Abstract
The melanocortin-1 receptor (MC1R) is a key regulator of pigmentation in mammals and is tightly linked to an increased risk of skin cancers, including melanoma, in humans. Physiologically activated by α-melanocyte stimulating hormone (αMSH), MC1R function can be antagonized by a secreted factor, agouti signal protein (ASP), which is responsible for the lighter phenotypes in mammals (including humans), and is also associated with increased risk of skin cancer. It is therefore of great interest to characterize the molecular effects elicited by those MC1R ligands. In this study, we determined the gene expression profiles of murine melan-a melanocytes treated with ASP or αMSH over a 4-day time course using genome-wide oligonucleotide microarrays. As expected, there were significant reductions in expression of numerous melanogenic proteins elicited by ASP, which correlates with its inhibition of pigmentation. ASP also unexpectedly modulated the expression of genes involved in various other cellular pathways, including glutathione synthesis and redox metabolism. Many genes up-regulated by ASP are involved in morphogenesis (especially in nervous system development), cell adhesion, and extracellular matrix-receptor interactions. Concomitantly, ASP enhanced the migratory potential and the invasiveness of melanocytic cells in vitro. These results demonstrate the role of ASP in the dedifferentiation of melanocytes, identify pigment-related genes targeted by ASP and by αMSH, and provide insights into the pleiotropic molecular effects of MC1R signaling that may function during development and may affect skin cancer risk.
Keywords: pigmentation, skin cancer
A major determinant of the pigment phenotype of the skin is the melanocortin-1 receptor (MC1R), a G protein coupled receptor (GPCR) that regulates many functional aspects of melanocytes, including their dendricity, melanogenesis, and proliferation (1, 2). MC1R function can be activated by α-melanocyte-stimulating hormone (αMSH), a POMC-derived peptide whose production in the skin (3) is increased by exposure to UV radiation (UV) via a p53-dependent mechanism (4). This interaction triggers increased melanin production (5, 6) and stimulates the repair of DNA photoproducts caused by UV (7–9), a phenomenon also induced by forskolin (10). It is therefore not surprising that MC1R polymorphisms are associated with a lighter pigment phenotype, including red hair/fair skin (11), with poor tanning responses (12) and an increased risk for melanoma and other skin cancers (13, 14).
MC1R signaling can be inhibited by the product of the agouti locus, agouti signal protein (ASP in mice, ASIP in humans), which acts as an inverse agonist of the murine Mc1r (15, 16). In mice, ASP is expressed in skin and testis and during embryogenesis. The spatiotemporal expression of ASP in skin is responsible for the agouti hair phenotype (a pheomelanic band against a dark eumelanic background) and for the pale coloration on the neck, breast, and ventral surface (17). A high degree of polymorphism in the promoter of the agouti gene (18) and epigenetic modifications (19) result in a wide diversity of mutant phenotypes, ranging from all black (a/a) to a uniform yellow coat (Ay/a). Ubiquitous overexpression of ASP in lethal yellow mice (Ay/a) is responsible for a pleiotropic syndrome characterized by obesity, enhanced linear growth, metabolic derangements, earlier mortality, and tumorigenesis (20). The mechanism(s) by which ASP induces tumorigenesis has not been elucidated, but several models have been proposed (21–24).
ASIP is widely expressed in human tissues (skin, heart, reproductive tract, fat tissue, liver, and kidney) (25), and although its functions in humans are poorly characterized, as in mice, the agouti locus regulates human pigmentation (26–29). In light of the correlation of MC1R variants with increased skin cancer risk, and considering the tumorigenic effects of ASP, one can expect that ASP would contribute to melanoma and/or other skin cancer risk. Interestingly, 2 different groups reported an association between variants on chromosome 20q11.22, which lies in the vicinity of ASIP, and cutaneous melanoma and basal cell carcinoma (30, 31). Although further studies will be necessary to confirm whether ASIP is the causal variant, a growing body of evidence supports this hypothesis.
Therefore, it is of major interest to further characterize the role played by ASP in regulating melanocyte function and the mechanism(s) of MC1R signaling through its ligand interactions. A recent expression profiling study was performed in the skin of mice carrying a loss of function mutation for Mc1r (Mc1re/e) after UV irradiation and identified a set of Mc1r-dependent genes involved in the cell cycle and oncogenesis (32). Earlier, Voisey et al. reported the effects of ASIP on human melanoma cells transfected to over-express ASIP (33). Only 5 new targets of ASIP were identified which may be due, at least in part, to the A2058 melanoma cells used in their study, which express low levels of MC1R (34), or the efficacy of ASP secretion, which was not reported. In this study, we used oligonucleotide microarray technology to identify previously undescribed downstream targets of ASP and αMSH in melanocytes over a 4-day time course.
ASP down-regulated an impressive panel of genes encoding proteins required for the pigmentary functions of melanocytes, uncovering the dedifferentiation process that occurs, whereas αMSH stimulated the expression of those genes. Surprisingly, the gene expression profile of melanocytes treated with ASP also revealed significant increases in the expression of genes involved in developmental and cell adhesion processes. Thus, the database generated in this study is useful not only to identify targets involved in melanogenesis but also to provide insights of the role of ASP in other cellular functions and thus a better understanding of MC1R signaling.
Results and Discussion
Overview of Transcriptome Changes Induced by ASP or αMSH.
To elucidate molecular mechanisms controlled by Mc1r, we undertook gene expression profiling of melanocytes cultured in the presence of ASP or αMSH for 3 h, 1 d, 2 d, 3 d, or 4 d. Gene expression levels in ASP- or αMSH-treated cells relative to untreated controls were determined using microarrays that contained a total of 38,784 oligos targeting murine genes. Transcripts were selected as significantly altered when the average ratio calculated from the 6 replicates per time point was ≥1.5 (false discovery rate, <1%) for at least 1 time point out of the 5 (the entire DNA microarray database is deposited at www.ncbi.nlm.nih.gov/geo, GSE 14089). A total of 1,487 and 255 unique probes were significantly modulated by ASP or by αMSH, respectively (Fig. 1A). Even though fewer genes were significantly altered by αMSH compared with ASP (Fig. S1), both ligands had overall opposite effects, as highlighted by principal component analysis (PCA) mapping (Fig. 1B), by heat map clustering of the complete set of altered genes (Fig. 1C), or by comparing genes significantly and inversely regulated by both ligands (Fig. S2).
Fig. 1.
