Abstract
Diverse cellular stress responses are linked to phosphorylation of serine 51 on the alpha subunit of translation initiation factor 2. The resultant attenuation of protein synthesis and activation of gene expression figure heavily in the adaptive response to stress, but dephosphorylation of eIF2(αP), which terminates signaling in this pathway, is less well understood. GADD34 and CReP, the products of the related mammalian genes Ppp1r15a and Ppp1r15b, can recruit phosphatase catalytic subunits of the PPP1 class to eIF2(αP), but the significance of their contribution to its dephosphorylation has not been explored systematically. Here we report that unlike Ppp1r15a mutant mice, which are superficially indistinguishable from wild type, Ppp1r15b−/− mouse embryos survive gestation but exhibit severe growth retardation and impaired erythropoiesis, and loss of both Ppp1r15 genes leads to early embryonic lethality. These loss-of-function phenotypes are rescued by a mutation, Eif2aS51A, that prevents regulated phosphorylation of eIF2α. These findings reveal that the essential process of eIF2(αP) dephosphorylation is the predominant role of PPP1R15 proteins in mammalian development.
Keywords: mouse genetics, phosphatase regulation, protein synthesis, unfolded protein response
Regulated phosphorylation of serine 51 of the alpha subunit of translation initiation factor 2 (eIF2α) attenuates rates of translation initiation and thereby protein synthesis in response to diverse stressful conditions (1). The protein kinases, PERK, GCN2, PKR, and HRI, respectively, couple the stress of protein misfolding in the endoplasmic reticulum (ER stress), amino acid deprivation, viral infection, and heme deficiency to eIF2α phosphorylation (2). The phenotypes associated with loss of these kinases are well characterized and indicate that the ability to downregulate protein synthesis favors survival of cells experiencing ER stress (3), amino acid starvation (4), or heme deficiency (5) and the survival of the host during viral invasion (6).
Dephosphorylation of eIF2(αP) is less well studied. Somatic cell genetic screens have led to the identification of 2 related genes, Ppp1r15a and Ppp1r15b, encoding the proteins GADD34 and CReP, whose overexpression promotes eIF2(αP) dephosphorylation (7, 8). Both proteins use their related C-terminal domain (of ≈200 aa) to recruit a catalytic subunit from one of several related protein phosphatase I (PPP1) isoforms to form a holophosphatase complex that can dephosphorylate eIF2(αP) in vitro (7–10). GADD34 levels are low in unstressed cells, but the Ppp1r15a/GADD34 gene is transcriptionally induced by rising levels of eIF2(αP) (7, 11, 12). GADD34 induction then correlates with the declining phase of eIF2(αP) later in the recovery phase of the stress response. Consequently, cells lacking Ppp1r15a/GADD34 phosphatase activity exhibit impaired recovery of protein synthesis (13, 14). Remarkably, basal levels of eIF2(αP) are little affected by the mutation, and apart from a measure of resistance to the lethal affects of ER stress, the Ppp1r15a/GADD34 mutant mice are superficially indistinguishable from the wild type (12). CReP, in contrast to GADD34, is constitutively expressed, and knockdown of Ppp1r15b/CReP (by siRNA) led to a mild defect in basal levels of eIF2(αP) dephosphorylation in cultured cells (8). However, until now, the significance of Ppp1r15b/CReP to mammalian physiology remained unexplored.
Although an important adaptation to a variety of stressful conditions, sustained elevation of eIF2(αP) is poorly tolerated (1, 15, 16). However, the role of dephosphorylation in protecting against the consequences of deregulated elevation in eIF2(αP) has not been studied. Though it is clear that the PPP1R15 family members GADD34 and CReP can promote eIF2(αP) dephosphorylation, neither their contribution to this process in vivo nor the potential existence of other, redundant mechanisms to control levels of eIF2(αP) have been fully explored. Furthermore, the functional importance of other activities of PPP1R15 proteins has not been addressed experimentally. Here we report on a phenotypic analysis of mice with induced mutations in Ppp1r15a and Ppp1r15b that lack functional GADD34 or CReP and compound mice lacking both genes. Our findings indicate that inadequate eIF2(αP) dephosphorylation dominates the phenotype of the mutants and that eIF2(αP) dephosphorylation is the essential function provided by the PPP1R15 family.
Results
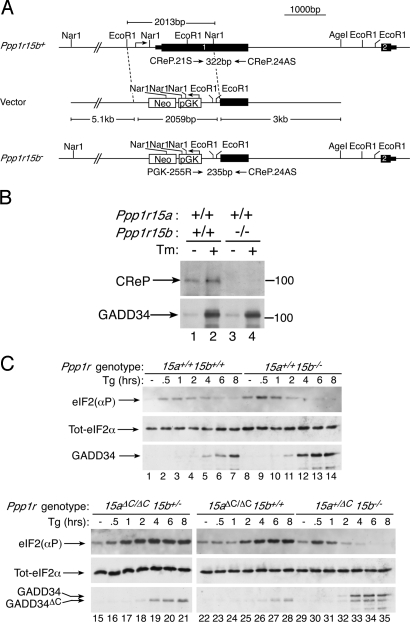
A deletion encompassing 768 bases of the promoter and the portion of exon 1 encoding the N-terminal 417 aa of the Ppp1r15b gene (which includes all of CReP's AUG codons) was created by homologous recombination in mouse embryonic stem cells, and the mutant allele was transmitted through the germline of chimeric mice (Fig. 1A). Mouse embryo fibroblasts (MEFs) derived from homozygous mutant embryos had no CReP protein detectable by sequential immunoprecipitation and immunoblotting with anti-CReP serum, consistent with nullizygosity (Fig. 1B).
