Abstract
Many studies have shown that individuals from invasive populations of many different plant species grow larger than individuals from native populations and that this difference has a genetic basis. This increased vigor in invasive populations is thought to be due to life history tradeoffs, in which selection favors the loss of costly defense traits, thereby freeing resources that can be devoted to increased growth or fecundity. Despite the theoretical importance of such allocation shifts for invasions, there have been no efforts to understand apparent evolutionary shifts in defense-growth allocation mechanistically. Reallocation of nitrogen (N) to photosynthesis is likely to play a crucial role in any growth increase; however, no study has been conducted to explore potential evolutionary changes in N allocation of introduced plants. Here, we show that introduced Ageratina adenophora, a noxious invasive plant throughout the subtropics, appears to have evolved increased N allocation to photosynthesis (growth) and reduced allocation to cell walls, resulting in poorer structural defenses. Our results provide a potential mechanism behind the commonly observed and genetically based increase in plant growth and vigor when they are introduced to new ranges.
Keywords: defense, invasiveness
Invasive alien plants generally experience lower numbers and impacts of herbivore and parasite consumers in their introduced ranges than in their native ranges than native plants in their new ranges (1–3). Such release from enemies may allow plants to compete with reduced ecological restrictions, which in turn may promote evolutionary changes. For example, the striking competitive abilities of some invasive plants may be achieved or enhanced by evolving to reduce allocation to costly structural and chemical defenses while increasing allocation to growth or reproduction (4) - the “Evolution of Increased Competitive Ability, or EICA hypothesis (5). Evidence for increased size in invasive plants is common (5–9), but less so for the full tradeoff-based hypothesis (10–12). Despite an intense focus on comparing growth rates and herbivore responses among invasive and native populations of exotic plants, few studies have examined defense compound concentrations or relevant physiological traits (but see 6, 7, 9, 10). To our knowledge, no study has compared defense compounds and photosynthetic traits between populations of an invader in both the native and invaded ranges, and attempted to explain the increased vigor of invasive plants by an evolutionary tradeoff between allocation of nitrogen (N) to photosynthesis versus defense (cell walls). To grow faster, plants must allocate more resources, primarily N, to photosynthesis. N is one of the most important limiting resources for plant growth in nature, and most leaf N is allocated to photosynthesis. Small changes in N allocation can greatly influence light-saturated photosynthetic rate (Pmax) and photosynthetic N-use efficiency (PNUE), and therefore plant performance (13–18). Leaf N that is not allocated to photosynthesis is generally used structurally in cell walls, a component of plant defense and chemical defenses. Allocation of large proportions of N to structural cell wall toughness and chemical defense may be selected for more strongly in the native range where consumer pressure is intense, but this allocational strategy may be selected against in the absence of strong consumer selective pressure in the introduced range. Concomitantly, the absence of strong consumer selective pressure in the introduced range may favor allocation of N to growth.
Ageratina adenophora (Sprengel) R. M. King & H. Robinson [Syn. Eupatorium adenophorum, Asteraceae] is native to Mexico but a noxious invasive perennial forb in southern and south-eastern Asia, eastern Australia, New Zealand, southwestern Africa, and the United States of America (19). It spread into Yunnan Province, southwest China from Burma in the 1940s. Now it occurs in 6 provinces of southwest China and is currently spreading into northern and eastern China (20). It invades roadsides, abandoned fields, agricultural fields, pastures, and disturbed forest, and replaces native species with dense monocultures in many habitats. Individual A. adenophora plants in the field grow much taller in the invasive ranges of China and India than in the native range in Mexico (Table S1). To test the hypothesis that introduced plant species might evolve to grow larger in the context of a tradeoff between increased N allocation to photosynthesis and reduced N allocation to defenses, we compared individuals from 10 invasive populations and 5 native populations of this plant in a common garden.