Overview of transcriptome changes after treatment of melanocytes with ASP or αMSH. The microarray experimental design was based on competitive hybridizations of ASP- or αMSH-treated vs. untreated melanocytes after 3 h, 1 d, 2 d, 3 d, and 4 d of treatment. (A) Number of genes differentially altered in response to ASP or αMSH by at least 1.5-fold for at least 1 time point of the 5 assessed. (B) PCA analysis of 1,715 differentially expressed genes after treatment with ASP or αMSH (P < 0.01 and 1.5-fold at any 1 time point) for which data were present for all time points. Projection of the 3 principal components covering the highest variance (84% of the total variance) shows drug-dependent variation along PC#1 (70.7%), whereas time-dependent changes are observed along PC#2 (7.7%) and PC#3 (5.5%). (C) Hierarchical clustering of 1,638 genes altered either by ASP or by αMSH using an average linkage algorithm, underscores the overall inverse regulation of the genes by αMSH relative to ASP. Distance metric is 1 correlation. Horizontal stripes represent genes, and columns show experimental samples. Clustering was performed only on genes, whereas samples were ordered by drug and time. Logarithmic values of treated to untreated expression ratios are shown in the heat map using red and green color codes for up- and down-regulation, respectively. Black indicates no change, and gray represents missing data.
The database for annotation, visualization, and integrated discovery (DAVID), which is based on Gene Ontology molecular function (MF), biological process (BP), cellular component (CC), and KEGG pathways, was then used to identify enriched function-related gene groups characterizing the different datasets generated. Data mining analysis of genes significantly altered by αMSH (271 unique IDs) or commonly altered by both ASP and αMSH (110 unique IDs) led to similar functional categories compared with the ASP signature (see Table S1 for a list of genes).
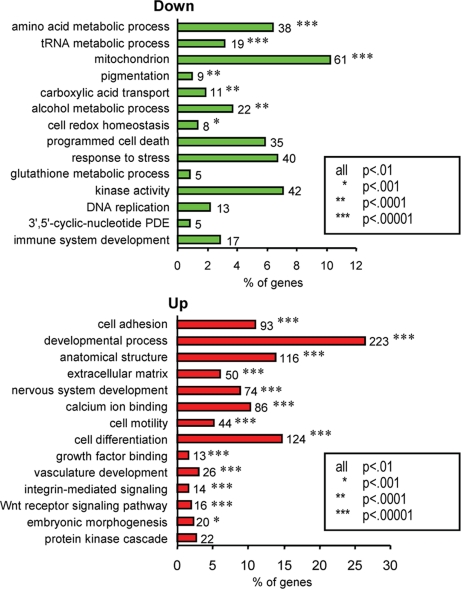
The majority of genes differentially expressed in ASP-treated cells were regulated in a similar manner within most of the identified categories, except for apoptosis, cell proliferation, calcium ion binding, MAPK signaling, kinase activity, and cyclic-nucleotide phosphodiesterase activity, where variable numbers of genes were up- or down-regulated. Therefore, we report in Fig. 2 the enriched functional groups of genes altered by ASP, within each of the 2 sets of up- or down-regulated genes. Genes down-regulated by ASP included those involved in biosynthesis, e.g., pigmentation, translation, transporters, alcohol metabolism, DNA replication, GSH anabolism, and mitochondrial redox abilities. However, genes up-regulated by ASP had a significant enrichment in differentiation and developmental biological processes, in cell adhesion and motility, and in extracellular matrix–receptor interactions.
Fig. 2.
Summary of the functional categories of genes significantly enriched in response to ASP. Analyses were performed individually on 609 and 878 significantly (P < 0.01) down- or up-regulated genes by ASP, respectively, using DAVID. All GO groups demonstrated enhanced statistical representation (EASE score value <0.01). Bars represent the proportion of genes involved by each category, for which statistical significance and number of genes is indicated. For complete breakdown of significantly enriched gene ontology groups, see Table S1.
ASP Down-Regulates Pigment-Related Genes and Their Encoded Proteins.
Given the primary role of Mc1r in controlling pigmentation and melanocyte function (35–37), we initially validated the microarray data by characterizing the effects of ASP on known pigment-specific genes. Among the 279 mapped loci known to influence color in mice (38), 113 have been cloned to date, and 24 of those are significantly altered by ASP in melanocytes (Table 1). Mitf, Tyr, Tyrp1, Dct, and Si were down-regulated, whereas Tcf4 was up-regulated. Previous studies reporting a similar effect of ASP on those genes (15, 16, 39, 40) were consistent with our data. We identified 16 other pigment-related genes whose transcription was repressed by ASP and 2 transcription factors which were up-regulated (Lef1 and Tcfap2a/Ap2a).
Table 1.