Fig. 1.
Targeted deletion of Ppp1r15b, encoding CReP, increases basal and stress-induced eIF2α phosphorylation. (A) Cartoon depicting the strategy for targeted deletion of the promoter and portion of exon 1, encoding amino acids 1–416 of Ppp1r15b, by replacement with a pGK-Neo cassette. The targeting vector, wild-type (Ppp1r15b+), the targeted allele (Ppp1r15b−), and the PCR primers used to detect both alleles are depicted. (B) Immunoblots of the products of the Ppp1r15a or Ppp1r15b genes (GADD34 and CReP) immunopurified from wild-type or Ppp1r15b−/− cells that were untreated or treated with tunicamycin (Tm, 2 μg/ml) for 6 h to induce ER stress. (C) Immunoblots of phospho-eIf2α [eIF2(αP)], total eIF2α, or GADD34 from cells of the indicated Ppp1r15a (15a+/+, 15aΔC/ΔC, or 15a+/ΔC) and Ppp1r15b genotypes (15b+/+, 15b−/−, or 15b+/−) following exposure to thapsigargin (Tg 400 nM) to induce ER stress.
Basal levels of phosphorylated eIF2α, measured by immunoblot with a phospho-specific antiserum, were slightly higher in the Ppp1r15b−/− compared with wild-type cells and increased transiently in cells of both genotypes after exposure to thapsigargin, an agent that promotes ER stress and activates the eIF2α kinase PERK. In both genotypes, the declining phase of eIF2α phosphorylation coincided with the induction of GADD34 protein (Fig. 1C Upper), as previously described (7). MEFs derived from embryos homozygous for a Ppp1r15a mutation that deletes the C-terminal PPP1c-interacting domain of GADD34 (Ppp1r15aΔC) also showed a slight increase in basal levels of phosphorylated eIF2α. Unlike the wild-type and Ppp1r15b−/− MEFs, the Ppp1r15aΔC/ΔC MEFs exhibited sustained increase in phosphorylated eIF2α throughout the stress response (Fig. 1C Lower), as previously noted (13, 14). A single copy of functional Ppp1r15a was sufficient to promote the decline in levels of phosphorylated eIF2α at later time points of the stress response even in cells lacking CREP, indicating that feedback regulation of eIF2α phosphorylation in the unfolded protein response is maintained by GADD34 (Fig. 1C Lower).
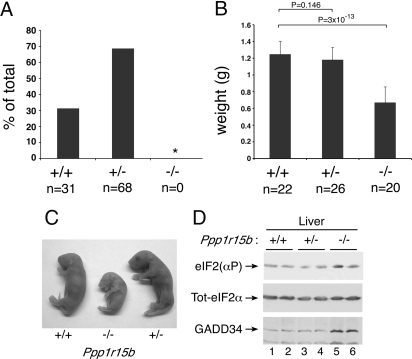
Ppp1r15b−/− embryos were recovered at the expected ratio up to the moment of birth; however, homozygous mutant newborns were about half the size of their wild-type littermates, notably pale (see next paragraph), and failed to nurse, and none survived the first day of postnatal life (Fig. 2A−C). Embryos heterozygous for the Ppp1r15b mutation were indistinguishable from wild type. Consistent with the observations made in cultured MEFs, levels of phosphorylated eIF2α were only modestly elevated in tissues of Ppp1r15b−/− embryos (data not shown), but in some tissues, such as liver, constitutively elevated levels of GADD34 protein may have compensated for CReP deficiency (Fig. 2D).
Fig. 2.
Stunted growth and perinatal lethality of Ppp1r15b−/− mice. (A) Graph depicting the distribution of genotypes found in 10-day-old pups born of Ppp1r15b+/− parents. (*Probability of finding no Ppp1r15b−/− progeny among 99 individuals by chance; P = 2.5 × 10−60.) (B) Graph depicting the mean weights + SD of e18.5 embryos conceived by Ppp1r15+/− parents. Paired two-tailed t-test results with P values are indicted above brackets. (C) Photograph of newborn Ppp1r15b+/+, Ppp1r15b+/−, and Ppp1r15b−/− mice. (D) Immunoblot of eIF2(αP), total eIF2α, and GADD34 from cytoplasmic extracts prepared from the liver of newborn mice of the indicated genotype.
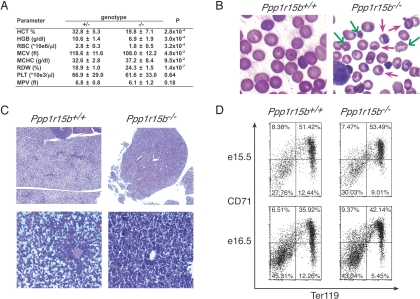
The pallor of the Ppp1r15b−/− embryos seemed well explained by low hematocrit and red blood cell count (Fig. 3A). These quantitative abnormalities in red cell mass were associated with significant qualitative abnormalities in red cell size and shape (Fig. 3 A and B), and histological examination of the liver was consistent with compensatory proliferation of blood precursors (Fig. 3C). These findings were further supported by FACS analysis, which showed a reduced percent of Ter119-positive, CD71-negative late erythroid precursors in the mutant liver (17, 18) (Fig. 3D). A similar but much milder defect had been reported previously in homozygous Ppp1r15a mutant mice (19), suggesting that an activity common to both of these homologous proteins was important to fetal erythropoiesis.