Results and Discussion
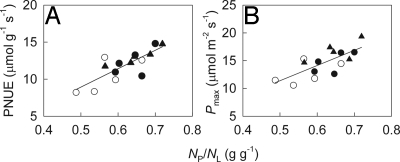
In the common garden, plants from invasive populations were significantly larger in height and diameter than plants from native populations (Table 1), which is consistent with the patterns observed in the field (Table S1). Corresponding with these differences in growth, plants from invasive populations allocated 13.0% more leaf N to photosynthesis (NP/NL), and had 24.4% higher Pmax and 20.2% higher PNUE than plants from native populations, even though total leaf N content (NL) was not significantly different among invasive and native populations (Table 1). Pmax and PNUE increased significantly with increased NP/NL (Fig. 1), indicating that the higher NP/NL of the invasive populations contributes to their higher Pmax and PNUE, and therefore, potentially to growth and invasive success (15–18).
Table 1.
Differences among populations of Ageratina adenophora originating from invasive (China and India) and native (Mexico) ranges
| Variable | Population origin |
F | ||
|---|---|---|---|---|
| Mexico | China | India | ||
| NP/NL, g·g−1 | 0.57 ± 0.029b | 0.63 ± 0.016a | 0.65 ± 0.021a | 3.58* |
| Pmax, μmol·m−2·s−1 | 12.72 ± 0.769c | 14.91 ± 0.51b | 16.63 ± 0.74a | 8.74*** |
| PNUE, μmol·g−1·s−1 | 10.39 ± 0.71b | 12.05 ± 0.47a | 13.01 ± 0.55a | 5.04** |
| NL, g·m−2 | 1.25 ± 0.04 | 1.26 ± 0.04 | 1.31 ± 0.05 | 0.58 |
| PCW, g·m−2 | 0.69 ± 0.045a | 0.23 ± 0.035b | 0.54 ± 0.083b | 16.75*** |
| PCW/PL, g·g−1 | 0.124 ± 0.010a | 0.065 ± 0.008b | 0.089 ± 0.008b | 10.80** |
| NCW/NL, g·g−1 | 0.093 ± 0.006a | 0.035 ± 0.007c | 0.064 ± 0.008b | 18.56*** |
| LMA, g·m−2 | 61.54 ± 2.11a | 50.85 ± 1.11b | 53.32 ± 1.34b | 13.27*** |
| Plant height, cm | 69.48 ± 1.03b | 79.18 ± 1.17a | 81.10 ± 1.29a | 30.851*** |
| Stem diameter, mm | 5.75 ± 0.12b | 6.45 ± 0.12a | 6.09 ± .016ab | 6.26** |
| Leaf density, kg·m−3 | 36.55 ± 0.78a | 25.68 ± 0.85c | 31.08 ± 1.00b | 40.98*** |
| Leaf length, cm | 6.18 ± 0.16c | 8.00 ± 0.10a | 6.85 ± 0.14b | 40.302*** |
| Leaf width, cm | 3.95 ± 0.10c | 5.07 ± 0.10a | 4.28 ± 0.09b | 36.002*** |
| Leaf area, cm2 | 12.92 ± 0.55c | 20.29 ± 0.60a | 15.08 ± 0.53b | 45.302*** |
Country mean value ± SE is given [five populations per country and five (10 for height and diameter) individuals per population]. Different letters in the same row indicate significant differences among countries (one-way ANOVA, Duncan test).
*, P ≤ 0.05;
**, P ≤ 0.01;
***, P ≤ 0.001.
NP/NL, the proportion of leaf nitrogen allocated to the photosynthetic machinery; Pmax, light-saturated photosynthetic rate; PNUE, photosynthetic nitrogen use efficiency; NL, leaf nitrogen content; PCW, cell wall protein content; PCW/PL, the ratio of cell wall proteins to total leaf proteins; NCW/NL, the proportion of leaf nitrogen allocated to cell walls; LMA, leaf mass per area.
Fig. 1.
Physiological traits versus the proportion of leaf nitrogen in photosynthesis (NP/NL). (A) Photosynthetic nitrogen use efficiency (PNUE) against NP/NL (P < 0.001, r = 0.798). (B) Light-saturated photosynthetic rate (Pmax) against NP/NL (P = 0.002, r = 0.729). Mean values (n = 5) are given for populations of Ageratina adenophora originating from invasive (China, closed circles and India, closed triangles) and native (Mexico, open circles) ranges.