Genes encoded by coat-color loci that are significantly altered by ASP
| Gene | Mutant Phenotype | Function | ASP 3 h P value | ASP 1 d P value | ASP 2 d P value | ASP 3 d P value | ASP 4 d P value |
|---|---|---|---|---|---|---|---|
| Mitf, microphthalmia transcription factor | Small eyes, white hair | Melanocyte development | −1.13 < 0.01 | −0.06 0.74 | −0.35 0.10 | 0.04 0.67 | −0.10 0.41 |
| Sfxn1, sideroflexin | Flexed tail | Melanocyte development | −0.01 0.93 | −0.14 0.36 | −0.47 0.10 | −0.96 < 0.01 | −0.68 0.06 |
| Gpnmb, Glycoprotein | Iris pigment dispersion | Melanosome component | −0.18 0.32 | −0.76 < 0.01 | −1.16 < 0.01 | −1.37 < 0.01 | −1.16 < 0.01 |
| Rab38, RAS oncogene family | Chocolate hair | Melanosome component | 0.16 0.32 | −0.43 0.01 | −0.47 0.04 | −0.79 < 0.01 | −0.76 < 0.01 |
| Matp, membrane-associated transporter | White hair | Melanosome component | −0.33 0.15 | −1.15 < 0.01 | −0.79 < 0.01 | −1.33 < 0.01 | −1.06 < 0.01 |
| Pmel17, silver protein | Silver hair | Melanosome component | −0.23 0.18 | −0.90 < 0.01 | −0.89 < 0.01 | −1.40 < 0.01 | −1.13 < 0.01 |
| Slc24a5, solute carrier family 24 # 5 | Golden hair | Melanosome component | −0.01 0.94 | −1.01 < 0.01 | −1.10 < 0.01 | −1.45 < 0.01 | −1.32 < 0.01 |
| Tyr, tyrosinase | White hair | Melanosome component | −0.32 0.05 | −0.56 < 0.01 | −0.68 < 0.01 | −0.82 < 0.01 | −1.18 < 0.01 |
| Tyrp1, tyrosinase-related protein | Brown hair | Melanosome component | −0.12 0.53 | −0.66 < 0.01 | −0.65 < 0.01 | −0.91 < 0.01 | −0.68 0.03 |
| Tyrp2/Dct, tyrosinase-related protein 2 | Gray hair | Melanosome component | −0.16 0.31 | −1.21 < 0.01 | −0.99 < 0.01 | −1.39 < 0.01 | −1.06 < 0.01 |
| Ggt1, γ-glutamyltranspeptidase 1 | Melanin synthesis/switch | −0.14 0.14 | −0.19 0.22 | −0.43 0.03 | −0.60 < 0.01 | −0.27 0.12 | |
| Slc7a11, solute carrier family 7 #11 | Subtle gray hair | Melanin synthesis/switch | −0.19 0.61 | −0.90 < 0.01 | −1.18 < 0.01 | −2.04 < 0.01 | −1.18 0.12 |
| Hps3, Hermansky-Pudlak syndrome gene 3 | Cocoa hair | Melanosome trafficking | 0.02 0.84 | −0.28 < 0.01 | −0.44 0.01 | −0.72 < 0.01 | −0.56 0.04 |
| Melan-A (Mlana, Mart1) | Melanosome trafficking | 0.16 0.50 | −0.27 0.27 | −0.72 < 0.01 | −0.81 < 0.01 | −0.61 < 0.01 | |
| Oca1, ocular albinism type 1 | Unpigmented eyes | Melanosome trafficking | 0.04 0.29 | −0.63 < 0.01 | −0.71 < 0.01 | −0.72 < 0.01 | −0.77 < 0.01 |
| Oca2, ocular albinism type 2 (P) | Pink eyes, grey hair | Melanosome trafficking | 0.09 0.51 | −0.51 0.02 | −0.75 0.04 | −0.79 < 0.01 | −0.73 < 0.01 |
| Rab27a, RAS-associated protein | Ashen hair | Melanosome transport | 0.12 0.35 | −0.40 0.03 | −0.54 0.02 | −0.92 < 0.01 | −0.65 0.04 |
| Cited1, Cbp/p300-interacting transactivator 1 | Unknown | 0.07 0.41 | −0.65 < 0.01 | −0.59 0.07 | −0.78 nd | −0.78 < 0.01 | |
| Brca1, breast cancer 1 | Unknown | 0.01 0.98 | −0.49 0.04 | −0.37 0.04 | −0.67 < 0.01 | −0.81 < 0.01 | |
| Tcfap2a, transcription factor AP-2α | Neural crest development | 0.03 0.88 | 0.00 0.98 | 0.47 0.03 | 0.70 < 0.01 | 1.01 0.10 | |
| Itf2/Tcf4, transcription factor 4 | Unknown | 0.13 0.12 | 1.20 < 0.01 | 0.83 < 0.01 | 1.23 < 0.01 | 0.97 < 0.01 |
Coat-color genes significantly modulated by ASP for at least 1 time point of the 5 assessed. Values indicate averaged logarithmic ratios to the base 2 of 6 replicates for each time point, followed by P values.
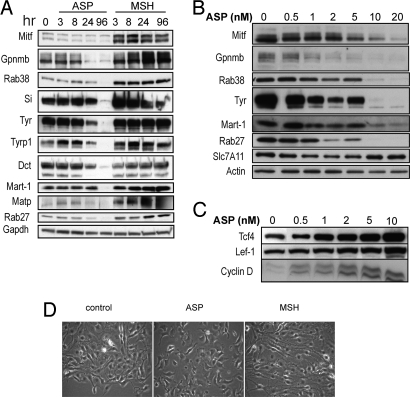
The levels of most proteins encoded by those genes were evaluated in a time- and/or dose-dependent manner where specific antibodies were available, and they confirmed the microarray results (Fig. 3). Mitf, the master melanogenic transcription factor, was significantly decreased by ASP within 3 h and remained much lower than the control at all other time points analyzed, whereas it was up-regulated by αMSH. Among the Mitf targets characterized, the melanogenic enzymes (Tyr, Tyrp1, and Dct) and other proteins participating in melanosome function (Si, Mart-1, Gpr143, and Rab27a) were significantly down-regulated by ASP, as were Met and Cdk2. The inhibition by ASP of other pigment genes, including Rab38, P and Gpnmb, raises interesting speculation concerning their possible regulation by Mitf.
Fig. 3.
Effects of ASP and αMSH on pigment-related protein expression levels. The ASP-induced down-regulation of gene products known to play roles in melanogenesis were assessed by Western blot analysis to confirm time- (A) and dose-dependent (after 3 d of treatment) (B) effects of ASP on Mitf, Dct, Tyr, Tyrp1, Gpnmb, Rab38, Slc7A11, Matp, Mart-1, Rab27a, and Si. The time-dependent effects of αMSH were analyzed and showed the up-regulation of the same genes compared with ASP. (C) Modulation of components of the Wnt pathway in response to ASP. Immunoblotting of Tcf4, cyclin-D, and Lef-1 showing the dose-dependent increase at the protein level of selected targets found up-regulated in the microarray dataset. (D) Effects of ASP on cell morphology. αMSH induced a more dendritic phenotype compared with control, whereas ASP modulated the shape of melanocytes toward a melanoblast-like shape [20x magnification after 4 d treatment with ASP (10 nM) or with αMSH (100 nM)].
A growing number of solute carrier (Slc) genes have been identified that play important roles in regulating mammalian pigmentation and in determining skin color (41–44). Several recent studies are in agreement with the inhibitory role of ASP on many of the Slc genes that were differentially expressed. Newton et al. reported the up-regulation of MATP by NDP-MSH, which is abolished in melanocytes expressing RHC variants for MC1R (45). Slc24a5, also regulates melanogenesis (42, 46) and is down-regulated in yellow quails overexpressing agouti (47). In addition to the inhibitory effects of ASP on proteins related to melanosome maturation and function, ASP also modulated genes encoding proteins involved in melanosome transport, such as Rab27a, Rab38, and Hps3.
