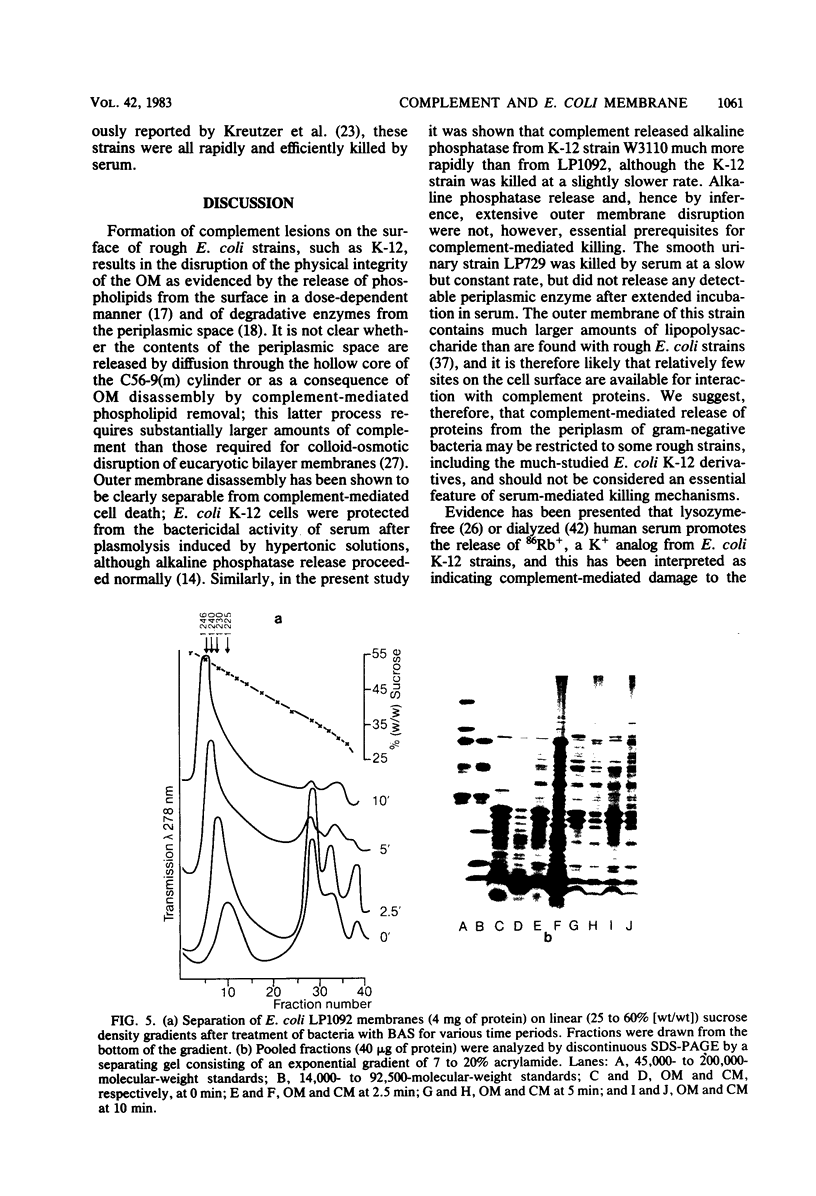
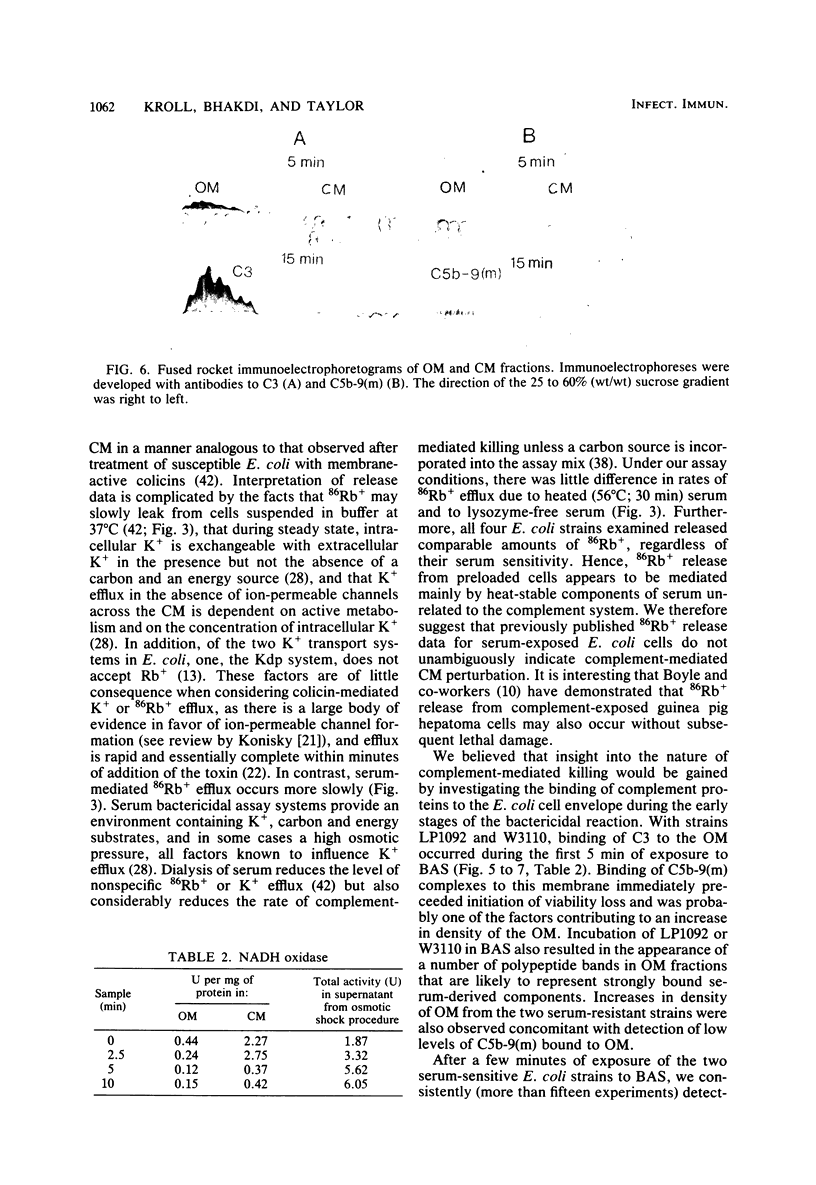
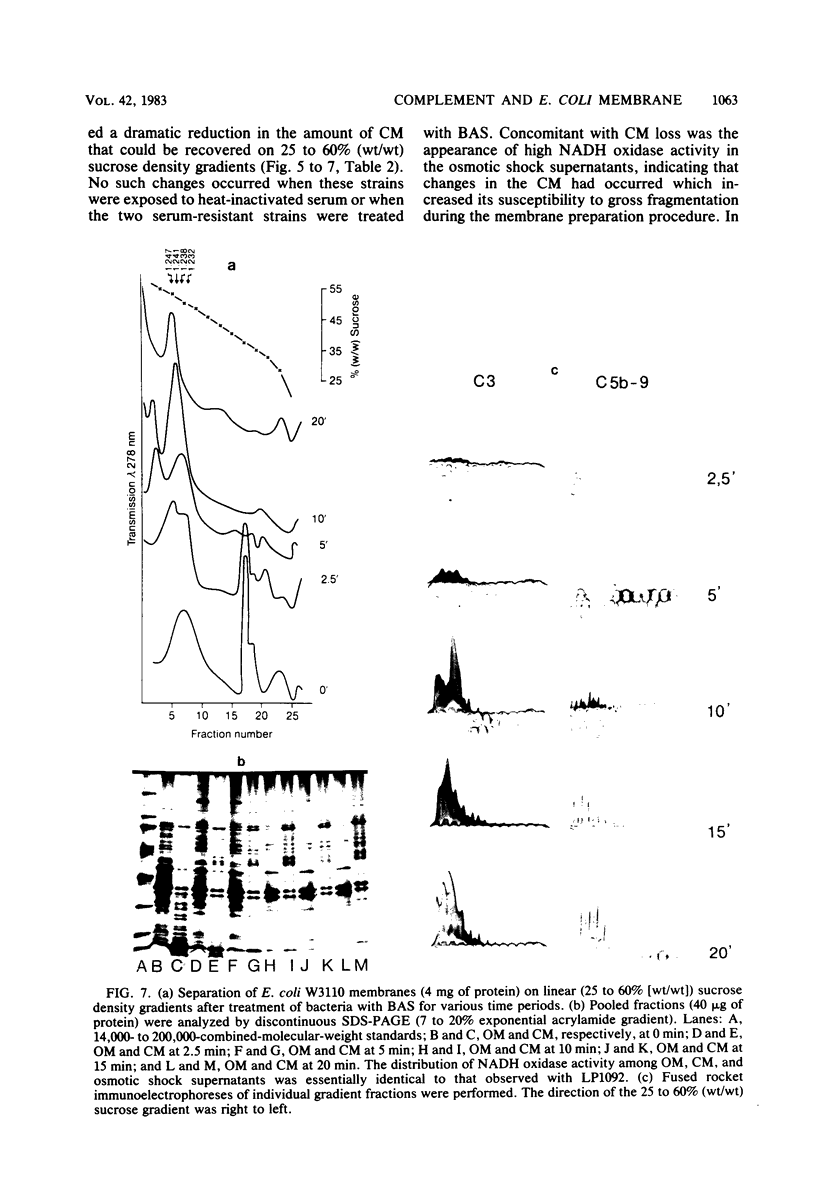
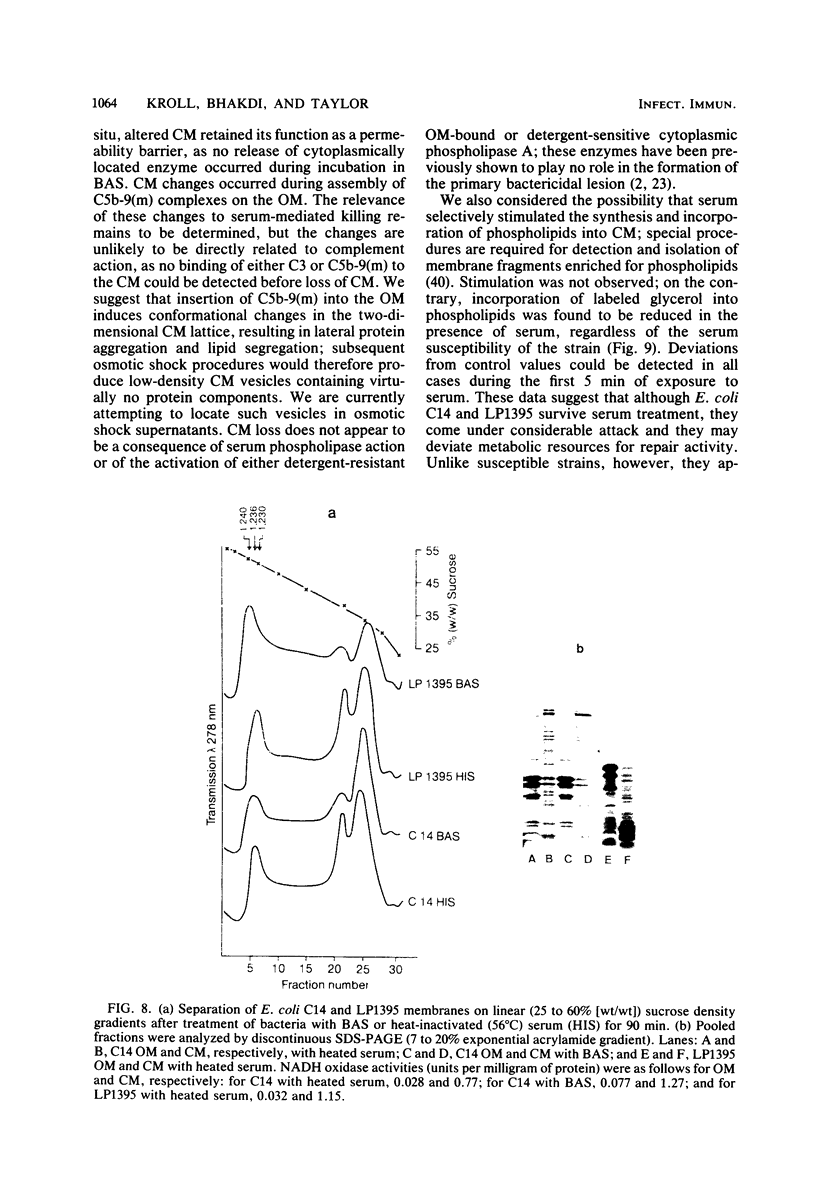
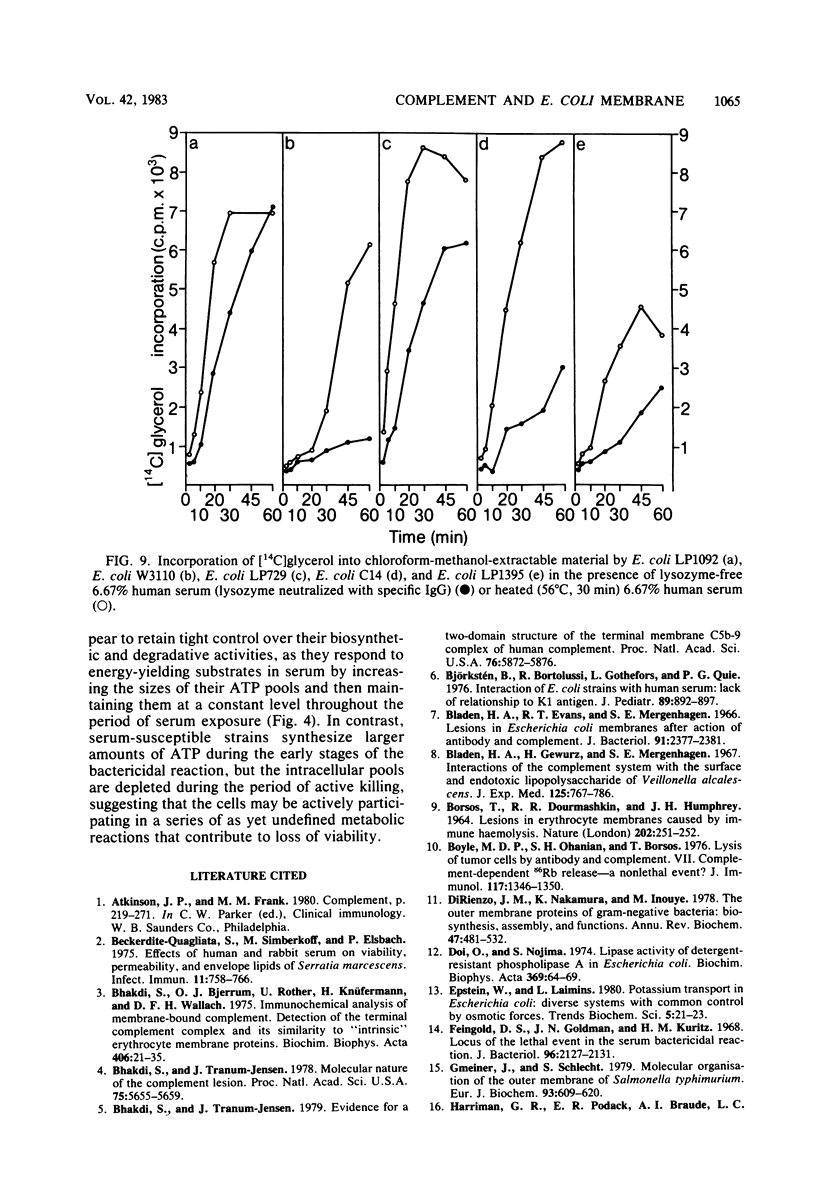
The effect of bactericidal concentrations of lysozyme-free human serum on parameters of membrane integrity has been studied in serum-susceptible and serum-resistant Escherichia coli strains. Serum treatment released all of the alkaline phosphatase from the periplasmic space of two rapidly serum-susceptible strains but did so at different rates. In contrast, no periplasmic enzyme was released from two serum-resistant strains or from one moderately susceptible smooth strain. Lysozyme-free serum and heat-inactivated serum released comparable amounts of 86Rb+ from preloaded cells at comparable rates, regardless of serum susceptibility. Serum decreased the rate of phospholipid biosynthesis in both serum-susceptible and serum-resistant strains. In susceptible but not in resistant strains, intracellular ATP pools were depleted after serum exposure. Outer membranes and cytoplasmic membranes were prepared from serum-treated E. coli, and assays for C3 and C5b-9(m) were performed. With rapidly susceptible strains, C3 deposition on the outer membrane without attachment of C5b-9(m) occurred during the short prekilling phase. Subsequent bacterial killing was accompanied by deposition of C5b-9(m), which was recovered with C3 exclusively in outer membrane fractions with increased density and by eventual total loss of recoverable cytoplasmic membranes. Minimal deposition of complement components, without accompanying cytoplasmic membrane loss, occurred with serum-resistant strains. Loss of recoverable cytoplasmic membrane was not due to