Fig. 3.
Impaired erythropoeisis in Ppp1r15b−/− mice. (A) Hematological profile of e18.5 embryos. The mean ± SD for the following parameters are displayed: HCT, hematocrit (the ratio of volume of all blood cells to whole blood); HGB, hemoglobin concentration in whole blood; RBC, red blood cell count; MCV, mean corpuscular volume; MCHC, mean corpuscular hemoglobin concentration; RDW, red blood cell distribution width (a measure of size heterogeneity); PLT, platelet count; MPV, mean platelet volume. P values for paired Student's t test comparing both genotypes are displayed. Ppp1r15b+/− (n = 10), Ppp1r15b−/− mice (n = 9). (B) Photograph of Wright's stained blood smears from e18.5 wild-type and Ppp1r15b−/− mice. Barbed arrows indicate nucleated cells; plain arrows indicate echinocytic (deformed) cells. (C) Photomicrograph of hematoxylin- and eosin-stained liver sections of e16.5 wild-type and Ppp1r15b−/− mice. (Magnification: Upper, 5×; Lower, 20×.) (D) Dual-color FACS analysis of freshly isolated fetal liver cells from Ppp1r15b+/+ and Ppp1r15b−/− e15.5 and e16.5 embryos stained with antisera to the erythroid-specific marker Ter119 and CD71 (the transferin receptor).
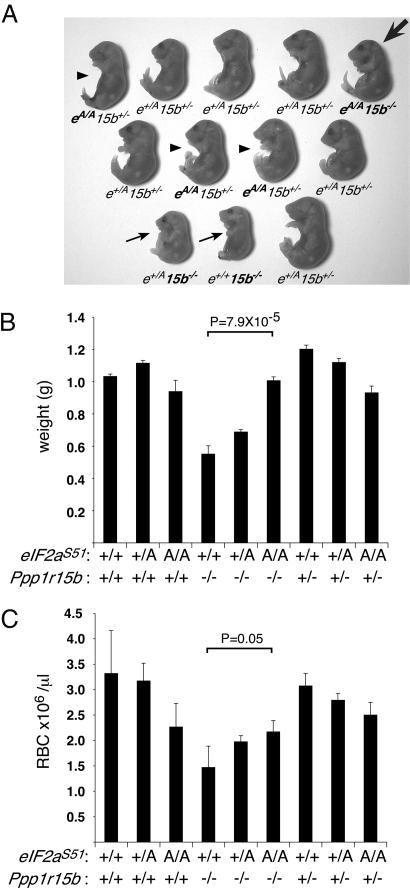
To examine the role of unmitigated eIF2(αP) levels in the phenotype of the Ppp1r15b−/− embryos, we crossed Ppp1r15b−/+ mice with mice carrying a mutant allele of Eif2a that replaces serine 51 with alanine. Although homozygosity for the Eif2aS51A allele abolishes all regulation of protein synthesis by eIF2α phosphorylation and markedly sensitizes cells to a variety of stresses, homozygous mutant Eif2aS51A embryos survive gestation and are superficially indistinguishable at birth from wild type (20). At embryonic day 18.5, Ppp1r15b−/−; Eif2aS51A/S51A progeny of the transheterozygous cross were significantly larger (P = 7.9 × 10−5, one-sided t test) than Ppp1r15b−/− embryos with a wild-type Eif2a allele and were indistinguishable from Eif2aS51A/S51A with either one or more wild-type Ppp1r15b alleles (Fig. 4 A and B). The rescue in embryo size was mirrored by restoration of red blood cell counts in the compound mutant embryos, to the level seen in the Eif2aS51A/S51A (P = 0.05, one-sided t test). The latter were noted to have a trend toward lower RBC counts than animals having at least one wild-type eIF2a allele, limiting the magnitude of the rescue attainable (Fig. 4C). Homozygous Eif2aS51A/S51A mice succumb to hypoglycemia hours after birth (20), thus precluding analysis of any postnatal features of the CReP deficiency that might be rescued in the compound mutants.
Fig. 4.
Rescue of Ppp1r15b−/− by an eIF2aS51A mutation that eliminates the phosphorylation site on eIF2α. (A) Photo micrograph of e18.5 embryos isolated from intercrosses of eIF2a+/S51A; Ppp1r15b+/− mice (e+/A; 15b+/−). Genotypes are indicated below each embryo with bold type to highlight those embryos with homozygous mutations in one or both genes. The arrowheads indicate eIF2a+/A; 15b+/−; the narrow arrows indicate eIF2a+/A; Ppp1r15b−/− or eIF2a+/+; Ppp1r15b−/−; and the bold arrow indicates the double eIF2aA/A; Ppp1r15b−/− individuals from one litter. (B) Mean weights + SD at e18.5 observed among progeny of 10 litters of e+/A; 15b+/− intercrosses. The number of individuals of each genotype scored (left to right) is n = 2, 12, 3, 3, 12, 5, 12, 19, 15. The P value for the one-sided t test assessing the rescue of the Ppp1r15b−/− weight defect by the eIF2aA/A genotype is indicated above the bracket. (C) Mean red blood cell counts + SD observed from the embryos shown in (B). The P value for the one-sided t test assessing the rescue of the Ppp1r15b−/− RBC defect by the eIF2aA/A genotype is indicated above the bracket.