It is tempting to speculate that most melanosomal proteins implicated in melanin production are also likely down-regulated by ASP. Thus, we report in Tables S2 and S3 genes differentially expressed in response to ASP that encode melanosomal proteins identified by proteomics (48). For instance, Slc24a4 was consistently decreased by ASP and up-regulated by αMSH and thus appears to be a strong candidate to regulate melanogenesis. Indeed, 2 genome-wide association studies (44, 49) have recently linked this gene with human hair and skin pigmentation.
Therefore, ASP alters the basic function of melanocytes by inhibiting the expression of factors involved in various steps of the pigmentary cascade, i.e., melanosome biogenesis and maturation, pigment synthesis, and intracellular transport. The effects of ASP observed on coat color genes are Mc1r-dependent, because the products of those genes were not down-regulated by ASP in melanocytes carrying a loss-of-function mutation at the Mc1r locus (Mc1re/e) (data not shown). Concomitantly, ASP severely altered melanin synthesis within 1 d in melan-a melanocytes (35) and elicited noticeable morphological changes (Fig. 3C). Overall, these data confirm the inhibitory effect of ASP on melanocyte differentiation, a phenomenon observed in murine melanoblasts (39, 50). Thus, the activation of MC1R by αMSH stimulates the differentiation of melanocytes to augment their biological functions whereas ASP turns off this process, reversing Mc1r signaling and triggering melanocyte dedifferentiation.
Potential Effects of ASP on Intracellular Signaling Pathways, Redox Function, and DNA Repair.
Interestingly, among the genes regulated by ASP were a number of intermediates in the MAPK pathway, the majority of them being up-regulated, whereas their inhibitors [such as the dual specificity protein phosphatases (DUSP)] were down-regulated. The up-regulation of MAPK signaling is known to influence the coordinated loss of expression of several melanocytic antigens in melanoma cells (51). Our analysis also revealed significant increases in expression of genes belonging to the Wnt signaling pathway (Table S3) in response to ASP. Among those genes, we used immunoblotting to confirm the up-regulation of Tcf4, Lef-1, and cyclin D (Fig. 3). This induction of Wnt-related genes by ASP is quite surprising, because Wnt has not only been shown to promote melanocytic differentiation, but also to serve as a major factor in development during embryogenesis (52–54). Tcf4 has been shown to be overexpressed in agouti mice during the transient expression of ASP and to be induced by ASP in melanocytes, where it acts as a negative transcriptional regulator of Mitf, Tyr, Tyrp1, and Dct (55). Therefore, Tcf4 up-regulation seems to favor Mitf inhibition triggered by ASP, although the mechanisms of induction remain unclear.
The decrease in expression of Mitf and its downstream targets elicited by ASP may also arise at least in part from the down-regulation of the SRY HMG box Sox9 (56) and from the inactivation of PKA. The microarray dataset reveals the up-regulation by ASP of genes encoding (i) Prkar1a and Prkar1b, 2 regulatory subunits of PKA inactivating its catalytic domains; (ii) Pkia and Pkib, 2 competitive inhibitors of PKA which interact with the catalytic subunit of the enzyme (both down-regulated by αMSH); and (iii) Cri, the inhibitor of Crebbp (or its homologue P300), which is required in the transcriptional activation complex induced by PKA. The regulatory domain of PKA (RIIB) mediates the secondary effects of ASP in the lethal yellow syndrome (obesity and linear growth) (57), and the catalytic domain of PKA (Prkaca) has been associated with the shift from pheomelanin to eumelanin synthesis (58). Furthermore, the induction of Mitf and Tyr by cAMP in neural crest cells prompts their differentiation into melanocytes (59), a phenomenon mainly dependent on PKA, which further supports the concept that the inhibition of PKA signaling plays a key role in the inhibition of differentiation induced by ASP.
The altered transcription of genes related to oxidative stress and mitochondria, including ATF4, suggests that ASP affects the redox abilities of melanocytes. For instance, ASP down-regulated genes involved in synthesis (Gclc, Nags), reduction (Gsr) or transfer (Gsta1, Gsta2, Gstp1) of glutathione, which is relevant to the imbalance of thiols that correlates with ASP expression in mice (60). In light of the ability of αMSH to reduce oxidative stress and DNA damage after UV exposure (61), those previously unidentified targets become of great interest in understanding this phenomenon.
Also regulated by ASP were 12 distinct transcripts that encode proteins involved in the repair of DNA damage. BRCA1 and Ddit3 (also known as Gadd153) were down-regulated by ASP and might play important roles in the nonpigment related effects of MC1R function on DNA repair (7–9). We recently showed that treatment of reconstructed skin with forskolin, which increases cAMP and activates PKA, enhanced the expression of XPC and the repair of UV-induced DNA damage (10). Thus, identification of genes/proteins down-regulated by ASP, whose functions are related to DNA repair, are of obvious interest. In support of this concept is the down-regulation of NR4A3. Members of the NR4A family play a role in the repair of UV-induced DNA damage after stimulation of human melanocytes by a superpotent αMSH analogue (9). Further, the induction of NR4A by that MSH derivative was abrogated in RHC variants of MC1R (9).
Genes Up-Regulated by ASP: Insights into MC1R Functions?
Genes that are normally expressed during morphogenesis/organogenesis or are involved in cell adhesion/communication were significantly enriched among the transcripts up-regulated by ASP (Fig. 2 and Table S4). Examples of genes contributing to various processes during embryonic morphogenesis, including Tcfap2α, CTGF, ErbB3, Efnb2, and N-cadherin, were confirmed to be up-regulated by ASP at the protein and mRNA levels (Fig. S3). Tcfap2a is a retinoic acid-inducible gene required during neural crest development and formation of its derivatives (62) and has been implicated in cancer. The ASP-mediated induction of Tcfap2a observed in melan-a melanocytes also occurred in B16F1 murine melanoma cells, and interestingly, Tcfap2a was dramatically increased in human melanocytes carrying the mutation R160W or D294H (2 MC1R variants associated with melanoma) compared with wild-type MC1R (data not shown). ErbB3, a member of the epidermal growth factor receptor (EGFR) family implicated with causation or sustenance of various cancers, including those originating from melanocytes and from brain cells (63), was not only increased by ASP but was also decreased by αMSH.
