Abstract
Targeting protein kinase C (PKC) isoforms by the small molecule inhibitor enzastaurin has shown promising preclinical activity in a wide range of tumor cells. We further delineated its mechanism of action in multiple myeloma (MM) cells and found a novel role of β-catenin in regulating growth and survival of tumor cells. Specifically, inhibition of PKC leads to rapid accumulation of β-catenin by preventing the phosphorylation required for its proteasomal degradation. Microarray analysis and small-interfering RNA (siRNA)–mediated gene silencing in MM cells revealed that accumulated β-catenin activates early endoplasmic reticulum stress signaling via eIF2α, C/EBP-homologous protein (CHOP), and p21, leading to immediate growth inhibition. Furthermore, accumulated β-catenin contributes to enzastaurin-induced cell death. Sequential knockdown of β-catenin, c-Jun, and p73, as well as overexpression of β-catenin or p73 confirmed that accumulated β-catenin triggers c-Jun–dependent induction of p73, thereby conferring MM cell apoptosis. Our data reveal a novel role of β-catenin in endoplasmic reticulum (ER) stress-mediated growth inhibition and a new proapoptotic mechanism triggered by β-catenin on inhibition of PKC isoforms. Moreover, we identify p73 as a potential novel therapeutic target in MM. Based on these and previous data, enzastaurin is currently under clinical investigation in a variety of hematologic malignancies, including MM.
Introduction
Multiple myeloma (MM) is the second most common hematologic neoplasm in the United States and is characterized by the malignant transformation of a terminally differentiated plasma cell within the bone marrow. Although clear incremental improvements in progression-free and overall survival have followed the recent introduction of the novel targeted therapies thalidomide, lenalidomide, and bortezomib, MM remains an incurable malignancy. Further delineation of mechanisms underlying the therapeutic specificity of these and other new agents will provide insight into disease pathogenesis and identify novel therapeutic targets to improve patient outcome.
The macrocyclic bisindolylmaleimide enzastaurin (LY317615.HCL) is a novel orally available inhibitor of protein kinase C (PKC) isoforms.1 Preclinical activity has been demonstrated in a wide spectrum of tumor types.2 Importantly, our own and other previous data strongly support a therapeutic role for enzastaurin in MM.3–6 Specifically, enzastaurin blocks activation of classic and novel PKC isoforms triggered by growth factors and cytokines and thereby inhibits MM cell proliferation, survival, migration, and drug resistance. Moreover, enzastaurin blocks angiogenesis both in vitro as well as in vivo in a murine xenograft model of human MM.4 Interestingly, growth inhibition of MM cells was detectable within a few hours of treatment, whereas actual cell death did not occur until 48 hours. Functionally, enzastaurin's antitumor activity has been primarily attributed to the inhibition of Akt and its downstream targets.1,4,5,7,8 However, very limited data are available on its precise mechanism of action.
β-Catenin is a key component of the WNT pathway, as well as a signal integrator for the Ras and phosphatidylinositol 3-kinase (PI3K) pathways.9,10 As a mediator of the WNT signaling in MM, β-catenin has been implicated in cell proliferation, migration, and bone disease, and was investigated as a promising therapeutic target.11–14 Cytoplasmic levels of β-catenin are tightly regulated by phosphorylation at serine residues (S33, S37, and S45) and by formation of a complex with glycogen synthase kinase (GSK)–3β, adenomatous polyposis of the colon (APC), axin, and β-TrCP (transducin repeat-containing protein).9,15,16 Phosphorylation occurs first at S45, which is required for subsequent phosphorylation at S33 and S37. Fully phosphorylated β-catenin is rapidly ubiquitinated and degraded by the proteasome.15,17 Importantly, PKC isoforms have been reported to contribute to the phosphorylation and degradation of β-catenin. Consequently, a marked accumulation of β-catenin has been seen after treatment with bisindoylmaleimide inhibitors of PKC (eg, BIM I).18,19 Whereas β-catenin may be best known for its oncogenic role in the pathogenesis of colon cancer,20,21 several reports have described an additional role in the induction of apoptosis. Proposed mechanisms include activation of p53 signaling, p14 ARF, and cyclin D1.22–25 However, other studies found β-catenin–mediated apoptosis to be independent of these factors.23 Thus, the proapoptotic mechanism of β-catenin still remains elusive.
Another promising therapeutic target in MM is the endoplasmic reticulum (ER)–associated stress pathway, also known as the unfolded protein response (UPR).26,27 Under physiologic conditions, the UPR is an adaptive response, activated by the accumulation of misfolded proteins in the ER, to maintain cell survival.28 Specifically, ER stress leads to the activation of 3 major UPR sensors: pancreatic eIF2-α kinase (PERK), high inositol-requiring 1 (IRE1-α), and ATF6. First, PERK phosphorylates the eukaryotic translation initiation factor-2a (eIF2a), which results in both the initial decrease in general translation initiation and the selective translation of the transcription factor ATF4. In turn, ATF4 induces growth arrest and DNA damage-inducible protein (GADD153/CHOP), resulting in cell cycle arrest and thereby preventing further damage to the cell.29,30 Second, IRE1-α mediates splicing of x-box–binding protein (Xbp1), which increases transcription of ER-resident chaperones, folding enzymes, and components of the protein degradation machinery. Third, ATF6, after activating cleavage, contributes to both induction of CHOP and up-regulation of protein folding and degradation.30 In addition to the 3 UPR branches, the transcription factors ATF2 and ATF3 have been reported to regulate CHOP.28
Prolonged, nonresolvable ER stress overrides the salvage mechanisms of the initial UPR and eventually leads to apoptosis involving CHOP signaling, JNK activation, bcl-2 phosphorylation and depletion, and caspase cleavage (eg, caspase 4). Importantly, MM cells display constitutively elevated baseline activity of the UPR because of the extensive protein synthesis associated with immunoglobulin production and secretion. MM cells are consequently highly sensitive to any perturbation of protein folding, for example, by inhibition of either chaperone proteins (eg, by heat shock protein 90 [HSP90] inhibitors) or proteasomal degradation (eg, by proteasome inhibitors).31–33
c-Jun is a central component of the AP-1 family of transcription factors and has been implicated in cell differentiation, growth, survival, and apoptosis.34,35 Importantly, various mechanisms mediating proapoptotic functions of c-Jun have been reported.36–38 For example, c-Jun mediates apoptosis by regulating the stability and activity of p73, a p53 family member.39
In the present study, we demonstrate that pharmacologic inhibition of PKC isoforms triggers rapid accumulation of β-catenin by preventing phosphorylation necessary for its proteasomal degradation. Accumulated β-catenin leads to early growth arrest by inducing an ER stress-related UPR. Furthermore, accumulated β-catenin also induces c-Jun, leading to increased protein levels of p73 and thereby triggering apoptotic cell death.