The experiments discussed herein indicate that defective eIF2α dephosphorylation accounts for the conspicuous growth defect of the Ppp1r15b−/− embryo and supports a role for regulated levels of eIF2(αP) in erythropoiesis. However, reports have linked GADD34 (the better-studied member of the 2-membered PPP1R15 family) to other cellular functions (e.g., signaling by TGFβ and TSC/TOR pathways) (21−23). Therefore, we wished to exploit the conspicuous embryonic phenotype of the mutation to gain further insight into the relative importance of defective eIF2α dephosphorylation versus other proposed functions of PPP1R15 family members.
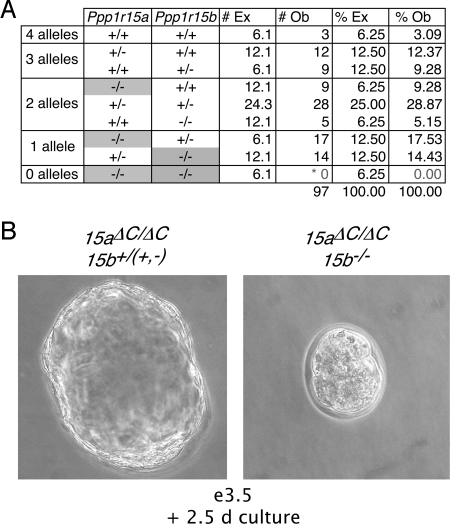
Crosses of Ppp1r15a+/ΔC; Ppp1r15b+/− transheterozygotes yielded no compound homozygous mutant embryos at e13.5 (n = 97, P = 5.78 × 10−25), consistent with early lethality of mice lacking all PPP1R15 function (Fig. 5A). Mutants carrying a single functional allele of a PPP1R15 gene displayed phenotypes similar to the single homozygous mutants such that Ppp1r15a+/ΔC; Ppp1r15b−/− embryos perish in the perinatal period and Ppp1r15aΔC/ΔC; Ppp1r15b+/− pups survive to adulthood and are fertile. The Ppp1r15aΔC/ΔC; Ppp1r15b+/− mice were intercrossed to examine the timing of embryonic lethality. Preimplantation embryos were isolated from uteri on e3.5 and cultured for 2.5 days in ES cell medium. PCR genotyping revealed that all 25 embryos that hatched were positive for the wild-type Ppp1r15b allele (P = 8.9 × 10−16), indicating that embryos lacking all PPP1R15 function failed to form a blastocyst cavity, grow, or hatch from the zona pellucida, and do not develop past the preimplantation period (Fig. 5B).
Fig. 5.
Compound homozygous Ppp1r15aΔC/ΔC; Ppp1r15b−/− embryos fail to develop past the preimplantation period. (A) Genotypes of embryos isolated at day e12.5 from matings of Ppp1r15a+/ΔC; Ppp1r15b+/− mice with the number and percent of expected (#Ex, %Ex) and observed (#Ob, %Ob) genotypes indicated. (*Probability of finding no compound Ppp1r15aΔC/ΔC; Ppp1r15b−/− progeny among 97 individuals by chance; P = 5.78 × 10−25.) (B) Photograph of e3.5 blastocysts isolated from matings of Ppp1r15aΔC/ΔC; Ppp1r15b+/− mice cultured for 2.5 days before genotyping.
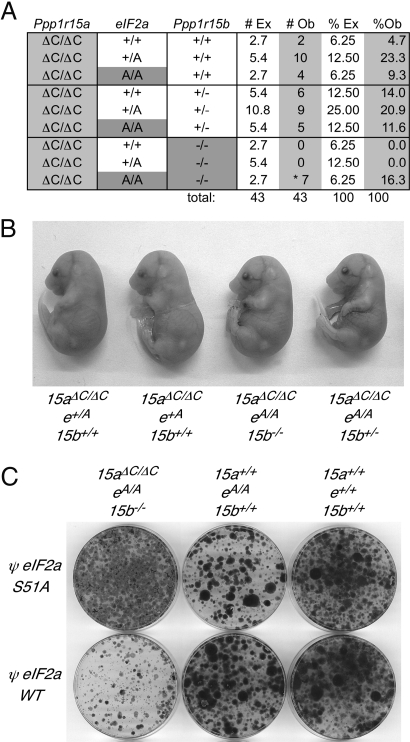
To estimate the role of defective eIF2α dephosphorylation in this lethal phenotype, we intercrossed Ppp1r15aΔC/ΔC; Ppp1r15b+/−; Eif2aS51A/+ mice and scored the genotypes in embryos late in gestation (at e17.5). With the Eif2aS51A allele in the background, compound homozygous mutant Ppp1r15aΔC/ΔC; Ppp1r15b−/− individuals were recovered. Remarkably, all 7 Ppp1r15aΔC/ΔC; Ppp1r15b−/− embryos identified were of the Eif2aS51A/S51A genotype (P = 6.1 × 10−05), indicating that the lethal phenotype of loss of PPP1R15 function can be rescued by a block in eIF2α phosphorylation (Fig. 6A). Furthermore, the compound mutant Ppp1r15b−/−; Ppp1r15aΔC/ΔC; Eif2aS51A/S51A embryos were indistinguishable in size and appearance from their littermates carrying at least one wild-type PPP1R15 allele (Fig. 6B), indicating that a mutation that prevents regulated phosphorylation of eIF2α can bypass those functions of PPP1R15 proteins that are critical to mouse embryogenesis.