Surprisingly, 74 of the developmental genes increased by ASP are involved in the development of the nervous system, including axon guidance molecules and genes involved in neurogenesis, such as ErbB3, Ablim1, Foxd1, Uchl1, Amigo1, and EphB2, and genes expressed during migration of neural crest cells (NCC), such as sema3f, sema3c, Nrp2, gbx2, and Efnb1. Other developmental categories include genes involved in skeletal, bone and cartilage development, such as Sox4, Sox6, and Postn, and kidney, muscle development, and vasculogenesis (Ctgf, Pdgfa, Pdgfb, Mef2c, and Efnb2).
ASP-treated melanocytes seem to have undergone a switch from their differentiated melanocytic state toward a pattern of genes displaying neuronal specification and other developmental features characteristic of their precursors, the NCC (64). In a similar manner, melanocytes isolated from quail embryos and cultured in the presence of endothelin 3, which is crucial for the development of 2 NC-derived lineages (melanocytes and enteric nerve cells), are able to revert into glia through a neural crest-derived glial-melanocytic progenitor, as elegantly shown by Dupin et al. (65). The plasticity of NCC has been well established and interestingly, the reminiscence of a multipotent phenotype is observed in the most aggressive melanoma (66). Indeed, it is thought that highly metastatic melanoma cells reexpress embryonic genes and by doing so acquire the ability to behave like their precursors did, i.e., proliferate or migrate and invade. Further, Gupta et al. showed that the metastatic proclivity of melanoma can be attributed to the expression of NCC-associated genes (like ErbB3) in human nevi before neoplastic transformation (67). The origin of melan-a melanocytes may at least partially account for the effects of ASP seen in this study, because they were derived from epidermal melanoblasts in embryos of C57BL6 mice (68) and are cultured in the presence of a tumor promoter, TPA. Therefore, they are likely to be prone to display such plasticity. However, the increased expression of developmental genes by ASP is relevant to the potential role of ASP during embryogenesis because ASP is expressed in mouse embryos starting at E10.5 in mesenchymal tissues (69, 70). The precise role of ASP during embryogenesis could be further investigated based on the data presented here.
Many of the genes increased by ASP have been implicated in various cancers, which is consistent with previous studies reporting the increased risk of induced or spontaneous tumors due to high levels of ASP in mice (21–24). In addition, the implication of MC1R in melanoma development and the suggested association between ASIP polymorphism and melanoma in humans (30) supports those findings. Interestingly, April and Barsh compared the expression levels of genes in mice carrying the recessive allele for Mc1r (Mc1re/e) with their littermates (Mc1r+/+) after UV exposure (32). Based on microarray analysis, they reported a set of Mc1r-dependent UVB-responsive genes greatly enriched for processes and functions involved in oncogenesis and the cell cycle, which supports the importance of Mc1r signaling in such events. Among those genes, Gnb4, Mmp15, S100a10, Pdlim1, and Mbnl1 are present in our dataset of genes up-regulated by ASP, and Ptgds, whose expression was lower in Mc1re/e mice compared with Mc1r+/+ mice, was decreased by ASP but increased by αMSH. Thus, genetic changes consequent to the Mc1re/e mutation or to the response of melanocytes to ASP may confer an increased susceptibility to tumor development.
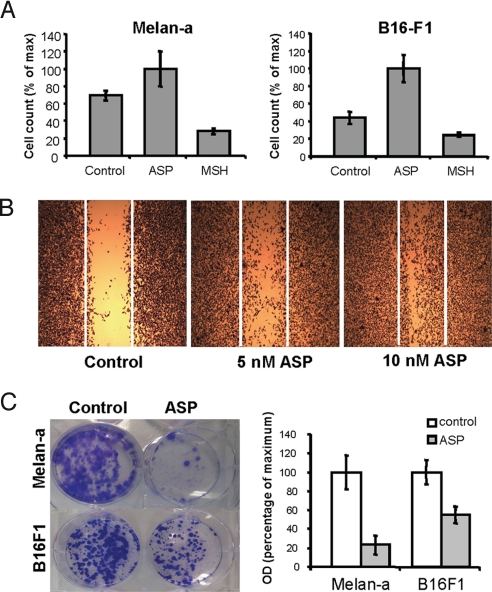
In addition, Wnt-related molecules and genes involved in ECM-receptor interactions (both critically involved in regulating differentiation and key events determining cell fate such as apoptosis, growth, and migration) were modulated by ASP. Indeed, various cell adhesion molecules like integrins, protocadherins, ECM components, and metallopeptidases were mostly up-regulated (Table S4). The enhanced expression of N-cadherin and Mel-Cam, known to be overexpressed in melanoma cells, and of the focal adhesion kinase 2 (Pyk2/Fak2), was confirmed in melan-a melanocytes (Fig. S3). These findings prompted us to investigate whether ASP affected cell migration. Indeed, treatment with ASP significantly enhanced the migration of melan-a melanocytes and of B16-F1 murine melanoma cells (Fig. 4 A and B) and their invasion through an artificial matrix (Fig. S4). In contrast, αMSH had the opposite effect on cell migration. ASP induced the directional migration of B16-F1 cells compared with untreated cells in a dose-dependent manner (Fig. 4B). The significant decrease in Mitf level in ASP-treated cells is relevant to the increased invasiveness of low Mitf melanoma cells (71). Indeed, low Mitf function can lead to a p27-dependent cell cycle arrest and increased ROCK-dependent invasiveness (72). Similarly, ASP inhibits cell proliferation, as shown in prior studies, that we observed as well (Fig. 4C), and increased invasiveness (Fig. S4).
Fig. 4.
ASP increases cell migration. (A) Chemotactic migration in Transwell Boyden-chamber assay. Melan-a and B16-F1 cells were plated in the presence of 10 nM ASP, 100 nM αMSH, or were left untreated (Control) and were allowed to migrate for 8 h. Differences were statistically significant at P < 0.001. Error bars indicate standard deviation (SD). Data are representative of 3 experiments. (B) Wound-healing (scratch) assay of B16-F1 melanoma cells. Confluent B16-F1 cells were serum-deprived and treated with ASP (5 or 10 nM) for 24 h or were left untreated (Control). Once they reached confluence, a single wound was made in the center of the monolayer using a pipet tip; fresh medium containing 5% FBS with or without ASP was added, and 24 h later, cells were stained and representative micrographs were taken at 20× magnification. (C) Effect of ASP on growth of melan-a melanocytes and B16F1 melanoma cells. Melan-a and B16F1 cells were cultured for 10 and 8 days, respectively, in the presence or absence of ASP. Quantification of the cells by optic densitometry revealed a significant decrease in cell growth when treated with ASP.