Taken together, this study delineates 2 novel mechanisms whereby enzastaurin-induced β-catenin accumulation triggers both the early UPR as well as c-Jun up-regulation, leading to MM cell growth inhibition and apoptosis, respectively. Moreover, it identifies p73 as a potential novel therapeutic target in MM. Based on our own and other previous reports and the current studies, several phase 1 to 3 clinical trials with enzastaurin have been initiated in both solid tumors and hematologic malignancies including MM (www.clinicaltrials.gov).
Methods
Materials
Enzastaurin (LY317615.HCL) was provided by Eli Lilly (Indianapolis, IN). BIM I was purchased from Calbiochem (San Diego, CA). All antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), except for p–β-cateninS33,37, p–β-cateninS45, p-eIF2aS51 (Cell Signaling Technology, Danvers, MA), and full-length p73 (GC15; BD Biosciences, San Jose, CA).
Cells and cell culture
The human MM cell line MM.1S and primary patient MM cells were cultured in RPMI 1640. HeLa cells were cultured in Dulbecco modified Eagle medium. Both media were supplemented with 10% heat-inactivated fetal bovine serum (Harlan, Indianapolis, IN), 100 units/mL penicillin, 10 g/mL streptomycin, and 2 mM l-glutamine (Cellgro, Herndon, VA).
Isolation of patients' tumor cells
After appropriate informed consent was obtained in accordance with the Declaration of Helsinki and under the auspices of protocols approved by the institutional review board of the Dana-Faber Cancer Institute, primary patient MM cells (CD38+CD45RA−) were obtained from bone marrow aspirate samples, as previously described.40 The purity of MM cells was more than 95%.
Cell lysis, immunoprecipitation, and Western blotting
Cell lysis, immunoprecipitation, and Western blot analysis were done as previously described.40
Quantitative real-time PCR
Cells were treated as indicated. RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA). TaqMan real-time polymerase chain reaction (PCR) for human CTNNB1 was performed using forward primer: GCAAGGCTTTTCCCAGTC; reverse primer: GAGCCCTAGTCATTGCATA; probe (Fam/Tamra labeled): TCACGCAAGAGCAAGTAG. Values were normalized using 18S RNA (Applied Biosystems, Foster City, CA) and expressed as the percentage of values of untreated controls.
Cell fractionation
Cells were washed 3 times with phosphate-buffered saline and then transferred into 400 μL hypotonic lysis buffer followed by cell fractionation, as previously described.41
Plasmids and transfections
pCMV6-β-catenin was purchased from Origene (Rockville, MD); full-length pcDNA3-transcriptionally active p73 (TAp73) was kindly provided by K. Sagapathy (National Cancer Institute, Singapore).39 Mutations were confirmed by sequencing. For β-catenin–, CHOP-, c-Jun-, and p73-specific knockdown experiments, cells were transiently transfected with small-interfering RNA (siRNA) SMARTpool or nonspecific control duplexes (pool of 4; Dharmacon RNA Technologies, Lafayette, CO) using the Cell Line Nucleofector Kit V Solution (Amaxa Biosystems, Gaithersburg, MD) for MM.1S cells (100-200 nM) or the Lipofectamine 2000 reagent (Invitrogen) for HeLa cells (10 nM) according to the manufacturer's instructions.
DNA synthesis and cell proliferation assay
Cell growth was assessed by measuring [3H]thymidine uptake, as in prior studies.40
Microarray assay
Total RNA was isolated from enzastaurin-treated or vehicle-treated MM.1S cells using TRIzol reagent (Invitrogen). Affymetrix U133A 2.0 arrays were hybridized with biotinylated in vitro transcription products (10 μg/chip), as per the manufacturer's instructions (Affymetrix, Santa Clara, CA), within the DFCI Microarray Core Facility. The DNA chips were then analyzed with a Gene Array Scanner (Affymetrix). CEL files were obtained using the Affymetrix Microarray Suite 5.0 software. The DNA Chip Analyzer (DChip)25 was used to normalize all CEL files to a baseline array with overall median intensity, and the model-based expression (perfect match minus mismatch) was used to compute the expression values. Probes showing at least 2-fold difference between control and enzastaurin-treated cells at 3, 6, and 12 hours were included in the analysis. The same data were also analyzed through the use of Ingenuity Pathways Analysis (Ingenuity Systems, Redwood City, CA). Cell viability, as assessed by trypan blue exclusion, was at least 85% in all enzastaurin-treated cells during the times indicated. The microarray data are available in the Gene Expression Omnibus (accession number GSE13514).42
Inhibition of protein translation
MM cells were treated with 5 μM enzastaurin, 50 μM cycloheximide, or a combination for 24 hours, followed by immunoblot analysis with the indicated antibodies.
MTT colorimetric survival assay
Cell survival using methyl-thiazol-tetrazolium (MTT) assay was estimated as a percentage of the values of untreated controls, as previously described.41
DNA fragmentation assay
Cell Death Detection ELISAplus (Roche Applied Science, Indianapolis, IN) was used to quantitate DNA fragmentation, as per the manufacturer's instructions.
Luciferase reporter assay
Cells were transfected with TOP-FLASH or FOP-FLASH Wnt reporter plasmids (Upstate Biotechnology, Charlottesville, VA) containing wild-type or mutant CTNNB1-TCF DNA-binding sites using the Cell Line Nucleofector Kit V Solution (Amaxa Biosystems) for MM.1S cells or Lipofectamine 2000 (Invitrogen) for HeLa and HEK293 cells, according to the manufacturer's protocol. After 24 hours, cells were treated with the indicated concentrations of enzastaurin for the indicated times. Reporter activity was assayed with the Bright-Glo Luciferase Assay System (Promega, Madison, WI). Results were normalized to total protein amounts. No significant FOP-FLASH activity was detected. The reporter assay results represent the average of 3 independent transfection experiments.
Statistical analysis
Two-way analysis of variance test with a Bonferroni posttest was performed to calculate the significance of the effects on survival of cells exposed to specific siRNAs or enzastaurin concentrations. The effect was judged as statistically significant with P less than .05.
Results
Enzastaurin triggers accumulation of β-catenin
The specific PKC-inhibitor enzastaurin effectively blocks growth, survival, and migration of MM cells both in vitro and in vivo.4,5 However, enzastaurin-induced changes in signaling sequelae downstream of PKC are undefined.