the action of either serum or bacterial phospholipase A. The results raise the possibilities that C5b-9(m) primarily damages the outer membrane and that the bacteria themselves actively participate in the ensuing, as yet unclarified, metabolic reactions that finally lead to their death.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORSOS T., DOURMASHKIN R. R., HUMPHREY J. H. LESIONS IN ERYTHROCYTE MEMBRANES CAUSED BY IMMUNE HAEMOLYSIS. Nature. 1964 Apr 18;202:251–252. doi: 10.1038/202251a0. [DOI] [PubMed] [Google Scholar]
- Beckerdite-Quagliata S., Simberkoff M., Elsbach P. Effects of human and rabbit serum on viability, permeability, and envelope lipids of Serratia marcescens. Infect Immun. 1975 Apr;11(4):758–766. doi: 10.1128/iai.11.4.758-766.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Bjerrum O. J., Rother U., Knüfermann H., Wallach D. F. Immunochemical analyses of membrane-bound complement. Detection of the terminal complement complex and its similarity to "intrinsic" erythrocyte membrane proteins. Biochim Biophys Acta. 1975 Sep 16;406(1):21–35. doi: 10.1016/0005-2736(75)90039-5. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Evidence for a two-domain structure of the terminal membrane C5b-9 complex of human complement. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5872–5876. doi: 10.1073/pnas.76.11.5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Molecular nature of the complement lesion. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5655–5659. doi: 10.1073/pnas.75.11.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkstén B., Bortolussi R., Gothefors L., Quie P. G. Interaction of E. coli strains with human serum: lack of relationship to K1 antigen. J Pediatr. 1976 Dec;89(6):892–897. doi: 10.1016/s0022-3476(76)80592-6. [DOI] [PubMed] [Google Scholar]