We demonstrate in this study the complex effects of ASP, which triggers the dedifferentiation of melanocytes by reducing the expression of a wide range of melanogenic proteins. Numerous genes down-regulated by ASP have been shown in other studies to be up-regulated by αMSH, but those responses were abrogated in RHC-variants. Consequently, whereas functional alterations of some MC1R variants prevent the positive effects of αMSH, it seems that ASP triggers the opposite signal and thus dramatically affects the protective effects of a functional MC1R. Thus, our results provide important insights into the mechanisms of MC1R function. Concomitant with the loss of melanocyte-specific factors, ASP up-regulates genes that are typically expressed during morphogenesis, which are reactivated in cancer cells (including aggressive melanomas), and promotes cellular migration and invasiveness in vitro suggesting a role for ASP beyond regulation of pigmentation.
Experimental Procedures
Cell Culture and Treatment.
Melan-a melanocytes (68) were a gift from Dorothy C. Bennett (St. George's Hospital Medical School, London) and were cultured as detailed (35). Cells were treated for 3, 24, 48, 72, and 96 h with 100 nM αMSH (Sigma) or with 10 nM murine recombinant ASP synthesized and purified as described (35).
Total Cytoplasmic RNA Extraction and cDNA Preparation.
Total cytoplasmic RNA was isolated from melanocytes using the RNeasy Mini Kit (Qiagen) according to the manufacturer's recommendations and were retrotranscribed into cDNA using SuperScript II RNase H- Reverse transcriptase (Invitrogen).
Microarray Hybridization and Data Analysis, Principal Component Analysis (PCA), and Hierarchical Clustering.
Full details are published in SI Materials and Methods.
Immunoblotting.
Full details and the list of antibodies used are published in SI Materials and Methods.
Cell Migration/Invasion and Wound-Healing Assays.
Full details are published in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Dr. Chandramouli Gadisetti for helpful advice regarding the statistical analyses of the microarrays and Drs. Heinz Arnheiter and Rainer Schmidt for very helpful discussions and suggestions. This work was supported in part by the Intramural Research Program of the National Cancer Institute at National Institutes of Health. The Ap-2a antibody (2B5-c), developed by Trevor Williams, was obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the National Institute of Child Health and Human Development, and maintained by the Department of Biological Sciences, University of Iowa, Iowa City.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.C. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806753106/DCSupplemental.
References
- 1.Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445:843–850. doi: 10.1038/nature05660. [DOI] [PubMed] [Google Scholar]
- 2.Rees JL. Genetics of hair and skin color. Annu Rev Genet. 2003;37:67–90. doi: 10.1146/annurev.genet.37.110801.143233. [DOI] [PubMed] [Google Scholar]
- 3.Rousseau K, et al. Proopiomelanocortin (POMC), the ACTH/melanocortin precursor, is secreted by human epidermal keratinocytes and melanocytes and stimulates melanogenesis. FASEB J. 2007;21:1844–1856. doi: 10.1096/fj.06-7398com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui R, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128:853–864. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 5.Abdel-Malek ZA, et al. Mitogenic and melanogenic stimulation of normal human melanocytes by melanotropic peptides. Proc Natl Acad Sci USA. 1995;92:1789–1793. doi: 10.1073/pnas.92.5.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunt G, Todd C, Cresswell JE, Thody AJ. α-Melanocyte stimulating hormone and its analogue Nle4DPhe7α-MSH affect morphology, tyrosinase activity and melanogenesis in cultured human melanocytes. J Cell Sci. 1994;107:205–211. doi: 10.1242/jcs.107.1.205. [DOI] [PubMed] [Google Scholar]
- 7.Bohm M, et al. α-Melanocyte-stimulating hormone protects from ultraviolet radiation-induced apoptosis and DNA damage. J Biol Chem. 2005;280:5795–5802. doi: 10.1074/jbc.M406334200. [DOI] [PubMed] [Google Scholar]
- 8.Kadekaro AL, et al. alpha-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res. 2005;65:4292–4299. doi: 10.1158/0008-5472.CAN-04-4535. [DOI] [PubMed] [Google Scholar]
- 9.Smith AG, et al. Melanocortin-1 receptor signaling markedly induces the expression of the NR4A nuclear receptor subgroup in melanocytic cells. J Biol Chem. 2008;283:12564–12570. doi: 10.1074/jbc.M800480200. [DOI] [PubMed] [Google Scholar]
- 10.Passeron T, Passeron H, Le Pape E, Hearing VJ. Forskolin protects keratinocytes from UVB-induced apoptosis by increasing DNA repair. J Invest Dermatol. 2009;129:162–166. doi: 10.1038/jid.2008.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flanagan N, et al. Pleiotropic effects of the melanocortin 1 receptor (MC1R) gene on human pigmentation. Hum Mol Genet. 2000;9:2531–2537. doi: 10.1093/hmg/9.17.2531. [DOI] [PubMed] [Google Scholar]
- 12.Healy E, et al. Melanocortin-1 receptor gene and sun sensitivity in individuals without red hair. Lancet. 2000;355:1072–1073. doi: 10.1016/S0140-6736(00)02042-0. [DOI] [PubMed] [Google Scholar]
- 13.Landi MT, et al. Science. 2006;323:521–522. [Google Scholar]
- 14.Palmer JS, et al. Melanocortin-1 receptor polymorphisms and risk of melanoma: Is the association explained solely by pigmentation phenotype? Am J Hum Genet. 2000;66:176–186. doi: 10.1086/302711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakai C, et al. Modulation of murine melanocyte function in vitro by agouti signal protein. EMBO J. 1997;16:3544–3552. doi: 10.1093/emboj/16.12.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki I, et al. Agouti signalling protein inhibits melanogenesis and the response of human melanocytes to α-melanotropin. J Invest Dermatol. 1997;108:838–842. doi: 10.1111/1523-1747.ep12292572. [DOI] [PubMed] [Google Scholar]