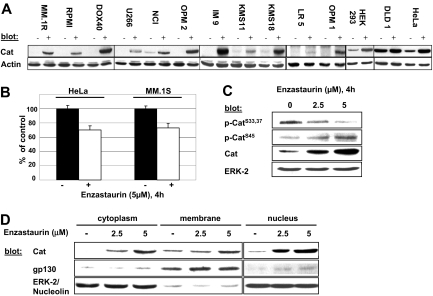
There is recent evidence that PKC isoforms directly phosphorylate β-catenin, leading to its ubiquitination and proteasomal degradation.18,19 We therefore first assessed protein levels of β-catenin after treatment with enzastaurin in several MM cell lines, as well as cell lines derived from viral-transformed lymphocytes (IM-9), colon cancer (DLD-1), human embryonic kidneys (HEK293), and cervical cancer (HeLa) (Figure 1A). Our results show a marked enzastaurin-induced increase in the levels of β-catenin in all tested cell lines. In contrast, quantitative real-time PCR revealed moderate inhibition of β-catenin–specific transcription in MM and HeLa cells (Figure 1B). Enzastaurin also blocked phosphorylation of β-catenin at S33,37 phosphorylation sites essential for PKC-regulated proteasomal degradation (Figure 1C).18,19 Indeed, inhibition of translation using cycloheximide did not abrogate enzastaurin-induced up-regulation of β-catenin. These data support the hypothesis that enzastaurin inhibits degradation of β-catenin via abrogation of ubiquitination (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). Furthermore, β-catenin accumulated predominantly in the nuclear and cytosolic compartment of MM.1S cells, whereas the membrane-bound content remained unchanged (Figure 1D). In addition, enzastaurin-induced β-catenin coimmunoprecipitated with its cofactor T-cell factor 4 (Figure S1B), subsequently activating the T-cell factor 4 promoter in MM.1S, HEK293, and HeLa cell lines, as shown by the TOP-FLASH reporter assay (Figure S1C). Taken together, these results demonstrate that enzastaurin triggers accumulation of transcriptionally active β-catenin because of inhibition of its degradation, rather than induced overexpression.
Figure 1.
Enzastaurin triggers β-catenin accumulation in MM and other tumor cells by inhibiting its degradation. (A-C) Indicated cell lines were treated with enzastaurin for 4 hours and subjected either to cell lysis (A,C) followed by immunoblotting with indicated antibodies or RNA extraction (B) followed by specific quantitative real-time PCR for β-catenin. (D) MM.1S cells were exposed to the indicated concentrations of enzastaurin for 4 hours followed by cell fractionation. Immunoblots were probed with the indicated antibodies.
Enzastaurin induces ER stress signaling in myeloma cells
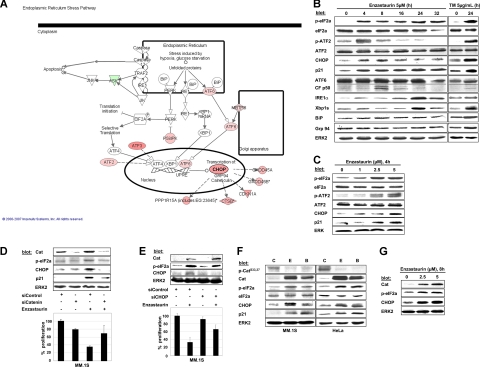
In an attempt to define the potential functional role of accumulated β-catenin in enzastaurin-treated MM cells, we performed serial expression profiling at 3, 6, and 12 hours, followed by analysis with the Ingenuity Systems software.43 Among differentially regulated genes with changes in expression levels more than or equal to 2-fold, 102 were up- and 94 down-regulated (Table S1) with striking up-regulation of genes associated with ER stress response signaling (Figure 2A).
Figure 2.
Enzastaurin-induced accumulation of β-catenin triggers ER stress response in tumor cells. (A) Differentially regulated genes of the ER stress pathway in MM cells treated with enzastaurin vs control were delineated by oligonucleotide microarray analysis (human U133A 2.0 Affymetrix GeneChip), followed by data analysis and modified graphical display using the Ingenuity Pathway software (for further information, see also www.ingenuity.com). Analysis with Ingenuity Systems was performed under the institutional license of Dana-Faber Cancer Institute. Gene overexpression is shown in pink, down-regulation in green. (B,C) Protein profiling of MM cells exposed to enzastaurin. MM.1S cells were exposed to enzastaurin for increasing time periods (B) or concentrations (C), followed by immunoblot analysis with the indicated antibodies. Tunicamycin (TM) was used as a positive control for ER stress signaling. (D) Knockdown of β-catenin protects against enzastaurin-induced growth inhibition. MM.1S cells were transiently transfected with mock or β-catenin siRNA and treated with enzastaurin (5 μM, 6 hours), followed by immunoblotting with the indicated antibodies (top) or proliferation assay ([3H]dT uptake, bottom). [3H]dT uptake was measured during the last 5 hours of treatment. Columns represent mean of experiments done in triplicate; bars represent SD. (E) Knockdown of CHOP/GADD153 partly attenuates enzastaurin-induced growth arrest. MM.1S cells were transiently transfected with mock or CHOP/GADD153 siRNA, treated with enzastaurin (2.5 μM), and immunoblotted with indicated antibodies (top) or assayed for 3H[dT] (bottom). (F) Indicated cells were treated with enzastaurin or BIM I (both 5 μM, 4 hours), followed by immunoblotting with the indicated antibodies. (G) Primary MM cells were treated with the indicated concentrations of enzastaurin (8 hours), followed by immunoblotting with the indicated antibodies.
We next confirmed these data by immunoblot assays. Enzastaurin induces all 3 major branches of the UPR in a time-dependent fashion. Specifically, PERK-dependent phosphorylation of eIF2a and induction of its downstream target CHOP were detected within 4 hours of treatment and maintained over time. In addition, the stress-related transcription factor ATF2 was also phosphorylated within 4 hours of treatment.44 Furthermore, the cell cycle inhibitor p21(WAF), a known downstream effector of CHOP, was up-regulated and sustained. Activating cleavage of ATF6 and up-regulation of IRE1-α, followed by an increase of the spliced variant of Xbp1 (∼ 50 kDa), were seen at later time points and appeared to be transient (Figure 2B). No significant change was observed in the level of the chaperone proteins immunoglobulin-binding protein/glucose-regulated protein (BiP/grp94). Dose-dependent activation of the PERK-eIF2a-ATF4-CHOP cascade and the induction of p21(WAF) at 4 hours were also observed (Figure 2C). These data demonstrate that enzastaurin increases ER stress in MM cells, thereby activating the UPR that leads to phosphorylation of eIF2a and up-regulation of CHOP, followed by ATF6-cleavage and IRE1a-mediated Xbp1 splicing.