- Bladen H. A., Evans R. T., Mergenhagen S. E. Lesions in Escherichia coli membranes after action of antibody and complement. J Bacteriol. 1966 Jun;91(6):2377–2381. doi: 10.1128/jb.91.6.2377-2381.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladen H. A., Gewurz H., Mergenhagen S. E. Interactions of the complement system with the surface and endotoxic lipopolysaccharide of Veillonella alcalescens. J Exp Med. 1967 May 1;125(5):767–786. doi: 10.1084/jem.125.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M. D., Ohanian S. H., Borsos T. Lysis of tumor cells by antibody and complement. VII. Complement-dependent 86Rb release--a nonlethal event? J Immunol. 1976 Oct;117(4):1346–1350. [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Doi O., Nojima S. Lipase activity of detergent-resistant phospholipase A in Escherichia coli. Biochim Biophys Acta. 1974 Oct 16;369(1):64–69. [PubMed] [Google Scholar]
- Feingold D. S., Goldman J. N., Kuritz H. M. Locus of the lethal event in the serum bactericidal reaction. J Bacteriol. 1968 Dec;96(6):2127–2131. doi: 10.1128/jb.96.6.2127-2131.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmeiner J., Schlecht S. Molecular organization of the outer membrane of Salmonella typhimurium. Eur J Biochem. 1979 Feb 1;93(3):609–620. doi: 10.1111/j.1432-1033.1979.tb12861.x. [DOI] [PubMed] [Google Scholar]
- Harriman G. R., Podack E. R., Braude A. I., Corbeil L. C., Esser A. F., Curd J. G. Activation of complement by serum-resistant Neisseria gonorrhoeae. Assembly of the membrane attack complex without subsequent cell death. J Exp Med. 1982 Oct 1;156(4):1235–1249. doi: 10.1084/jem.156.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Kinoshita T., Okada M., Akiyama Y. Release of phospholipids from complement-mediated lesions on the surface structure of Escherichia coli. J Immunol. 1977 Jul;119(1):65–72. [PubMed] [Google Scholar]