Fig. 6.
Rescue of the early lethality of compound mutant Ppp1r15aΔC/ΔC; Ppp1r15b−/− embryos by the S51A mutation that eliminates the phosphorylation site on eIF2α. (A) Table of genotypes observed in e17.5 embryos isolated from intercrosses of Ppp1r15aΔC/ΔC; eIF2a+/S51A; Ppp1r15b+/− parents with the number and percent of expected (#Ex, %Ex) and observed (#Ob, %Ob) indicated. (*Probability of all 7 Ppp1r15aΔC/ΔC; Ppp1r15b−/− embryos inheriting an eIF2aA/A genotype by chance; P = 6.1 × 10−05.) (B) Photomicrograph of e17.5 embryos isolated from intercrosses of Ppp1r15aΔC/ΔC; eIF2a+/S51A; Ppp1r15b+/− parents (abbreviated 15aΔC/ΔC; e+/A; 15b+/−). Genotypes are indicated below each embryo. (C) Photograph of crystal violet-stained mouse embryonic fibroblasts of the indicated genotype 10 days after transduction with a Puror-marked retrovirus expressing either a wild-type or a S51A mutant allele of human eIF2α and selection with puromycin.
To confirm the previous observations and determine if the dephosphorylation of eIF2(αP) is an important cell-autonomous function of the PPP1R15 proteins, we procured MEFs from compound Ppp1r15b−/−; Ppp1r15aΔC/ΔC; Eif2aS51A/S51A mutant embryos, control Ppp1r15b+/+; Ppp1r15a+/+; Eif2aS51A/S51A mutant embryos, and wild-type Ppp1r15b+/+; Ppp1r15a+/+; Eif2a+/+ embryos and transduced each with either a wild-type Eif2a+ or mutant Eif2aS51A allele linked in a retroviral vector to the dominant Puror selection marker. Clonal outgrowth assays of puromycin-resistant cells transduced with either retroviral vector revealed that the mutant Ppp1r15b−/−; Ppp1r15aΔC/ΔC; Eif2aS51A/S51A cells were selectively compromised in their ability to tolerate acquisition of the wild-type (phosphorylatable) Eif2a+ allele (Fig. 6C). This observation indicates that the ability to dephosphorylate eIF2α is critical for cell viability.
Discussion
GADD34 (PPP1R15a) and CReP (PPP1R15b), which share the ability to associate with the catalytic phosphatase PPP1 subunit and repress eIF2α phosphorylation when overexpressed, have less than 22% overall identity at the amino acid level. This study proves that despite their relatively weak homology, both proteins have important overlapping functions in mouse embryogenesis, as loss of CReP renders GADD34 essential and vice versa. Furthermore, the critical function of GADD34 and CReP can be rescued by a mutation (Eif2aS51A) that prevents eIF2α phosphorylation. Together, these observations prove that eIF2(αP) dephosphorylation is the common critical function of the PPP1R15 family of phosphatase regulatory subunits in mouse embryogenesis.
The arrest of embryos lacking both PPP1r15 genes at the preimplantation stage is consistent with a role for the encoded proteins in eIF2(αP) dephosphorylation, as failure of this process would plausibly frustrate the marked increase in protein synthesis that normally occurs at the early 2- to 8-cell stage (24). This phase of embryonic development entails changes in expression of genes affecting translation initiation, which include activation of genes encoding eIF2 subunits, Ppp1r15a and Ppp1r15b, as well as repression of genes that inhibit translation, such as those encoding the eIF2α kinases PERK and GCN2 (25). The subsequent degeneration of the mutant embryos is consistent with the known role of uncontrolled eIF2α phosphorylation in promoting cell death (15, 26, 27). The latter cell-autonomous phenotype nicely explains the deleterious, dominant affect of expression of a wild-type (phosphorylatable) eIF2α allele in cells lacking both PPP1r15 genes.
Both inadequate signaling by eIF2(αP)—exemplified by the deficiency in ATF4, a translationally induced target of eIF2α phosphorylation (28), or by homozygosity for eIF2aS51A allele (Fig. 4C)—and its converse, excessive signaling by eIF2(αP) of the Ppp1r15b−/− mice (Fig. 4C), are associated with fetal anemia. Together, these findings call attention to the fact that eIF2(αP) must be regulated within a narrow range for normal fetal erythropoiesis. Nonetheless, though growth retardation often accompanies fetal anemia of similar magnitude of other causes, the uniform perinatal lethality of embryos lacking CReP is not consistently observed in such cases (28–31). Further evidence for the multifactorial basis of the growth retardation of the Ppp1r15b−/− mice is provided by the observation that whereas the rescue of the fetal anemia by homozygosity for eIF2aS51A is incomplete (presumably the rescue is capped by the defect imposed by eIF2aS51A mutation), the rescue of the growth defect is nearly complete (compare Fig. B and C). We also lack a clear view on the role of specific eIF2α kinases in promoting unsustainable levels of eIF2α phosphorylation in the mutant embryos. It is notable in this regard that the conspicuous anemia of the embryos lacking CReP is not reversed by deletion of HRI, the predominant eIF2α kinase of adult erythoblasts, or by deletion of PERK, the predominant eIF2α kinase of the adult liver parenchyma (data not shown), suggesting that the erythropoietic defect, too, might be imposed by more than one kinase.