Enzastaurin-induced ER stress is caused by the accumulation of β-catenin and contributes to early growth arrest
In contrast to agents that directly interfere with protein degradation or protein folding, such as bortezomib or HSP90 inhibitors, respectively, the mechanism of induction of the ER stress response by enzastaurin is undefined. We therefore used siRNA to specifically deplete β-catenin to investigate the potential of accumulated β-catenin to trigger activation of the UPR. Despite treatment with enzastaurin, phosphorylation of eIF2a and induction of CHOP, 2 key markers of the early UPR, were markedly inhibited on depletion of β-catenin (Figure 2D top). Moreover, enzastaurin-induced up-regulation of p21(WAF) was also abrogated on depletion of β-catenin. These data are consistent with abrogation of enzastaurin-induced up-regulation of CHOP. Importantly, these events were associated with marked attenuation of early growth inhibition, as evidenced by [3H]dT uptake (Figure 2D bottom). Conversely, specific knockdown of CHOP by siRNA markedly attenuated enzastaurin-induced inhibition of proliferation (Figure 2E) but not survival (data not shown), confirming the growth inhibitory role of CHOP in MM cells. Similar to enzastaurin, the PKC inhibitor BIM I also induced β-catenin accumulation and triggered activation of the UPR in both MM.1S as well as in HeLa cells (Figure 2F). Moreover, accumulation of β-catenin with activation of the UPR was also found in enzastaurin-treated OPM-2 and RPMI 8226 cells (Figure S2), as well as primary MM cells treated with enzastaurin (Figure 2G). These findings indicate that enzastaurin-induced accumulation of β-catenin activates the PERK-eIF2a-CHOP branch of the UPR and thereby contributes to early growth arrest induced by enzastaurin via p21(WAF).
Enzastaurin induces c-Jun up-regulation and p73 induction
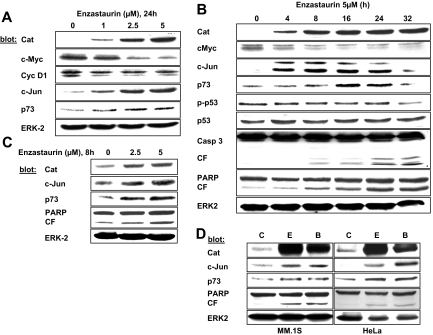
Given the surprising effects of β-catenin as the mediator of enzastaurin-induced ER stress and early growth inhibition, we next investigated target genes of β-catenin, in particular, c-Jun, c-Myc, and cyclin D1, and their possible contribution to the anti-MM effect of enzastaurin. Our results show that β-catenin induces c-Jun, whereas c-Myc was markedly down-regulated. In contrast, cyclin D1 remained unchanged (Figure 3A). Consistent with these data, induction of JUN gene transcription (> 2-fold) was found in the microarray analysis, whereas down-regulation of MYC was less than 2-fold. No significant change of CCND1 gene transcription was detected (Table S1). c-Jun stabilizes p73, a p53 family member leading to apoptotic cell death.39 Consistently, enzastaurin induced p73, but not p53, in a dose- and time-dependent manner (Figure 3B). Importantly, enzastaurin also induced β-catenin, c-Jun, and p73 in OPM-2 and RPMI 8226 cells (Figure S2), as well as primary patient MM cells (Figure 3C) and HeLa cells (Figure 3D). Similar findings were observed using the PKC inhibitor BIM I (Figure 3D). These data led us to the hypothesis that enzastaurin-induced accumulation of β-catenin not only contributes to early MM cell growth inhibition via activation of the UPR but also plays a role in cell death via c-Jun and p73 induction.
Figure 3.
Protein profiling of downstream targets of β-catenin. (A,B) MM.1S cells were exposed to enzastaurin with increasing concentrations (A) or time periods (B), followed by immunoblotting with the indicated antibodies. (C) Primary MM patient cells were exposed to enzastaurin and immunoblotted as indicated. (D) Indicated cells were treated with enzastaurin or BIM I (both 5 μM, 24 hours), followed by immunoblotting with indicated antibodies. C indicates control; E, enzastaurin; B, BIM I.
Enzastaurin-induced cell death is triggered in part by β-catenin via c-Jun–mediated p73 induction
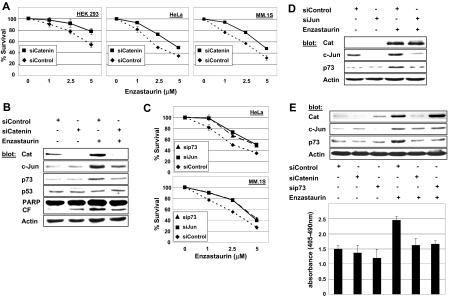
To test the relevance of β-catenin accumulation to enzastaurin-induced cell death, we specifically knocked down β-catenin using siRNA, followed by assessment of cell survival. Depletion of β-catenin resulted in a partial rescue of enzastaurin-induced cell death in HEK 293, HeLa, and MM.1S cells (Figure 4A). Furthermore, β-catenin knockdown was associated with attenuation of enzastaurin-induced c-Jun up-regulation and p73 induction, indicating that c-Jun and p73 are downstream of β-catenin (Figure 4B). Depletion of c-Jun by siRNA showed a similar rescue of MM.1S and HeLa cell survival after enzastaurin treatment (Figure 4C). Immunoblotting confirmed c-Jun to be downstream of β-catenin because enzastaurin-induced β-catenin accumulation was not affected by knockdown of c-Jun. Moreover, enzastaurin-triggered p73 induction was abrogated by c-Jun depletion (Figure 4D). Finally, p73 siRNA proved to be as effective as knockdown of either β-catenin or c-Jun in rescuing both cell lines from enzastaurin-induced cell death (Figure 4C). Moreover, the accumulation of β-catenin and up-regulation of c-Jun triggered by enzastaurin strongly suggest p73 to be a downstream effector within this cascade. In addition, attenuation of enzastaurin-induced apoptosis by knockdown of either β-catenin or p73 was specifically confirmed by DNA fragmentation assay (Figure 4E). Conversely, transient overexpression of full-length p73, but not empty vector, resulted in marked cell death in MM cells (Figure S3).
Figure 4.
β-Catenin contributes to enzastaurin-induced cell death via induction of c-Jun and p73. (A) After knockdown of β-catenin by siRNA, indicated cell lines were exposed to enzastaurin at increasing concentrations and cell survival was assessed by MTT assay during the last 4 hours of 48-hour cultures (siControl vs siCatenin, P < .001). (B) Knockdown of β-catenin inhibits induction of c-Jun and p73 in MM cells. MM.1S cells were exposed to enzastaurin (5 μΜ, 24 hours) after transient transfection with mock or β-catenin siRNA; immunoblots were probed with indicated antibodies. (C) After knockdown of c-Jun or p73 by siRNA, indicated cell lines were exposed to enzastaurin at increasing concentrations, and cell survival was assessed by MTT assay during the last 4 hours of 48-hour cultures (siControl vs siJun/p73, P < .001). (D) Depletion of c-Jun inhibits induction of p73 but does not change β-catenin accumulation in MM cells. (E) Inhibition of β-catenin accumulation or p73 induction by siRNA rescues MM cells from enzastaurin-induced apoptosis. MM.1S cells were exposed to enzastaurin (5 μΜ, 24 hours) after depletion of β-catenin or p73 by siRNA. Cell lysates were subjected to immunoblotting with indicated antibodies (top), and induction of apoptosis was assessed by DNA fragmentation (bottom).