- Inoue K., Takamizawa A., Kurimura T., Yomemasu K. Studies on the immune bacteriolysis. 13. Leakage of enzymes from Escherichia coli during immune bacteriolysis. Biken J. 1968 Sep;11(3):193–201. [PubMed] [Google Scholar]
- Inoue K., Takamizawa A., Yano K., Amano T. Chemical studies on damages of Escherichia coli by the immune bactericidal reaction. I. Release and degradation of phospholipids from damaged bacteria. Biken J. 1974 Dec;17(4):127–134. [PubMed] [Google Scholar]
- Inoue K., Yonemasu K., Takamizawa A., Amano T. [Studies on the immune bacteriolysis. XIV. Requirement of all nine components of complement for immune bacteriolysis]. Biken J. 1968 Sep;11(3):203–206. [PubMed] [Google Scholar]
- Konisky J. Colicins and other bacteriocins with established modes of action. Annu Rev Microbiol. 1982;36:125–144. doi: 10.1146/annurev.mi.36.100182.001013. [DOI] [PubMed] [Google Scholar]
- Kopecky A. L., Copeland D. P., Lusk J. E. Viability of Escherichia coli treated with colicin K. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4631–4634. doi: 10.1073/pnas.72.11.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzer D. L., Vandermaten M., Buller C. S., Robertson D. C., Hirata A. A. Role of bacterial phospholipases in serum-mediated killing of Escherichia coli. Infect Immun. 1977 Oct;18(1):183–188. doi: 10.1128/iai.18.1.183-188.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. K., Levine R. P. Molecular transport via the functional complement lesion. Mol Immunol. 1980 Dec;17(12):1465–1474. doi: 10.1016/0161-5890(80)90172-8. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Martinez R. J., Carroll S. F. Sequential metabolic expressions of the lethal process in human serum-treated Escherichia coli: role of lysozyme. Infect Immun. 1980 Jun;28(3):735–745. doi: 10.1128/iai.28.3.735-745.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. M. Membrane damage by complement. Johns Hopkins Med J. 1981 Jun;148(6):243–258. [PubMed] [Google Scholar]
- Meury J., Kepes A. The regulation of potassium fluxes for the adjustment and maintenance of potassium levels in Escherichia coli. Eur J Biochem. 1981 Sep;119(1):165–170. doi: 10.1111/j.1432-1033.1981.tb05589.x. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Plate C. A., Suit J. L., Jetten A. M., Luria S. E. Effects of colicin K on a mutant of Escherichia coli deficient in Ca 2+, Mg 2+-activated adenosine triphosphatase. J Biol Chem. 1974 Oct 10;249(19):6138–6143. [PubMed] [Google Scholar]
- Schreiber R. D., Morrison D. C., Podack E. R., Müller-Eberhard H. J. Bactericidal activity of the alternative complement pathway generated from 11 isolated plasma proteins. J Exp Med. 1979 Apr 1;149(4):870–882. doi: 10.1084/jem.149.4.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims P. J., Lauf P. K. Analysis of solute diffusion across the C5b-9 membrane lesion of complement: evidence that individual C5b-9 complexes do not function as discrete, uniform pores. J Immunol. 1980 Dec;125(6):2617–2625. [PubMed] [Google Scholar]