Previous studies implicated GADD34 (the better-studied member of the PPP1R15 family) in TGFβ signaling and in regulating the activity of the tuberous sclerosis complex (TSC) (21, 23). However, the near-complete rescue of the combined GADD34 and CReP deficiency by the Eif2aS51A mutation argues against a prominent role for the PPP1R15 proteins in regulating the activity of these pathways, as the severe perturbation of mammalian development associated with deregulated TGFβ (32) or TSC activity (33–35) would not be rescued by the Eif2aS51A mutation. Furthermore, we detected no differences in the activity of S6 kinase, a downstream target of the TSC complex, between wild-type cells and those lacking both PPP1r15 genes (Fig. S1).
Though a subtle role for PPP1R15 proteins in regulating processes other than levels of eIF2(αP) could have been missed in a study reliant on detecting perturbation to mouse embryonic development, the evidence at hand does not support the previously published hints for pleiotropy in PPP1R15 protein action. In this regard, the proteins involved in eIF2(αP) dephosphorylation are similar to the known eIF2α kinases in their commitment to a simple linear pathway with a single integrating node: the phosphorylation of eIF2α on serine 51.
Though this study highlights the untoward consequences of a complete loss of the ability to dephosphorylate eIF2(αP), other experiments have indicated that more-modest increases in the levels of phosphorylated eIF2(αP) and in the activity of the downstream gene expression program may promote resistance to various stressful conditions (36, 37). Indeed, both genetic and pharmacological interventions that modestly reduce the activity of PPP1R15 family members protect cells against stress (12, 38). This study indicates that the salubrious features of partial inhibition of GADD34 and CReP are indeed mediated by their effects on levels of eIF2(αP) and not some other function. Furthermore, the evidence that the PPP1R15 proteins contribute mainly to a linear signaling pathway that hinges on levels of eIF2(αP) suggests that specific inhibitors of this class of phosphatase regulatory subunits may have narrow and predictable consequences on animals' physiology that may be cautiously exploited to useful ends.
Materials and Methods
Gene Targeting and Mouse Breeding.
The murine Ppp1r15b (CReP) gene was targeted in E14 ES cells with a positive-negative selection vector based on pTNL in which a weak PGK::neor cassette on the antisense strand replaced a 2,013-bp genomic region encompassing the proximal promoter and the part of exon 1 encoding amino acids 1–417 (which include all of the in-frame methionines of CReP). Once homologous recombination was confirmed, a short-range PCR strategy was used to detect a wild-type 322-bp fragment, derived by PCR with CReP.21S (5′ GGAACATAACCTTCTCCGGATGGAC 3′) and CReP.24AS (5′ CAGAATCAGAGCTGGCTTCCAAGTC 3′) and a mutant 235-bp fragment, derived by PCR with Neo.255R (5′ GCCTACCGGTGGATGTGGAATGTG 3′) and CReP.24AS. Germline transmission was obtained following injection of the heterozygous Ppp1r15b+/− targeted ES line 1G7. Ppp1r15aΔC (GADD34 ΔC), HRI−, and eIF2aS51A mice have been described previously (13, 20, 39). All experiments in mice were approved by the New York University Institutional Animal Care and Use Committee.
Analysis of Embryonic Phenotypes.
Postcoital day 18.5 embryos were rinsed in PBS, dabbed dry, and weighed. Blood was isolated from the carotid artery using heparinized capillary tubes diluted 1:10 and analyzed on a Cell-Dyn 4000 (Abbott Labs). Blood smears were stained with the Wright Stain Kit from Fisher Scientific. Manual red blood cell counts were done on heparinized samples diluted 1:100 using a hemocytometer. Histological analysis was performed on paraformaldehyde-fixed tissues using standard methods.
Embryos were isolated from intercrossed Ppp1r15aΔC/ΔC; Ppp1r15b+/− mice by flushing the uteri of superovulated females 3.5 days postcoitum (40). The isolated embryos were scored as having normal multicellular blastocyst morphology (i.e., a blastocoel) (25), abnormal multicellular morphology (no clear blastocoel) (14), or the granular/pebbled appearance of unfertilized eggs (29). No genotypes were obtainable from the last category, and they were discarded. The 39 multicellular embryos were cultured in ES cell medium for an additional 2.5 days and scored for hatching from the zona pellucida. All 25 blastocysts (of normal morphology at isolation) hatched, whereas none of the 14 embryos of abnormal morphology hatched. PCR genotyping revealed that all 25 embryos that hatched were positive for the wild-type allele, as was one of the nonhatched embryos.
Cell Culture and Analysis of Cellular Phenotypes.
Cell lines from Ppp1r15b+/+, Ppp1r15b−/−, Ppp1r15aΔC/ΔC, eIF2aS51A, and combined genotypes were obtained by serial passage of SV40 T-antigen transfected MEFs. The cells were cultured in DMEM supplemented with 10% FetalClone II serum (HyClone), 1× MEM nonessential amino acids, 55 μM β-mercaptoethanol, penicillin-streptomycin, and glutamine. For immunoblot analysis, cytoplasmic proteins were isolated from cell lines using detergent lysis as previously described (41).