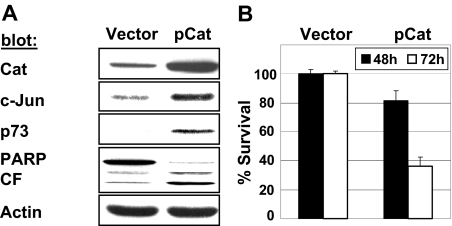
Having shown the contribution of β-catenin to enzastaurin-mediated cell death, we finally asked whether ectopic overexpression of β-catenin in untreated MM cells also induces c-Jun and p73 associated with apoptosis. We therefore transiently transfected MM cells with β-catenin. As shown in Figure 5A, overexpressed β-catenin induced c-Jun as well as p73 protein levels. Moreover, transient overexpression of β-catenin resulted in time-dependent reduction of cell viability (Figure 5B). It is worth noting that overexpressed β-catenin also induced phosphorylation of eIF2a and up-regulation of CHOP in this setting (data not shown).
Figure 5.
Ectopic overexpression of β-catenin induces cell death via induction of c-Jun and p73. MM.1S cells were transiently transfected with plasmids expressing β-catenin (pCat) or empty vector only. (A) Cell lysates (48 hours after transfection) were immunoblotted with the indicated antibodies. (B) Cell survival was measured by MTT assay at indicated times.
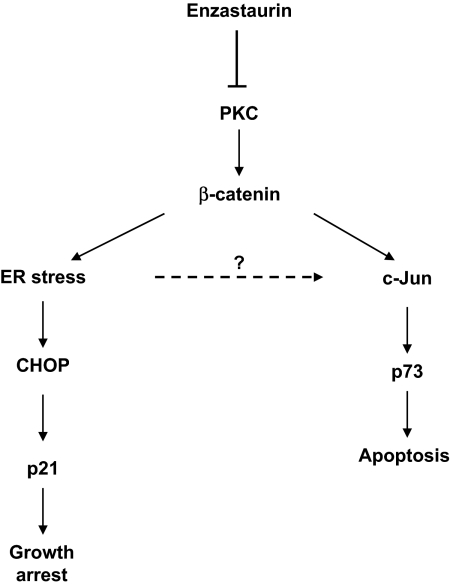
Taken together, these findings demonstrate that enzastaurin-induced accumulation of β-catenin not only activates the PERK-eIF2a-CHOP branch of the UPR and thereby contributes to early growth arrest but also contributes to cell death via c-Jun up-regulation followed by p73 induction (Figure 6).
Figure 6.
Enzastaurin-induced accumulation of β-catenin triggers UPR-mediated growth arrest and c-Jun-mediated apoptosis. Enzastaurin inhibits phosphorylation of β-catenin, thereby inducing accumulation of β-catenin leading to ER stress response-mediated growth arrest, as well as triggering apoptosis via c-Jun-stabilized p73.
Discussion
PKC isoforms are involved in MM-cell apoptosis, proliferation, and migration.4,13,41,45 Our previous study showed that specific inhibition of PKC isoforms by enzastaurin, a bisindoylmaleimide-derived, clinical-grade small molecule, blocks proliferation and induces apoptosis in MM cells.4 Our present study further characterizes the downstream effects of therapeutic PKC inhibition. We report a previously unknown role of β-catenin in the induction of ER stress-mediated inhibition of proliferation and a new mechanism whereby β-catenin induces apoptosis via up-regulation of c-Jun.
β-Catenin is best known as a key signal transducer in the canonical WNT pathway, which is dysregulated in many cancers.46 We here show that PKC inhibition by enzastaurin prevents phosphorylation of β-catenin at S33,37 leading to rapid and marked accumulation of the protein. Whereas phosphorylation and degradation of β-catenin are mainly regulated by a complex of GSK3β, APC, axin, and other proteins, PKC isoforms represent another family of kinases that regulate β-catenin stability by phosphorylation. Consequently, bisindoylmaleimide-derived PKC inhibitors lead to accumulation of β-catenin.18,19 Although β-catenin can be phosphorylated at S45 irrespective of treatment with enzastaurin, inhibition of phosphorylation at S33,37 is sufficient for stabilization of β-catenin, despite activating dephosphorylation of GSK3β.1 In contrast, general inhibition of proteasomal degradation by low-dose bortezomib is not sufficient to cause accumulation of β-catenin (Figure S4), indicating that enzastaurin-induced inhibition of ubiquitination of β-catenin might also block alternative ubiquitin-dependent pathways of protein degradation. However, we do not directly show the mechanism by which specific PKC isoforms contribute to the phosphorylation and thereby to the accumulation of β-catenin.
Under physiologic conditions, stabilized β-catenin enhances proliferation and protects against apoptosis.47–49 In this regard, inhibition of the transactivating activity of β-catenin has been reported to be a promising therapeutic target in MM.12 In contrast, we here demonstrate that accumulated β-catenin blocks proliferation and induces apoptosis in MM and HeLa cells. These opposing effects have previously been reported for β-catenin as well as for other transcription factors, such as c-Jun.23–25,34,35,50
We describe 2 mechanisms whereby accumulated β-catenin leads to inhibition of cell growth and induction of apoptosis, respectively. First, we demonstrate that accumulated β-catenin triggers the activation of the UPR, thereby conferring enzastaurin-induced inhibition of proliferation. In contrast to the cell-protective effects of the UPR under physiologic conditions, drug-induced ER stress can also result in growth inhibition of tumor cells. Indeed, MM cells are characterized by a constitutively high protein turnover because of the processing and secretion of immunoglobulin and are highly sensitive to additional ER stress.31,32,51–53 Consistent with our results, inhibition of protein folding (eg, by HSP90 inhibitors) or degradation (eg, by bortezomib) has been shown to result in the accumulation of cytosolic proteins, thereby inducing the UPR as a feedback inhibition that prevents the retrograde translocation of misfolded proteins from the ER.32,33,54,55 This explains, at least in part, the therapeutic success of these agents in MM. Specifically, we demonstrate that accumulated β-catenin leads to PERK-mediated phosphorylation of eIF2a and up-regulation of CHOP and p21(WAF), thereby conferring enzastaurin-induced inhibition of proliferation. The delayed and only transient activation of ATF6 and IRE1-α-induced splicing of Xbp1 indicates that the associated inhibition of proliferation is predominantly mediated by the first branch of the UPR. This is further supported by the lack of significant induction of ER chaperone proteins, such as BiP/grp78 or grp94, mainly mediated by ATF6 and Xpb1. However, we cannot exclude additional regulatory or supporting roles of these 2 branches of the UPR.