- 17.Vrieling H, Duhl DMJ, Millar SE, Miller KA, Barsh GS. Differences in dorsal and ventral pigmentation result from regional expression of the mouse agouti gene. Proc Natl Acad Sci USA. 1994;91:5667–5671. doi: 10.1073/pnas.91.12.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bultman SJ, Michaud EJ, Woychik RP. Molecular characterization of the mouse agouti locus. Cell. 1992;71:1195–1204. doi: 10.1016/s0092-8674(05)80067-4. [DOI] [PubMed] [Google Scholar]
- 19.Cropley JE, Suter CM, Beckman KB, Martin DI. Germ-line epigenetic modification of the murine A vy allele by nutritional supplementation. Proc Natl Acad Sci USA. 2006;103:17308–17312. doi: 10.1073/pnas.0607090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolff GL, Roberts DW, Mountjoy KG. Physiological consequences of ectopic agouti gene expression: The yellow obese mouse syndrome. Physiol Genom. 1999;1:151–163. doi: 10.1152/physiolgenomics.1999.1.3.151. [DOI] [PubMed] [Google Scholar]
- 21.Hsiao WL, et al. Differential spontaneous transformation in vitro of newly established mouse fibroblast lines carrying or lacking the viable yellow mutation (Avy) of the mouse agouti locus. Mol Carcinogenet. 1996;15:70–80. doi: 10.1002/(SICI)1098-2744(199601)15:1<70::AID-MC10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 22.Kuklin AI, et al. Liver-specific expression of the agouti gene in transgenic mice promotes liver carcinogenesis in the absence of obesity and diabetes. Mol Cancer. 2004;3:17. doi: 10.1186/1476-4598-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vlahakis GE, Heston WE. Increase of induced skin tumors in the mouse by the lethal yellow gene (Ay) J Natl Cancer Inst. 1963;31:189–195. [PubMed] [Google Scholar]
- 24.Wolff GL, et al. Tumorigenic responses to lindane in mice: potentiation by a dominant mutation. Carcinogenesis. 1987;8:1889–1897. doi: 10.1093/carcin/8.12.1889. [DOI] [PubMed] [Google Scholar]
- 25.Wilson BD, et al. Structure and function of ASP, the human homolog of the mouse agouti gene. Hum Mol Genet. 1995;4:223–230. doi: 10.1093/hmg/4.2.223. [DOI] [PubMed] [Google Scholar]
- 26.Bonilla C, et al. The 8818G allele of the agouti signaling protein (ASIP) gene is ancestral and is associated with darker skin color in African Americans. Hum Genet. 2005;116:402–406. doi: 10.1007/s00439-004-1251-2. [DOI] [PubMed] [Google Scholar]
- 27.Kanetsky PA, et al. A polymorphism in the agouti signaling protein gene is associaed with human pigmentation. Am J Hum Genet. 2002;70:770–775. doi: 10.1086/339076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sulem P, et al. Two newly identified genetic determinants of pigmentation in Europeans. Nat Genet. 2008;40:835–837. doi: 10.1038/ng.160. [DOI] [PubMed] [Google Scholar]
- 29.Voisey J, et al. A polymorphism in the agouti signalling protein (ASIP) is associated with decreased levels of mRNA. Pigment Cell Res. 2006;19:226–231. doi: 10.1111/j.1600-0749.2006.00301.x. [DOI] [PubMed] [Google Scholar]
- 30.Gudbjartsson DF, et al. ASIP and TYR pigmentation variants associate with cutaneous melanoma and basal cell carcinoma. Nat Genet. 2008;40:886–891. doi: 10.1038/ng.161. [DOI] [PubMed] [Google Scholar]
- 31.Brown KM, et al. Common sequence variants on 20q11.22 confer melanoma susceptibility. Nat Genet. 2008;40:838–840. doi: 10.1038/ng.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.April CS, Barsh GS. Distinct pigmentary and melanocortin 1 receptor-dependent components of cutaneous defense against ultraviolet radiation. PLoS Genet. 2007;3:e9. doi: 10.1371/journal.pgen.0030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voisey J, Kelly G, van Daal A. Agouti signal protein regulation in human melanoma cells. Pigment Cell Res. 2003;16:65–71. doi: 10.1034/j.1600-0749.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 34.Smith AG, et al. The human melanocortin-1 receptor locus: Analysis of transcription unit, locus polymorphism and haplotype evolution. Gene. 2001;281:81–94. doi: 10.1016/s0378-1119(01)00791-0. [DOI] [PubMed] [Google Scholar]
- 35.Le Pape E, Wakamatsu K, Ito S, Wolber R, Hearing VJ. Regulation of eumelanin and pheomelanin synthesis by MC1R ligands in melanocytes. Pigment Cell Res. 2008;21:477–486. doi: 10.1111/j.1755-148X.2008.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wakamatsu K, et al. Diversity of pigmentation in cultured human melanocytes is due to differences in the type as well as quantity of melanin. Pigment Cell Res. 2006;19:154–162. doi: 10.1111/j.1600-0749.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- 37.Rees JL. The melanocortin 1 receptor (MC1R): More than just red hair. Pigment Cell Res. 2000;13:135–140. doi: 10.1034/j.1600-0749.2000.130303.x. [DOI] [PubMed] [Google Scholar]
- 38.Bennett DC, Lamoreux ML. The color loci of mice - a genetic century. Pigment Cell Res. 2003;16:333–344. doi: 10.1034/j.1600-0749.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 39.Aberdam E, et al. Involvement of microphthalmia in the inhibition of melanocyte lineage differentiation and of melanogenesis by agouti signal protein. J Biol Chem. 1998;273:19560–19565. doi: 10.1074/jbc.273.31.19560. [DOI] [PubMed] [Google Scholar]
- 40.Furumura M, et al. Characterization of genes modulated during pheomelanogenesis using differential display. Proc Natl Acad Sci USA. 1998;95:7374–7378. doi: 10.1073/pnas.95.13.7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norton HL, et al. Genetic evidence for the convergent evolution of light skin in Europeans and East Asians. Mol Biol Evol. 2007;24:710–722. doi: 10.1093/molbev/msl203. [DOI] [PubMed] [Google Scholar]
- 42.Lamason RL, et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science. 2005;310:1782–1786. doi: 10.1126/science.1116238. [DOI] [PubMed] [Google Scholar]
- 43.Chintala S, et al. Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci USA. 2005;102:10964–10969. doi: 10.1073/pnas.0502856102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sulem P, et al. Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat Genet. 2007;39:1443–1452. doi: 10.1038/ng.2007.13. [DOI] [PubMed] [Google Scholar]