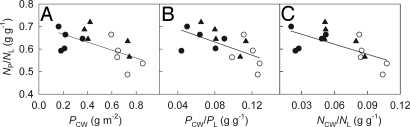
Also as expected, plants from invasive populations had 45.2% lower cell wall protein content (PCW), a 37.8% lower ratio of cell wall proteins to total leaf proteins (PCW/PL), and 46.5% lower proportions of leaf N allocated to cell walls (NCW/NL) than plants from native populations (Table 1). NP/NL increased significantly with the decrease of PCW, PCW/PL, and NCW/NL (Fig. 2), indicating that the lower PCW, PCW/PL, and NCW/NL of the invasive populations contributes to their higher NP/NL.
Fig. 2.
The proportion of leaf nitrogen in photosynthesis (NP/NL) versus measures of cell wall proteins. (A) NP/NL against cell wall protein content (PCW; P < 0.020, r = −0.593). (B) NP/NL against the ratio of cell wall proteins to total leaf proteins (PCW/PL; P < 0.014, r = −0.617). (C) NP/NL against the proportion of leaf nitrogen in cell walls (NCW/NL; P < 0.013, r = −0.624). Mean values (n = 5) are given for populations of Ageratina adenophora originating from invasive (China, closed circles and India, closed triangles) and native (Mexico, open circles) ranges.
Our study shows a tradeoff between N allocation to photosynthesis versus allocation to cell walls in invasive and native populations of a noxious invasive plant. However, the change in NCW/NL may not fully explain the variation in NP/NL as the range of variation for NCW/NL was smaller than that for NP/NL (0.04–0.13 vs. 0.54–0.68). Therefore, differences between the invasive and native populations in the proportion of leaf N allocated to defense chemicals may also contribute to their difference in NP/NL. For example, accumulation of cyanogenic glycosides decreases N allocation to photosynthesis and net assimilation rate in Eucalyptus trees (17, 21). Alkaloids have been detected in A. adenophora (22), but we did not measure N allocated to defense compounds.
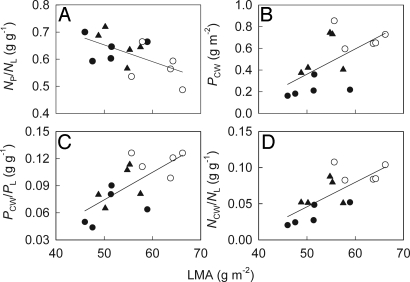
For plants in general, leaf mass per area (LMA) is highly positively correlated with cell wall mass, which accounts for 13.7–28.9% of total cell mass (13). Primary cell walls contain 0.4–2.2% N (23). We found that LMA was positively correlated with PCW, PCW/PL, and NCW/NL, but negatively with NP/NL (Fig. 3), indicating that the lower LMA of the invasive populations (Table 1) contributes to their lower PCW, PCW/PL, and NCW/NL, and therefore, to their higher NP/NL (Fig. 2). LMA is positively correlated with leaf toughness (24), which is a fundamental defensive trait for plants (12, 25). Cell wall proteins may also directly function in defense (26). Thus, invasive populations may reduce their defenses by decreasing PCW and LMA, consistent with their significantly lower leaf density and larger leaf size compared with the native populations (Table 1).
Fig. 3.
Nitrogen allocation to photosynthesis and cell walls versus leaf mass per area (LMA). (A) The proportion of leaf nitrogen in photosynthesis (NP/NL) against LMA (P < 0.020, r = −0.593). (B) Cell wall protein content (PCW) against LMA (P < 0.014, r = 0.616). (C) The ratio of cell wall proteins to total leaf proteins (PCW/PL) against LMA (P < 0.004, r = 0.698). (D) The proportion of leaf nitrogen in cell walls (NCW/NL) against LMA (P = 0.001, r = 0.744). Mean values (n = 5) are given for populations of Ageratina adenophora originating from invasive (China, closed circles and India, closed triangles) and native (Mexico, open circles) ranges.