Although ER stress-induced apoptosis is strongly associated with CHOP induction, we did not observe activation of key components of the terminal UPR, such as JNK or caspase 4, or inhibition of bcl-2 (data not shown). Indeed, our previous work showed that enzastaurin inhibits phosphorylation of JNK.4 These data do not exclude a role for the terminal UPR in enzastaurin-induced cell death. However, the lack of these proapoptotic markers suggests that β-catenin-mediated ER stress predominantly blocks cell proliferation.
Second, we describe a novel mechanism of induction of apoptosis by accumulated β-catenin. Our results demonstrate that β-catenin-related c-Jun up-regulation contributes to enzastaurin-mediated cell death via increased protein levels of p73.
Although best known as an enhancer of proliferation and survival in tumor cells, overexpressed or accumulated β-catenin has also been reported to induce apoptosis in fibroblasts, as well as in a variety of tumor cell lines.23–25,50 Damalas et al showed that inducible β-catenin harboring mutations preventing its phosphorylation and consequent degradation triggers p53-dependent growth arrest in fibroblasts.25 Similar findings for a stabilized mutant form of β-catenin have been reported by Saegusa et al in endometrial carcinoma.24 However, Kim et al observed induction of apoptosis independent of p53 status and transactivating function of β-catenin, when β-catenin is overexpressed in colon cancer or HeLa cells.23 We here show that the proapoptotic effect of accumulated β-catenin in enzastaurin-treated MM cells is dependent on the up-regulation of c-Jun and p73, rather than p53. Specifically, the transcription factor c-Jun has been implicated in cell differentiation, growth, survival, and apoptosis.34,35 Importantly, various mechanisms mediating proapoptotic functions of c-Jun have been reported, including transcriptional regulation as well as activation of caspase-mediated cleavage of important components of cell survival and apoptosis pathways.36–38 For example, c-Jun mediates cisplatin-induced apoptosis by regulating the stability and activity of p73, a p53 family member.39 However, we did not establish whether c-Jun transcription is directly activated by β-catenin or whether the activation of ER stress pathways additionally contributes to c-Jun induction. Nevertheless, our results clearly demonstrate that β-catenin-related c-Jun up-regulation leads to increased protein levels of p73 in MM and Hela cells. Furthermore, overexpression of a full-length p73 leads to pronounced cell death in MM cells, suggesting that p73 isoforms may represent a novel and promising therapeutic target in MM.
p73 is a structural and functional homologue of the p53 tumor-suppressor gene with homologies in transactivation and DNA-binding domains.56 In contrast to p53, p73 is rarely mutated in human cancers. Because of distinct promoters, the TP73 gene allows formation of 2 proteins, a transactivation-proficient TAp73 with proapoptotic effects, as well as a transactivation-deficient, amino-deleted ΔNp73 with opposing effects.57 In addition, there are several isoforms of p73 resulting from extensive splicing, resulting in a complicated array of functional effects.58–60 Although p73 isoforms have been implicated in chemosensitivity of tumor cells, only very limited data on p73 exist in MM.61–63 Therefore, ongoing work is aimed at further evaluating the differential role of p73 isoforms in the context of PKC inhibition, as well as at identifying novel agents that specifically induce p73-dependent apoptosis.
The described novel role of β-catenin in drug-mediated inhibition of proliferation and survival is only seemingly contradictory to other reports on WNT signaling in MM.12–14 In contrast to physiologic conditions, under which β-catenin contributes to MM cell proliferation and survival, enzastaurin triggers an immediate and rapid accumulation of this transcription factor to highly unphysiologic levels. It may thereby change the availability of internal and external cofactors or binding partners, thereby triggering antiproliferative and antisurvival signaling cascades that are still functional, even in malignant cells.
In conclusion, our present study describes a novel mechanism by which β-catenin mediates both growth inhibition via induction of ER stress and induces apoptosis via the c-Jun/p73 pathway in MM, as well as in other tumor cells. Further exploration of these mechanisms holds promise for new treatments to improve patient outcome in MM and other malignant diseases.
Supplementary Material
Acknowledgments
The authors acknowledge the contribution of Dr P. G. Richardson and Dr R. Schlossman, as well as the patients, nursing staff, and clinical research coordinators of the Jerome Lipper Multiple Myeloma Center/Dana-Farber Cancer Institute, for their help in providing primary tumor specimens for this study.
This work was supported by a fellowship grant of the Fritz-Thyssen Foundation (M.S.R.), a Multiple Myeloma Research Foundation (MMRF) Senior Research grant award and a Dunkin Donuts Rising Stars Award (K.P.), the National Institutes of Health (grants RO CA50947, PO-1 CA78378, and P50 CA100707), and the LeBow Fund to Cure Myeloma (K.C.A.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.S.R. designed and performed research, analyzed data, and wrote the manuscript; K.P. performed and analyzed research; I.B., G.T., J.Z., T.N., and J.H.F. performed research; P.J.H., B.K.L., N.C.M., T.H., D.C., and K.C.A. analyzed data; and all authors reviewed and approved the final manuscript.
Conflict-of-interest disclosure: M.S.R., K.C.A., and K.P. are members of the myeloma advisory board of Eli Lilly and Company. B.K.L. is an employee of Eli Lilly and Company. The remaining authors declare no competing financial interests.
Correspondence: Marc S. Raab, Dana-Farber Cancer Institute, Department of Medical Oncology, 44 Binney Street, Boston, MA 02115; e-mail: marc_raab@dfci.harvard.edu; and Kenneth C. Anderson, Dana-Farber Cancer Institute, Department of Medical Oncology, 44 Binney Street, Boston, MA 02115; e-mail: kenneth_anderson@dfci.harvard.edu.