Puromycin-resistant (Puror) retroviruses encoding wild-type and S51A mutant human eIF2α were constructed in the pBABEpuro vector and packaged in 293T cells as described. Virally transduced immortalized MEFs were subjected to 10 days of selection in puromycin (2 μg/ml), at which point the plates were fixed and stained with crystal violet.
Liver extracts were made by homogenization of fresh tissue in a Teflon-glass homogenizer in 4 volumes of extract buffer (20 mM Tris-HCl [pH 7.5], 300 mM KCl, 10 mM MgCl2, 1 mM DTT, 1 μg/ml pepstatin and aprotinin, and 1 mM PMSF) followed by clearing at 14,000 rpm. CReP from 2 mg of total protein was immunopurified using 2 μl of antiserum and protein A Sepharose. Washed immunopreciptates or 50 μg of total proteins were separated by PAGE and transferred to nitrocellulose and probed with previously described (CReP, GADD34, total eIF2α) (7, 8) or commercially available eIF2(αP) (BioSource/Invitrogen) antisera.
Statistical Analysis.
All numerical data are displayed as mean ± SD or graphed as mean + SD. Differences in the mean values between groups (Figs. 2B and 3A) were determined by paired two-tailed Student's t test. Evaluation of the significance of the rescue of the CReP phenotype by the eIF2aS51A/S51A genotype was evaluated by paired one-tailed Student's t test (Fig. 4 B and C). Calculated probabilities (P) of allele distribution among progeny (Figs. 2A, 5A, and 6A) assume Mendelian segregation of unlinked loci.
Supplementary Material
Acknowledgments.
We thank Ross Bash for the CD71 antibody, Takeshi Egawa for the pTNL vector, Tony Chin for initial analysis of eIF2α phosphorylation in CReP KO cells, and Lawrence Gardner and Gregory David (all from New York University) for reagents and helpful discussions. This work was supported by National Institutes of Health Grants DK47119 and ES08681 (to D.R.), and DK42394, HL52173, and PO1 HL057346 (to R.J.K., an investigator of the Howard Hughes Medical Institute).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809632106/DCSupplemental.
References
- 1.Hinnebusch AG. Mechanism and regulation of initiator methionyl-tRNA binding to ribosomes. In: Sonenberg N, Hershey JWB, Mathews MB, editors. Translational Control of Gene Expression. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2000. pp. 185–243. [Google Scholar]
- 2.Ron D, Harding H. eIF2a phosphorylation in cellular stress responses and disease. In: Sonenberg N, Hershey J, Mathews M, editors. Translational Control. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2007. pp. 345–368. Cold Spring Harbor Monograph Series. [Google Scholar]
- 3.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kilberg MS, Pan YX, Chen H, Leung-Pineda V. Nutritional control of gene expression: How mammalian cells respond to amino acid limitation. Annu Rev Nutr. 2005;25:59–85. doi: 10.1146/annurev.nutr.24.012003.132145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen JJ. Regulation of protein synthesis by the heme-regulated eIF2alpha kinase: Relevance to anemias. Blood. 2007;109:2693–2699. doi: 10.1182/blood-2006-08-041830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadler AJ, Williams BR. Structure and function of the protein kinase R. Curr Top Microbiol Immunol. 2007;316:253–292. doi: 10.1007/978-3-540-71329-6_13. [DOI] [PubMed] [Google Scholar]
- 7.Novoa I, Zeng H, Harding H, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J Cell Biol. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jousse C, et al. Inhibition of a constitutive translation initiation factor 2α phosphatase, CReP, promotes survival of stressed cells. J Cell Biol. 2003;163:767–775. doi: 10.1083/jcb.200308075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connor JH, Weiser DC, Li S, Hallenbeck JM, Shenolikar S. Growth arrest and DNA damage-inducible protein gadd34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol Cell Biol. 2001;21:6841–6850. doi: 10.1128/MCB.21.20.6841-6850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brush MH, Weiser DC, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol Cell Biol. 2003;23:1292–1303. doi: 10.1128/MCB.23.4.1292-1303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma Y, Hendershot LM. Delineation of a negative feedback regulatory loop that controls protein translation during endoplasmic reticulum stress. J Biol Chem. 2003;278:34864–34873. doi: 10.1074/jbc.M301107200. [DOI] [PubMed] [Google Scholar]
- 12.Marciniak SJ, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes and Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novoa I, et al. Stress-induced gene expression requires programmed recovery from translational repression. EMBO J. 2003;22:1180–1187. doi: 10.1093/emboj/cdg112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kojima E, et al. The function of GADD34 is a recovery from a shutoff of protein synthesis induced by ER stress-elucidation by GADD34-deficient mice. FASEB J. 2003;17:1573–1575. doi: 10.1096/fj.02-1184fje. [DOI] [PubMed] [Google Scholar]
- 15.Scheuner D, et al. Double-stranded RNA-dependent protein kinase phosphorylation of the α-subunit of eukaryotic translation initiation factor 2 mediates apoptosis. J Biol Chem. 2006;281:21458–21468. doi: 10.1074/jbc.M603784200. [DOI] [PubMed] [Google Scholar]