- Sims P. J. Permeability characteristics of complement-damaged membranes: evaluation of the membrane leak generated by the complement proteins C5b-9. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1838–1842. doi: 10.1073/pnas.78.3.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Goldschneider I. The serum bactericidal system: ultrastructural changes in Neisseria meningitidis exposed to normal rat serum. J Exp Med. 1969 Jan 1;129(1):51–79. doi: 10.1084/jem.129.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W. Immunochemical investigations on lipopolysaccharides and acidic polysaccharides from serum-sensitive and serum-resistant strains of Escherichia coli isolated from urinary-tract infections. J Med Microbiol. 1976 Nov;9(4):405–421. doi: 10.1099/00222615-9-4-405. [DOI] [PubMed] [Google Scholar]
- Taylor P. W., Kroll H. P. Killing of an encapsulated strain of Escherichia coli by human serum. Infect Immun. 1983 Jan;39(1):122–131. doi: 10.1128/iai.39.1.122-131.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranum-Jensen J., Bhakdi S., Bhakdi-Lehnen B., Bjerrum O. J., Speth V. Complement lysis: the ultrastructure and orientation of the C5b-9 complex on target sheep erythrocyte membranes. Scand J Immunol. 1978;7(1):45–46. doi: 10.1111/j.1365-3083.1978.tb00425.x. [DOI] [PubMed] [Google Scholar]
- WARDLAW A. C. The complement-dependent bacteriolytic activity of normal human serum. I. The effect of pH and ionic strength and the role of lysozyme. J Exp Med. 1962 Jun 1;115:1231–1249. doi: 10.1084/jem.115.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Levine R. P. How complement kills E. coli. I. Location of the lethal lesion. J Immunol. 1981 Sep;127(3):1146–1151. [PubMed] [Google Scholar]
- van Heerikhuizen H., Kwak E., van Bruggen E. F., Witholt B. Characterization of a low density cytoplasmic membrane subfraction isolated from Escherichia coli. Biochim Biophys Acta. 1975 Dec 1;413(2):177–191. doi: 10.1016/0005-2736(75)90102-9. [DOI] [PubMed] [Google Scholar]