Müller-Schärer et al. (27) argued that the most prominent change experienced by introduced plants in terms of natural enemies is a shift in the composition toward an assemblage that is dominated by generalists rather than specialists. They therefore proposed a refinement of the EICA hypothesis in which predictions about qualitative (generally toxic secondary compounds) and quantitative (generally digestion-inhibiting structural compounds) are explicit. Only a few studies of qualitative defenses have found significant direct (allocation) costs (28), whereas quantitative defenses appear to incur much higher costs because they constrain the inherent relative growth rate of plants (29). In China, one of the invasive ranges of A. adenophora, field surveys found an absence of specialists on A. adenophora (with the exception of a galling insect, Procecidochares utilis Stone, which was introduced into China in 1984), found that virtually no native generalists attack the plant. In the context of the ideas of Müller-Schärer et al. (27), inexpensive qualitative defenses may keep local generalist consumers at bay, whereas the absence of leaf-attacking specialists may lead to a decrease in costly quantitative defenses, as indicated by decreased LMA, PCW, and leaf density for plants from invasive populations of A. adenophora.
Our results suggest that plants from introduced populations of A. adenophora may have experienced selection for increased N allocation to photosynthesis and reduced N allocation to defenses (cell walls), contributing to the species' invasive success by selecting for genotypes with high specific leaf area, photosynthetic rate, and N-use efficiency. These results provide a mechanistic basis for the tradeoff between growth and defense, contribute to understanding the differences in the effects of specialist and generalist insects on invasions, and help to explain why studies of the evolution of greater size and competitive ability in invasive plants vary so much in their conclusions.
Materials and Methods
Materials.
Seeds of A. adenophora were collected in 2006 from 5 populations in its native range Mexico and 5 populations in each of 2 invasive ranges, China and India (Table S1). The detailed physiological comparisons between the invasive and native populations limited our ability to work with larger numbers of populations, and our low replication of native populations is an important caveat for our interpretation of evolved biogeographic differences. For example, a low population sample size raises the probability of founder effects rather than evolved differences. For each population, seeds were collected from a minimum of 15 individuals, at least 20 m apart from one another, and mixed. The seeds were germinated in a seedbed in December 2006 and in February 2007, when the seedlings were ≈10 cm tall, 300 similar-sized seedlings (20 per population) were transplanted to five 2 m × 2 m plots established at an open site in Xishuangbanna Tropical Botanical Garden (21°56′ N, 101°15′ E) of the Chinese Academy of Sciences located in Mengla County, Yunnan Province, southwest China. Each plot contained 4 seedlings per population. No water, fertilizer, or pesticides were added to the plots during the experiment. In this Garden, the mean annual temperature is 21.7 °C, the mean temperature of the hottest month (July) is 25.3 °C, and 15.6 °C during the coolest month (January). The mean annual precipitation is 1557 mm with a dry period lasting from November until April.
Measurements.
Two months after transplanting, length, width, and area of the 4th leaf from the tip of the shoot were measured with a Li-3000C leaf area meter (Li-Cor) on 5 plants per population, 1 per each plot. Then each leaf was oven-dried at 60 °C for 48 h and weighed, and density was calculated as the ratio of leaf mass to the product of leaf area and thickness. Three months after transplanting, plant height and diameter were measured on 10 plants per population, 2 per each plot. In October 2007, leaf N content and N allocation to photosynthesis and cell walls were measured on fully expanded leaves of 5 plants per population, 1 per each plot.
Under saturated photosynthetic photon flux density (PPFD), photosynthetic rate was measured at 380, 300, 260, 220, 180, 140, 110, 80, 50, and 0 μmol·mol−1 CO2 in the reference chamber with a Li-6400 Portable Photosynthesis System (Li-Cor). Relative humidity of the air in the leaf chamber was controlled at ≈70% and leaf temperature at 25 °C. The constant values of photosynthetic rate and intercellular CO2 concentration of each sample leaf were recorded after 200 s under each PPFD and CO2 step. Pmax was the value measured at 380 μmol·mol−1 CO2 and 2,000 μmol·m−2·s−1 PPFD. Afterward, light- and CO2-saturated photosynthetic rate was measured after 500 s under 2,000 μmol·m−2·s−1 PPFD and 1,500 μmol·mol−1 CO2. Before measurement, each sample leaf was illuminated with a saturating level of PPFD provided by the LED light source of the equipment for 5–20 min to achieve fully photosynthetic induction. No photoinhibition occurred during the measurements.