- 16.Rutkowski DT, et al. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4:e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGrath K, Palis J. Ontogeny of erythropoiesis in the mammalian embryo. Curr Top Dev Biol. 2008;82:1–22. doi: 10.1016/S0070-2153(07)00001-4. [DOI] [PubMed] [Google Scholar]
- 18.Socolovsky M, et al. Ineffective erythropoiesis in Stat5a(−/−)5b(−/−) mice due to decreased survival of early erythroblasts. Blood. 2001;98:3261–3273. doi: 10.1182/blood.v98.12.3261. [DOI] [PubMed] [Google Scholar]
- 19.Patterson AD, Hollander MC, Miller GF, Fornace AJ., Jr Gadd34 requirement for normal hemoglobin synthesis. Mol Cell Biol. 2006;26:1644–1653. doi: 10.1128/MCB.26.5.1644-1653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheuner D, et al. Translational control is required for the unfolded protein response and in-vivo glucose homeostasis. Mol Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 21.Shi W, et al. GADD34-PP1c recruited by Smad7 dephosphorylates TGFβ type I receptor. J Cell Biol. 2004;164:291–300. doi: 10.1083/jcb.200307151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yagi A, et al. GADD34 induces p53 phosphorylation and p21/WAF1 transcription. J Cell Biochem. 2003;90:1242–1249. doi: 10.1002/jcb.10711. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe R, et al. GADD34 inhibits mammalian target of rapamycin signaling via tuberous sclerosis complex and controls cell survival under bioenergetic stress. Int J Mol Med. 2007;19:475–483. [PubMed] [Google Scholar]
- 24.Schultz RM, Letourneau GE, Wassarman PM. Program of early development in the mammal: Changes in patterns and absolute rates of tubulin and total protein synthesis during oogenesis and early embryogenesis in the mouse. Dev Biol. 1979;68:341–359. doi: 10.1016/0012-1606(79)90209-4. [DOI] [PubMed] [Google Scholar]
- 25.Zeng F, Baldwin DA, Schultz RM. Transcript profiling during preimplantation mouse development. Dev Biol. 2004;272:483–496. doi: 10.1016/j.ydbio.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman RJ, Davies MV, Pathak VK, Hershey JW. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol Cell Biol. 1989;9:946–958. doi: 10.1128/mcb.9.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srivastava SP, Kumar KU, Kaufman RJ. Phosphorylation of eukaryotic translation initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA-dependent protein kinase. J Biol Chem. 1998;273:2416–2423. doi: 10.1074/jbc.273.4.2416. [DOI] [PubMed] [Google Scholar]
- 28.Masuoka HC, Townes TM. Targeted disruption of the activating transcription factor 4 gene results in severe fetal anemia in mice. Blood. 2002;99:736–745. doi: 10.1182/blood.v99.3.736. [DOI] [PubMed] [Google Scholar]
- 29.Chan JY, et al. Targeted disruption of the ubiquitous CNC-bZIP transcription factor, Nrf-1, results in anemia and embryonic lethality in mice. EMBO J. 1998;17:1779–1787. doi: 10.1093/emboj/17.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Humbert PO, et al. E2F4 is essential for normal erythrocyte maturation and neonatal viability. Mol Cell. 2000;6:281–291. doi: 10.1016/s1097-2765(00)00029-0. [DOI] [PubMed] [Google Scholar]
- 31.Socolovsky M, Fallon AE, Wang S, Brugnara C, Lodish HF. Fetal anemia and apoptosis of red cell progenitors in Stat5a−/−5b−/− mice: A direct role for Stat5 in Bcl-X(L) induction. Cell. 1999;98:181–191. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 32.Kitisin K, et al. Tgf-Beta signaling in development. Sci STKE. 2007;2007:cm1. doi: 10.1126/stke.3992007cm1. [DOI] [PubMed] [Google Scholar]
- 33.Huang J, Manning BD. The TSC1-TSC2 complex: A molecular switchboard controlling cell growth. Biochem J. 2008;412:179–190. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobayashi T, et al. Renal carcinogenesis, hepatic hemangiomatosis, and embryonic lethality caused by a germ-line Tsc2 mutation in mice. Cancer Res. 1999;59:1206–1211. [PubMed] [Google Scholar]
- 35.Kobayashi T, et al. A germ-line Tsc1 mutation causes tumor development and embryonic lethality that are similar, but not identical to, those caused by Tsc2 mutation in mice. Proc Natl Acad Sci USA. 2001;98:8762–8767. doi: 10.1073/pnas.151033798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan S, Somia N, Maher P, Schubert D. Regulation of antioxidant metabolism by translation initiation factor 2alpha. J Cell Biol. 2001;152:997–1006. doi: 10.1083/jcb.152.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu PD, et al. Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. EMBO J. 2004;23:169–179. doi: 10.1038/sj.emboj.7600030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boyce M, et al. A selective inhibitor of eIF2a dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 39.Han AP, et al. Heme-regulated eIF2alpha kinase (HRI) is required for translational regulation and survival of erythroid precursors in iron deficiency. EMBO J. 2001;20:6909–6918. doi: 10.1093/emboj/20.23.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagay A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the Mouse Embryo: A Laboratory Manual. 3rd Ed. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2003. [Google Scholar]
- 41.Harding H, et al. Diabetes mellitus and exocrine pancreatic dysfunction in Perk−/− mice reveals a role for translational control in survival of secretory cells. Mol Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.