References
- 1.Graff JR, McNulty AM, Hanna KR, et al. The protein kinase Cbeta-selective inhibitor, Enzastaurin ( LY317615.HCl), suppresses signaling through the AKT pathway, induces apoptosis, and suppresses growth of human colon cancer and glioblastoma xenografts. Cancer Res. 2005;65:7462–7469. doi: 10.1158/0008-5472.CAN-05-0071. [DOI] [PubMed] [Google Scholar]
- 2.Podar K, Raab MS, Chauhan D, Anderson KC. The therapeutic role of targeting protein kinase C in solid and hematologic malignancies. Expert Opin Investig Drugs. 2007;16:1693–1707. doi: 10.1517/13543784.16.10.1693. [DOI] [PubMed] [Google Scholar]
- 3.Baumann P, Armann J, Mandl-Weber S, et al. Inhibitors of protein kinase C sensitise multiple myeloma cells to common genotoxic drugs. Eur J Haematol. 2008;80:37–45. doi: 10.1111/j.1600-0609.2007.00977.x. [DOI] [PubMed] [Google Scholar]
- 4.Podar K, Raab MS, Zhang J, et al. Targeting PKC in multiple myeloma: in vitro and in vivo effects of the novel, orally available small-molecule inhibitor enzastaurin ( LY317615.HCl). Blood. 2007;109:1669–1677. doi: 10.1182/blood-2006-08-042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizvi MA, Ghias K, Davies KM, et al. Enzastaurin ( LY317615), a protein kinase Cbeta inhibitor, inhibits the AKT pathway and induces apoptosis in multiple myeloma cell lines. Mol Cancer Ther. 2006;5:1783–1789. doi: 10.1158/1535-7163.MCT-05-0465. [DOI] [PubMed] [Google Scholar]
- 6.Neri A, Marmiroli S, Tassone P, et al. The oral PKC-beta inhibitor enzastaurin ( LY317615) suppresses signalling through the AKT pathway, inhibits proliferation and induces apoptosis in multiple myeloma cell lines. Leuk Lymphoma. :20081–20010. doi: 10.1080/10428190802078289. [DOI] [PubMed] [Google Scholar]
- 7.Moreau AS, Jia X, Ngo HT, et al. Protein kinase C inhibitor enzastaurin induces in vitro and in vivo antitumor activity in Waldenstrom macroglobulinemia. Blood. 2007;109:4964–4972. doi: 10.1182/blood-2006-10-054577. [DOI] [PubMed] [Google Scholar]
- 8.Lee KW, Kim SG, Kim HP, et al. Enzastaurin, a protein kinase C beta inhibitor, suppresses signaling through the ribosomal S6 kinase and bad pathways and induces apoptosis in human gastric cancer cells. Cancer Res. 2008;68:1916–1926. doi: 10.1158/0008-5472.CAN-07-3195. [DOI] [PubMed] [Google Scholar]
- 9.Willert K, Shibamoto S, Nusse R. Wnt-induced dephosphorylation of axin releases beta-catenin from the axin complex. Genes Dev. 1999;13:1768–1773. doi: 10.1101/gad.13.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espada J, Perez-Moreno M, Braga VM, Rodriguez-Viciana P, Cano A. H-Ras activation promotes cytoplasmic accumulation and phosphoinositide 3-OH kinase association of beta-catenin in epidermal keratinocytes. J Cell Biol. 1999;146:967–980. doi: 10.1083/jcb.146.5.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards CM, Edwards JR, Lwin ST, et al. Increasing Wnt signaling in the bone marrow microenvironment inhibits the development of myeloma bone disease and reduces tumor burden in bone in vivo. Blood. 2008;111:2833–2842. doi: 10.1182/blood-2007-03-077685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sukhdeo K, Mani M, Zhang Y, et al. Targeting the beta-catenin/TCF transcriptional complex in the treatment of multiple myeloma. Proc Natl Acad Sci U S A. 2007;104:7516–7521. doi: 10.1073/pnas.0610299104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiang YW, Walsh K, Yao L, et al. Wnts induce migration and invasion of myeloma plasma cells. Blood. 2005;106:1786–1793. doi: 10.1182/blood-2005-01-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derksen PW, Tjin E, Meijer HP, et al. Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc Natl Acad Sci U S A. 2004;101:6122–6127. doi: 10.1073/pnas.0305855101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubinfeld B, Albert I, Porfiri E, et al. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 17.Liu C, Li Y, Semenov M, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 18.Gwak J, Cho M, Gong SJ, et al. Protein-kinase-C-mediated beta-catenin phosphorylation negatively regulates the Wnt/beta-catenin pathway. J Cell Sci. 2006;119:4702–4709. doi: 10.1242/jcs.03256. [DOI] [PubMed] [Google Scholar]
- 19.Orford K, Crockett C, Jensen JP, Weissman AM, Byers SW. Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin. J Biol Chem. 1997;272:24735–24738. doi: 10.1074/jbc.272.40.24735. [DOI] [PubMed] [Google Scholar]
- 20.Morin PJ, Sparks AB, Korinek V, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 21.Korinek V, Barker N, Morin PJ, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 22.Damalas A, Ben-Ze'ev A, Simcha I, et al. Excess beta-catenin promotes accumulation of transcriptionally active p53. EMBO J. 1999;18:3054–3063. doi: 10.1093/emboj/18.11.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim K, Pang KM, Evans M, Hay ED. Overexpression of beta-catenin induces apoptosis independent of its transactivation function with LEF-1 or the involvement of major G1 cell cycle regulators. Mol Biol Cell. 2000;11:3509–3523. doi: 10.1091/mbc.11.10.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saegusa M, Hashimura M, Kuwata T, Hamano M, Okayasu I. Beta-catenin simultaneously induces activation of the p53-p21WAF1 pathway and overexpression of cyclin D1 during squamous differentiation of endometrial carcinoma cells. Am J Pathol. 2004;164:1739–1749. doi: 10.1016/s0002-9440(10)63732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Damalas A, Kahan S, Shtutman M, Ben-Ze'ev A, Oren M. Deregulated beta-catenin induces a p53- and ARF-dependent growth arrest and cooperates with Ras in transformation. EMBO J. 2001;20:4912–4922. doi: 10.1093/emboj/20.17.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patil C, Walter P. Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr Opin Cell Biol. 2001;13:349–355. doi: 10.1016/s0955-0674(00)00219-2. [DOI] [PubMed] [Google Scholar]
- 27.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 28.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 29.Rahmani M, Davis EM, Crabtree TR, et al. The kinase inhibitor sorafenib induces cell death through a process involving induction of endoplasmic reticulum stress. Mol Cell Biol. 2007;27:5499–5513. doi: 10.1128/MCB.01080-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim R, Emi M, Tanabe K, Murakami S. Role of the unfolded protein response in cell death. Apoptosis. 2006;11:5–13. doi: 10.1007/s10495-005-3088-0. [DOI] [PubMed] [Google Scholar]
- 31.Meister S, Schubert U, Neubert K, et al. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res. 2007;67:1783–1792. doi: 10.1158/0008-5472.CAN-06-2258. [DOI] [PubMed] [Google Scholar]