Leaf discs were taken from each sample leaf and oven-dried at 60 °C for 48 h. Leaf mass per area (LMA) was calculated as the ratio of leaf mass to area. Leaf N content was determined with a Vario MAX CN Element Analyser (Elementar Analysensysteme GmbH). Leaf chlorophyll content was measured with chemical methods (30). The same leaf of each sample plant was used, if possible, for measurements of photosynthesis, chlorophyll, LMA, and N content (NL). With the data for photosynthesis, chlorophyll, and NL, N allocation to photosynthesis was calculated using the methods described in references 15–18. PNUE was calculated as the ratio of Pmax to NL.
Leaf proteins can be divided into water-soluble, detergent-soluble, and detergent-insoluble fractions. Water-soluble proteins include soluble enzymes such as Rubisco; the detergent-soluble fraction includes membrane-associated proteins such as enzymes and electron transport; and the detergent-insoluble fraction measures the proteins in cell walls, which contribute to leaf toughness (14). The contents of water-soluble, detergent-soluble, and detergent-insoluble (cell wall proteins, PCW) fractions were determined using another leaf from each sample plant (13, 14). Total leaf protein content (PL) was calculated as the sum of the contents of the 3 protein fractions; the ratio of cell wall proteins to total leaf proteins (PCW/PL) was also calculated. N content in cell walls (NCW) was calculated from PCW with the conversion coefficient (0.16 g·N·g−1 wall proteins); the proportion of leaf N allocated to cell walls was calculated as NCW/NL.
Statistical Analyses.
A primary reason for collecting seeds in India and including them in the common garden in China was to deal with the potential of the invasive Chinese populations to perform better than the Mexican populations (native range) due to local acclimation or adaptation over the 60 years since invasion, rather than adaptation due to invasion per se. Plants from India were also invasive but could not have locally adapted to China. If the invasive Indian populations performed better than the Mexican populations, the potential of local adaptation to confound the effects of invasion would be reduced. Differences among the populations originating from Mexico, China, and India in the variables evaluated in this study were analyzed with a 1-way ANOVA followed by a Duncan's test to differentiate between each of the 3 regions. The significance of the correlation between each pair of variables in Figs. 1–3 was tested with a Pearson correlation (two-tailed). All analyses were carried out using SPPS 13.0 (SPSS Inc).
Supplementary Material
Acknowledgments.
This work was supported by Project of National Natural Science Foundation of China Grants 30830027 and 30670394, Applied Basic Study Project of Yunnan Province Grant 2007C108M, Key Project of Knowledge Innovation Engineering of the Chinese Academy of Sciences Grant KSCX1-SW-13-0X-0X, the University of Delhi, and the National Science Foundation and the Office of Sponsored Research at the University of Montana (R.M.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808434106/DCSupplemental.
References
- 1.Keane RM, Crawley MJ. Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol. 2002;16:199–204. [Google Scholar]
- 2.Mitchell CE, Power AG. Release of invasive plants from fungal and viral pathogens. Nature. 2003;421:625–627. doi: 10.1038/nature01317. [DOI] [PubMed] [Google Scholar]
- 3.Liu H, Stiling P. Testing the enemy release hypothesis: A review and meta-analysis. Biol Inv. 2006;8:1535–1545. [Google Scholar]
- 4.Herms DA, Mattson WJ. The dilemma of plants: To growth or to defend? Q Rev Biol. 2002;67:283–335. [Google Scholar]
- 5.Blossey B, Nötzold R. Evolution of increased competitive ability in invasive non-indigenous plants: A hypothesis. J Ecol. 1995;83:887–889. [Google Scholar]
- 6.Siemann E, Rogers WE. Genetic differences in growth of an invasive tree species. Ecol Lett. 2001;4:514–518. [Google Scholar]
- 7.Joshi J, Vrieling K. The enemy release and EICA hypothesis revisited: Incorporating the fundamental difference between specialist and generalist herbivores. Ecol Lett. 2005;8:704–714. [Google Scholar]