- 45.Newton RA, Roberts DW, Leonard JH, Sturm RA. Human melanocytes expressing MC1R variant alleles show impaired activation of multiple signaling pathways. Peptides. 2007;28:2387–2396. doi: 10.1016/j.peptides.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Ginger RS, et al. SLC24A5 encodes a trans-Golgi network protein with potassium-dependent sodium-calcium exchange activity that regulates human epidermal melanogenesis. J Biol Chem. 2008;283:5486–5495. doi: 10.1074/jbc.M707521200. [DOI] [PubMed] [Google Scholar]
- 47.Nadeau NJ, et al. Characterization of Japanese quail yellow as a genomic deletion upstream of the avian homolog of the mammalian ASIP (agouti) gene. Genetics. 2008;178:777–786. doi: 10.1534/genetics.107.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chi A, et al. Proteomic and bioinformatic characterization of the biogenesis and function of melanosomes. J Proteome Res. 2006;5:3135–3144. doi: 10.1021/pr060363j. [DOI] [PubMed] [Google Scholar]
- 49.Han J, et al. A genome-wide association study identifies novel alleles associated with hair color and skin pigmentation. PLoS Genet. 2008;4:e1000074. doi: 10.1371/journal.pgen.1000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sviderskaya EV, Hill SP, Balachandar D, Barsh GS, Bennett DC. Agouti signaling protein and other factors modulating differentiation and proliferation of immortal melanoblasts. Dev Dyn. 2001;221:373–379. doi: 10.1002/dvdy.1153. [DOI] [PubMed] [Google Scholar]
- 51.Kono M, et al. Role of the mitogen-activated protein kinase signaling pathway in the regulation of human melanocytic antigen expression. Mol Cancer Res. 2006;4:779–792. doi: 10.1158/1541-7786.MCR-06-0077. [DOI] [PubMed] [Google Scholar]
- 52.Christiansen JH, Coles EG, Wilkinson DG. Molecular control of neural crest formation, migration and differentiation. Curr Opin Cell Biol. 2000;12:719–724. doi: 10.1016/s0955-0674(00)00158-7. [DOI] [PubMed] [Google Scholar]
- 53.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 54.Larue L, Delmas V. The WNT/Beta-catenin pathway in melanoma. Front Biosci. 2006;11:733–742. doi: 10.2741/1831. [DOI] [PubMed] [Google Scholar]
- 55.Furumura M, et al. Involvement of ITF2 in the transcriptional regulation of melanogenic genes. J Biol Chem. 2001;276:28147–28154. doi: 10.1074/jbc.M101626200. [DOI] [PubMed] [Google Scholar]
- 56.Passeron T, et al. SOX9 is a key player in UVB-induced melanocyte differentiation and pigmentation. Proc Natl Acad Sci USA. 2007;104:13984–13989. doi: 10.1073/pnas.0705117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Czyzyk TA, Sikorski MA, Yang L, McKnight GS. Disruption of the RIIbeta subunit of PKA reverses the obesity syndrome of Agouti lethal yellow mice. Proc Natl Acad Sci USA. 2008;105:276–281. doi: 10.1073/pnas.0710607105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alizadeh A, Fitch KR, Niswender CM, McKnight GS, Barsh GS. Melanocyte-lineage expression of Cre recombinase using Mitf regulatory elements. Pigment Cell Melanom Res. 2008;21:63–69. doi: 10.1111/j.1755-148X.2007.00425.x. [DOI] [PubMed] [Google Scholar]
- 59.Ji M, Andrisani OM. High-level activation of cyclic AMP signaling attentuates bone morphogenetic protein 2-induced sympathoadrenal lineage development and promotes melanogenesis in neural crest cultures. Mol Cell Biol. 2005;25:5134–5145. doi: 10.1128/MCB.25.12.5134-5145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Granholm DE, Reese RN, Granholm NH. Agouti alleles alter cysteine and glutathione concentrations in hair follicles and serum of mice (Ay/a, AwJ/AwJ, and a/a) J Invest Dermatol. 1996;106:559–563. doi: 10.1111/1523-1747.ep12344031. [DOI] [PubMed] [Google Scholar]
- 61.Abdel-Malek ZA, Knittel JJ, Kadekaro AL, Swope VB, Starner R. The melanocortin 1 receptor and the UV response of human melanocytes - a shift in paradigm. Photochem Photobiol. 2008;84:501–508. doi: 10.1111/j.1751-1097.2008.00294.x. [DOI] [PubMed] [Google Scholar]
- 62.Knight RD, et al. lockjaw encodes a zebrafish tfap2a required for early neural crest development. Development. 2003;130:5755–5768. doi: 10.1242/dev.00575. [DOI] [PubMed] [Google Scholar]
- 63.Sithanandam G, Anderson LM. The ERBB3 receptor in cancer and cancer gene therapy. Cancer Gene Ther. 2008;15:413–448. doi: 10.1038/cgt.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Le Douarin NM, Dupin E. Multipotentiality of the neural crest. Curr Opin Genet Dev. 2003;13:529–536. doi: 10.1016/j.gde.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 65.Dupin E, Glavieux C, Vaigot P, Le Douarin NM. Endothelin 3 induces the reversion of melanocytes to glia through a neural crest-derived glial-melanocytic progenitor. Proc Natl Acad Sci USA. 2000;97:7882–7887. doi: 10.1073/pnas.97.14.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bittner M, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406:536–540. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- 67.Gupta PB, et al. The melanocyte differentiation program predisposes to metastasis after neoplastic transformation. Nat Genet. 2005;37:1047–1054. doi: 10.1038/ng1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bennett DC, Cooper PJ, Hart IR. A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumor promoter for growth. Int J Cancer. 1987;39:414–418. doi: 10.1002/ijc.2910390324. [DOI] [PubMed] [Google Scholar]
- 69.Millar SE, Miller MW, Stevens ME, Barsh GS. Expression and transgenic studies of the mouse agouti gene provide insight into the mechanisms by which mammalian coat color patterns are generated. Development. 1995;121:3223–3232. doi: 10.1242/dev.121.10.3223. [DOI] [PubMed] [Google Scholar]
- 70.Candille SI, et al. Dorsoventral patterning of the mouse coat by Tbx15. PLoS Biol. 2004;2:E3. doi: 10.1371/journal.pbio.0020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goodall J, et al. Brn-2 represses microphthalmia-associated transcription factor expression and marks a distinct subpopulation of microphthalmia-associated transcription factor-negative melanoma cells. Cancer Res. 2008;68:7788–7794. doi: 10.1158/0008-5472.CAN-08-1053. [DOI] [PubMed] [Google Scholar]
- 72.Carreira S, et al. Mitf regulation of Dia1 controls melanoma proliferation and invasiveness. Gene Dev. 2006;20:3426–3439. doi: 10.1101/gad.406406. Le Pape et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.