- 32.Obeng EA, Carlson LM, Gutman DM, et al. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107:4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davenport EL, Moore HE, Dunlop AS, et al. Heat shock protein inhibition is associated with activation of the unfolded protein response pathway in myeloma plasma cells. Blood. 2007;110:2641–2649. doi: 10.1182/blood-2006-11-053728. [DOI] [PubMed] [Google Scholar]
- 34.Jochum W, Passegue E, Wagner EF. AP-1 in mouse development and tumorigenesis. Oncogene. 2001;20:2401–2412. doi: 10.1038/sj.onc.1204389. [DOI] [PubMed] [Google Scholar]
- 35.Vogt PK. Jun, the oncoprotein. Oncogene. 2001;20:2365–2377. doi: 10.1038/sj.onc.1204443. [DOI] [PubMed] [Google Scholar]
- 36.Bossy-Wetzel E, Bakiri L, Yaniv M. Induction of apoptosis by the transcription factor c-Jun. EMBO J. 1997;16:1695–1709. doi: 10.1093/emboj/16.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Podar K, Raab MS, Tonon G, et al. Up-regulation of c-Jun inhibits proliferation and induces apoptosis via caspase-triggered c-Abl cleavage in human multiple myeloma. Cancer Res. 2007;67:1680–1688. doi: 10.1158/0008-5472.CAN-06-1863. [DOI] [PubMed] [Google Scholar]
- 38.Whitfield J, Neame SJ, Paquet L, Bernard O, Ham J. Dominant-negative c-Jun promotes neuronal survival by reducing BIM expression and inhibiting mitochondrial cytochrome c release. Neuron. 2001;29:629–643. doi: 10.1016/s0896-6273(01)00239-2. [DOI] [PubMed] [Google Scholar]
- 39.Toh WH, Siddique MM, Boominathan L, Lin KW, Sabapathy K. c-Jun regulates the stability and activity of the p53 homologue, p73. J Biol Chem. 2004;279:44713–44722. doi: 10.1074/jbc.M407672200. [DOI] [PubMed] [Google Scholar]
- 40.Podar K, Shringarpure R, Tai YT, et al. Caveolin-1 is required for vascular endothelial growth factor-triggered multiple myeloma cell migration and is targeted by bortezomib. Cancer Res. 2004;64:7500–7506. doi: 10.1158/0008-5472.CAN-04-0124. [DOI] [PubMed] [Google Scholar]
- 41.Podar K, Tai YT, Lin BK, et al. Vascular endothelial growth factor-induced migration of multiple myeloma cells is associated with beta 1 integrin- and phosphatidylinositol 3-kinase-dependent PKC alpha activation. J Biol Chem. 2002;277:7875–7881. doi: 10.1074/jbc.M109068200. [DOI] [PubMed] [Google Scholar]
- 42.Gene Expression Omnibus. [Accessed November 11, 2008]; http://www.ncbi.nlm.nih.gov/geo/
- 43.Calvano SE, Xiao W, Richards DR, et al. A network-based analysis of systemic inflammation in humans. Nature. 2005;437:1032–1037. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- 44.van der Sanden MH, Meems H, Houweling M, Helms JB, Vaandrager AB. Induction of CCAAT/enhancer-binding protein (C/EBP)-homologous protein/growth arrest and DNA damage-inducible protein 153 expression during inhibition of phosphatidylcholine synthesis is mediated via activation of a C/EBP-activating transcription factor-responsive element. J Biol Chem. 2004;279:52007–52015. doi: 10.1074/jbc.M405577200. [DOI] [PubMed] [Google Scholar]
- 45.Chauhan D, Pandey P, Ogata A, et al. Cytochrome c-dependent and -independent induction of apoptosis in multiple myeloma cells. J Biol Chem. 1997;272:29995–29997. doi: 10.1074/jbc.272.48.29995. [DOI] [PubMed] [Google Scholar]
- 46.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 47.He TC, Sparks AB, Rago C, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 48.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 49.Nateri AS, Spencer-Dene B, Behrens A. Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature. 2005;437:281–285. doi: 10.1038/nature03914. [DOI] [PubMed] [Google Scholar]
- 50.Ghosh JC, Altieri DC. Activation of p53-dependent apoptosis by acute ablation of glycogen synthase kinase-3beta in colorectal cancer cells. Clin Cancer Res. 2005;11:4580–4588. doi: 10.1158/1078-0432.CCR-04-2624. [DOI] [PubMed] [Google Scholar]
- 51.Lee AH, Iwakoshi NN, Anderson KC, Glimcher LH. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc Natl Acad Sci U S A. 2003;100:9946–9951. doi: 10.1073/pnas.1334037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knittler MR, Dirks S, Haas IG. Molecular chaperones involved in protein degradation in the endoplasmic reticulum: quantitative interaction of the heat shock cognate protein BiP with partially folded immunoglobulin light chains that are degraded in the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1995;92:1764–1768. doi: 10.1073/pnas.92.5.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Melnick J, Dul JL, Argon Y. Sequential interaction of the chaperones BiP and GRP94 with immunoglobulin chains in the endoplasmic reticulum. Nature. 1994;370:373–375. doi: 10.1038/370373a0. [DOI] [PubMed] [Google Scholar]
- 54.Fiebiger E, Story C, Ploegh HL, Tortorella D. Visualization of the ER-to-cytosol dislocation reaction of a type I membrane protein. EMBO J. 2002;21:1041–1053. doi: 10.1093/emboj/21.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.VanSlyke JK, Musil LS. Dislocation and degradation from the ER are regulated by cytosolic stress. J Cell Biol. 2002;157:381–394. doi: 10.1083/jcb.200111045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang A, Kaghad M, Caput D, McKeon F. On the shoulders of giants: p63, p73 and the rise of p53. Trends Genet. 2002;18:90–95. doi: 10.1016/s0168-9525(02)02595-7. [DOI] [PubMed] [Google Scholar]
- 57.Grob TJ, Novak U, Maisse C, et al. Human delta Np73 regulates a dominant negative feedback loop for TAp73 and p53. Cell Death Differ. 2001;8:1213–1223. doi: 10.1038/sj.cdd.4400962. [DOI] [PubMed] [Google Scholar]
- 58.Kaghad M, Bonnet H, Yang A, et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809–819. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- 59.De Laurenzi V, Costanzo A, Barcaroli D, et al. Two new p73 splice variants, gamma and delta, with different transcriptional activity. J Exp Med. 1998;188:1763–1768. doi: 10.1084/jem.188.9.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ueda Y, Hijikata M, Takagi S, Chiba T, Shimotohno K. New p73 variants with altered C-terminal structures have varied transcriptional activities. Oncogene. 1999;18:4993–4998. doi: 10.1038/sj.onc.1202817. [DOI] [PubMed] [Google Scholar]
- 61.Lin KW, Nam SY, Toh WH, Dulloo I, Sabapathy K. Multiple stress signals induce p73beta accumulation. Neoplasia. 2004;6:546–557. doi: 10.1593/neo.04205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Irwin MS, Kondo K, Marin MC, et al. Chemosensitivity linked to p73 function. Cancer Cell. 2003;3:403–410. doi: 10.1016/s1535-6108(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 63.Gong JG, Costanzo A, Yang HQ, et al. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 1999;399:806–809. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.