- 8.Rogers WE, Siemann E. Herbivory tolerance and compensatory differences in native and invasive ecotypes of Chinese tallow tree (Sapium sebiferum) Plant Ecol. 2005;181:57–68. [Google Scholar]
- 9.Zou J-W, Rogers WE, Siemann E. Differences in morphological and physiological traits between native and invasive populations of Sapium sebiferum. Funct Ecol. 2007;21:721–730. [Google Scholar]
- 10.Maron J, Vilà M, Arnason J. Loss of enemy resistance among introduced populations of St. John's wort (Hypericum perforatum) Ecology. 2004;85:3243–3253. [Google Scholar]
- 11.Bossdorf O, et al. Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia. 2005;144:1–11. doi: 10.1007/s00442-005-0070-z. [DOI] [PubMed] [Google Scholar]
- 12.Ridenour WM, Vivanco JM, Feng Y-L, Horiuchi J, Callaway RM. No evidence for trade-offs: Centaurea plants from America are better competitors and defenders. Ecol Monogr. 2008;78:369–386. [Google Scholar]
- 13.Onoda Y, Hikosaka K, Hirose T. Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Funct Ecol. 2004;18:419–425. [Google Scholar]
- 14.Takashima T, Hikosaka K, Hirose T. Photosynthesis or persistence: Nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Environ. 2004;27:1047–1054. [Google Scholar]
- 15.Feng Y-L, Auge H, Ebeling SK. Invasive Buddleja davidii allocates more nitrogen to its photosynthetic machinery than five native woody species. Oecologia. 2007;153:501–510. doi: 10.1007/s00442-007-0759-2. [DOI] [PubMed] [Google Scholar]
- 16.Feng Y-L. Photosynthesis, nitrogen allocation and specific leaf area in invasive Eupatorium adenophorum and native Eupatorium japonicum grown at different irradiances. Physiol Plant. 2008;133:318–326. doi: 10.1111/j.1399-3054.2008.01072.x. [DOI] [PubMed] [Google Scholar]
- 17.Feng Y-L, Fu G-L. Nitrogen allocation, partitioning and use efficiency in three invasive plant species in comparison to their native congeners. Biol Inv. 2008;10:891–902. [Google Scholar]
- 18.Feng Y-L, Fu G-L, Zheng Y-L. Specific leaf area relates to the differences in leaf construction cost, photosynthesis, nitrogen allocation and use efficiencies between invasive and noninvasive alien congeners. Planta. 2008;228:383–390. doi: 10.1007/s00425-008-0732-2. [DOI] [PubMed] [Google Scholar]
- 19.Cronk QCB, Fuller JL. Plant Invaders: The Threat to Natural Ecosystems. London, UK: Chapman and Hall; 1995. [Google Scholar]
- 20.Wang R, Wang Y-Z. Invasion dynamics and potential spread of the invasive alien plant species Ageratina adenophora (Asteraceae) in China. Divers Distrib. 2006;12:397–408. [Google Scholar]
- 21.Goodger JQD, Gleadow RM, Woodrow IE. Growth cost and ontogenetic expression patterns of defense in cyanogenic Eucalyptus spp. Trees-Struct Funct. 2006;20:757–765. [Google Scholar]
- 22.Liu Y-P. The Study on Acaricide Activation of Eupatorium Adenophorum. China: A masters degree dissertation, Sichuan University; 2005. [Google Scholar]
- 23.Lamport DTA. The protein component of primary cell walls. Adv Bot Res. 1965;2:151–218. [Google Scholar]
- 24.Wright IJ, Cannon K. Relationships between leaf lifespan and structural defenses in a low-nutrient, sclerophyll flora. Funct Ecol. 2001;15:351–359. [Google Scholar]
- 25.Coley PD. Herbivory and defensive characteristics of tree species in a low land tropical forest. Ecol Monogr. 1983;53:209–233. [Google Scholar]
- 26.Showalter AM. Structure and function of plant cell wall proteins. Plant Cell. 1993;5:9–23. doi: 10.1105/tpc.5.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Müller-Schärer H, Schaffner U, Steinger T. Evolution in invasive plants: Implications for biological control. Trends Ecol Evol. 2004;19:417–422. doi: 10.1016/j.tree.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Strauss SY, Rudgers JA, Lau JA, Irwin RE. Direct and ecological costs of resistance to herbivory. Trends Ecol Evol. 2002;17:278–285. [Google Scholar]
- 29.Poorter H, de Jong RA. A comparison of specific leaf area, chemical composition and leaf construction costs of field plants from 15 habitats differing in productivity. New Phytol. 1999;143:163–176. [Google Scholar]
- 30.Lichtenthaler HK, Wellburn AR. Determination of total carotenoids and chlorophyll a and b of leaf extracts in different solvents. Biochem Soc T. 1983;603:591–592. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.